Potential kidney protective effects of glucagon-like peptide-1 receptor agonists
Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have gained increasing attention for their potential benefits in people with type 2 diabetes (T2DM) with chronic kidney disease (CKD). This class of medication has demonstrated promising results in reducing albuminuria, preserving estimated glomerular filtration rate (eGFR), and mitigating cardiovascular (CV) risk, making them potential therapeutic options for individuals with CKD. The kidney protective effects of GLP-1RAs extend beyond glycaemic control, and are thought to be attributed to their anti-inflammatory, antioxidant, and natriuretic properties. Despite these promising findings, the use of GLP-RAs has yet to be definitively shown to slow progression to chronic kidney failure, or reduce CV and kidney related death in people with T2DM and CKD. The Research Study to See How Semaglutide (a once weekly subcutaneous administered GLP-1RA) Works Compared to Placebo in People with Type 2 Diabetes and Chronic Kidney Disease (FLOW trial) was recently stopped because of efficacy. The primary end point for the FLOW trial consists of a composite endpoint of (i) onset of chronic kidney failure; (ii) death from kidney failure; (iii) cardiovascular death; and (iv) onset of a persistent ≥50% reduction in eGFR from baseline. It has also been reported by the sponsors of the trial that the primary end point of the trial was reduced by 24% with both CKD and CV outcomes contributing to risk reduction. In anticipation of the results of the FLOW trial being published, we review the current evidence surrounding kidney outcomes and proposed kidney protective pathways associated with GLP-1RA use.
1 INTRODUCTION
Diabetic kidney disease (DKD) is a leading cause of end stage kidney disease (ESKD) and is a frequent microvascular complication of type 2 (T2DM) diabetes mellitus.1 DKD is associated with substantial morbidity and mortality, including a significant contribution to cardiovascular (CV) disease risk.2 Currently, the pharmacological cornerstones of treatment for DKD for people with T2DM include renin-angiotensin system (RAS) blockade, sodium glucose cotransporter-2 (SGLT2) inhibition and mineralocorticoid receptor antagonists (MRA), specifically finerenone.2, 3 Despite these therapies, there is ongoing burden of DKD, and therefore the need to develop new potential therapies to delay the onset and progression of DKD is a priority.
While kidney outcomes have been explored as secondary endpoints in GLP-1RA cardiovascular outcome trials (CVOTs), there is currently no evidence to definitively show an effect on slowing the progression of kidney function loss to ESKD or a reduction in CV or kidney-related mortality in people with T2DM and CKD. The research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW trial) was recently stopped because of efficacy.4 In anticipation of the results of this trial being published in 2024, we review the current evidence surrounding kidney outcomes and proposed kidney protective pathways associated with GLP-1RA use.
2 PATHOGENESIS OF DIABETIC KIDNEY DISEASE
Hyperglycaemia, hypertension, dyslipidaemia, albuminuria, obesity and inflammation are all involved in the initiation and promotion of CKD in people with T2DM.5 The first sign of DKD is hyperfiltration of individual nephrons, with albuminuria usually being an early clinical manifestation, but the phenomenon of non-albuminuria renal insufficiency is T2DM is well described.5, 6 Mesangial expansion and basement membrane thickening are very early structural manifestations of DKD. The main pathological characteristics as DKD progresses are fibrotic-driven damage to the glomerulus and tubular-interstitial tissues. Hyperglycaemia and obesity drive this tissue damage through the stimulation of multiple inflammatory pathways.7 Specifically, hyperglycemia results in the generation of advanced glycation end products (AGEs) that via activation of the receptor the AGEs (RAGE) promote activation of NF-kappaB and mitochondrial production of reactive oxygen species (ROS).5, 6
3 INCRETINS, MECHANISM OF ACTION AND CLINICAL BENEFITS
Incretins are gut-derived peptide hormones that modulate glucose homeostasis as well as other physiologic pathways.8 GLP-1 and GIP are incretin hormones produced by the intestinal mucosa by L-cells and K-cells, respectively, in response to ingestion of a meal.8 These hormones play an important role in regulating glucose homeostasis via regulating insulin and glucagon secretion and are also key players in body weight regulation. Both hormones are usually very quickly inactivated by dipeptidyl peptidase-4 (DPP-4).8 The role of GIP will only be discussed further in the context of its combined effects with other molecules on kidney health.
The therapeutic use of endogenous GLP-1 has been achieved by the manipulation of the molecule to prolong its biological action and through the development of specific DPP-4 inhibitors. The key advantage of DPP-4 inhibition is its safety profile and sustained efficacy in all stages of CKD.9 However, their clinical use is restricted to their modest glucose lowering effect as they do not have any major impact on kidney or CV health.9, 10 In clinical practice, the GLP-1RAs are used as glucose lowering agents (HbA1c decrease of 0.8%–1.5%) and as weight control agents (mean weight loss of ~3 kg).11 Multiple studies have also shown that they offer CV protection in people with T2DM.12-18 Recently, the GLP-1 RA semaglutide, at a dose of 2.4 mg per week, reduced CV and kidney related (primarily through the reduction in albuminuria) events in people without diabetes who had pre-existing atherosclerotic CV disease and a body mass index (BMI) ≥27 kg/m2 in the Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) trial.19
In addition to the above effects, GLP-1RAs reduce multiple inflammatory and oxidative stress markers, as discussed later, and inhibit RAGE-dependent pathways that result in tissue damage. However, the exact atherosclerotic and kidney protective effects of the GLP-1RAs still remain to be fully elucidated.
4 OVERVIEW OF INCRETINS AND KIDNEY HEALTH
Additionally, some GLP-1RAs have consistently been shown to reduce albuminuria and most likely slow the expected decline in estimated glomerular filtration rate (eGFR) in people with T2DM, indicating a potential kidney protective effect.10, 20 Indirect effects of GLP-1RAs that may be related to improved measures of kidney health include reductions in systolic blood pressure and HbA1c, and the promotion of weight loss.20 There is also evidence to suggest that GLP-1RAs directly affect kidney physiology, having positive effects on intra-renal haemodynamics, hormone modulation, and modulation of RAGE-induced inflammation and oxidative stress, as mentioned above, among other effects.20, 21 The effects of GLP-1RAs on clinical parameters that possibly mediate some of the kidney protective effects of this class of medication are specifically discussed in later sections of this review.
5 KIDNEY PHYSIOLOGY AND GLP-1
The effect of GLP-1 on kidney physiology is a current area of interest as pathways and mechanisms to explain the potential kidney-protective effects of GLP-1RAs are actively being investigated. GLP-1 has a natriuretic effect in humans, both healthy individuals and those with T2DM, but this effect only appears to occur in the presence of extracellular fluid expansion.22-24 This effect is independent of changes in renal plasma flow and GFR.22 GLP-1 activates renal afferent nerves in rats, and the modulation of this nerve activity may regulate this natriuretic effect, although this proposed relationship has not been demonstrated in humans.25 In addition, inhibition of the sodium-hydrogen exchanger-3, which results in a reduction in sodium and bicarbonate reabsorption in the proximal tubule, has been implicated as a promoter of GLP-1 induced natriuresis and improvements in blood pressure.26 Indeed, injection of the GLP-1RA liraglutide in humans reduces proximal tubule sodium reabsorption (as measured by lithium clearance) and increases urinary pH,27 with similar effects on acute infusion of the GLP-1RA exenatide.28, 29 GLP-1RAs may also reduce systemic blood pressure via vasodilation, effects on central BP control and effects on the renin-angiotensin system.30 However, studies of GLP-1RA effects on GFR, effective renal plasma and intra-renal arterial tone have yielded inconsistent results.28, 29, 31
GLP-1, when administered as a 7–36 amide (an active form of GLP-1) at physiologic levels, also induces a significant reduction in circulating levels of angiotensin II, without corresponding changes in renin or aldosterone in healthy adults, an important finding given the well-established kidney-protective effects of RAS blockade.31, 32 The mechanism of angiotensin II suppression in this setting is not yet known. The natriuretic effect and suppression of angiotensin II, as described above, depends on GLP-1 receptor activation as demonstrated by the fact that these effects are abolished by co-administration of a GLP-1 receptor antagonist.33 In contrast, when the GLP-1RA semaglutide is administered to people with T2DM already taking SGLT2 inhibitors, aldosterone levels are reduced without any changes in renin levels, angiotensin II levels were not measured in this study.34 In summary, the effect of GLP-1RAs on the RAS and aldosterone levels remains to be fully established.
Both renal cortical and medullary perfusion, as well as renal oxygen tension, are increased in response to infusion of GLP-1 (achieving slightly supraphysiologic levels) in volume loaded individuals, in the absence of changes in renal arterial blood flow.23 As angiotensin II has a vasoconstrictor effect on medullary vessels, this increased perfusion may reflect the GLP-1 induced reduction of angiotensin. It has been proposed that this improved renal perfusion may contribute to kidney-protective effects of GLP-1RAs.23 The mechanistic REMODEL trial (NCT04865770) will investigate the effect of semaglutide on inflammatory and hypoxia-related pathways in the kidney via the use of functional kidney magnetic resonance imaging and the interrogation of kidney biopsy samples as discussed later in this article.35
6 GLP-1 EXPRESSION AND GLP-1 RECEPTORS IN THE KIDNEY
The mapping of GLP-1 receptors within the kidney has been challenging and the exact localization of receptors remains to be established. This is partly related to the lack of specificity of GLP-1 receptor antibodies. However, GLP-1 receptor messenger RNA has been identified in vascular smooth muscle cells of preglomerular arterioles in both human and animal kidneys.36 GLP-1 receptor activation leads to stimulation of the cyclic adenosine monophosphate–protein kinase A pathway that most likely produces anti-oxidative and anti-inflammatory effects. Indeed, the GLP-1RA liraglutide reduces albuminuria and mesangial expansion which is associated with decreased levels of glomerular superoxide and renal NAD(P)H oxidase in diabetic mice, an effect that is abolished by the administration of adenylate cyclase and protein kinase A inhibitors.36
7 GLP-1 RECEPTOR SIGNALLING AND PATHWAYS THAT HAVE KIDNEY PROTECTIVE EFFECTS
Murine studies, using an adeno-associated viral vector system to overexpress GLP-1 and its cleavage products, have demonstrated that GLP-1 constructs have systemic immunomodulatory effects, reduce tubulointerstitial kidney damage, lower expression of tubular injury markers and attenuate kidney tissue accumulation of macrophages and T cells.37 Of interest, these effects were seen both in a diabetic mouse model but also in an acute kidney ischaemia/perfusion mouse model.37 In clinical studies, the use of the GLP-1RA dulaglutide has also been associated with a possible reduction in kidney fibrosis, especially those related to a reduction in type VI collagen formation and an increased rate of degradation.38
More recently, other potential glucose-independent kidney protective pathways mediating the actions of GLP-1 have been described in a series of elegant experimental mouse-based studies. First, in mice without diabetes, global genetic deletion of the receptor of GLP-1 resulted in kidney injury, as gauged by the development of albuminuria and glomerulosclerosis.21 The induction of diabetes through the injection of streptozotocin exacerbated albuminuria in the above model. In contrast, the deletion of the gene for RAGE (the receptor for advanced glycation end-products) attenuated the above indices of kidney damage in the setting of diabetes. In addition, treatment of wild-type diabetic mice with the liraglutide, reduced kidney damage, down regulated kidney RAGE expression, and attenuated inflammatory signals in macrophages.21
8 KIDNEY OUTCOMES WITH GLP-1RA CVOTs
Cardiovascular (CV) outcome trials have provided insights into the potential kidney protective effects of the GLP-1RAs. These trials have involved the comparison of a particular GLP-1RA versus placebo in participants with T2DM with and without a clinical history of CV disease. The aim of these trials has been to assess the CV safety of the GLP-1RA class of medication on a background of normal clinical care. They have shown that the GLP-1RA class of medications is not only safe from the CV point of view but also has the potential to provide CV protection. Participants in these trials have generally not had established chronic kidney disease (CKD) and hence kidney health outcomes reported from these trials need to be interpreted with caution. GLP-1RA CVOTs that have reported on an exploratory composite primary kidney outcome endpoint are presented in Tables 1 and 2. Most CVOTs (LEADER-liraglutide, SUSTAIN-6-semaglutide, REWIND-dulaglutide and AMPLITUTE-O-efpeglenatide), have reported a significant reduction in the trial's primary composite kidney outcome with GLP-1RA use versus placebo, but this outcome has mainly been driven by a slowing of progression of albuminuria.12, 13, 15, 18
| Trial (Year) | Drug | Duration | Major inclusion criteria | Kidney status at baseline | Primary composite kidney outcome components | Composite kidney function outcome (excluding albuminuria) components |
|---|---|---|---|---|---|---|
| LEADER (2016) | Liraglutide subcut 1.8 mg/day versus placebo | Median follow up 3.8 years | T2DM with HbA1c ≥7.0% and: age ≥ 50 years with ≥1 cardiovascular comorbidity, or age ≥ 60 years with ≥1 cardiovascular risk factor | eGFR: 80 mL/min/1.73m2 UACR: 21 mg/g |
New-onset persistent macroalbuminuria, persistent doubling of the serum creatinine level and eGFR of ≤45 mL/min/1.73m2, RRT, or death due to renal disease | NR |
| SUSTAIN-6 (2016) | Semaglutide subcut 0.5 or 1.0 mg/week versus placebo | 104 weeks | T2DM with HbA1c ≥7% and: age ≥50 years with established cardiovascular disease or chronic kidney disease of Stage 3 or higher, or age ≥60 years with ≥1 cardiovascular risk factor | eGFR: 80 mL/min/1.73m2 UACR: 37 mg/g |
New onset persistent macroalbuminuria, or persistent doubling of serum creatinine level and creatinine clearance <45 mL/min/1.73 m2, or the need for continuous RRT (in the absence of an acute reversible cause) or death due to renal disease. | NR |
| EXSCEL (2017) | Exenatide subcut 2 mg/week versus placebo | Median follow up 3.2 years | T2DM with HbA1c 6.5%–10% and eGFR≥30; 70% had previous cardiovascular event and 30% had no previous cardiovascular event | eGFR: 77 mL/min/1.73m2 UACR: 23 mg/g |
Incident macroalbuminuria plus an increase in the UACR of ≥30% from baseline, a sustained decrease in the eGFR of ≥40% for ≥30 days, RRT for ≥90 days, or a sustained eGFR of <15 mL/min/1.73 m2 for ≥30 days) | Outcome 1: Sustained (two or more consecutive measurements) eGFR reduction of ≥40% or ESKD (chronic dialysis or kidney transplantation) Outcome 2: Sustained eGFR reduction of ≥30% or ESKD (chronic dialysis or kidney transplantation)a |
| REWIND (2019) | Dulaglutide subcut 1.5 mg/week versus placebo | Median follow up 5.4 years | T2DM, age ≥ 50 years with a previous cardiovascular event or cardiovascular risk factors | eGFR: 75 mL/min/1.73m2 UACR: 16 mg/g |
New macroalbuminuria, sustained ≥30% decline in eGFR (ie, based on two consecutive eGFR concentrations), or new chronic RRT comprising dialysis or renal transplantation | eGFR reduction ≥40% from baseline at two or more consecutive visits or ESKD (kidney replacement therapy, kidney transplant or adverse events reported as ESKD) or kidney related deathb |
| AMPLITUDE-O (2021) | Efpeglenatide subcut 4 or 6 mg/week versus placebo | Median follow up 1.8 years | T2DM and either history of CV disease or current kidney disease (eGFR 25–59.9 mL/min/1.73m2) plus additional CV risk factor | eGFR: 72 mL/min/1.73m2 UACR: 28 mg/g |
Incident macroalbuminuria (UACR >300 mg/g) plus an increase in the UACR of at least 30% from baseline, a sustained decrease in eGFR of ≥40% for 30 days or more, RRT for 90 days or more, or a sustained eGFR of <15 mL/min/1.73 m2 for 30 days or more | eGFR reduction of ≥40% for ≥30 days, end-stage kidney disease (defined as dialysis for ≥90 days, kidney transplantation, or an eGFR of <15 mL/min/1.73 m2 for ≥30 days), or death from any cause |
| Trial (year) | Effect of GLP-1 RA on primary composite kidney outcome endpoint | Effect of GLP-1 RA on albuminuria | Effect on composite kidney function endpoint (excluding albuminuria) | Effect of GLP-1 RA on change in eGFR | Effect of GLP-1 RA on ESKD |
|---|---|---|---|---|---|
| LEADER (2016) | Significant reduction (HR = 0.78, 95% CI: 0.67–0.92, p = .003 for liraglutide versus placebo) | New onset macroalbuminuria reduced by 26% (HR 0.74, 95% CI: 0.67–0.92), p = .003 for liraglutide versus placebo | NR | 2% reduction in rate of decline of eGFR with liraglutide versus placebo, p = .01 Doubling of serum creatinine non-significantly reduced by 11% (HR 0.89, 95% CI: 0.67–1.19) |
No significant difference for liraglutide versus placebo, (HR = 0.87, 95% CI: 0.61–1.24) |
| SUSTAIN-6 (2016) | Significant reduction (HR 0.64, 95% CI: 0.46–0.88 for semaglutide versus placebo) | New onset macroalbuminuria reduced by 46% (HR 0.54, 95% CI: 0.37–0.77), p = .003 for semaglutide versus placebo | NR | Significant reduction in annual change in eGFR: estimated treatment difference 0.6 mL/min/1.73m2 (95% CI: 0.24–0.96, p = .001) Doubling of serum creatinine not reduced (HR 1.28, 95% CI: 0.64–2,58) |
No significant difference for semaglutide versus placebo, (HR = 0.91, 95% CI: 0.40–2.07) |
| EXSCEL (2017) | Non-significant reduction (HR = 0.88, 95% CI: 0.76–1.01) | Albuminuria reduced compared to placebo in the exenatide group: −21.6% (95% CI: −32.3% to −9.1%) for baseline UACR ≤30 mg/g, −28.2% (−39.2% to −15.2%) for baseline UACR >30 mg/g and − 34.5% (95%CI: −54.4 to −6%) for UACR >200 mg/g |
Outcome 1: Non-significant reduction (HR = 0.86, 95% CI 0.72–1.04) Outcome 2: Significant reduction (HR = 0.87, 95% CI 0.77–0.99) for exenatide versus placeboa |
Rate of eGFR decline was reduced in the exenatide group for patients with a baseline UACR >100 mg/g (treatment effect 0.79 mL/min/1.73m2, p = .005) and UACR >200 mg/g (treatment effect 1.32 mL/min/1.7m2, p = .0005), but not in those with lower UACRa | NR |
| REWIND (2019) | Significant reduction (HR 0.85, 95% C: I 0.77–0.93 for dulaglutide versus placebo) | New onset macroalbuminuria reduced by 23% (HR = 0.77, 95% CI: 0.68–0.87) For dulaglutide versus placebo |
Significant reduction (HR = 0.72, 95% CI 0.58–0.88) for dulaglutide versus placebob | Rate of sustained eGFR reduction of ≥30% was non-significantly reduced in dulaglutide versus placebo groups (HR = 0.89, 95% CI: 0.78–1.01) Rate of sustained eGFR reduction of ≥40% was significantly reduced in dulaglutide versus placebo groups (HR = 0.72, 95% CI: 0.58–0.88), p = .002b |
No significant difference for dulaglutide versus placebo, (HR = 0.75, 95% CI: 0.39–1.44) |
| AMPLITUDE-O (2021) | Significant reduction (HR 0.68, 95% CI: 0.57–0.79, p < .001 for efpeglenatide versus placebo) | Significant reduction compared to placebo by 21% (HR 0.79 95% CI: 0.72–0.86) | Non-significant reduction (HR = 0.77, 95% CI: 0.57–1.02) for efpeglenatide versus placebo | Non-significant reduction (HR = 0.89, 95% CI: 0.27–151) for efpeglenatide versus placebo | NR |
However, a subsequent analysis from the REWIND CVOT has shown that the use of dulaglutide is associated with a reduction in a composite kidney function endpoint that does not include albuminuria (≥40% decline in eGFR, ESKD or kidney-related death) by 28% (HR 0.72, 95% CI: 0.58–0.88, p = .002) versus placebo treatment.39 In contrast, the primary kidney endpoint of the EXSCEL CVOT was not significantly reduced with exenatide, but some favourable effects of exenatide on albuminuria, GFR loss and a combined kidney function been reported in sub-studies from this trial.17, 40-42 In the AMPLITUDE-O CVOT the composite kidney function endpoint (excluding albuminuria) was reduced by 23% with efpeglenatide therapy, but this reduction failed to reach statistical significance.12
Other major GLP-1RA CVOTs that have reported on separate kidney outcomes are presented in Tables 3 and 4. The ELIXA trial did not report on a composite kidney outcome, but the use of lixisenatide in the trial was associated with favourable effects on albuminuria without any effect on GFR compared to placebo.16, 43 HARMONY and PIONEER-6 reported that Albiglutide and oral Semaglutide, respectively, had no effect on eGFR values compared to placebo.44, 45 A 2021 meta-analysis of eight CVOTs found that GLP-1 RAs reduced the risk of a composite kidney outcome including new albuminuria, worsening renal function and end stage kidney disease by 21%.46
| Trial (Year) | Drug | Duration | Major inclusion criteria | Kidney status at baseline |
|---|---|---|---|---|
| ELIXA (2015) | Lixisenatide subcut 20 μg/day versus placebo | 25 months | T2DM, age ≥ 30 years, eGFR>30 mL/min/1.73m2 and an acute coronary event in the 180 days prior to screening | eGFR: 78 mL/min/1.73m2 UACR: 10 mg/g |
| Harmony (2018) | Albiglutide subcut 15 mg/week versus placebo | Median follow up 1.6 years | T2DM, age ≥40 years, HbA1c >7.0%, eGFR >30 mL/min/1.73m2, established coronary, cerebrovascular or peripheral artery disease | eGFR: 79 mL/min/1.73m2 UACR: NR |
| PIONEER-6 (2019) | Semaglutide oral 14 mg/day versus placebo | Median follow up 15.9 months | T2DM, eGFR >30 and: age ≥ 50 years with established cardiovascular disease or chronic kidney disease, or age ≥ 60 years with ≥1 cardiovascular risk factor | eGFR: 74 mL/min/1.73m2 UACR: NR |
| AWARD-7 (2018)a | Dulaglutide once weekly 0.75 mg or 1.5 mg subcut versus titrated insulin glargine | 52 weeks | Adults with T2DM, HbA1c 7.5–10.5% and CKD stages 3–4 Who were already treated with insulin +/− oral anti-hyperglycaemic drug and RAS blockade |
eGFR: 38 mL/min/1.73m2 UACR: 214 mg/g |
| SURPASS-4 kidney outcomes (2022) | Tirzepatide subcut weekly (does 5,10,15 mg) versus titrated insulin glargine | 104 weeks | Adults with T2DM, HbA1c 7–10.5%, eGFR >30 mL/min/1.73m2 | eGFR: 81 mL/min/1.73m2 UACR: 15 mg/g |
- Note: ELIXA, evaluation of lixisenatide in acute coronary syndrome.43 Harmony outcomes, albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease.44 PIONEER 6, oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes.45 AWARD-7, dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease.53 SURPASS, tirzepatide versus semaglutide once weekly in patients with type 2 diabetes.71 RAS, renin-angiotensin-system; NR, not reported.
- a A composite kidney function endpoint was also reported from this study as discussed in the text.54
| Trial (Year) | Effect of GLP-1 RA on albuminuria | Effect of GLP-1 RA on change in eGFR |
|---|---|---|
| ELIXA (2015) | Lixisenatide reduced UACR compared to placebo in patients with baseline macroalbuminuria (−39%, p = .007) and microalbuminuria (−21%, p = .05), but not with normoalbuminuria | No significant difference eGFR values for lixisenatide versus placebo |
| Harmony Outcomes (2018) | NR | Not significant: Mean eGFR difference at 16 months between albiglutide and placebo −0.43 mL/min/1.73m2 (95% CI: −1.26 to 0.41) |
| PIONEER-6 (2019) | NR | Significant reduction in annual change in eGFR: estimated treatment difference 0.55 mL/min/1.73m2 (95% CI: 0.04–1.06, p = .03) |
| AWARD-7 (2018)a | Overall, no significant difference in UACR lowering effect of insulin glargine versus dulaglutide. However, UACR decreases were significantly larger with dulaglutide 1∙5 mg than with insulin glargine in patients with macroalbuminuria: 0.1% for insulin glargine versus −29.0% for dulaglutide 1∙5 mg, p = .020 versus insulin glargine | At 52 weeks, eGFR did not change with dulaglutide 1.5 mg (−1.1 mL/min/1.73m2, but declined from baseline with insulin glargine (−2.9 mL/min/1.73m2, p < .0001). The difference in eGFR values between insulin glargine versus dulaglutide 1.5 mg, but not dulaglutide 0.75 mg, was significantly different |
| SURPASS-4 Kidney outcomes (2022) | Pooled tirzepatide (5,10,15 mg) doses reduced UACR by 39% (95% CI: 37.7 to −25.7) versus insulin glargine | eGFR slope difference was +2.21 (95% CI: 1.59–2.83), for pooled tirzepatide doses versus insulin glargine. |
In general, GLP-1RAs CVOTs have reported favourable outcomes related to kidney function loss but this has not been a universal finding. To date, no CVOT has shown a reduction in the development of the need for chronic kidney replacement therapy associated with the use of GLP-1RAs.
9 KIDNEY-PROTECTIVE MECHANISM OF GLP-1RAs: EVIDENCE FROM CVOTs
A meta-regression analysis of CVOTs reporting composite kidney outcomes was carried out to determine whether reduction in body weight or HbA1c was responsible for the kidney outcome improvements.47 For both cardiovascular outcomes and the secondary outcomes, including kidney outcomes, it was HbA1c reduction rather than body weight reduction which was associated with reduced CV and kidney risk. There was a 35% decrease in the log-HR of the composite kidney outcome for every 1.0% normalized HbA1c reduction, while normalized body weight reduction was not associated with this outcome.47 It should be appreciated that, as mentioned previously, changes in albuminuria were the main driver of a decrease in the composite primary kidney outcomes endpoint reported in CVOTs involving GLP-1RAs and that improved glycaemic control has been linked to improvements in albuminuria in multiple studies.48
Interestingly, in LEADER, the level of mediation of HbA1c, blood pressure and body weight differed according to baseline eGFR subgroup. In those patients with a baseline eGFR of ≥60 mL/min/1.73m2, HbA1c mediated the probability of a kidney endpoint occurring by 57%, while in the baseline eGFR <60 mL/min/1.73m2 group, HbA1c did not mediate the likelihood of this outcome.49 Similarly, bodyweight mediated the likelihood of a kidney event by 29% in patients with higher baseline eGFR and did not have a mediating effect in the group with eGFR <60 mL/min/1.73m2. Systolic blood pressure, conversely, did have a modest mediatory effect in both eGFR groups (8% for eGFR ≥60 mL/min/1.73m2 and 15% for eGFR <60 mL/min/17.3m2).49 This suggests that, particularly in patients with lower eGFR at baseline, other direct factors are more responsible for the improvement in composite kidney outcomes with GLP-1RAs than improvements in blood pressure, glycaemic control, and weight management. Overall, the above findings reinforce the importance of achieving early tight metabolic control as a means of slowing down kidney function loss in people with T2DM.
10 KIDNEY OUTCOMES OF GLP-1RAs IN REAL-WORLD STUDIES
A number of real-world studies have examined renal outcomes with GLP-1RA use, with promising results. A large Scandinavian cohort study comparing new GLP-1RA (dulaglutide, liraglutide, exenatide or lixisenatide) users with new users of DPP-4 inhibitors showed a significant 24% reduction in a combined serious renal outcome comprising the need for renal replacement therapy, death from renal causes, and hospitalization for renal events.50 Another cohort study followed up patients with T2DM for 3 years and compared outcomes in those using sulfonylureas, SGLT2 inhibitors, DPP-4 inhibitors and GLP-1RAs. A composite outcome of eGFR decline >50%, ESKD, or all-cause mortality was significantly less common in GLP-1RA users than users of sulfonylureas (HR = 0.72, 95% CI 0.67–0.77) and DPP-4 inhibitors (HR = 0.79, 95% CI 0.74–0.85) but was not reduced compared to SGLT2 inhibitor users.51 A small observational study found that liraglutide was associated with a non-significant reduction in albuminuria of 17 mg/L over a 36-month period, with stable eGFR.52 While these data must be interpreted with caution, they complement the CVOTs with interesting insight into the real world effects of GLP-1RAs in clinical practice.
11 GLP-1RAs COMPARED TO INSULIN
The AWARD-7 trial compared glycaemic control and kidney outcomes for patients (n = 576) with T2DM and moderate to severe CKD (normo-[22%], micro-[33%] and macroalbuminuria [45%]) treated with the GLP-1RA dulaglutide (0.75 mg and 1.5 mg doses) versus titrated insulin glargine (U100), both in combination with the quick-acting insulin lispro.53 This trial showed that overall, dulaglutide and insulin glargine produced similar glycaemic control and reduced albuminuria to a similar extent, but in macroalbuminuric patients, dulaglutide (1.5 mg but not 0.75 mg) had a greater albuminuria reducing effect. Dulaglutide (1.5 mg dose only) was also more efficacious in slowing down eGFR decline over the 52 weeks of the trial compared to insulin glargine (Tables 3 and 4).53 In addition, as shown in Figure 1, the risk of a composite kidney endpoint comprising of reduction of eGFR by ≥40% or persistent kidney replacement therapy was significantly lower in the dulaglutide (1.5 mg per week) versus insulin group (5.2% vs. 10.8%, p = .04) with this effect being dose-dependent (the 1.5 mg group having a lower rate of endpoint events than the 0.75 mg group).54

12 GLP-1RAs IN COMBINATION WITH SGLT2 INHIBITORS
Overall, there is no definitive evidence of additional kidney benefit with combined GLP-1RAs and SGLT2 inhibitor use, although there is a suggestion of potential benefit from some studies.55-57 The role and potential additive kidney benefit of GLP-1RAs in combination with SGLT2 inhibitors is an area of ongoing research interest.
13 ADVERSE EFFECTS OF GLP-1RAs
The gastrointestinal (GI) side-effects of GLP-1RAs usually improve after a few weeks of therapy and it is recommended to start with low-dose GLP-1RA therapy to minimize GI side-effects.58 Hypoglycaemia is not a feature of GLP-1RA use if this class of medication is not combined with insulin and/or sulfonylurea use.58
As post-marketing surveillance has suggested a possible increased risk of pancreatitis with GLP-1RAs, these agents should be used with extreme caution in patients with prior history of pancreatitis.59 An increase in heart rate and a decrease in blood pressure has also been reported with the use of GLP-1RAs.60 While studies have explored the potential link between GLP-1RAs and thyroid cancer, especially medullary thyroid cancer, current evidence suggests conflicting results. Current prescribing guidelines have medullary thyroid cancer as a contraindication to GLP-1RA use, but it would be prudent to very carefully assess the risks versus benefits of GLP-1RA use for people with a history of any form of thyroid cancer.61-65
Another practice point to consider is that GLP1-RAs should not be used in combination with DPP-4 inhibitors as their use becomes redundant due to the fact that these medications increase endogenous GLP-1 levels.66
14 USING GLP-1RAs IN THE SETTING OF CKD
Although experience with GLP-1RAs is limited in people with eGFR levels <30 mL/min/1.73m2, most can be safely used in the setting of moderate to severe CKD with the appreciation of some precautions. Reassuringly, a recent observational study has suggested that the presence of CKD does not influence the safety profile or efficacy, in terms of improvements in glycaemic control or body weight, in people with T2DM.67 Of note, liraglutide, semaglutide and dulaglutide are not primarily excreted by the kidneys, so no dose adjustment is required for these medications as eGFR declines.68 However, the well described side effects of nausea, vomiting and possibly diarrhoea with the GLP-1RAs and the associated dehydration could contribute to a worsening in kidney function and the development of acute kidney injury. A patient's uraemic symptoms should also be evaluated carefully as this may also contribute to a patient's degree of nausea and decreased appetite.
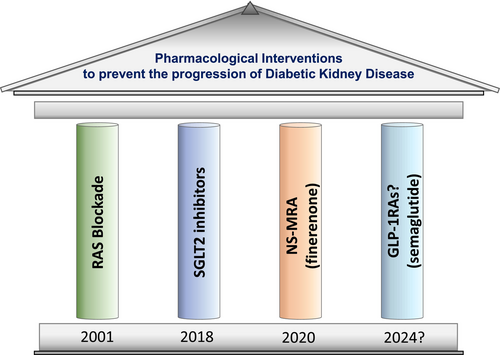
Current Kidney Disease Improving Global Outcomes (KDIGO) guidelines for managing diabetes in patients with CKD recommend GLP1-RAs as the next-in-line therapy after initiation of metformin and SGLT2 inhibitors use for further HbA1c lowering and/or reduction in CV risk.69 It will be of great interest to see where GLP-1RAs are positioned in CKD treatment algorithms after the release of the FLOW trial results. Indeed, recent reviews have suggested that in addition to the existing three pillars of treatment to slow progression of DKD in the setting on T2DM, those being RAS blockade, SGLT2 inhibitors and finerenone, GLP-1RAs may possibly be an additional ‘fourth pillar’ in the quest to further slow kidney function loss (Figure 2).2
15 POTENTIAL KIDNEY BENEFITS OF INCRETIN-BASED THERAPIES BEYOND GLP-1RAs
Tirzepatide is a dual GLP-1 and GIP agonist that also possibly has kidney protective benefits (Tables 3 and 4).70-72 This medication has already been shown to cause unprecedented reductions in HbA1c, clinically significant weight loss and other metabolic benefits in people with T2DM.73 Tirzepatide has consistently been shown to reduce albuminuria in the setting of T2DM.70 A post hoc analysis of the SURPASS-4 trial, which compared the effects of tirzepatide versus titrated insulin glargine, showed that tirzepatide slowed the rate of eGFR decline (−1·4 vs. −3·6 mL/min/1·73 m2 per year) and reduced a composite kidney endpoint (time to first occurrence of eGFR decline of at least 40% from baseline, end-stage kidney disease, death owing to kidney failure, or new-onset macroalbuminuria) compared to insulin glargine treatment (HR: 0.58, 95% CI: 0.43–0.83), respectively, during a mean treatment duration of 85 weeks.71 A small mechanistic study that will also focus on changes in albuminuria and measured GFR in people with obesity with or without T2DM is currently underway (TREASURE-CKD; NCT05536804).
Triple receptor agonists that bind to GLP-1, GIP and glucagon receptors and dual amylin and GLP-1 receptor agonists have been developed, and are currently being tested as therapies for T2DM and obesity.74 Currently, no information regarding the kidney benefits of these medications is available. However, a study that will look at changes in urinary albumin and estimated GFR in people with T2DM and CKD who are overweight or obese treated with cargrilintide, semaglutide (2.4 mg weekly) or the combination of both medications (CargriSema) is just about to start recruitment (NCT06131372).
16 FUTURE DIRECTIONS
While secondary composite kidney endpoints and post hoc analyses from CVOTs have been useful in highlighting a likely kidney benefit of GLP-1RA use, the call to specifically design trials to determine clinically important kidney-related outcomes and to further delineate potential kidney protective pathways associated with the use of GLP-1RAs is in the process of being answered.
The FLOW trial (NCT03819153), which started in 2019, will be the first GLP-1RA dedicated kidney outcome trial and will assess whether weekly treatment with semaglutide protects against kidney function loss and ultimately slows progression to chronic kidney failure.3 This is an international, randomized, double-blind, placebo-controlled trial in which participants have T2DM and established kidney disease (defined as eGFR 50-75 mL/min/1.73m2 and UACR 300-5000 mg/g, or eGFR 25-50 mL/min/1.73m2 and UACR 100-5000 mg/g).3 Participants have been randomized 1:1 to weekly subcutaneous semaglutide (1 mg dose) or placebo in addition to standard of care treatment including RAS inhibition. The primary endpoint of the trial, after 3–5 years of exposure, will be time to onset of a composite endpoint comprising onset of kidney failure (need for ongoing kidney replacement therapy or persistent eGFR <15 mL/min/1.73m2), death from kidney failure or cardiovascular causes and persistent ≥50% decrease in eGFR. Secondary endpoints will include annual rate of change of eGFR, a composite cardiovascular endpoint and time to onset of the individual components of the primary composite endpoint (Figure 3).3
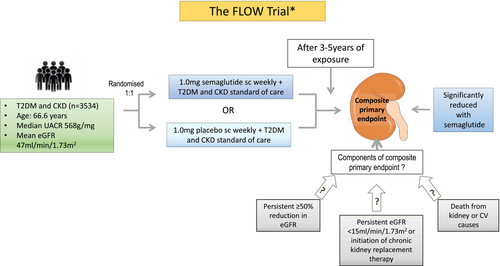
A detailed description of the baseline characteristics of participants in the FLOW trial has already been published.3 However, in summary, 3534 participants were randomized in the trial, who have a mean age of 67 years, 70% were male and 66% were white. Baseline characteristics of trial participants included an HbA1c of 7.8% (69% had a baseline level >7%), diabetes duration of 17 years, eGFR of 47 mL/min/1.73 m2, UACR of 568 mg/g (macroalbuminuria was present in 68%), body mass index of 32.0 kg/m2, systolic and diastolic blood pressure were 138.6 and 76.4 mmHg, respectively, history of CV disease was present in 52% and only 16% were receiving SGLT2 inhibitors.3
On 10 October 2023, the sponsor of the FLOW trial, Novo Nordisk, announced the decision to stop the trial due to efficacy.75 It has also subsequently been reported by the sponsors of the trial that the primary end point of the trial was reduced by 24% (Hazard Ratio: 0.76, 95% CI 0.66–0.88) with both CKD and CV components of the primary endpoint contributing to risk reduction.76 After the results of the FLOW trial are reflected on, it will be interesting to see what further potential benefits of GLP-1RAs in DKD are explored. For example, the use of GLP-1RAs in people with type 1 diabetes has not yet been studied, but could be a future focus. Mechanistic studies such as the Trial of semaglutide for diabetic kidney disease in type 1 diabetes (RT1D) have already been planned (NCT05822609), as well as the type 1 diabetes impacts of semaglutide on cardiovascular outcomes (T1-DISCO) Study (NCT05819138). A small study has suggested a potential benefit of GLP-1RAs in people with T2DM post-kidney transplant in terms of protecting against kidney function loss but currently there is no definitive evidence to support their use in this population for kidney protection.77
Another GLP-1RA outcomes trial has completed randomization and should also provide some extra useful information regarding the potential kidney protective effects of semaglutide when it is completed. The SOUL trial (Semaglutide cardiOvascular oUtcomes triaL) will investigate the effects of daily oral semaglutide on the risk of CV events in 9650 individuals with T2DM and established atherosclerotic CV disease and/or CKD.78 The main secondary outcome for the trial will be a kidney composite outcome of CV death, kidney-related death, persistent ≥50% reduction from baseline in eGFR, persistent eGFR <15 mL/min/1.73m2 or the initiation of chronic kidney replacement therapy.78
The international REMODEL study, which is currently in progress, will complement the above outcome trials through its aim to elucidate the mechanism of action of semaglutide on the kidney.35 Adults with T2DM and kidney disease (eGFR 40-75 mL/min/1.73m2 and UACR 30-5000 mg/g), who are already stable on RAS inhibition, will be randomized 2:1 to weekly subcutaneous semaglutide 1 mg or placebo in addition to standard of care.35 Subjects enrolled in this study will have baseline and follow-up MRIs and kidney biopsies. The primary endpoint of the study will be functional MRI-derived changes in kidney oxygenation (BOLD MRI R2*), perfusion (phase-contrast MRI), and inflammation (T1 Mapping MRI). MRIs will be repeated at weeks 4 and 52. Secondary MRI derived endpoints will include the apparent diffusion coefficient which estimates renal fibrosis evaluated from diffusion-weighted MRI. Kidney biopsies will also be obtained at baseline and at 52 weeks and other secondary endpoints of interest in the trial derived from these biopsies will include change from baseline to week 52 in intra-renal mRNA expression, assessed by single-nucleus transcriptomics and glomerular basement membrane width. Clinical parameters that will also be measured include changes in natriuresis, albumin excretion rate and creatinine clearance.35 This study will provide new mechanistic insights into potential hypoxia and inflammation related kidney-protective mechanisms of GLP-1RAs.
17 CONCLUSION
It has been demonstrated, through secondary analyses of clinical trials focusing on CV safety, that GLP-1RAs reduce albuminuria and may reduce rate of eGFR decline in people with T2DM. Reduced progression to chronic kidney failure, on the other hand, has not yet been proven. GLP-1RAs maintain their favourable metabolic effects in people with T2DM and CKD and provide CV protection. We await the results of the FLOW trial to guide the optimal use of GLP-1RAs in the clinical care of people with T2DM and CKD, and the REMODEL trial to clarify the possible mechanisms of kidney protection with GLP-1RAs.
ACKNOWLEDGEMENTS
RM: Is a principal investigator and a member of the expert global panel for the FLOW trial. Has received research grants from Novo Nordisk, Servier, Medtronic, The Rebecca Cooper Medical Research Foundation, St Vincent's Research Foundation, The Juvenile Diabetes Research Foundation, Grey Innovations, The Diabetes Australia Research Trust/Program and The National Health and Medical Research Council of Australia. Also received honoraria for lectures from Eli Lilly, Novo Nordisk, Sanofi Aventis, Astra Zeneca, Merck Sharp & Dohme, Norvartis and Boehringer Ingelheim and has been or is on the advisory boards for Novo Nordisk, Boehringer Ingelheim-Eli Lilly Diabetes Alliance, Astra Zeneca and Merck Shape and Dohme. Travel support has been supplied by Novo Nordisk, Sanofi, Boehringer Ingelheim and Astra Zeneca. Has been a principal investigator for industry-sponsored clinical trials run by Novo Nordisk, Sanofi, Bayer, Johnson-Cilag and Abbive. EIE's institution receives research funding from Eli Lilly, Novo Nordisk, Amgen, Versanis, Endogenex, Novartis, Astra Zeneca and EIE is on advisory boards and gives presentations for Eli Lilly, Astra Zeneca, Boehringer and these funds are donated towards diabetes research at EIE's institution. The Australian Centre for Accelerating Diabetes Innovations (ACADI) was established through MRFF Funding from the Australian Government's Targetted Translational Research Accelerator Program Delivered by MTP Connect. Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.




