Surgical Strategy for Recurrent Giant Cell Tumor in the Thoracolumbar Spine
Ao Leng, Minglei Yang, and Haitao Sun these authors contributed equally to this work.
Abstract
Objective
Recurrent giant cell tumor (RGCT) of the spine represents a clinical challenge for surgeons, and the treatment strategy remains controversial. This study aims to describe the long-term follow-up outcomes and compare the efficacy of en bloc spondylectomy versus piecemeal spondylectomy in treating RGCT of the thoracolumbar spine.
Methods
A total of 32 patients with RGCT of the thoracolumbar spine treated from June 2012 to June 2019 were retrospectively reviewed. A total of 15 patients received total en bloc spondylectomy (TES) with wide or marginal margin while 17 patients received total piecemeal spondylectomy (TPS) with intralesional margin. Postoperative Eastern Cooperative Oncology Group Performance Score (ECOG-PS), Frankel classification and recurrence-free survival (RFS) were evaluated after surgery. Survival curves were estimated by the Kaplan–Meier method and differences were analyzed with the log-rank test. Multivariate analysis was performed with Cox regression to identify the independent prognostic factors affecting RFS.
Results
During a median follow-up of 41.9 ± 17.5 months, all patients with compromised neurologic functions exhibit significant improvement, with the mean ECOG-PS decreasing from 1.5 ± 1.3 to 0.13 ± 0.3 (p < 0.05). Among the 17 patients treated with TPS, eight patients developed local recurrence after a median time of 15.9 ± 6.4 months and four patients died from progressive disease. On the other hand, local recurrence were well managed with TES, since only one out of 15 patients experienced local relapse and all patients are alive with satisfied function at the latest follow-up. The median RFS for patients receiving TES and TPS are 75.0 months (95% CI: 67.5–82.5 m) and 38.3 months (95% CI: 27.3–49.3 m) respectively (p = 0.008). Multivariate analysis shows that the Ki67 index (p = 0.016), resection mode (p = 0.022), and denosumab (p = 0.039) are independent risk factors affecting RFS.
Conclusions
TES with wide/marginal margin should be offered to patients with RGCT whenever feasible, given its long-term benefits in local control and symptom alleviation. Additionally, patients with lower Ki67 index and application of denosumab tend to have a better prognosis.
Background
Giant cell tumor (GCT), accounting for 4%–5% of all primary bone tumors, is a benign tumor with local aggressiveness and an inclination for distant metastasis.1 A total of 85% of GCT arises in the meta-epiphyseal region of long bones, but they may also arise in the axial skeleton (10%) or occasionally in the small bones of hands and feet (5%).2 Although there is much published research on appendicular GCT, there are only a few focusing on GCT of the mobile spine, and even these have limited numbers of cases. However, as opposed to those arising in the extremities, GCT of the spine inclines to afflict younger patients and is predisposed to relapse or metastasizing.3
Surgery is currently the mainstay of treatment for GCT, even in the setting of recent therapeutic advances such as denosumab. Appendicular GCT can be well managed through intralesional curettage combined with intraoperative adjuvants including high-speed burring, ethanol and cryosurgery, etc.4 However, such adjuvants are generally not adopted in the treatment of spinal lesions for fear of iatrogenic damage to adjacent neural elements and vessels.3 Therefore, the goal of surgical treatment for spinal GCT is to remove the tumor in one piece without violation of the tumor border. TES can provide oncologically adequate resection margin, and is therefore recommended by the Spine Oncology Study Group in treating primary spinal GCT.5 However, up to 14% of patients still had local recurrence even after en bloc resection, which poses another challenge for spinal surgeons due to the deranged anatomy and extensive tissue adhesion resulting from previous procedures.5 Revision surgery, through en bloc spondylectomy, is therefore technically challenging and has only been reported in individual cases.6 Therefore, the long-term outcome and prognostic factors of surgical treatment for RGCT is unclear, and whether TES or TPS can better benefit the patients remains controversial.
Denosumab, a fully human monoclonal antibody against receptor activator of nuclear factor kappa-Β ligand, was approved for the treatment of adults and skeletally mature adolescents with GCT that is unresectable or when surgical resection is likely to result in severe morbidity.7 Up to date, the use of denosumab before surgery remains controversial. Some believe that this may aid in defining a peripheral rim around the tumor, thus benefiting intraoperative tumor resection,8 while others debate that this may cause difficulty in scraping the tumor and increase the recurrence rate.9 In general, there is no consensus on the preoperative use of denosumab, and its efficacy is, to a certain extent, related to the selected surgical method. This further complicates the treatment strategy for RGCT and requires further research.
The objectives of our study are: (i) to compare the efficacy of TES versus TPS in treating RGCT of the thoracolumbar spine; (ii) to analyze the prognostic factors affecting RFS; and (iii) to demonstrate the clinical features and long-term follow-up with the application of denosumab based on our clinical practice.
Methods
Patients
We performed a retrospective review of 183 patients with spinal GCT treated in our center between June 2012 and June 2019. The inclusion criteria of this study are: (i) patients with pathologically confirmed RGCT in the thoracolumbar spine treated in our center; (ii) patients who received either TES or TPS for the recurrent lesion; and (iii) patients who were followed up for more than 1 year. The exclusion criteria of this study are: (i) patients with lesions located at the cervical spine or the sacrum; (ii) patients who refused to receive surgery or received palliative surgery; and (iii) patients who were lost to follow-up. After screening, 32 patients reached the above criteria and were enrolled in our study. Relevant clinical data were collected including patients' demographic characteristics, radiographic manifestations, surgical records, and pathologic results, etc. The research was approved by the hospital ethics committee (2019SL045) and written informed consent was obtained from all patients or their legal guardians.
Therapeutic Regime
Individualized surgical strategy was decided on a case-by-case basis in reference to the Tomita classification and the Weinstein–Boriani–Biagini (WBB) classification. According to the WBB classification, TES was applied in patients without a large mass in layer A and sectors 5–8, for avoiding damage to adjacent vascular structures.10 Meanwhile, the target population of TES for thoracolumbar GCT included those with type 1–4 or some type 5 and 6 lesions according to the Tomita classification, without major vascular involvement.11 Additionally, TPS is recommended in patients that already had pulmonary metastases or multiple lesions in the spine. For patients undergoing TES, posterior fixation is performed before the vertebral osteotomy, in order to work on a stabilized spine while doing the distraction/contraction maneuver.12 Afterwards, posterior structures including the spinous process, lamina and flavum ligament are resected with rongeurs and scalpels until the dural sac can be fully exposed and rigorously liberated. Unilateral or bilateral thoracic nerves are sacrificed if necessary, and the anterior structures are bluntly separated. Subsequently, the posterior longitudinal ligament and contiguous intervertebral discs are dissected, until the tumor is fully removed and a definitive rotation maneuver is performed to pass the resected segments around the dural sac. Finally, a circumferential reconstruction is performed with a titanium mesh cage or artificial vertebrae filled with allogenous bone chips, together with posterior instrumentation. After surgery, all patients were followed every 3 months for the first year and once a year afterwards with either computed tomography (CT) or magnetic resonance imaging (MRI) examination.
As presented in Fig. 1, multi-level TES was performed for RGCT of T7-T8 in a 28-year-old male patient. The patient received intralesional surgery for the primary GCTB at another hospital. A total of 12 months after surgery, the patient developed local recurrence. Later on, the patient was referred to our center and started on neoadjuvant denosumab for a month (120 mg) on days 1, 8, 15, and 22 before surgery. After 1 month, CT examination revealed a significant tumor shrinkage with peripheral bone formation. Subsequently, the patient underwent TES of GCT located in T7 and T8 with internal fixation. After surgery, the patient was followed as an outpatient, and no recurrence was observed at 1 and 2 years after surgery.
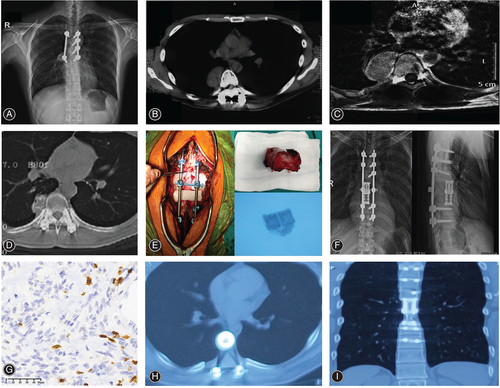
ECOG-PS and Frankel classification were adopted to access the general status and the neurologic functions of all patients at 6 months after surgery. Relevant time points including the detection of recurrence and the date of death were also recorded. RFS was calculated as the time period from revision surgery till the detection of recurrence or last follow-up.
Statistics
In this study, we specifically focused on the postoperative recurrence and overall well-being of patients after surgical treatments. RFS was defined as the interval between surgery and the notification of local recurrence. Survival curves were estimated by the Kaplan–Meier method and differences were analyzed with the log-rank test. Factors with p values ≤0.1 were subjected to multivariate analysis by Cox regression. p values less than 0.05 were considered statistically significant. All statistic calculations were performed using SPSS Statistics, version 21.0 (IBM corp., Armonk, NY, USA).
Results
Patient Description
Enrolled in this study were 15 male patients and 17 female patients with an average age of 33.0 ± 10.0 years. Four patients received their primary operations in our center, while 28 patients were initially treated elsewhere and later referred to our center after recurrence. The median interval between primary surgery and later recurrence was 19.8 ± 22.5 months. A total of 30 patients presented recurrence-related symptoms, such as pain or neurologic deficits, while the other two patients remained asymptomatic, and recurrence was not noticed until routine examination. Recurrent lesions were located in the thoracic spine in 22 patients and the lumbar spine in 10 patients. According to the Enneking staging system, 30 patients had aggressive GCT, defined as symptomatic and extending beyond the compartment (S3), while two patients had active GCT, defined as asymptomatic and remaining intracompartmental (S2).13 Baseline characteristics, such as patients' age, gender, preoperative functioning, histologic type, preoperative radiological parameters and application of adjuvant therapies were not significantly different between patients receiving TES or TPS, as presented in Table 1.
| Variables | TPS (n = 17) | TES (n = 15) | Statistical value (F/χ2) | p |
|---|---|---|---|---|
| Age | 33.6 ± 8.7 | 32.3 ± 11.6 | 0.021 | 0.886 |
| Gender (M/F) | 7/10 | 8/7 | - | 0.723 |
| Preoperative ECOG score (0–2/3–4) | 12/5 | 11/4 | - | 1.000 |
| Preoperative Frankel classification (B-C/D-E) | 5/12 | 4/11 | - | 1.000 |
| Enneking stage (II/III) | 1/16 | 1/14 | - | 1.000 |
| Ki-67(%) | 11.8 ± 7.9 | 9.6 ± 6.3 | 1.378 | 0.250 |
| Number of resected segments (1/2/3) | 6/7/4 | 4/7/4 | - | 0.906 |
| Denosumab (no/yes) | 7/10 | 8/7 | - | 0.723 |
| Radiotherapy (no/yes) | 10/7 | 10/5 | - | 0.726 |
| Bisphosphonate (no/yes) | 9/8 | 9/6 | - | 0.735 |
| Operation time (minutes) | 369.4 ± 112.4 | 416.7 ± 110.4 | 0.225 | 0.638 |
| Intraoperative bleeding (ml) | 3194.1 ± 2559.4 | 2313.3 ± 1426.2 | 5.822 | 0.022* |
| Complications (no/yes) | 14/3 | 6/9 | - | 0.027* |
| Recurrence at 2 years after salvage surgery (no/yes) | 9/8 | 14/1 | - | 0.018* |
| Radiological parameters | ||||
| Height (cm) | 3.5 ± 0.9 | 3.5 ± 1.3 | 0.143 | 0.887 |
| Length (cm) | 3.3 ± 0.5 | 3.2 ± 0.6 | 0.508 | 0.615 |
| Width (cm) | 2.8 ± 0.6 | 2.6 ± 0.6 | 0.797 | 0.432 |
- Notes: -: Statistic value is immeasurable with Fisher Exact Test.
- * p values less than 0.05 are deemed statistically significant.
Treatment Strategy
Individualized surgical strategy was decided on a case-by-case basis in reference to the Tomita classification, the Weinstein–Boriani–Biagini (WBB) classification and physical condition of each patient. The posterior approach was adopted for 27 patients while the one-stage combined anteroposterior approach was adopted for five patients. A total of 15 patients received TES with wide or marginal margin while 17 patients received TPS with intralesional margin. Patients receiving TES had less intraoperative blood loss (2313.3 ± 1426.2 ml vs. 3194.1 ± 2559.4 ml, p = 0.022) but were exposed to higher risk of developing postoperative complications (9/15 vs. 3/17, p = 0.027). Among patients receiving TPS, two patients developed wound infection after surgery while one developed cerebrospinal fluid (CSF) leak. For patients receiving TES, two patients developed CSF leak, five had hemothorax or pneumothorax, one patient underwent hardware failure and two had wound infection. After proper treatment, including debridement for wound infection, lumbar drainage for CSF leak, and closed chest drainage for hemothorax or pneumothorax, full recovery was achieved for all patients by the time of discharge.
Apart from surgery, 12 patients received radiotherapy before they were transferred to our center for surgical intervention. Six patients received neoadjuvant denosumab for a month (120 mg) on days 1, 8, 15, and 22 before surgery, while 11 patients only received denosumab as a postoperative adjuvant (120 mg/4 weeks for 2 years), with one patient developing osteonecrosis of the jaw during the follow-up. In addition, 14 patients were treated with bisphosphonate after surgery with no side effects being observed.
Follow-up and Outcomes
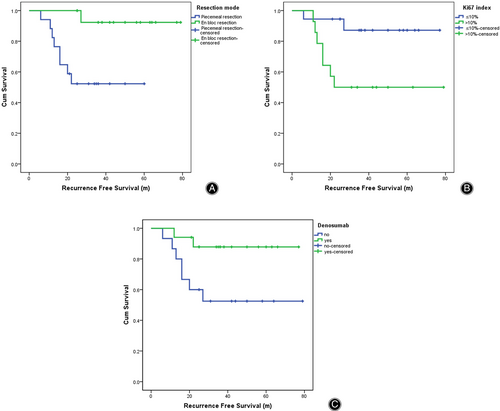
During a median follow-up of 41.9 ± 17.5 months (all surviving patients were followed for a minimum of 24 months), all patients with compromised neurologic functions showed significant improvements, with the mean ECOG-PS decreasing from 1.5 ± 1.3 to 0.13 ± 0.3 (p < 0.05). Nine patients developed local recurrence, and four died from tumor progression. In general, local recurrence was well managed with TES, since only one patient experienced local relapse after TES and all patients were alive with satisfactory functioning at the most recent follow-up. As for the 17 patients receiving TPS, eight patients developed local recurrence after an average time of 15.9 months (15.9 ± 6.4 m), and four died from progressive disease. The median RFS for patients receiving TES and TPS were 75.0 months (95% CI: 67.5–82.5 m) and 38.3 months (95% CI: 27.3–49.3 m) respectively, as shown in Fig. 2.
Multivariate Analysis
We first performed univariate analysis for all possible factors that may affect the patients' prognosis (Table 2). Factors with p values less than 0.1 were later subjected to multivariate analysis by using the COX regression (Table 3). Ki67 index (RFS: 69.4 m ± 5.0 m vs. 47.4 m ± 8.5 m, p = 0.016), resection mode (RFS: 38.3 m ± 5.6 m vs. 75.0 m ± 3.8 m, p = 0.022), and denosumab (RFS: 49.0 m ± 8.3 m vs. 69.7 m ± 4.8 m, p = 0.039) are found to be independent risk factors affecting postoperative recurrence (Fig. 2).
| Factors | Numbers | Recurrence free survival (m) | Statistical value (χ2) | p value |
|---|---|---|---|---|
| Gender | 15/17 | 52.6 (37.5–67.7)/67.1 (54.8–79.3) | 1.546 | 0.214 |
| Age (≤30 years/>30 years) | 16/16 | 55.5 (40.7–70.4)/64.6 (52.0–77.3) | 0.988 | 0.320 |
| Preoperative ECOG (0–2/ 3–4) | 23/9 | 46.6 (36.9–56.4)/72.0 (59.1–84.9) | 1.806 | 0.179 |
| Resection mode (piecemeal/en bloc) | 17/15 | 38.3 (27.3–49.3)/75.0 (67.5–82.5) | 6.942 | 0.008* |
| Denosumab (no/yes) | 15/17 | 49.0 (32.7–65.3)/69.7 (60.3–79.2) | 4.659 | 0.031* |
| Radiotherapy (no/yes) | 20/12 | 55.2 (45.7–64.7)/53.8 (36.8–70.7) | 1.156 | 0.282 |
| Bisphosphonate | 18/14 | 60.6 (46.9–74.3)/59.4 (44.7–74.0) | 0.001 | 0.974 |
| Enneking stage (II/III) | 2/30 | 27.0 (11.8–42.2)/61.7 (51.5–72.0) | 0.443 | 0.510 |
| Ki-67 (≤10%/>10%) | 18/14 | 69.4 (59.6–79.3)/47.4 (30.7–64.0) | 5.617 | 0.018* |
- * p values less than 0.1 are subjected to multivariate analysis for RFS by Cox regression.
| Factors | 95% CI for Exp (B) | HR | p value |
|---|---|---|---|
| Resection mode (piecemeal/en bloc) | 0.005–0.657 | 0.055 | 0.022* |
| Denosumab (no/yes) | 00.013–0.897 | 0.107 | 0.039* |
| Ki-67 (≤10%/>10%) | 1.106–8.998 | 1.978 | 0.016* |
- * p values less than 0.05 are deemed statistically significant.
Discussion
GCTs are locally aggressive benign tumors, constituting 4%–5% of all primary bone tumors.1 A variable incidence of spinal involvement has been reported, with some series demonstrating up to 15% occurring in the spine and the sacrum.14 In general, patients with spinal GCTs have considerably worse prognosis than patients with appendicular lesions, with the recurrence rate reported to be 22%–61% after surgery.15, 16 Furthermore, surgery for recurrent lesions can be technically challenging. In this study, we demonstrate that TES with wide/marginal margin should be offered to patients with RGCT whenever feasible, given its long-term benefits in local control and symptom alleviation. Additionally, patients with lower Ki67 index and application of denosumab tend to have a better prognosis.
Surgical Treatment
Surgery is the definitive treatment for GCTs. For appendicular GCTs, local adjuvants including high-speed burring, ethanol and cryosurgery etc. have been used in conjunction with intralesional curettage to improve local control rates.4 However, such adjuvants are generally not adopted in the treatment of spinal lesions for fear of iatrogenic damage to adjacent neural elements and vessels. In addition, owing to the locally aggressive nature of GCT, insufficient excision is associated with increased risk of recurrence. Therefore, en bloc resection is expected to remove the tumor in a single, intact piece, without violating the tumor border. The overall recurrence rate after en bloc vertebrectomy and intralesional resection are reported to be 0%–14% and 0%–71% respectively.5 Treatment for recurrent lesions remains another challenge for spinal surgeons, due to the deranged anatomy and extensive tissue adhesion resulting from previous operations.17 The morbidity of en bloc resection on recurrent cases is reported to be higher, since there will be a significant risk of loss of function due to the sacrifice of anatomical elements to achieve an appropriate margin.18 Additionally, based on our experience and literature reports, for patients who have received intralesional surgery before, the oncological margins may be unreliable due to tumor contamination by previous surgeries, particularly in the epidural space, and this can cause an increased risk for a second recurrence.19
RGCT tends to have a larger area of compromise compared to primary lesions, therefore a multi-level spondylectomy is often in need to achieve a total resection. While the isolated posterior approach is achievable for the majority of thoracic tumors, a combined posterior–anterior approach is expected for lesions in the lower lumbar spine or if there is serious invasion or adhesion to the anterior structures.20 Although TES has been widely accepted as an ideal option to control local recurrence for spinal malignancies, the indication to perform TES should be individually weighed on the risk-to-benefit ratio.13, 14 WBB classification is one of the references to decide on the surgical method. TES could be applied in patients without a large mass in layer A and sectors 5–8 based on the WBB system, or with type 1–4 or some type 5 and 6 lesions based on the Tomita classification, for avoiding damage to the vascular structures.7 Additionally, TPS is more appropriate if the tumor compromises the thoracic/abdominal aorta or esophagus, since to perform TES in such patients could represent a serious sacrifice especially given the relatively benign nature of the GCT. Moreover, for patients with pulmonary metastases or multiple lesions in the spine, the main purpose of surgery is to relieve nerve compression and restore normal function. In this scenario, TPS should be given priority since it can achieve the above purpose at a minimum cost. In our study, 17 patients received TPS, while 15 patients received TES for RGCT. The median RFS for patients receiving TES and TPS were 75.0 months (95% CI: 67.5–82.5 m) and 38.3 months (95% CI: 27.3–49.3 m), respectively. In addition, local recurrence is well managed with TES, since only one patient experienced local relapse, while eight out of 17 patients receiving TPS developed local recurrence after an average time of 15.9 months (15.9 ± 6.4 m), and four of them died from progressive disease by the time of the latest follow-up. With the results, we demonstrate the superiority of TES over TPS in extending patients' RFS, and advocate the application of TES despite the complications and complicated techniques. Furthermore, despite the fact that TES is correlated with more complications in our study and previous research (ranging from 17.1% to 65.2%),21, 22 most of the surgery-related complications are salvageable and patients can achieve full recovery after proper treatment. Therefore, only when the postoperative morbidity is unacceptable, should an intentional transgression of oncological principle, such as TPS, be considered.23
Denosumab
Denosumab, a fully human monoclonal antibody against receptor activator of nuclear factor kappa-Β ligand, was approved for the treatment of adults and skeletally mature adolescents with GCT that is unresectable or when surgical resection is likely to result in severe morbidity.7 Based on pathological research, denosumab treatment can cause marked reduction of both multinucleated giant cells and neoplastic stromal cells, replacing them with reactive collagen matrix and densely woven bones.24, 25 These histopathologic changes correlated with patients' radiologic manifestations, including substantial reduction of the tumor size, central sclerosis and peripheral bone formation.24 However, whether denosumab, in the neoadjuvant setting, can reduce the local recurrence rate after surgery remains debatable, and this is, to some extent, influenced by the surgical technique adopted in the subsequent treatment.26 Studies revealed that denosumab administered before en bloc resection may harden the tumor, reduce tumor spillage and therefore decreases the local recurrence rate; while administration of denosumab before curettage causes osteosclerosis, which makes it difficult to intraoperatively identify the tumor area and increases the local recurrence rate.27 Despite the controversies over its influence on local recurrence, neoadjuvant denosumab proves to be beneficial in surgical downstaging, and it is suggested for the treatment of local advanced tumors to facilitate a less morbid surgical resection.28 In four of the six patients receiving neoadjuvant denosumab, we observed an incomplete peripheral rim around the tumor, and the margin of the tumor was relatively easy to identify. Additionally, we did not notice serious peripheral adhesion, which may be attributed to using only loading doses instead of long-term denosumab before surgery.29 In all six cases, there are ossifications inside the mass and while the intensity of the tumor increased, the blood supply within the lesion decreased, facilitating surgical resection with less intraoperative bleeding. Of the 15 patients receiving TES, five received neoadjuvant denosumab before surgery while two patients received denosumab during the follow-up due to development of pulmonary metastases. Of the 17 patients receiving TPS, one patient received neoadjuvant denosumab before surgery and continued after the operation, while nine patients only used denosumab after surgery. Overall, patients receiving denosumab tend to have better local control compared to those receiving no denosumab (49.0 m (32.7–65.3 m) vs. 69.7 m (60.3–79.2 m), p = 0.039). With the effects of denosumab being reported in the literature, such benefits are to be expected.30 Based on our experience, subcutaneous injection of denosumab (120 mg/4 weeks) is recommended within 2 years after the operation for spinal GCT patients, especially for those received TPS. Whether continuous use of denosumab after 2 years postoperatively is based on the recurrence risk.
Other Treatment Options
Other treatment options for GCT include radiotherapy (RT), selective arterial embolization, cryotherapy and medications such as bisphosphonate and interferon alpha.28 Specialized techniques such as 3-D conformal RT, intensity-modulated RT and stereotactic body RT have also been associated with good local control rates in patients with GCTs that are not amenable to complete surgical resection.31 However, the findings from studies evaluating adjuvant radiotherapy with and without surgery are inconsistent, and radiation-induced malignant transformations are observed.28 Therefore, some believe that RT should be reserved for cases where surgery is infeasible and denosumab, zoledronic acid, or embolization is not available.26 In our study, 12 patients received radiotherapy and the estimated RFS was 53.8 months (36.8–70.7 m), showing no significant difference from patients receiving no radiotherapy (55.2 m (45.7–64.7 m)).
Strength and Limitations
Given the rarity of spine GCT, we enrolled a relatively large cohort of patients with RGCT of the thoracolumbar spine, and consistently compared the efficacy of TES versus TPS. In addition, we performed multivariate analysis and found that patients with lower Ki67 index and application of denosumab tend to have a better prognosis. Several limitations of this study should be addressed. First, our study is a nonrandomized retrospective study. Given its retrospective nature, the choice of the two surgical methods is not randomly assigned. Despite us trying to consistently follow the abovementioned criteria during the decision-making process, selection bias still exists. Second, the small amount of patients precludes us from drawing more definite conclusions. As a next step, multi-center evaluation of a larger group of patients is critical for improving our understanding of the treatment for this group of patients.
Conclusions
RGCT of the spine should be managed with en bloc spondylectomy whenever feasible, as TES provides better local control and extended RFS compared to patients who received TPS. Additionally, Ki67 index and postoperative application of denosumab are independent prognostic factors affecting patients' RFS.
Author Contributions
Ao Leng and Minglei Yang: conceptualization, methodology and writing the original draft. Haitao Sun: validation, software and formal analysis. Zhi Zhu: pathologic examination. Zeyu Dai and Wei Wan: resources and data curation, Jianru Xiao: supervision, project administration, review and editing.
Acknowledgments
This study was generously supported by the National Key Research and Development Project of China (2016YFC0902100), the Shanghai Science and Technology Committee (Grant No. 21MMC1930100 and 19411962700), the Logistics Support Department of PLA (21QNPY044) and Youth Doctor Assistance Program Funds of Shanghai Changzheng Hospital.
Conflict of Interest Statement
The Authors report no conflict of interest in this work.
Ethical Statement
This research has been approved by the Institutional Review Boards of the authors' affiliated institutions (2019SL045), with written informed consent obtained from all patients or their legal guardians.




