TGF-β1 Inhibits Osteoclast Differentiation and Abnormal Angiogenesis in Intervertebral Disc Degeneration: Evidence from RNA Sequencing and Animal Studies
Abstract
Objective
Mechanisms involved in developing intervertebral disc degeneration (IDD) are poorly understood, thus making developing effective therapies difficult. This study aimed to suggest a possible molecular mechanism, based on transcriptome sequencing-identified transforming growth factor (TGF-β), underlying the effects on bone homeostasis in IDD.
Methods
A mouse model for IDD was established. Transcriptome sequencing of nucleus pulposus tissue from mice (n = 3) identified differentially expressed mRNAs and key genes impacting bone homeostasis. A protein–protein interaction network pinpointed core genes. GO and KEGG analysis revealed gene functions. Expression levels of TGF-β1, tartrate-resistant acid phosphatase (TRAP), and cathepsin K (CTSK) were measured. Micro-CT evaluated vertebral structures and vascular imaging. Western Blot measured expression levels of Vegf, Opn, MMP3, and MMP13. Safranin O-Fast Green and TRAP staining were performed on intervertebral discs and endplates.
Results
Transcriptomic analysis found 1790 differentially expressed mRNAs in IDD mice. Twenty-eight genes related to bone homeostasis in IDD were identified. TGF-β1 was confirmed as the core gene. GO and KEGG showed TGF-β1 regulates osteoclast markers like CTSK and TRAP through pathways including NF-κB and MAPK. Experimental validation revealed lower TGF-β1 expression in IDD mice than controls, and increased TRAP and CTSK expression. Micro-CT showed decreased bone mass and intervertebral disc space in IDD mice. Vascular imaging showed increased vascular volume in IDD cartilaginous endplates. Western blot displayed increased VEGF and OPN levels, but decreased MMP3 and MMP13 in IDD mice. Safranin O-fast green staining revealed severe IDD degeneration. However, TGF-β1 injection improved bone parameters in IDD mice. In vitro experiments confirmed TGF-β1 inhibits bone marrow macrophages differentiation into osteoclasts.
Conclusion
From our data, we conclude that TGF-β1 repressed osteoclast differentiation and aberrant bone-associated angiogenesis in cartilage endplates (EPs) to alleviate IDD, which may be instrumental for the therapeutic targeting of IDD.
Introduction
The intervertebral disc degeneration (IDD) is mainly characterized by apoptosis of intervertebral disc (IVD) cells, synthesis, and degradation of extracellular matrix components (proteoglycans, type II collagen), loss of water in the nucleus pulposus, reduction of IVD height, redistribution of IVD stresses.1-3 It eventually leads to degeneration of the IVD tissue, rupture of the annulus fibrosus, compression of the internal nucleus pulposus tissue, and protrusion from the rupture of the annulus fibrosus, resulting in a series of clinical symptoms due to stimulation or compression of the nerve roots or nerve of cauda equina at the corresponding level.4, 5 The synthesis and structural maintenance of the IVD matrix are inseparable from cell metabolism. The nucleus pulposus cells are the main cells of the IVD, and its synthesis of proteoglycans, collagen, and other matrix components ensures the structural, biological, and mechanical properties of the nucleus pulposus. Therefore, its apoptosis and reduction are one of the main causes of progressive IDD.6 The cell viability and metabolism of IVD are affected by many factors, including inflammation, nutrition, mechanics, extracellular matrix, etc., inflammatory stimulation, nutritional deficiencies, and abnormal stresses will directly contribute to the activation and amplification of apoptotic signals inducing apoptosis in the nucleus pulposus cells.7 However, the regulation of bone homeostasis plays an important role in these mechanisms. Therefore, understanding the role of bone homeostasis regulation in IDD is important for investigating the mechanisms of disc degeneration.
Several growth factors, such as transforming growth factor β (TGF-β), insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), and fibroblast growth factor (FGF), have the potential to inhibit disc degeneration.8 TGF-β is involved in a variety of cellular processes, including cell proliferation, differentiation, angiogenesis, apoptosis, motility, and death,9 with TGF-β1 (encoded by TGF-β1) being the most abundant factor subtype in the TGF-β superfamily.9, 10 As a powerful immunosuppressive factor, TGF-β1 can inhibit inflammatory reactions of various cell types.11 Recent studies have shown that TGF-β1 has protective effects against disc degeneration, and TGF-β1 promotes the proliferation of nucleus pulposus cells, stimulates extracellular matrix synthesis, and inhibits the expression of MMP and ADAMTS.12-16 Furthermore, TGF-β1 has been shown to have an anti-inflammatory effect on nucleus pulposus cells.17 In addition, TGF-β1 plays a key role in the bone environment, affecting both osteoblast and osteoclast functions, thus helping to maintain the dynamic balance between bone formation and bone resorption, which may play an important role in the development of disc degeneration.10 TGF-β1 plays a critical role in various biological processes, such as cell proliferation, differentiation, and collagen synthesis, making it the subject of extensive research in IDD.18, 19 Previous studies have shown that TGF-β1 could promote cell proliferation and extracellular matrix synthesis in intervertebral disc cells, particularly collagen and proteoglycans, which may be beneficial for restoring intervertebral disc structure and function.20-22 However, abnormal vascular invasion may occur during the degeneration process of the intervertebral disc, and the role of TGF-β1 in this process remains unclear. Furthermore, there is an association between TGF-β1 and the activation, differentiation, and function of osteoclasts, but further exploration is needed to understand how this specifically impacts intervertebral disc degeneration. Current challenges include understanding the exact mechanisms of TGF-β1 in intervertebral disc degeneration, precise utilization of TGF-β1 for treatment, its interaction with other factors, and potential side effects and safety issues. Despite some understanding of the role of TGF-β1, there are still many unresolved issues in the treatment of intervertebral disc degeneration.
In this study, we aim to investigate the key gene TGF-β1, identified through transcriptome sequencing, which plays a crucial role in intervertebral disc degeneration. We will explore the specific molecular mechanisms by which TGF-β1 regulates bone homeostasis and alleviates the occurrence of intervertebral disc degeneration, providing a theoretical foundation for identifying potential diagnostic and therapeutic targets. Our research objectives include: (i) screening key genes involved in bone homeostasis regulation during intervertebral disc degeneration through transcriptome sequencing of intervertebral disc tissues in an experimental mouse model of IDD; and (ii) investigating the molecular regulatory mechanisms by which the key gene TGF-β1 mitigates intervertebral disc degeneration through bioinformatics analysis and animal experiments, aiming to elucidate the pathogenesis of intervertebral disc degeneration and provide a theoretical basis for identifying suitable diagnostic and therapeutic targets.
Materials and Methods
Establishment of Mouse Model of IDD
Twelve-week-old SPF-healthy C57BL/6J female mice were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). The mice were raised under standard laboratory conditions, and the experiments were performed after approval by the Animal Ethics Committee of the Second Hospital of Lanzhou University (No. D2023-228). A total of 30 mice were randomly divided into two groups: the control group (n = 15) and the IDD group (n = 15). A mouse model of IDD was constructed by inducing mice to stand bipedally using their water-fearing habits. After 1 week of acclimatization, each mouse was placed individually in a 500 mL transparent beaker. Except for the control group, a small amount of normal-temperature water was added to the beakers in the treated group to induce the mice to stand bipedally. The amount of water was appropriate not to pass the ankle joint of the mice. The mice in the control group had the same treatment (food, space, light, etc.), except that no water was added to the beaker.23 The mice were allowed to stand for 8 h per day, with a 1-h rest period during which they were free to forage for food.24-26 The experiment lasted 10 weeks, and each group of mice was anesthetized with 3% pentobarbital sodium (P3761, Sigma) intraperitoneally and then euthanized, and samples were obtained for subsequent studies. Fig. S1 presents the flowchart of the in vivo animal experiment.
IVD Tissue Collection for Transcriptome Sequencing
Three mice in the control and IDD groups were randomly selected, and the L4/L5 IVD tissues were broken in liquid nitrogen. After total RNA extraction, rRNA was removed from the total RNA using the MGIEasy rRNA removal kit (1000005953, MGI, China). Next, the RNA was broken into a fragment of about 300 bp in length using ion interruption. Next, the first strand of cDNA was synthesized with a 6-base random primer and reverse transcriptase using the RNA as a template, and the second strand of cDNA was synthesized using the first strand of cDNA as a template. After the library was constructed, PCR amplification was used to enrich the library fragments, and a library of 450 bp in size was selected. The library was then quality checked by an Agilent 2100 Bioanalyzer, and the total and effective library concentrations were detected. Then according to the effective concentration of the library and the amount of data required by the library (the library contains different index sequences, each sample plus a different index, and finally, the raw data of each sample is differentiated according to the index) are mixed proportionally. Finally, the mixed libraries are uniformly diluted to 2 nM and denatured by the base to form single-stranded libraries.
After RNA extraction, purification, and library construction, these libraries were sequenced using Next-Generation Sequencing (NGS) technology based on the Illumina HiSeq sequencing platform for Paired-end (PE) sequencing. First, the software cutadapt (v1.18) cut the sequencing adapter contained in the Raw Reads obtained from Illumina sequencing. Then the internal software tools (v.0.1.6) were used to filter out the low-quality sequences to obtain high-quality data (Clean Reads). All subsequent analysis is based on Clean Reads. At the same time, the Q30 quality score was also calculated for the Clean Reads. HiSAT2 (v.2.1.0) aligned filtered Clean Reads to the mouse genome (Mus musculus GRCm38.90). The r package “Deseq” was used to analyze the differences in data, and the heat map and volcano plot were drawn. The screening criteria for differentially expressed genes (DEGs) were |logFC| > 1 and FDR <0.05. Finally, mRNAs differentially expressed in IDD were obtained.27
Screening for Core Genes of IDD in the Mouse Model
Differentially expressed mRNAs from mice with IDD were obtained based on the transcriptome sequencing difference analysis. the GeneCard database was used, and with “Intervertebral disc degeneration” as the search condition, the top 1000 genes were selected as the genes related to IDD by “Relevance score.” “Bone homeostasis” was used as the keyword, and “Relevance score” >10 as the search condition to screen the genes related to bone homeostasis. The intersection of the above three genes was identified as a key gene in regulating bone homeostasis in IDD by using the academic online analysis tool named Xiantao database.
These key genes were further imported into the STRING database for protein–protein interaction (PPI) analysis: the minimum required interaction score was set to 0.4 and, discrete proteins were removed, the PPI network and node files were exported, statistical node graph based on PPI node data was drawn using R software, then the connected node statistics and relevant literature were combined, and appropriate genes were selected as core genes for subsequent analysis.28
Functional Enrichment Analysis of Core Genes
GO and KEGG enrichment analyses were performed on key genes in the PPI network of IDD mice using the R language “ClusterProfiler” package. The GO enrichment analysis included three levels: biological process (BP), cellular component (CC), and molecular function (MF), with p < 0.05 as the screening condition. KEGG enrichment analysis, with p < 0.05 as the significant enrichment screening condition, was conducted to analyze the cell function and signal pathway mainly affected by key DEGs in mice.28 In addition, the KEGG database was used to retrieve the relevant pathways of core genes in regulating bone homeostasis.
Isolation, Culture and Differentiation Induction of Mouse Bone Marrow-derived Macrophages (BMMs)
Five normal mice in the control group were anesthetized and euthanized, the femur and tibia were separated, the bone marrow was washed with pre-cooled PBS (79378, Sigma) containing 2% FBS and placed in serum-free ɑ-MEM medium (M4655, Sigma) for later use. The cell suspension was filtered through a 100 mesh sieve, centrifuged at 250 g for 5 min, and the supernatant was discarded; 10 times the cell volume of red blood cell lysis buffer (R7757, Sigma) was added and centrifuged at 250 g for 5 min, and the supernatant was discarded. Bone marrow cells were suspended in ɑ-MEM medium containing 10% FBS and incubated at 37°C in the incubator of 5% CO2 for 24 h. The supernatant was collected; the supernatant was discarded after centrifugation at 250 g for 5 min and then incubated in ɑ-MEM medium containing 25 ng/mL M-CSF (SRP3221, Sigma) and 10% FBS for 3 days. The adherent cells were used as osteoblastic progenitor cells. Finally, ɑ-MEM medium containing 25 ng/mL M-CSF, 50 ng/mL RANKL (R0525, Sigma), and 10% FBS was used to incubate for 6 days to induce osteoblast differentiation. During the induction of differentiation, 5 ng/mL TGF-β1 was added or not as TGF-β1+ group or TGF-β1− group to observe the effect of TGF-β1 on osteoclast differentiation.29
Drug Treatment and Grouping of IDD Mice
IDD mice were divided into the following three groups: IDD group (n = 15), IDD + TGF-β1 group (n = 15), and TGF-β1 + Vegf group (n = 15). During IDD modeling, the IDD + TGF-β1 group mice were injected with 0.5 μg of TGF-β1 (PHG9214, Invitrogen, Thermo Fisher) daily in the IVD, while the TGF-β1 + Vegf mice were additionally injected with 100 ng/g of Vegf (752-VC-025, R&D Systems) every 2 days, and the experiment lasted 10 weeks for subsequent experiments.26
Micro-computed Tomography (micro-CT)
Ten mice from each group were subjected to micro-CT analysis for IDD mouse model assessment and vertebral endplate (EP) elated detection. After the mice were anesthetized and euthanized, the L4/5 spinal segments were taken and immediately immersed in 4% paraformaldehyde solution (V900894, Sigma), fixed at 4°C for 72 h and washed three times with PBS for 15 min each time. Five coronal 3D images were used for morphological analysis of the EPs and IVDs. The spine samples were scanned with high-resolution micro-CT (Skyscan 1272, Bruker, Belgium). Three-dimensional images of L4/5 coronal planes were used for morphological analysis of EP and IVD. The CTAn (v1. 9) software-defined regions of interest for the vertebral body, EPs, and discs. The disc was defined as the invisible area between the L4/5 vertebral bodies, and the bony EPs were defined as the visible structures covering the surface of the vertebral body. The disc height index (DHI) of the L4/5 segment was used to assess the degree of disc degeneration to verify the success of IDD modeling in mice; the 3D structural parameters of the vertebral body included bone mineral density (BMDtv, bone volume per unit total volume), bone volume fraction (BV/TV, bone volume/total volume), trabecular number (Tb. N, mean of the number of intersections between bone and non-bone tissue per mm in the region of interest) and trabecular connection density (Conn.D, number of connections between trabecular meshwork per unit volume); parameters of the EP included bone volume fraction (BV/TV) and total pore volume (Po. V[tot]).
Another five mice from each group were imaged using micro-CT on the bone vessels. The specific methods are as follows: the mice were anesthetized and euthanized, the thoracic cavity was opened, a needle was inserted into the left ventricle as an inflow channel, and an outflow channel was set up in the right auricle. Subsequently, 5 mL of 100 U/mL heparin saline (H3149, Sigma), 3 mL of 10% formaldehyde, and 3 mL of silicone rubber compound contrast agent (Microfil MV-122, Flow-Tech) were injected. The specimens were stored overnight at 4°C, then fixed and decalcified for ~21 days. The micro CT setting parameters were set to a voltage of 60 kV and a current of 165 μA to scan images. Similarly, NRecon (v1. 6) software and CTAn (v1. 9) software were used for the reconstruction and analysis of the scanned images, and CTvox (v3. 0) software was used for 3D image reconstruction to calculate vessel volume and vessel surface area.30-34
RT-qPCR for Gene Expression
Total RNA was extracted from tissues and cells using TRIzol (15596026, ThermoFisher). First, the RNA was reverse transcribed into cDNA according to the instructions of PrimeScript RT reagent Kit (RR047A, Takara, Japan). Then, the synthesized cDNA was subjected to RT-qPCR using a Fast SYBR Green PCR kit (11736059, ThermoFisher). Gapdh was used as the internal reference gene, and the 2-ΔΔCt method was used to detect the expression of the target gene.29, 35 The primer sequences are shown in Table S1.
Western Blot for Protein Expression
Tissues were digested in RIPA lysis buffer (P0013B, Beyotime Biotechnology, Shanghai, China) to isolate the total protein. Protein concentrations were quantified using a BCA assay kit (A53226, Thermo Fisher Scientific, Rockford, IL). Proteins were separated by polyacrylamide gel electrophoresis and then transferred to PVDF membranes (IPVH85R, Millipore, Darmstadt, Germany) through the wet conversion method. The membranes were closed at room temperature for 1 h in 5% BS and incubated overnight at 4°C with primary antibodies (Table S2). The membranes were incubated with HRP-conjugated secondary antibody for 2 h. The blots were visualized by enhanced chemiluminescence. ImageJ 1.48 u software (V1.48, National Institutes of Health) was used for protein quantification with the internal reference Gapdh.30, 36, 37
Safranin O-fast Green Staining
The above L4/5 spinal segments fixed in 4% paraformaldehyde for 72 h were decalcified by immersion in 10% EDTA (E9884, Sigma) for 14 d and rinsed in running water for 4 h. After gradient dehydration and paraffin embedding, the samples were sectioned coronally at 4 μm and stained with safranin O-fast green. According to the reagent manufacturer's instructions, the safranin O-fast green staining (G1053, Xavier Company, Wuhan, China) was completed. The operations are as follows: paraffin slices were dewaxed in xylene (534056, Sigma) for 10 min, replaced by fresh xylene for 10 min dewaxing, and placed in anhydrous ethanol (PHR1070, Sigma) for 5 min, fresh anhydrous ethanol for 5 min, 90% ethanol for 5 min, 75% ethanol for 5 min, and distilled water for 3 min. Next, the slices were stained in fast green for about 1–5 min, rinsed in running tap water until the excess stain was colorless, treated with 1% hydrochloric acid for about 10–15 s, and washed slightly in tap water. The slices were then stained with safranin for about 5–10 s, dehydrated rapidly in four tanks of anhydrous ethanol for 3–5 s each time, and examined microscopically after the fourth dehydration until they were red with the colorless background. Next, the slices were transparent in xylene for 5 min, then in fresh xylene for 5 min, and sealed with neutral balsam.30, 38 Microscopic examination (AE-2000, MOTIC) was performed for image acquisition and analysis.
Tartrate Resistant Acid Phosphatase (TRAP) Staining
To identify osteoblasts, paraffin slices or cells were stained with TRAP. First, cell round coverslips were fixed using 4% paraformaldehyde and washed in PBS. Then according to the reagent manufacturer's instructions, an acid phosphatase kit (MAK446, Sigma) was used for TRAP activity staining: 5 mL of nitrite solution and 0.5 mL of hexaazo-fuchsin solution were mixed and left to stand for 2 min before using it. The staining incubation solution was prepared by adding 1 mL of pre-configured nitrite solution and hexaazo-fuchsin solution, 1 mL of tartrate solution, 0.5 mg of naphthol AS-BI phosphate, 2 mL of acetate buffer, and 45 mL of deionized water pre-warmed at 37°C to a 100 mL beaker. The beaker containing the staining incubation solution was water bath heated to 37°C. Round coverslips of cells were immersed in it, protected from light, and incubated at 37°C for 1 h. Then they were rinsed with deionized water at 37°C and re-stained with hematoxylin for 90 s. After rinsing with an alkaline solution for 3 min and drying, they were examined under a microscope. After paraffin sections were dewaxed and hydrated as described previously, the sections were immersed in TRAP staining solution (BC2135, Solebro, Beijing, China) according to the instructions of the reagent manufacturer and incubated at 37°C for 50 min; after washing in tap water, the specimens were stained with methyl green and examined microscopically; TRAP-positive (pink-purple) multinucleated cells were osteoblasts.29
Statistical Methods
All data were processed using SPSS 24.0 statistical software (Chicago, IL, USA), and all data were tested for normal distribution and homogeneity of variance. Measurement data conforming to normal distribution were expressed as mean ± standard deviation. The comparison between the two groups was conducted with an independent sample t-test. The comparison between multiple groups was conducted with one-way analysis of variance. In addition, Tukey's post-hoc test was conducted. A value of p < 0.05 indicated that the difference was statistically significant.
Results
Whole Transcriptome Sequencing Screens the Core Gene of IDD TGF-β1
We constructed an IDD mouse model to obtain the key genes responsible for the changes in bone homeostasis in IDD. The result showed that IDD mice had significantly lower DHI than mice in the control group and significantly reduced intervertebral space height (Fig. S2). Subsequent RNA-seq differential analysis of IVD tissues showed 1790 differentially expressed mRNAs (Fig. 1A,B), 1118 mRNAs were significantly up-regulated, and 672 were significantly down-regulated in the IDD group. Further, the GeneCard database was used to sort the top 1000 genes by “Relevance score” using “Intervertebral disc degeneration” as the search criteria. The 708 genes related to bone homeostasis were obtained by using “Bone homeostasis” as the keyword and “Relevance score” >10. The above three were intersected to obtain 28 genes associated with changes in bone homeostasis in IDD (Fig. 1C).
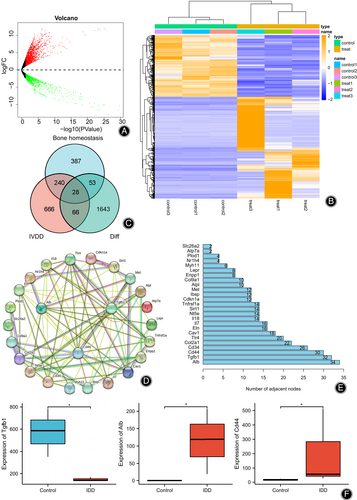
To further screen the core genes in IDD, the above 28 related genes were imported into the String database, and the PPI network (Fig. 1D) and protein connection node map (Fig. 1E) were drawn. The results showed that the three genes with the highest number of nodes were Alb, TGF-β1, and Cd44, and their expression levels are shown in Fig. 1F. The expression levels of TGF-β1 in IDD mice were significantly reduced, while the expression levels of Alb and Cd44 were significantly increased. Among them, TGF-β1 can be involved in the regulation of bone metabolism and is associated with osteoclast formation,29, 32 so we chose TGF-β1 for the subsequent study.
TGF-β1 May Regulate Bone Metabolic Homeostasis by Affecting Osteoblast Differentiation or Extracellular Matrix Production
To further understand the role played by TGF-β1 in IDD, we conducted GO functional analysis (Fig. 2A,B) and KEGG pathway analysis (Fig. 2C) on 28 screened DEGs related to bone homeostasis in IDD to explore their main enriched functions and pathways in IDD. The results of GO functional analysis showed that the PPI network with TGF-β1 as the involved core items, such as extracellular matrix organization, tissue remolding, and cytotoxic mediated signaling pathway in the biological process (BP). In the cell composition (CC), the PPI network was mainly enriched in the basal plasma membrane, basal part of the cell, and collagen-containing extracellular matrix; however, at the molecular function (MF) level, there was no significant enrichment pathway. The results of the KEGG pathway analysis showed that 28 DEGs related to IDD and bone homeostasis were involved in items of the PI3K-Akt signaling pathway, Proteoglycans in cancer and Malaria (Fig. 2C). These results suggested that TGF-β1 might be involved in IDD development through pathways such as regulating the extracellular matrix or cytokine interactions. To further understand the role of TGF-β1 in the regulation of bone homeostasis in IDD, we searched the KEGG database to obtain the signaling pathways involved in osteoclast differentiation (Fig. 2D), and the results showed that TGF-β1 could regulate the expression of osteoclast marker genes such as cathepsin K (CTSK) and tartrate-resistant acid phosphatase (TRAP) through signaling pathways such as NF-κB and MAPK.
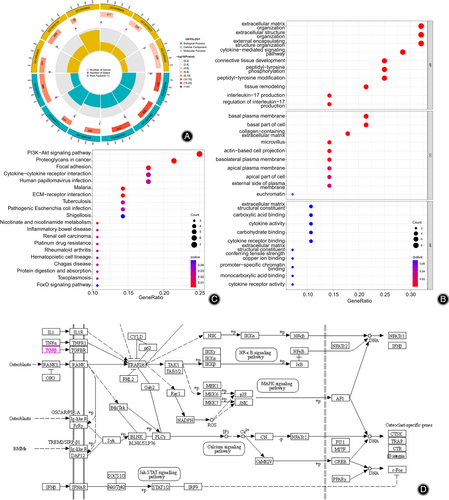
Based on the above bioinformatics analysis and literature search results, we speculate that TGF-β1 may mitigate IDD by affecting osteoclast differentiation or extracellular matrix, thereby regulating bone metabolic homeostasis and influencing IVD mechanotransduction or nutrient metabolism.
TGF-β1 Inhibits Osteoclast Differentiation and Reduces Bone Resorption in the Vertebral EPs of IDD Mice
To verify that TGF-β1 could affect the vertebral body and bony EP structure by inhibiting osteoclast differentiation in IDD mice, we examined the expression of TGF-β1, as well as osteoclast marker genes TRAP and CTSK in IVD tissues of IDD and control mice by RT-qPCR (Fig. 3A), and the results showed that the expression of TGF-β1 in IDD mice was significantly lower, while the expression of TRAP and CTSK was significantly higher than that of control mice. Furthermore, TRAP staining of vertebral EPs and quantitative analysis showed that many activated osteoclasts could be observed in IDD mice. In contrast, the number of osteoclasts in control mice was low (Fig. 3B), representing increased bone resorption.
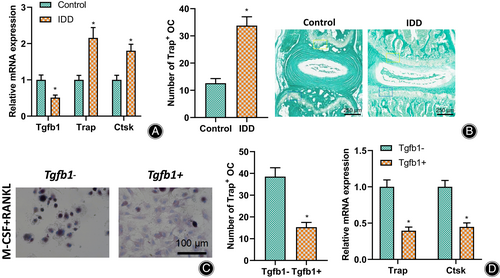
Since osteoclasts originate from bone marrow mononuclear macrophages, we isolated macrophages from normal mouse bone marrow to verify the effect of TGF-β1 on osteoclast differentiation. We added RANKL and M-CSF to induce their differentiation into osteoclast precursor cells. TRAP staining (Fig. 3C) and RT-qPCR (Fig. 3D) were performed, respectively, with or without the addition of TGF-β1. TRAP staining showed that the number and volume of osteoclasts were significantly reduced in macrophages treated with TGF-β1; the expression of osteoclast marker genes TRAP and CTSK were significantly reduced.
TGF-β1 Reduces Bone Resorption and Maintains Vertebral Body and Bony EP Bone Mass, Thereby Protecting Disc Mechanics Transmission
To further verify the effect of TGF-β1 low expression disrupting bone metabolic homeostasis on the vertebral body and EP structure in mice, micro-CT analysis was used to examine the changes in the vertebral body and EP structure as the changes in IVD volume in IDD mice and control mice. The results showed that compared to the control mice, the L5 vertebral trabeculae of IDD mice were more sparse (Fig. 4A). In addition, the bone mineral density (BMDtv), bone volume fraction (BV/TV), trabecular number (Tb. N), and trabecular connection density (Conn. D) (Fig. 4B) of the vertebrae were significantly reduced; In addition, a large number of cavities were observed in the L4/L5 caudal EPs of IDD mice (Fig. 4C), and the bone volume fraction BV/TV was significantly reduced. In contrast, the total pore volume Po.V (tot) (Fig. 4D) was significantly increased. It indicated a reduction in IDD mice's vertebral body and bony EP bone mass. As vertebrae and EPs were the objects of biological stress of the nucleus pulposus, their bone mass and structure inevitably affected the mechanical transmission of IVDs. It was shown that the IVD space and volume of IDD mice were significantly smaller than those of control mice (Fig. 4E).
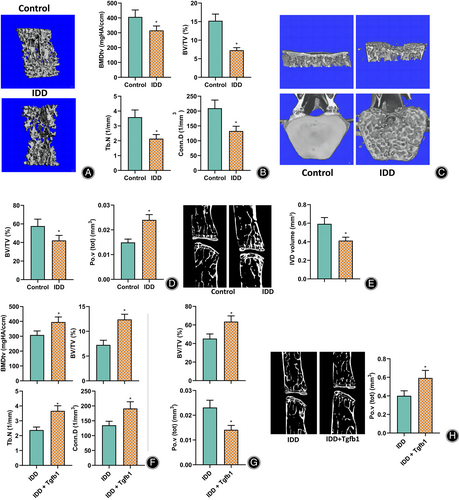
The IVDs of IDD mice were further injected with TGF-β1, and the vertebral EP-related indexes were examined by micro-CT analysis. The results showed that compared to IDD mice, the BMDtv, BV/TV, Tb.N, and Conn.D (Fig. 4F) of the vertebral body were significantly higher after TGF-β1 injection. Furthermore, the EP's bone volume fraction BV/TV was significantly higher, while the total pore volume Po.V (tot) (Fig. 4G) was significantly lower. In addition, the disc space and disc volume increased (Fig. 4H).
These results suggested that TGF-β1 could inhibit osteoclast differentiation and bone resorption, maintain bone mass in the vertebral body and EP of mice, and thus protect IVD mechanotransmission to alleviate IDD development.
TGF-β1 Inhibits Aberrant Bone-associated Angiogenesis in Cartilage EPs and Thereby Inhibits Cartilage EP Ossification
The IVD is an avascular organ whose nutrient supply and metabolism mainly depend on the cartilaginous EP-nucleus pulposus and peripheral annulus fibrosus pathways.39 It has been shown that cartilage EP calcification and bone remodeling are present in IDD and are associated with aberrant angiogenesis therein,30 while TGF-β1 can regulate H-type vessels associated with bone formation.32 Therefore, TGF-β1 may disrupt the nutrient supply and metabolism of the intervertebral disc by promoting its ossification by inhibiting the formation of blood vessels related to abnormal bone formation in the cartilage endplate. We performed capillary angiography using micro-CT on the cartilage EP vessels (Fig. 5A). The results showed that vessel volume and surface area were significantly higher in the cartilage EPs of IDD mice than in control mice.
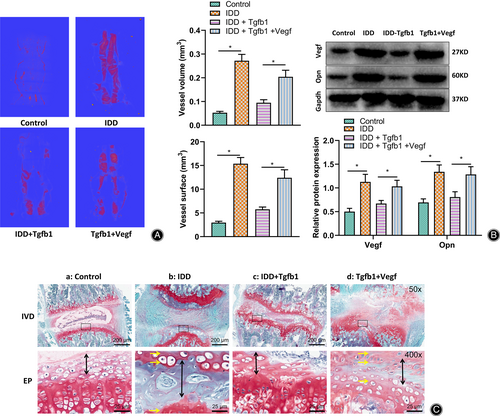
Furthermore, a western blot was used to detect the expression of vascular endothelial growth factor (VEGF) and osteoblast marker protein Opn in the IVDs (Fig. 5B). Both showed that they were significantly higher in IDD mice. In addition, the safranin O-fast green staining results (Fig. 5C) showed significant degeneration and structural disorders of the IVDs in IDD mice.
Further, in IDD mice injected with TGF-β1 or TGF-β1 + Vegf, the results showed that IDD mice injected with TGF-β1 had significantly lower expression of VEGF and Opn and significantly less cartilage EP osteogenesis compared to IDD mice. In contrast, the expression of VEGF and Opn and cartilage EP osteogenesis were not statistically different in IDD mice injected with TGF-β1 + VEGF simultaneously (Fig. 5B,C).
The above results suggested that low expression of TGF-β1 in IDD mice could promote bone-associated angiogenesis, increase bone formation and lead to ossification of cartilage EPs.
TGF-β1 Inhibits Cartilage EP Ossification and Maintains the Number of Nucleus Pulposus Cells
To verify the effect of cartilage EP ossification caused by TGF-β1 low expression on disc matrix metabolism and nucleus pulposus cells, we performed safranin O-fast green staining for disc tissues to observe histopathological changes (Fig. 6A) and Western blot to detect the expression of extracellular matrix (ECM)-associated proteins MMP3 and MMP13 (Fig. 6B). The results showed a significant decrease in the amount of nucleus pulposus cells and proteoglycans in the IVD tissue of IDD mice and a significant decrease in the expression of MMP3 and MMP13.
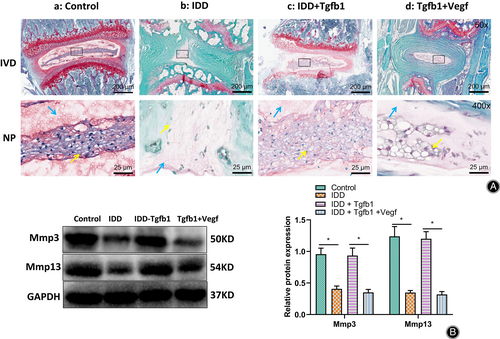
IDD mice were further treated with TGF-β1 or TGF-β1 + Vegf, and the contents of nucleus pulposus cells, proteoglycans, and the expression of MMP3 and MMP13 were detected with saffron O-fast green staining and Western blot (Fig. 6A,B). The results showed that compared with IDD mice, the contents of nucleus pulposus cells and proteoglycans and the expression of MMP3 and MMP13 increased significantly in TGF-β1-treated mice. In contrast, the number of ECM and nucleus pulposus cells were not statistically different in IDD mice with TGF-β1 + Vegf injection compared to IDD mice.
The above results suggested that TGF-β1 could inhibit cartilage EP ossification, protect the nutrient supply and matrix metabolism of the IVD and maintain the number of nucleus pulposus cells.
Discussion
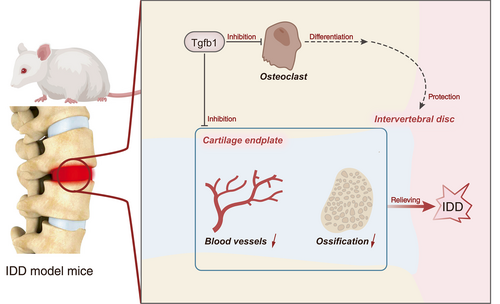
In this study, we elucidated the crucial molecular mechanisms by which TGF-β1 alleviates IDD through its involvement in bone homeostasis regulation. Based on transcriptome sequencing of intervertebral disc tissues, we identified TGF-β1 as a key gene involved in bone homeostasis regulation during the development of IDD in mice. Through bioinformatics analysis and mouse experiments, we validated the role of TGF-β1 in bone homeostasis regulation, including the following findings: downregulation of TGF-β1 promotes osteoclast differentiation, leading to a decrease in bone mass in vertebral bodies and endplates, consequently affecting the mechanical transmission of intervertebral discs. Furthermore, downregulation of TGF-β1 promotes abnormal generation of bone-related vessels in the cartilaginous endplate, inducing endplate ossification, and influencing intervertebral disc matrix metabolism, resulting in a decrease in nucleus pulposus cells and contributing to the occurrence of IDD.
The Role of TGF-β1 in IDD
In this study, we constructed an intervertebral disc degeneration (IDD) mouse model and conducted a differential analysis of transcriptomes in intervertebral disc tissues. By combining this analysis with the GeneCard database, we identified multiple genes related to bone homeostasis regulation in IDD mice.40 Subsequently, based on relevance scores, we selected 28 genes with higher correlations to bone homeostatic changes in intervertebral disc degeneration from a pool of IDD and bone homeostasis-related targets. Using protein–protein interaction analysis, we constructed a PPI network for these 28 genes and found that Alb, TGF-β1, and Cd44 were the three genes with the highest number of nodes.
Recent studies have revealed various aspects of TGF-β1. It is involved in the distribution of intervertebral disc tissues in individuals of different ages, particularly in the expression of TGF-β1 in the nucleus pulposus cells associated with fibroblast-like cells.41 TGF-β1 also participates in the regulation of bone metabolism and is associated with osteoclast formation.29, 32 In skeletal tissue, TGF-β1 binds to cell surface receptors and regulates osteoblast and osteoclast function by activating receptor-regulated Smads (R-Smads).42 Additionally, TGF-β1 acts through non-Smad effectors, including Rho GTPases, focal adhesion kinase (FAK), and mitogen-activated protein kinases (MAPKs).43 The research conducted by Yong Tang et al. indicated that macrophages promote bone formation by secreting TGF-β1, providing a potential therapeutic method for radiation-induced osteoporosis. Hongying Zhao et al. discovered that miR-155 regulates osteoclast differentiation through the TGFβ1/Smad4 signaling pathway. Considering these studies, we confirm that TGF-β1 is a key gene with potential importance in intervertebral disc degeneration.29, 32
To further understand the role of TGF-β1 in intervertebral disc degeneration, we performed GO functional analysis and KEGG pathway analysis on the 28 IDD-related differentially expressed genes. The results indicated that TGF-β1 may regulate bone metabolism balance, mechanical transmission of intervertebral discs, and nutrient metabolism by influencing osteoclast differentiation or the extracellular matrix, thus alleviating the occurrence of IDD.
Mechanisms of TGF-β1 in Bone and Cartilage Metabolism and Bone Homeostasis
Transforming growth factor β (TGF-β) participates in various cellular processes such as proliferation, differentiation, angiogenesis, apoptosis, locomotion, and death. Among the TGF-β superfamily, TGF-β1 is the most abundant subtype factor.9 Our GO functional enrichment analysis and KEGG pathway analysis of the aforementioned gene set further showed that TGF-β1 might participate in the occurrence of IDD through regulation of the extracellular matrix or cytokine interaction. Additionally, we discovered through the KEGG database that TGF-β1 can regulate the expression of osteoclast marker genes, such as CTSK and TRAP, through signal pathways including NF-κB and MAPK. Similar to these findings, Guo et al. reported that TGF-β1 acts by preventing extracellular matrix degradation during the cow follicle-luteal transition. Several studies have also shown that TGF-β1 promotes nucleus pulposus cell proliferation and ECM synthesis. Moreover, reported that TGF-β1 downregulates the expression of CCL4 via activation of the MAPK pathway, thus alleviating inflammation in the intervertebral disc.44 All these studies indicate the crucial role of TGF-β1 in the occurrence of IDD. However, these studies mainly focus on the direct effects of TGF-β1 on the intervertebral disc or the nucleus pulposus.
In IDD, there is limited research regarding cartilage or subchondral bone metabolism and remodeling. Our study confirms that downregulation of TGF-β1 promotes macrophage differentiation into osteoclasts, resulting in decreased vertebral body and endplate bone mass, thus affecting the mechanical transmission of intervertebral discs. Previous studies have shown that TGF-β1 non-covalently binds to latent-associated protein (LAP) in the bone matrix, acting as a primary coupling factor between osteoclast and osteoblast activity.45, 46 TGF-β1 can stimulate the differentiation of CD4+ T cells into Th17 cells and induce the secretion of IL-17, further triggering epithelial and endothelial cells to secrete pro-inflammatory factors such as IL-6 and IL-8, which play significant roles in osteoclast differentiation.47, 48 Additionally, our research demonstrates that downregulation of TGF-β1 promotes osteoclast differentiation, resulting in vertebral endplate bone loss and increased endplate porosity. As mentioned earlier, the biomechanical response of intervertebral discs and the energy supply between vertebral bodies and intervertebral discs rely on the normal structure of the vertebral endplate. An increase in endplate porosity at stress concentration points may lead to the formation of Modic changes, which disperses stress on the nucleus pulposus, disrupts load transfer, and increases annular fiber stresses, eventually causing intervertebral disc degeneration.49
Finally, we demonstrated through in vitro cell experiments that macrophages treated with TGF-β1 exhibited a significant reduction in the number and volume of osteoclasts, along with a marked decrease in the expression of osteoclast marker genes TRAP and CTSK. Our mouse experiments further confirmed that TGF-β1 could promote the abnormal formation of bone-related blood vessels in the growth plate, inducing its ossification and leading to a decrease in nucleus pulposus cells, thus contributing to the occurrence of intervertebral disc degeneration (IDD). This supports our previous hypothesis that bone-cartilage remodeling and aberrant vascularization in avascular cartilage are coupled processes and may be a crucial aspect of mouse intervertebral disc degeneration. Endothelial cells (ECs) have two subtypes, H-type and L-type, with H-type ECs being rich in growth factors required for the survival and proliferation of osteoblasts, thus facilitating the differentiation of progenitor cells into osteocytes.50, 51 TGF-β1, on the other hand, has been implicated in the regulation of bone-forming H-type vessels,32 promoting osteocyte differentiation and ultimately leading to bone-cartilage remodeling and growth plate ossification. To investigate this, we assessed the expression levels of Vegf and Opn as markers of angiogenesis and osteocytic differentiation, respectively, and validated their effects on the extracellular matrix (ECM) and nucleus pulposus cells. The results were evident: the growth plate, serving as a semi-permeable barrier, is the main conduit for nutrient uptake and waste excretion in the intervertebral disc, and any alterations in its permeability disrupt normal disc metabolism,52 resulting in reduced ECM proteoglycans, MMP3, MMP13 expression, and decreased nucleus pulposus cells.
Potential Clinical Implications of TGF-β1 in IDD Treatment
The identification of TGF-β1 as a key regulator of bone tissue stability and IDD provides a potential target for therapeutic interventions.17, 53, 54 Our study suggests that promoting TGF-β1 expression or developing strategies to enhance TGF-β1 signaling may have therapeutic benefits in preventing or alleviating IDD. This could be achieved through the use of TGF-β1-based therapies or by targeting downstream signaling pathways regulated by TGF-β1. Further research is needed to explore the feasibility and efficacy of these approaches in a clinical setting.
Limitations and Future Directions
Although our study provides valuable insights into the molecular mechanisms of TGF-β1 in IDD, there are certain limitations that should be acknowledged. First, the study was conducted in a mouse model, and the translation of these findings to humans requires further investigation. Second, the specific mechanisms underlying the regulation of osteoclast differentiation and CEP vascularization by TGF-β1 need to be further elucidated. Future studies could employ advanced techniques such as single-cell sequencing and gene knockout models to gain a deeper understanding of the involved cellular processes. Additionally, the therapeutic potential of targeting TGF-β1 should be evaluated in preclinical and clinical trials to assess its efficacy and safety.
Conclusion
In conclusion, our study highlights the critical role of TGF-β1 in preventing intervertebral disc degeneration by inhibiting osteoclast differentiation and abnormal blood vessel formation in the cartilage endplate (Fig. 7). The identification of TGF-β1 as a key regulator in bone tissue homeostasis offers potential avenues for developing novel therapeutic strategies for the prevention and treatment of IDD. Further research is warranted to validate these findings and explore their clinical implications.
Author Contributions
Keping Wang, Zuolong Wu, and Chaoyang Gong wrote the paper and conceived and designed the experiments. Guanghai Zhao and Haihong Zhang analyzed the data. Keping Wang and Zuolong Wu collected and provided the sample for this study. All authors have read and approved the final submitted manuscript.
Conflict of Interests
The authors declare that they have no competing interests.
Funding Information
This work was supported by Scientific Research Program of Gansu Provincial Department of Health (No. GSWSKY2021-07).
Ethics Statement
The mice were raised under standard laboratory conditions, and the experiments were performed after approval by the Animal Ethics Committee of the Second Hospital of Lanzhou University (No. D2023-228).
Open Research
Data Availability Statement
The data supporting this study's findings are available on request from the corresponding author.




