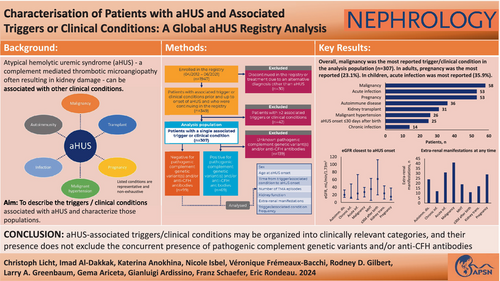
Characterization of patients with aHUS and associated triggers or clinical conditions: A Global aHUS Registry analysis
Abstract
Introduction
Atypical haemolytic uremic syndrome (aHUS) is a rare form of thrombotic microangiopathy (TMA) associated with complement dysregulation; aHUS may be associated with other ‘triggers’ or ‘clinical conditions’. This study aimed to characterize this patient population using data from the Global aHUS Registry, the largest collection of real-world data on patients with aHUS.
Methods
Patients enrolled in the Global aHUS Registry between April 2012 and June 2021 and with recorded aHUS-associated triggers or clinical conditions prior/up to aHUS onset were analysed. aHUS was diagnosed by the treating physician. Data were classified by age at onset of aHUS (< or ≥18 years) and additionally by the presence/absence of identified pathogenic complement genetic variant(s) and/or anti-complement factor H (CFH) antibodies. Genetically/immunologically untested patients were excluded.
Results
1947 patients were enrolled in the Global aHUS Registry by June 2021, and 349 (17.9%) met inclusion criteria. 307/349 patients (88.0%) had a single associated trigger or clinical condition and were included in the primary analysis. Malignancy was most common (58/307, 18.9%), followed by pregnancy and acute infections (both 53/307, 17.3%). Patients with an associated trigger or clinical condition were generally more likely to be adults at aHUS onset.
Conclusion
Our analysis suggests that aHUS-associated triggers or clinical conditions may be organized into clinically relevant categories, and their presence does not exclude the concurrent presence of pathogenic complement genetic variants and/or anti-CFH antibodies. Considering a diagnosis of aHUS with associated triggers or clinical conditions in patients presenting with TMA may allow faster and more appropriate treatment.
1 INTRODUCTION
Atypical haemolytic uremic syndrome (aHUS) is a rare form of thrombotic microangiopathy (TMA) commonly associated with dysregulation of the alternative pathway of the complement system.1, 2 aHUS results in thrombocytopenia, microangiopathic haemolytic anaemia and organ injury, most commonly of the kidneys.2, 3 Currently, aHUS remains a clinical diagnosis, typically following exclusion of other conditions such as thrombotic thrombocytopenic purpura and Shiga toxin-producing Escherichia coli haemolytic uremic syndrome.3, 4 However, diagnosis is often complicated by the heterogeneity of aHUS presentation. For example, some patients have a slower progression of disease, leading to recurrent end-organ injury without organ failure; such heterogeneity can result in a delayed diagnosis of aHUS.5
aHUS is often associated with unremitting dysregulation of the complement system due to pathogenic complement genetic variant(s) and/or anti-complement factor H (CFH) antibodies causing excess terminal complement activation.6 Approximately 50%–60% of patients with aHUS have been shown to carry rare variant(s) of complement genes.7 However, irrespective of the presence of any genetic predisposition, manifestation of aHUS is often preceded by a precipitating event, with clinical conditions or events including infections, pregnancy, kidney transplant and malignant hypertension all reported in the scientific literature.2, 8, 9 A recent study of patients with MHT and aHUS10 highlighted the difficulties, but also the importance, of establishing accurate and timely diagnoses in complex patient populations such as these to improve outcomes.
Despite the evidence available in the literature, substantial uncertainty remains around the conditions which can lead to complement cascade activation and subsequent development of aHUS. While proposed lists of conditions which appear to be associated with aHUS have been published, full elucidation of their pathophysiological and temporal relationships with aHUS development is still needed. Furthermore, standardization of terminology would also be beneficial. For example, it may be possible to differentiate an associated ‘trigger’ from a ‘clinical condition’ by the duration of an event. A ‘trigger’ may be acute, such as acute infection, surgery or drugs; while an ‘associated clinical condition’ may be a chronic condition commonly preceding the onset of aHUS, such as autoimmune diseases, malignancy and/or chronic infection.
The Global aHUS Registry lends itself to investigation of aHUS subtypes by associated triggers or clinical conditions, as it provides relatively sizeable subtype cohorts. In this report we sought to describe the types of associated triggers or clinical conditions occurring prior to the diagnosis of aHUS, as well as to explore differences in disease presentation, demographics, and/or clinical characteristics, including pathogenic complement genetic variant(s) and/or anti-CFH antibodies, by event.
2 METHODS
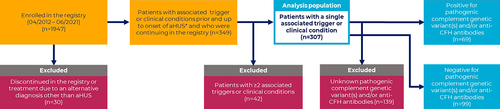
This analysis included patients enrolled in the Global aHUS Registry (NCT01522183) between April 2012 and June 2021 who had a recorded trigger or clinical condition prior/up to the initial onset of aHUS. Onset of aHUS was defined as the earliest of initial symptom presentation, initial diagnosis of aHUS, or first recorded TMA. Patients were excluded if an alternative diagnosis was subsequently made. Patients with multiple associated triggers or clinical conditions were also excluded from the primary analysis population owing to difficulties differentiating the associated triggers or clinical conditions primarily responsible for onset of aHUS.
Data were classified, where appropriate, by age at onset of aHUS—paediatric (<18 years) or adult (≥18 years)—and separately by the presence or absence of identified pathogenic complement genetic variant(s) and/or anti-CFH antibodies. Patients who were not tested or who had incomplete results recorded were excluded from this stratification. As recommended by Kidney Disease Improving Global Outcomes guidelines, genetic testing included the following genes commonly associated with aHUS: C3, CD46/MCP, FH, FI, FB and THBD.3 Further manual validation of all genetic data for included patients was also conducted by an author with substantial expertise in the genetics of aHUS.
Included associated triggers or clinical conditions were bone marrow transplant, autoimmune disease, chronic and acute infections (adjudicated based on data entered in the electronic case report form), malignancy, malignant hypertension, drug-induced, aHUS onset ≤30 days after birth, kidney transplantation, pregnancy and C3 glomerulopathy. All patients with acute infections occurring more than 90 days before aHUS onset were excluded from the analysis. Lists of identified acute and chronic infections and autoimmune diseases can be found in the Supplementary Material.
The following characteristics were investigated and stratified by associated trigger or clinical condition: sex, age at aHUS onset, time from associated trigger or clinical condition to aHUS onset, number of TMA episodes after onset of aHUS, kidney function (the closest estimated glomerular filtration rate [eGFR] values available in either direction to the time of aHUS onset), presence or absence of pathogenic complement genetic variant(s) and/or anti-CFH antibodies, and the frequency and affected organ classes of any extra-renal manifestations of aHUS arising at any time following onset of aHUS. For pregnant patients, the proportions experiencing onset of aHUS during pregnancy or during the postpartum period (≤60 days after delivery), as well as the time during gestation at which aHUS developed, were also reported.
Data are presented as n (%) or median (Q1, Q3), as appropriate. Data were analysed descriptively and no formal statistical comparisons were conducted as part of this analysis due to small sample sizes. All patients enrolled in the Global aHUS Registry were diagnosed with aHUS by the local assessing physician using their clinical experience; no standardized criteria for aHUS diagnosis were recommended in the Registry protocol and no central confirmation of diagnosis was conducted. Data accuracy and completeness within the Global aHUS Registry is reliant on the physician and/or the centre managing data entry. Only mutations in complement genes considered to be pathogenic were reported.
3 RESULTS
3.1 Patient disposition
A total of 1947 patients were enrolled in the Registry by June 2021 and 349 (17.9%) met the general inclusion criteria; 307/349 (88.0%) had one associated trigger or clinical condition and were included in the primary analysis. A further 34 (9.7%) had two associated triggers or clinical conditions and 8 (2.3%) had three, these patients were excluded from the primary analysis population (Figure 1). A listing of all patients with multiple associated triggers or clinical conditions and the frequencies of specific event combinations are reported in Supplementary Table 1.
3.2 Prevalence of associated triggers or clinical conditions
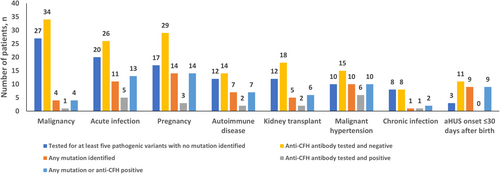
Table 1 shows the number of patients who experienced each trigger or condition by age at onset of aHUS and also by the presence or absence of pathogenic complement genetic variant(s) and/or anti-CFH antibodies. Overall, malignancy was the most common condition, seen in 58/307 (18.9%) patients, followed by the triggers pregnancy and acute infection (both 53/307, 17.3%). In paediatric patients (n = 78), the top two triggers were acute infection (28/78, 35.9%) and aHUS onset ≤30 days after birth (25/78, 32.1%). In adult patients (n = 229), pregnancy was the most common trigger (53/229, 23.1%), followed by malignancy (51/229, 22.3%). In the 69 patients positive for pathogenic complement genetic variant(s) and/or anti-CFH antibodies, pregnancy and acute infection were the most frequently observed triggers (14/69, 20.3% and 13/69, 18.8%, respectively). Lastly, in the 99 patients negative for pathogenic complement genetic variant(s) and/or anti-CFH antibodies, malignancy was the most frequently observed condition followed by pregnancy (26/99, 26.3% and 16/99, 16.2%, respectively).
| Associated trigger or clinical condition n (%) | All | Age at aHUS onset | Pathogenic complement genetic statusa | ||
|---|---|---|---|---|---|
| N = 307 | Paediatric (<18 years) | Adult (≥18 years) | Positive | Negative | |
| n = 78 | n = 229 | n = 69 | n = 99 | ||
| Malignancy | 58 (18.9) | 7 (9.0) | 51 (22.3) | 4 (5.8) | 26 (26.3) |
| Pregnancy | 53 (17.3) | 0 (0.0) | 53 (23.1) | 14 (20.3) | 16 (16.2) |
| Acute infectionb | 53 (17.3) | 28 (35.9) | 25 (10.9) | 13 (18.8) | 15 (15.2) |
| Autoimmune diseasec | 36 (11.7) | 4 (5.1) | 32 (14.0) | 7 (10.1) | 10 (10.1) |
| Kidney transplant | 31 (10.1) | 4 (5.1) | 27 (11.8) | 6 (8.7) | 12 (12.1) |
| Malignant hypertension | 26 (8.5) | 8 (10.3) | 18 (7.9) | 10 (14.5) | 9 (9.1) |
| aHUS onset ≤30 days after birth | 25 (8.1) | 25 (32.1) | 0 (0.0) | 9 (13.0) | 3 (3.0) |
| Chronic infectionb | 14 (4.6) | 1 (1.3) | 13 (5.7) | 2 (2.9) | 7 (7.1) |
| Bone marrow transplant | 5 (1.6) | 1 (1.3) | 4 (1.7) | 1 (1.4) | 1 (1.0) |
| Drug-induced aHUS | 4 (1.3) | 0 (0.0) | 4 (1.7) | 1 (1.4) | 0 (0.0) |
| C3 glomerulopathy | 2 (0.7) | 0 (0.0) | 2 (0.9) | 2 (2.9) | 0 (0.0) |
- Abbreviations: aHUS, atypical haemolytic uraemic syndrome; CFH, complement factor H.
- a Patients were classed as either positive or negative for pathogenic complement genetic variant(s) and/or anti-CFH antibodies. The remaining 139 patients were either not tested, had no results recorded, or were adjudicated to have incomplete genetic data recorded in the Registry and were classed as having an unknown genetic status.
- b Acute versus chronic infections were adjudicated based on data entered in the electronic case report form. All patients with acute infections occurring earlier than 90 days before aHUS onset were excluded from the analysis. A list of acute and chronic infections can be found in Supplementary Lists 1 and 2, respectively.
- c A list of autoimmune conditions identified in these patients can be found in Supplementary List 3.
3.3 Genetic screening
Figure 2 presents the results of screening for pathogenic complement genetic variant(s) and/or anti-CFH antibodies in patients with associated triggers or clinical conditions. Among patients who were tested and had results reported, the aHUS onset ≤30 days after birth group had the highest proportion of patients positive for pathogenic complement genetic variant(s) and/or anti-CFH antibodies (9/14, 64.3%). Patients with aHUS associated with chronic infections or malignancy had the lowest proportions of pathogenic genetic variant(s) and/or anti-CFH antibodies (2/9, 22.2% and 4/37, 10.8%; respectively). Further information on patients positive for pathogenic complement genetic variant(s) and/or anti-CFH antibodies is available in Supplementary Figure 1 and Supplementary Table 2.
3.4 Patient demographics
Baseline demographics and characteristics of all patients, as well as demographics stratified by the presence or absence of pathogenic complement genetic variant(s) and/or anti-CFH antibodies, are presented in Figure 3A–D. Further detail on these patients can be found in Supplementary Table 3 (baseline characteristics) and Supplementary Table 4 (number of patients enrolled, by country).
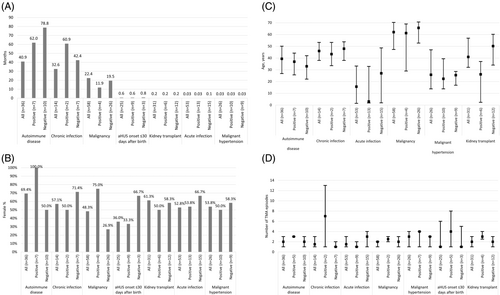
Patients with an associated trigger or clinical condition were more likely to be adults at onset of aHUS, unless they had an acute infection or aHUS onset ≤30 days after birth. The median time from an associated trigger or clinical condition to aHUS onset was shortest following acute infections, malignant hypertension and kidney transplant (all approximately 1 day). Patients with aHUS onset ≤30 days after birth also had a short median time from trigger to onset (18 days) (Figure 3A). After bone marrow transplant, autoimmune disease, chronic infection, malignancy or drug-induced aHUS, the median time from an associated trigger or clinical condition to onset of aHUS was ≥10 months.
Except for malignancy (48.3%) and aHUS onset ≤30 days after birth (36.0%), all associated triggers or clinical conditions had a greater proportion of females than males (Figure 3B). Most individuals were older at aHUS onset, with similar ages observed for all patients except those with acute infections or malignant hypertension, who tended to be younger on average than individuals with other associated triggers or clinical conditions (Figure 3C).
Over half of all patients with an associated trigger or clinical condition had a subsequent TMA episode after aHUS onset. Patients with malignant hypertension or drug-induced aHUS had the greatest number of subsequent TMA episodes, with a median of three episodes, while patients with aHUS onset ≤30 days after birth had the least, with a median of one (Figure 3D).
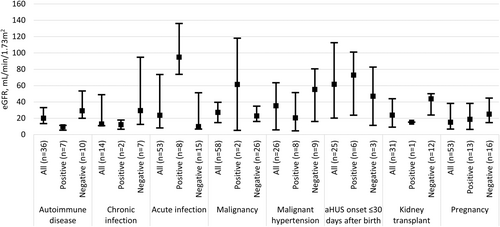
3.5 Kidney function
Overall, patients had severe kidney injury at aHUS onset as reflected by low median eGFR values (Figure 4). In general, patients negative for pathogenic complement genetic variant(s) and/or anti-CFH antibodies had higher median eGFR at aHUS onset across all associated triggers or clinical conditions, except those with acute infections and malignancy. This also applied to aHUS onset ≤30 days after birth; however, difficulties in accurately measuring eGFR in neonates may complicate interpretation of this specific cohort's data.
3.6 Extra-renal manifestations of aHUS
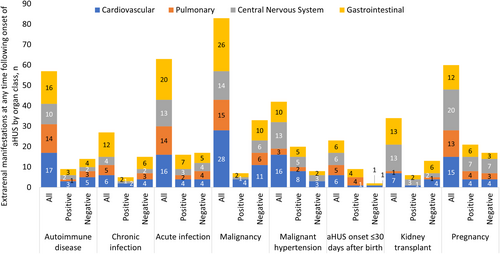
For all associated triggers or clinical conditions, except aHUS onset ≤30 days after birth and bone marrow transplant, ≥50% of patients within each group experienced an extra-renal manifestation of aHUS at any time at or after onset of aHUS (Supplementary Table 5). In the two named categories, 11/25 (44.0%) patients with aHUS onset ≤30 days after birth and 1/5 (20.0%) patients with a bone marrow transplant had an extra-renal manifestation of aHUS.
When assessed by affected organ class, cardiovascular and central nervous system manifestations were most frequent in patients with malignant hypertension (16/26, 61.5% and 13/26, 50.0%, respectively), pulmonary manifestations were most frequent in those with autoimmune diseases (14/36, 38.9%) or chronic infections (5/14, 35.7%) and gastrointestinal manifestations were most frequent in those with chronic infections (12/14, 85.7%) (Figure 5). Frequencies of extra-renal manifestations were determined as a proportion of the total number of patients in that trigger or clinical condition cohort. For all associated triggers or clinical conditions, patients generally experienced similar levels of extra-renal manifestations regardless of their pathogenic complement genetic status and/or presence of anti-CFH antibodies.
4 DISCUSSION
Characterization of patients with aHUS presenting together with associated triggers or clinical conditions is an area of active research. This study, using data from the Global aHUS Registry, is the largest real-world analysis of this population to date. It sought to identify and describe types of associated triggers or clinical conditions, as well as the demographics, presence or absence of pathogenic complement genetic variant(s) and/or anti-CFH antibodies, and clinical characteristics of these populations.
The population assessed in this study, which included patients with aHUS and an associated trigger or clinical condition, included approximately 18% of the total number of patients within the Global aHUS Registry. The 18% prevalence of patients with aHUS and associated triggers or conditions is notably lower than that reported in broader TMA cohorts.8 However, as the cohort is derived from an aHUS Registry, it is enriched with patients highly suspected of having aHUS, as determined by the enrolling physician. In this sense, it differs from other studies which have included cohorts with broader TMAs, such as the cohort assessed by Le Clech and colleagues.11
Due to the presence of an associated trigger or clinical condition, a portion of this study cohort may also be characterized as having secondary aHUS as defined by some research groups.12, 13 Complement dependent aHUS has been seen in certain patient groups including those with a viral infection, post-partum, organ transplant or malignant hypertension, however, complement dependency of TMAs has not been conclusively established in other settings such as metastatic cancer, autoimmunity and drug-induced TMA. Due to the heterogeneity of autoimmune diseases and variety of drugs which can lead to endothelial injury and subsequent TMA presentation, differentiation between aHUS and complement independent TMA in these settings is more complex. In this study, the subgroup with drug-induced aHUS was extremely small (4/307, 1.3%) likely indicating the rarity of drug-induced TMAs being categorized as aHUS. Subgroups with associated malignancy and autoimmune disease would need to be further studied to delineate underlying disease pathophysiology.
In our analyses, the frequencies of pathogenic complement genetic variant(s) and/or anti-CFH antibodies differed among clinical categories. Notably, within the 168 patients with available results for pathogenic complement genetic variant(s) and/or anti-CFH antibodies, pregnant patients as well as patients with an acute infection, autoimmune disease, kidney transplant, malignant hypertension or aHUS onset ≤30 days after birth had high proportions of identified pathogenic complement genetic variant(s) and/or anti-CFH antibodies (Table 1). This provides further support that alternative pathway dysregulation likely has a role in TMA associated with an underlying clinical condition. On the other hand, among patients with reported complement genetic status and/or anti-CFH antibodies, a high percentage of patients with malignancy and chronic infections were found to be negative for pathogenic complement genetic variant(s) and/or anti-CFH antibodies (Table 1; 26/30 [86.7%] and 7/9 [77.8%], respectively). The opposite was true for patients with aHUS onset ≤30 days after birth, with a high percentage (75.0%, 9/12) of patients having pathogenic complement genetic variant(s) and/or anti-CFH antibodies. This finding is consistent with the early presentation of many patients with pathogenic genetic variants.15
The time intervals between a preceding event and onset of aHUS also differed by type of event. As discussed, this difference may be a key distinguishing factor between associated ‘triggers’ and ‘clinical conditions’. Acute infection, malignant hypertension, kidney transplant and post-pregnancy categories each displayed relatively short time intervals between event and aHUS manifestation; these categories met the proposed definition of an acute ‘trigger’. Specifically, in the case of malignant hypertension, it is important to note that this can also occur secondary to TMA. In these cases, malignant hypertension would not be considered an acute trigger, however this occurrence is rare.16 Other conditions, such as malignancy, autoimmune disease and drug-induced aHUS were associated with a larger interval (in some cases years) between event and onset of aHUS and likely characterize a separate type of disease pathophysiology/progression. While this may be unsurprising for conditions such as autoimmune diseases, which are chronic, typically life-long diseases often presenting in a relapsing–remitting manner,17 the temporal relationship between other conditions and aHUS may be less discernible. Explanations for this include the possibility of ongoing, sub-clinical aHUS which developed close to the onset of the associated clinical condition, but which did not present clinically until much later in the disease course. Other possible explanations could include events, such as malignancy, bone marrow transplant and autoimmune disease, having clinical manifestations that are similar to TMA such as anaemia, thrombocytopenia and organ dysfunction (including acute kidney injury) even in the perceived absence of TMA. Cases such as those described could fall into the previously discussed group of patients with complex presentations and/or slower progression of disease, leading to delayed diagnosis.5
When kidney function was assessed, it was found that, irrespective of associated trigger or clinical condition, all patients had low eGFR measurements at the closest point to onset of aHUS. While some triggers/clinical conditions were associated with more severe reductions in eGFR than others, all except aHUS onset ≤30 days after birth (where eGFR measurements are particularly difficult) had median eGFR values of <60 mL/min/1.73 m2. These data illustrate the need for further research to explore if earlier diagnosis and treatment could decrease the severity of acute kidney injury at clinical presentation and, more importantly, whether it would lead to better long-term outcomes.
Limitations of this analysis include small sample sizes for some of the associated triggers or clinical conditions, which potentially limits the conclusions that can be drawn from these groups. A further limitation was underrepresentation of some geographical regions (Supplementary Table 4), which should be considered when extrapolating this analysis. Alongside a lack of complete genetic testing results in all patients, the small samples sizes also limited assessment of any associations between triggers or conditions and the presence or absence of pathogenic complement genetic variant(s) and/or anti-CFH antibodies. However, some signals which may warrant further investigation were observed. Additionally, only the index aHUS event was assessed; triggers or conditions preceding aHUS relapse were not investigated. Such future investigations may further enhance the understanding of relationships between associated triggers or clinical conditions and the onset/recurrence of aHUS. As this study is based on an aHUS registry, we are not able to comment on patients with secondary TMA which sometimes present with a similar clinical picture.
This study is the largest assessment of demographics and clinical characteristics in a real-world population of patients with aHUS and associated triggers or clinical conditions. The findings of this study suggest that associated triggers or clinical conditions may be organized into clinically relevant categories, and that the presence or absence of known pathogenic complement genetic variant(s) and/or anti-CFH antibodies does not substantially impact patient clinical profiles, as reflected in kidney function at diagnosis of aHUS. It also provides further support that alternative complement dysregulation is an important driver of initial disease development in this population, highlighting that the presence of associated triggers or clinical conditions does not exclude the concurrent presence of pathogenic complement genetic variant(s) and/or anti-CFH antibodies. Diagnosis of aHUS should be considered even in the presence of other associated triggers or clinical conditions, which is likely to facilitate faster implementation of targeted treatment, thereby improving prognoses in this population.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and/or design of the study, participated in the acquisition, analysis and/or interpretation of data, and in the writing, review and/or revision of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
Open Access funding was provided by Alexion, AstraZeneca Rare Disease, Boston, MA, USA. This analysis was funded by Alexion, AstraZeneca Rare Disease. Alexion, AstraZeneca Rare Disease was responsible for the collection, management and analysis of information contained in the Global aHUS Registry. Alexion, AstraZeneca Rare Disease contributed to data interpretation, preparation, review and approval of the manuscript for submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors would like to acknowledge Dr. Andrew Siedlecki for his contributions to this study, including data analysis and interpretation, as well as critical review of the manuscript. The sponsor and investigators thank the patients and their families for their participation in, and support for, this clinical study. The authors would also like to thank all the Global aHUS Registry investigators who have contributed data: Scientific Advisory Board members of the Global aHUS Registry: Christoph Licht, Véronique Frémeaux-Bacchi, Gema Ariceta, Larry Greenbaum, Sally Johnson, Franz Schaefer, Jeff Schmidt, Margriet Eygenraam and Katerina Anokhina; and National Coordinators of the Global aHUS Registry: Miquel Blasco (Spain), Donata Cresseri (Italy), Galina Generolova (Russia), Nicholas Webb (UK), Patricia Hirt-Minkowski (Switzerland), Natalya Lvovna Kozlovskaya (Russia), Danny Landau (Israel), Anne-Laure Lapeyraque (Canada), Chantal Loirat (France), Christoph Mache (Austria), Michal Malina (Czech Republic), Leena Martola (Finland), Annick Massart (Belgium), Eric Rondeau (France) and Lisa Sartz (Sweden). The authors would like to acknowledge Matthew D. Badham, PhD, of Bioscript Medical Communications, Macclesfield, UK for providing medical writing support with funding from Alexion, AstraZeneca Rare Disease, and Radha Narayan, PhD, Alexion, AstraZeneca Rare Disease, for critical review of the manuscript.
CONFLICT OF INTEREST STATEMENT
Imad Al-Dakkak and Katerina Anokhina are employees of Alexion, AstraZeneca Rare Disease. Nicole Isbel has received funding from Alexion, AstraZeneca Rare Disease with honoraria for advisory board membership and educational lectures. Véronique Frémeaux-Bacchi has received fees from Alexion, AstraZeneca Rare Disease, Roche, BioCryps, and Apellis for invited lectures and/or board membership and is the recipient of a research grant from Alexion, AstraZeneca Rare Disease. Larry A. Greenbaum has received research and consulting support from Alexion, AstraZeneca Rare Disease. Gema Ariceta is a member of the Scientific Advisory Board of the Global aHUS Registry supported by Alexion, AstraZeneca Rare Disease; received consulting fees from Alexion, AstraZeneca Rare Disease, Chiesi, Advicenne, Recordati Rare Diseases, Dicerna and Alnylam; and received lecture fees from Alexion, AstraZeneca Rare Disease, Chiesi, Advicenne, Kyowa Kirin, and Recordati Rare Diseases. Gianluigi Ardissino is a member of the Scientific Advisory Board of the Global aHUS Registry supported by Alexion, AstraZeneca Rare Disease; received honoraria from Alexion, AstraZeneca Rare Disease, Alnylam, Roche, Novartis, and Eligo Bioscience. Franz Schaefer has received honoraria from Alexion, AstraZeneca Rare Disease, Alnylam, Astellas, AstraZeneca, Bayer, Otsuka, Roche; and is Chair of the Global aHUS Registry. Eric Rondeau has received honoraria, fees and grants from Alexion, AstraZeneca Rare Disease. Christoph Licht is a member of the Scientific Advisory Board of the Global aHUS Registry, was the Canadian Coordinator for trials of eculizumab in patients with aHUS that were funded by Alexion, AstraZeneca Rare Disease; received lecture/consultancy honoraria from Alexion, AstraZeneca Rare Disease, Apellis, Catalyst Biosciences, Eleva, Novartis; and unrestricted research grants from Fresenius and Pfizer. Rodney D. Gilbert has nothing to disclose.
ETHICS STATEMENT
This clinical trial was evaluated and approved by the Institutional Review Board or Independent Ethics Committee at each participating centre, and the study was conducted in accordance with the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines. All participants provided informed consent prior to enrolling in the study.
Open Research
DATA AVAILABILITY STATEMENT
Alexion, AstraZeneca Rare Disease will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion, AstraZeneca Rare Disease-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion, AstraZeneca Rare Disease Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form: https://alexion.com/contact-alexion/medical-information.