PD-L1 in squamous cell carcinoma of the oral tongue shows gender-specific association with prognosis
Abstract
Objective
To use alternative quantitation approaches to clarify the clinical implication of programmed cell death ligand 1 (PD-L1) in squamous cell carcinoma of the oral tongue (SCCOT).
Materials and Methods
Ventana SP263 immunohistochemistry assay and a multiplicative QuickScore method were applied to quantify PD-L1 in tumor and surrounding immune cells from 101 patients with SCCOT. Tumor-infiltrating immune cells were estimated from bulk tissue transcriptional profiles of 25 patients. Circulating PD-L1 levels were measured in serum from 30 patients using an electrochemiluminescence assay platform.
Results
We found higher tumor cell PD-L1 levels in females than males (p = .019). For patients with low PD-L1 in tumor cells, better survival was seen in males than females (overall survival p = .021, disease-free survival p = .020). Tumor-infiltrating natural killer T cells, immature dendritic cells, and M1 macrophages were positively associated with tumor cell PD-L1 (p < .05).
Conclusions
Our data confirmed the significance of gender on tumor cell PD-L1 expression and demonstrated combined effects of gender and PD-L1 levels on clinical outcome in patients with SCCOT. The data also indicated the involvement of specific immune cell types in PD-L1-regulated immune evasion.
1 INTRODUCTION
Squamous cell carcinoma of the head and neck (SCCHN), derived from squamous epithelium at multiple anatomic sites, is a major cause of cancer-associated morbidity and mortality worldwide (Bray et al., 2018). Treatment of SCCHN is generally based on surgical resection of the primary tumor, neck dissection, and radio- and/or chemo-therapy (Lo Nigro, Denaro, Merlotti, & Merlano, 2017). Despite improvements in diagnostic and therapeutic approaches in recent years, the survival rate has not improved over the past decades (Leemans, Snijders, & Brakenhoff, 2018; Sgaramella et al., 2018). Novel agents targeting immune checkpoints have shown promising results in patients with advanced SCCHN (Lenouvel et al., 2019).
Tumor cells may activate immune checkpoint pathways to evade immune recognition and elimination (Chen & Mellman, 2013; Pardoll, 2012). The programmed cell death receptor (PD)-1 and its ligand PD-L1 are key players in immune checkpoint control, and elevated levels have been demonstrated in diverse cancer entities (Sun, Mezzadra, & Schumacher, 2018; Wei, Duffy, & Allison, 2018). Binding of PD-L1 to PD-1 on activated T cells suppresses antitumor immunity by counteracting T-cell-activating signals (Sun et al., 2018). Immune checkpoint inhibitors that target the PD1/PD-L1 axis are now approved for treatment of different cancer types. However, only a subset of patients shows clinical benefit (Sun et al., 2018; Wei et al., 2018). When assessing the association between PD-L1 expression and efficacy of immune checkpoint inhibitors for patients with recurrent or metastatic SCCHN, both PD-L1-positive and PD-L1-negative tumors showed treatment response, with higher response rates observed in PD-L1-positive compared to PD-L1-negative patients (Chow et al., 2016; Ferris et al., 2016, 2018; Mehra et al., 2018). It is therefore likely that factors such as tumor genomics, host germline genetics, and tumor microenvironment combine to affect the efficacy of checkpoint inhibitor-based immunotherapies (Havel, Chowell, & Chan, 2019).
As one of the major factors in immune checkpoint activity, the clinical relevance of PD-L1 in tumor cells and tumor microenvironments is currently the subject of intense investigation. It has been shown in some studies that high levels of PD-L1 in cancer cells correlate with SCCHN tumor size, presence of regional metastases (Moratin et al., 2019; Muller et al., 2017), and poor prognosis (de Vicente et al., 2019; Moratin et al., 2019; Muller et al., 2017; Ngamphaiboon et al., 2019; Yang, Zeng, et al., 2018), whereas other studies of SCCHN demonstrated no clinical significance (Cho, Yoon, Lee, Hong, & Hong, 2011; Mattox et al., 2017; Yoshida et al., 2018). Overall, a meta-analysis of 3,105 patients with SCCHN did not find significant differences in clinical outcome between patients with PD-L1-positive or PD-L1-negative tumors (Yang, Wong, Thomson, Li, & Su, 2018). Interestingly, high PD-L1 on tumor-infiltrating immune cells, but not on tumor cells, has been reported as a favorable prognostic factor for SCCHN (Kim et al., 2016). Obviously, no consensus has emerged on the impact of PD-L1 on clinicopathological features and clinical outcomes for patients with SCCHN (Lenouvel et al., 2019).
Accurate measurement and scoring of PD-L1 protein expression is difficult due to various technical and biological pitfalls (Topalian, Taube, Anders, & Pardoll, 2016), and the choice of immunohistochemical assays and scoring strategies could lead to discrepancies between different studies (Dodson et al., 2019; Lenouvel et al., 2019). Several PD-L1 diagnostic assays are commercially available, such as Ventana SP263 (durvalumab), Dako 22C3 (pembrolizumab), and Dako 28–8 (nivolumab). Among these three assays, while showing similar performance in tumor cell staining (Ratcliffe et al., 2017), the SP263 assay is superior in immune cell staining compared to the other two assays (Schats et al., 2018). Regarding PD-L1 scoring, the utility of TPS (tumor proportion score, % of PD-L1-positive tumor cells at any intensity) or CPS (combined positive score, % of tumor and mononuclear inflammatory cells within the tumor nests and adjacent supporting stroma expressing PD-L1 at any intensity) has been established as an index of PD-L1 expression levels in clinical practice (Burtness et al., 2019; Chow et al., 2016; Cohen et al., 2019). However, as staining intensity also should be considered, the QuickScore (QS) method taking both proportion of stained cells and staining intensity into consideration should preferably be used (Detre, Saclani Jotti, & Dowsett, 1995).
PD-L1 levels in SCCHN may differ depending on the anatomic origin of the tumor (Troeltzsch et al., 2017), similar to previous findings that showed distinct molecular and clinical features between tumors from different anatomical subsites within the head and neck region (Boldrup, Coates, Laurell, & Nylander, 2011; Boldrup, Coates, Wahlgren, Laurell, & Nylander, 2012; Cancer Genome Atlas, 2015; Leemans et al., 2018). Therefore, SCCHN subsite- and cell type-specific PD-L1 detection using appropriate scoring methodology is warranted. Squamous cell carcinoma of the oral tongue (SCCOT) (distinct from tumors arising in the base of the tongue) is the dominant SCCHN subtype showing high tumor PD-L1 (Troeltzsch et al., 2017). According to TPS, higher percentages of PD-L1-positive tumor cells correlate with worse clinical outcome in SCCOT (Naruse et al., 2019; Yoshida et al., 2018), whereas an earlier study did not identify any correlation (Mattox et al., 2017). In the present study, we sought to elucidate the clinical relevance of PD-L1 in a large series of SCCOT patients, according to the SP263 assay and the QS method. In addition, infiltrating immune cell types and their activation states were estimated from tumor tissue transcriptional profiles. Circulating PD-L1 protein levels were also investigated in a subset of patients.
2 MATERIALS AND METHODS
2.1 Patients
To quantify PD-L1 levels in tumor samples, a total of 101 patients (51 females and 50 males) with primary SCCOT were included in this study. Patient clinicopathological characteristics are summarized in Table 1. The mean age of the patients at diagnosis was 62 years, range 19–89 years. The mean follow-up time was 48.8 months, ranging from 1 to 179 months. Formalin-fixed, paraffin-embedded (FFPE) biopsies were available at Clinical Pathology, Umeå University Hospital, Sweden. Data on survival and cause of death were obtained from the clinical files or the Swedish Death Registry. Serum samples collected before treatment were available from 13 of the 101 patients. To quantify serum PD-L1 levels in more patients, another 17 patients with SCCOT were further recruited. Clinical information for patients included in the circulating PD-L1 study is shown in Table S1. All patient details were anonymized, and the project was performed according to the principles of the Declaration of Helsinki after approval by the local Ethical Committee (dnr 03–201, 08-003M). When using archived tissue, no written consent was required. The understanding and written consent was obtained from patients donating blood samples.
| Clinicopathological features | Total patients (N = 101) | |
|---|---|---|
|
FFPE samples (N = 101) |
Fresh frozen samples (N = 25) |
|
| Age at diagnosis | ||
| ≤40 years | 16 | 4 |
| 41–65 years | 40 | 11 |
| >65 years | 45 | 10 |
| Gender | ||
| Female | 51 | 12 |
| Male | 50 | 13 |
| Smoking | ||
| Non-smoker | 26 | 10 |
| Previous smoker | 23 | 5 |
| Smoker | 27 | 9 |
| Unknown | 25 | 1 |
| Alcohol | ||
| No | 3 | 1 |
| Normal | 67 | 20 |
| Previous abuser | 4 | 1 |
| Abuser | 6 | 3 |
| Unknown | 21 | 0 |
| T stage | ||
| 1 | 24 | 5 |
| 2 | 42 | 14 |
| 3 | 16 | 0 |
| 4 | 19 | 6 |
| Lymph node metastasis | ||
| No | 79 | 21 |
| Yes | 22 | 4 |
| Distant metastasis | ||
| No | 99 | 25 |
| Yes | 2 | 0 |
| TNM stage (7th edition) | ||
| 1 | 24 | 5 |
| 2 | 37 | 12 |
| 3 | 15 | 2 |
| 4 | 25 | 6 |
| Lymphocytic responsea | ||
| 1 Continuous dense rim of lymphoid tissue | 45 | 7 |
| 2 Patches of discontinuous dense lymphoid infiltrate | 39 | 13 |
| 3 Limited or no response | 17 | 5 |
| Treatment | ||
| Radiotherapy (RT) | 21 | 6 |
| Surgery (OP) | 10 | 2 |
| Pre-operative RT/OP | 53 | 10 |
| OP/postoperative RT | 14 | 7 |
| No treatment | 3 | 0 |
| Status | ||
| Disease-free | 49 | 14 |
| With disease | 52 | 11 |
- a Data from 79 of the samples have previously been published by Lundqvist, Stenlund, Laurell, & Nylander. (2012).
2.2 Immunohistochemistry (IHC) and scoring
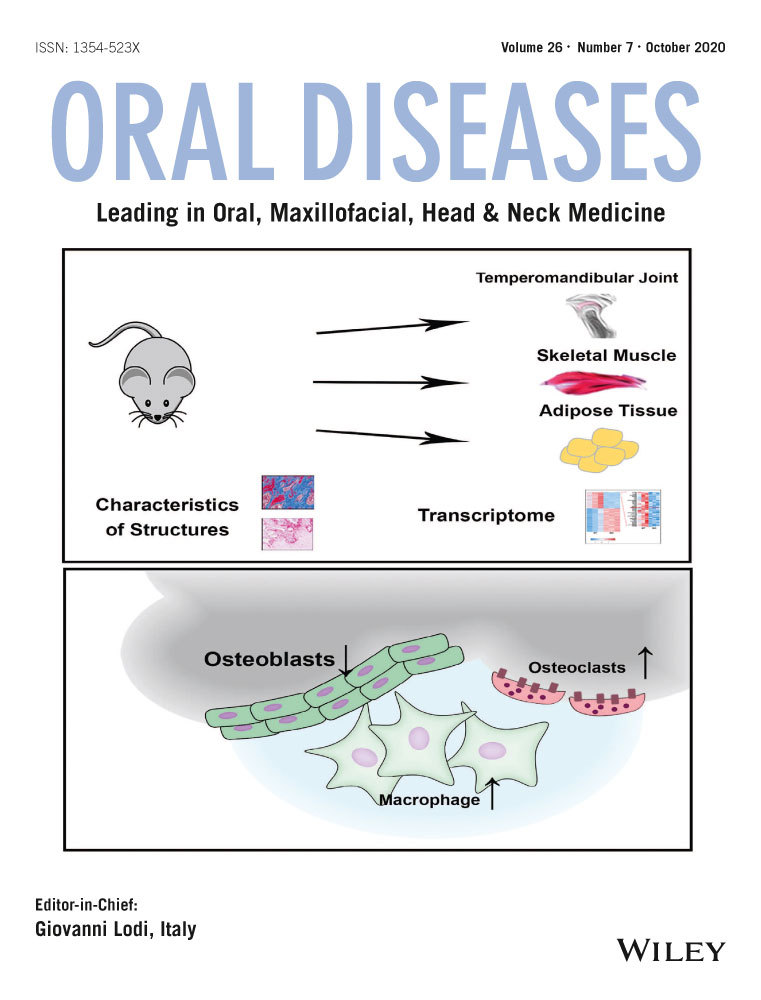
FFPE sections were stained with the Ventana PD-L1 (SP263) assay (Ventana Medical Systems Inc.) in a Ventana staining machine according to the supplier's recommendations. PD-L1 levels were evaluated separately in tumor cell and immune cell compartments using the QuickScore (QS) method (Detre et al., 1995). Briefly, the proportion of stained cells was divided into six categories: 1 = 0%–4%, 2 = 5%–19%, 3 = 20%–39%, 4 = 40%–59%, 5 = 60%–79%, and 6 = 80%–100%. Intensity of staining was divided into four levels: 0 = negative, 1 = weak, 2 = intermediate, and 3 = strong. The QS (ranging from 0 to 18) was calculated by multiplying the proportion score with the intensity score. Tumor cell PD-L1 was scored independently by two of the authors (LB and KN), and cases of disagreement were discussed to provide a consensus score. Immune cell PD-L1 was scored by KN only.
2.3 Immune cell type estimation
Gene expression profiling was performed previously (Boldrup, Gu, et al., 2017; Gu et al., 2019), and data were available for 25 of the 101 patients. Briefly, RNA was extracted from tumor biopsies collected before treatment of the patients and processed for gene expression profiling using Illumina HumanHT-12 v4 Expression BeadChip (Illumina Inc.) (Boldrup, Gu, et al., 2017; Gu et al., 2019). Raw data are available from ArrayExpress (accession numbers E-MTAB-4678 and E-MTAB-5534). To investigate the relationship between PD-L1 mRNA levels and immune infiltration, we applied the xCell method (https://xcell.ucsf.edu/) (Aran, Hu, & Butte, 2017) to estimate immune cell types according to gene expression profiling data. The generated xCell scores of 34 immune cell types were used for cell quantification.
2.4 Circulating PD-L1 protein quantification
Similar to previous descriptions (Boldrup, Troiano, et al., 2017), 3.5 ml of peripheral blood was collected into BD vacutainer SST II advance tubes with clot activator and separating gel using standardized venipuncture procedures. Separated serum was stored at −80°C until further use. Serum PD-L1 levels were analyzed using R-PLEX Human PD-L1 Antibody Set from Meso Scale Discovery (MSD) following the manufacturer's instructions. By making a dilution series of a human recombinant PD-L1 calibrator included in the assay, the concentration of PD-L1 can be calculated for each sample.
2.5 Statistical analysis
Patients were categorized into three groups: PD-L1-negative (QS = 0), PD-L1 low (QS from 1 to 5), or PD-L1-high (QS > 5). Associations between categorized clinicopathological variables and categorized PD-L1 levels were determined by chi-square test. The Kaplan–Meier method with log-rank test was used to compare patient survival between groups. For continuous variables, correlations were studied using non-parametric Spearman correlation analysis and the correlation coefficient (rho) was calculated to evaluate correlation strength. Non-parametric Mann–Whitney U test or Kruskal–Wallis H test was used to study the difference between two or several groups of continuous variables, respectively. All statistical tests were conducted in IBM SPSS Statistics 25 (IBM Corp.). A two-sided p-value < .05 was considered significant.
3 RESULTS
3.1 PD-L1 expression in SCCOT and tumor-infiltrating immune cells
PD-L1 staining was seen on the membrane and in the cytoplasm of tumor cells and immune cells (Figure 1). Quantitation of staining according to the QS method showed that PD-L1 levels ranged from 0 to 18 in tumor cells and 0 to 15 in immune cells. Tumor cell levels were directly correlated with immune cell PD-L1 levels in individual patients (rho = 0.427, p < .001). Considering the tumor cell compartment, 21 of 101 patients were negative for PD-L1 staining (QS = 0), whereas only 4 patients were PD-L1 negative regarding tumor-infiltrating immune cells. These 4 patients also showed negative PD-L1 tumor cell compartments.
3.2 PD-L1 and clinicopathological factors
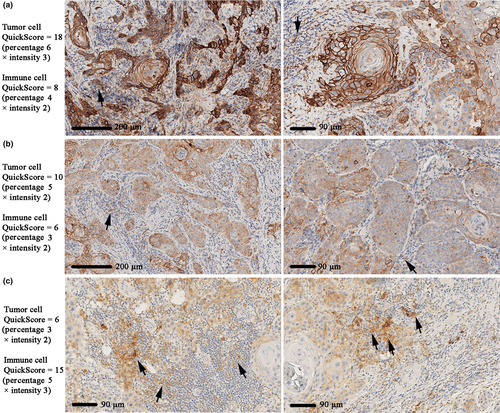
Based on PD-L1 staining of tumor cells, patients were classified into negative (QS = 0), low (QS from 1 to 5), and high (QS from 6 to 18) groups. Likewise, patients were divided into the same three groups based on immune cell PD-L1 levels. There were no significant associations (Chi2 p > .05) between PD-L1 in either tumor or immune cells with the clinicopathological characteristics investigated (Table 2). Although not statistically significant (p = .097), more PD-L1-negative tumors were found in males and more PD-L1-high tumors in females (Table 2). To investigate this further, we compared PD-L1 QS values between genders using Mann–Whitney U test on non-categorized scores. Results showed that tumor cell PD-L1 levels were significantly higher in females than in males (p = .019; Figure 2a). In immune cells, however, PD-L1 did not differ between genders (Figure 2b). As most previous studies used either cell proportion or intensity to investigate PD-L1, we therefore also evaluated the correlations between PD-L1 cell proportion scores or PD-L1 intensity levels and clinicopathological variables. These analyses demonstrated that female patients contained a higher percentage of PD-L1-positive tumor cells than males (p = .007), whereas no other correlation was found. Immune cell PD-L1 proportion or intensity scores did not correlate with any clinicopathological variable.
| Tumor cell PD-L1 QS | Immune cell PD-L1 QS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1–5 | 6–18 | Total | p-value | 0 | 1–5 | 6–18 | Total | p-value | ||
| Age at diagnosis (years) | 19–40 | 5 | 6 | 5 | 16 | .494 | 1 | 10 | 5 | 16 | .495 |
| 41–65 | 8 | 21 | 11 | 40 | 2 | 24 | 14 | 40 | |||
| 66–89 | 8 | 18 | 19 | 45 | 1 | 21 | 23 | 45 | |||
| Gender | Female | 7 | 22 | 22 | 51 | .097 | 2 | 27 | 22 | 51 | .95 |
| Male | 14 | 23 | 13 | 50 | 2 | 28 | 20 | 50 | |||
| T stage | T1, T2 | 13 | 30 | 23 | 66 | .929 | 3 | 32 | 31 | 66 | .254 |
| T3, T4 | 8 | 15 | 12 | 35 | 1 | 23 | 11 | 35 | |||
| Lymph node metastasis | N0 | 19 | 33 | 27 | 79 | .286 | 4 | 40 | 35 | 79 | .255 |
| N1–N3 | 2 | 12 | 8 | 22 | 0 | 15 | 7 | 22 | |||
| TNM stage | I, II | 13 | 26 | 22 | 61 | .888 | 3 | 31 | 27 | 61 | .608 |
| III, IV | 8 | 19 | 13 | 40 | 1 | 24 | 15 | 40 | |||
| Lymphocytic responsea | 1 | 9 | 17 | 19 | 45 | .529 | 0 | 25 | 20 | 45 | .447 |
| 2 | 7 | 20 | 12 | 39 | 3 | 20 | 16 | 39 | |||
| 3 | 5 | 8 | 4 | 17 | 1 | 10 | 6 | 17 | |||
| 2-year Survivalb | Yes | 11 | 25 | 18 | 54 | .929 | 3 | 26 | 25 | 54 | .331 |
| No | 10 | 20 | 17 | 47 | 1 | 29 | 17 | 47 | |||
| 5-year Survivalb | Yes | 9 | 17 | 11 | 37 | .855 | 3 | 20 | 14 | 37 | .351 |
| No | 10 | 24 | 17 | 51 | 1 | 32 | 18 | 51 | |||
| Status | Disease-free | 13 | 20 | 16 | 49 | .384 | 3 | 24 | 22 | 49 | .387 |
| With disease | 8 | 25 | 19 | 52 | 1 | 31 | 20 | 52 | |||
| Total | 21 | 45 | 35 | 101 | 4 | 55 | 42 | 101 | |||
- Abbreviation: QS, QuickScore.
- a Lymphocytic response was defined according to Brandwein-Gensler et al. (2005). 1, Continuous dense rim of lymphoid tissue; 2, patches of discontinuous dense lymphoid infiltrate, 3, limited or no response.
- b 2-year survival data are available for all 101 patients and 5-year survival for 88 patients.
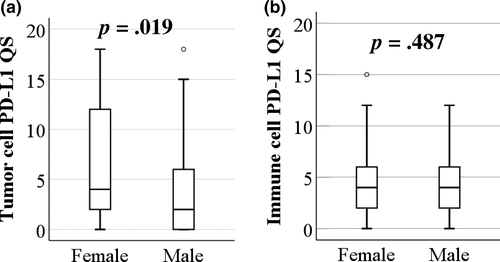
3.3 PD-L1 and patient survival
The impact of tumor cell PD-L1 levels on patient survival was determined using Kaplan–Meier with log-rank test. There was no significant difference in overall survival among patients with negative-, low- or high-PD-L1 levels on their tumor cells (p = .789; Figure 3a). Because PD-L1 levels were higher in females, we further investigated whether there was a gender-dependent impact of tumor cell PD-L1. Female patients with negative PD-L1 showed improved overall survival compared to female patients with positive PD-L1 staining (p = .050), whereas no difference was seen in overall survival for males with varying degrees of PD-L1 (p = .314; Figure 3a). We next investigated whether there were differences in overall survival between genders for patients with negative, low, or high levels of tumor cell PD-L1 (Figure 3b). Clearly, there was no overall effect of gender on survival outcome (p = .400; Figure 3b, all patients). Male patients with PD-L1-negative tumor cells showed a trend toward poorer survival than female patients, although this did not reach statistical significance (p = .070; Figure 3b, negative). A favorable outcome was demonstrated in male compared to female patients with low PD-L1 expression (QS = 1–5) in tumor cells (p = .021; Figure 3b, low). There was no significant difference in overall survival between genders for patients with high tumor cell PD-L1 levels (QS = 6–18) (p = .851; Figure 3b, high). For all patients, we further investigated gender differences in clinical variables. As shown in Table S2, female patients were significantly older than males (p = .003). However, focusing on the PD-L1-low group, there was no significant difference in age between genders (p = .097). We also studied the association between tumor cell PD-L1 and disease-free survival. Again, for patients with low PD-L1, better disease-free survival was seen in males than females (p = .020; Figure S1).
Similar survival analyses were performed to investigate the impact of immune cell PD-L1 levels, where no significance was identified even when gender was taken into consideration. Finally, there were no significant associations with patient survival according to PD-L1 cell proportion or intensity level.
3.4 PD-L1 and the presence of infiltrating immune cell types
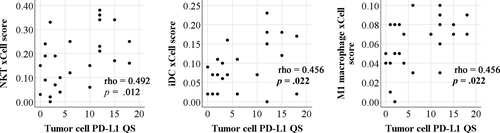
Microarray gene expression profiling of fresh frozen tumor samples had been performed previously for 25 of the 101 patients included in this study. To estimate immune cell types in these tumor samples, we uploaded the gene expression data to the xCell webtool. The resulting xCell scores for immune cell types were then correlated with PD-L1 IHC results. Among the 34 immune cell types identified by xCell, natural killer T cells (NKT), immature dendritic cells (iDC), and M1 macrophage showed increased accumulation in tumors with higher PD-L1 levels (Figure 4). In contrast, PD-L1 levels in immune cells in the tumor microenvironment did not correlate with accumulation of any specific immune cell types identified by xCell.
3.5 Circulating PD-L1
Serum PD-L1 levels were quantified in 30 patients. The median PD-L1 concentration was 49.97 pg/ml. Comparing serum PD-L1 levels among patients with various clinicopathological features, no significant difference was found (Table S1). As both FFPE sections and blood samples were available from 13 patients, we also investigated the correlation between tissue PD-L1 and serum PD-L1 levels. Neither tumor cell PD-L1 (rho = −0.505, p = .078) nor immune cell PD-L1 (rho = −0.123, p = .689) correlated significantly with serum PD-L1 levels.
4 DISCUSSION
PD-L1 is a transmembrane protein that interacts with the PD-1 receptor on T cells, thereby inhibiting T-cell activation and promoting tumor-mediated immune evasion (Dong et al., 2002). In this study, the clinical implications of PD-L1 expression were investigated in a cohort of SCCOT patients from Northern Sweden, using an FDA (Food and Drug Administration, USA) approved PD-L1 IHC assay. Sections were scored individually for proportion and intensity of staining in tumor cells and in infiltrating immune cells. Using our methodology, PD-L1-positive tumor cells were seen in 79% and PD-L1-positive immune cells in 96% of the samples. Previous studies focusing on patients with SCCOT have reported PD-L1 positivity in 23% (Yoshida et al., 2018), 57.9% (Naruse et al., 2019), or 79% (Mattox et al., 2017) of patients. In addition to intra-tumor heterogeneity of PD-L1 (Rasmussen et al., 2019), such differences between studies are most probably due to the different IHC antibodies/detection protocols and the different scoring criteria used. Notably, Mattox et al. (Mattox et al., 2017) reported two patterns of PD-L1 staining in SCCOT cells, either diffuse staining throughout the tumor or peripheral staining around the tumor. However, we did not find such patterns in our samples. It is also worth mentioning that PD-L1 staining in nuclei of tumor cells (Naruse et al., 2019) is likely an artifact resulting from inappropriate sample treatment (Polioudaki et al., 2019) and nuclear staining was not seen in our samples.
When evaluating the association of PD-L1 with clinicopathological characteristics and clinical outcomes in patients with SCCOT, different conclusions were also drawn from the above-mentioned studies. In line with our results (Table 2), Mattox et al. (Mattox et al., 2017) found no significant associations. Interestingly, in another study investigating 81 patients aged ≤45 years with oral cavity squamous cell carcinoma (86.4% of patients with SCCOT), high tumor PD-L1 and the presence of tumor-infiltrating lymphocytes were associated with better prognosis among females (Hanna et al., 2018). Considering all patients in our study (aged 19–89 years), we did not find any relevance of infiltrating immune cells or PD-L1-positive infiltrating immune cells for clinical features. However, we did find a gender-related impact of PD-L1 on overall and disease-free survival: Females lacking PD-L1-positive tumor cells have better survival outcome than males lacking PD-L1 on their tumor cells. In contrast, males with low PD-L1 IHC scores demonstrated better survival than PD-L1-low females. When comparing tumor PD-L1 between genders, significantly higher PD-L1 levels were seen in females. Gender differences in immune response have been well described (Capone, Marchetti, Ascierto, Malorni, & Gabriele, 2018; Klein & Flanagan, 2016), and a previous study of oral squamous cell carcinoma indicated a prognostic value of PD-L1 for males (Lin et al., 2015). Even though the efficacy of immune checkpoint inhibitors might not differ between females and males (Conforti et al., 2018; Wallis et al., 2019), this study further implicates that future studies on the clinical relevance of PD-L1 should consider gender as a critical biological variable.
For a subset of patients, we used the xCell tool to estimate tumor-infiltrating immune cell composition in bulk tumor samples. Unlike the widely used IHC staining approaches for immune cell detection and quantitation, xCell is a transcriptome-based computational method showing high accuracy for well-defined cell type signatures (Aran et al., 2017; Sturm et al., 2019). However, due to the lack of information regarding their location (Galon et al., 2014), xCell-estimated immune cells and their correlation with PD-L1 should be evaluated with caution. For example, IHC analysis has shown CD8+ T cells and CD4+ T cells to be correlated with PD-L1 in oral squamous cell carcinoma (Cho et al., 2011; Mattox et al., 2017; Satgunaseelan et al., 2016), whereas our study did not show any correlation. Instead, we found NKT, iDC, and M1 macrophages positively correlated with PD-L1 on tumor cells. Obviously, interaction between immune cells and PD-L1-positive tumor cells cannot be clarified using the xCell approach. Nonetheless, as engagement of PD-1 by PD-L1 can modify the effector functions of T cells in many ways, such as inhibiting T-cell proliferation, survival, or cytokine production (Sun et al., 2018), it is likely that cytotoxic T cells are delicately targeted by tumor cells by multiple mechanisms in different patients. The role of M1 macrophages in antitumor immunity has been described previously (Biswas & Mantovani, 2010; Paul, Chhatar, Mishra, & Lal, 2019), where M1 subtype cells promote inflammation compared to M2 macrophages that tend to predominate in tumor microenvironments and are anti-inflammatory. iDC, on the other hand, could facilitate tolerance toward cancer cells (Dudek, Martin, Garg, & Agostinis, 2013). NKT cells, depending on their T-cell receptor repertoire, have the potential to induce an antitumor response or promote tumor progression (Krijgsman, Hokland, & Kuppen, 2018). How these cells are involved in PD-L1-related immune evasion in SCCOT and how they respond to PD-L1 inhibitors will need to be investigated in the future.
In summary, this is the first work applying the QS and the xCell methods to investigate PD-L1 in SCCOT using a standardized FDA-approved IHC protocol. Our data contribute to a growing literature suggesting that the impact of PD-L1 on patient survival is gender-related. With the future development in assessing immune cell populations, transcriptome-based computational methods for cellular characterization provide additional information for understanding the complex tumor microenvironment and its response to tumor cells exhibiting varying degrees of immune checkpoint activity.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
T.W provided medical materials, analyzed data, and wrote the manuscript. X.G analyzed data and wrote the manuscript. L.B performed experiments and analyzed data. P.J.C analyzed data and wrote the manuscript. R.F wrote the manuscript. L.W analyzed data. N.H.N. analyzed clinical correlations. L.N.S provided medical materials. N.S analyzed data. K.N designed and supervised the project, performed experiments, and wrote the manuscript. All authors commented on the manuscript.
ETHICAL APPROVAL
This project was performed according to the principles of the Declaration of Helsinki after approval by the Regional Ethics Review Board, Umea, Sweden (dnr 03–201, 08-003M).
PATIENT CONSENT FOR PUBLICATION
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author XG on reasonable request.