Frequent hepatic steatosis in amyotrophic lateral sclerosis: Implication for systemic involvement
Abstract
Background
Abnormal energy metabolism has been reported in amyotrophic lateral sclerosis (ALS) that broadens the concept of ALS as a systemic disease. Dyslipidemia is likely to reflect the clinical outcome in ALS.
Aim
To study whether evidence of dysmetabolism can be detected non-invasively by imaging study, such as liver sonography.
Methods
Abdominal sonography, lipid profiles and liver enzymes were obtained in three groups of individuals: (i) the patients with ALS; (ii) other neurodegenerative diseases; and (iii) asymptomatic dyslipidemic persons. The sonographic steatosis grade was determined by a blinded technician. Statistical tests including one-way anova with post-hoc test, multiple linear regression were carried out.
Results
The lipid profiles and liver enzymes were similar between the ALS and dyslipidemic groups. The disease control group had lower low-density lipoprotein, low-density lipoprotein-to-high-density lipoprotein ratio and triglyceride than other groups. Hepatic steatosis was present in 76% of ALS patients, 19% of the disease control and 38% of the dyslipidemic group (P < 0.001 vs ALS). Multivariate analysis showed that only the patient group was correlated to the sonographic score.
Conclusion
The present study showed that hepatic steatosis is frequent and a unique phenomenon in ALS among neurodegenerative diseases. Hepatic steatosis might be a sensitive marker for dysmetabolism in ALS.
Introduction
Abnormal metabolism and dyslipidemia are frequently present in amyotrophic lateral sclerosis (ALS), and could be associated with the clinical outcome.1-7 The recent view is that dysmetabolism is not a mere consequence of decreased oral intake and muscle atrophy; rather, ALS is a systemic disease that affects metabolic pathways.8 A recent animal study suggested that the liver is the target organ in ALS, with this view being supported by the post-mortem evidence of frequent steatosis in patients.3, 9 However, scarce information has been available on hepatic abnormality in patients with ALS. Thus, the aim of the present study was to assess the severity and frequency of hepatic steatosis in patients with ALS, and to compare these with patients with other neurological diseases and asymptomatic subjects.
Method
The following three groups were recruited and prospectively assessed: (i) ALS: the patients who fulfilled either definite, clinically probable or laboratory-supported probable ALS criteria by revised El Escorial criteria; (ii) disease control: the patients with other neurodegenerative diseases were included, all of whom met the respective diagnostic criteria; and (iii) dyslipidemic controls: asymptomatic persons who showed abnormality in at least one of the lipid profiles (low-density lipoprotein, high-density lipoprotein or triglycerides) by annual health check-ups. At the time of the sonography, the ALS patients had the ALS Functional Rating Scale-Revised. The exclusion criteria were the following in all the groups: (i) evidence of infection against hepatitis virus by history taking and serology; (ii) excessive alcohol drinking (i.e. >500 mL of beer per day); (iii) positive antinuclear antibody; and (iv) abnormally high serum ferritin level. The study was approved by the institutional review board of Vihara Hananosato Hospital and Tokushima University. The participants received written informed consent.
Diagnostic abdominal ultrasound was carried out under fasting conditions by a single technician (NT) in a blinded manner for diagnosis using Toshiba Aprio (Tokyo, Japan) with a 5-MHz convex probe. The grading of hepatic steatosis was established and agreed with one of the experienced ultrasonography technicians (KU, SN) who reviewed the images independently. The following standard scoring system was used:10 grade 1 = the presence of abnormal hepato-renal contrast (A); grade 2 = the presence of (A), and either unclear vessels (B) or deep attenuation (C); grade 3 = the presence of (A), (B) and (C) (Figure S1). The abnormality in the most affected area was graded.
spss version 20 (SPSS, Tokyo, Japan) was used for statistical analysis, using one-way anova with Games–Howell post-hoc test, Fisher's exact test, χ2-test, Spearman's correlation coefficient and multiple linear regression where applicable. A statistically significant P-value was set at 0.05.
Results
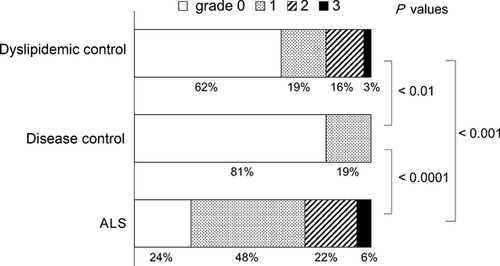
The patient characteristics and the laboratory findings are summarized in Tables 1–3. The ALS patients were younger than the disease control group, but older than the dyslipidemic controls. The body mass index of the ALS patients was comparable with the disease controls, but smaller than the dyslipidemic controls (Table 1). In the ALS patients, the disease duration was 3.3 ± 1.9 years (range 1–8), and the ALS Functional Rating Scale-Revised score was 32.5 ± 11.6 (range 0–48). Two patients (4%) were on a ventilator, and nine patients (17%) were fed by percutaneous endoscopic gastrostomy tube. The disease control group (n = 37) was comprised of the patients with the following diseases: Parkinson's disease (n = 30), Alzheimer's disease (n = 3), diffuse Lewy body disease (n = 2), multiple system atrophy-P (n = 1) and progressive supranuclear palsy (n = 1). The low-density lipoprotein, low-density lipoprotein-to-high-density lipoprotein ratio and triglyceride were the greatest in the dyslipidemic group, followed by the ALS group and the disease controls. The serum glutamic oxaloacetic transaminase and gamma-glutamyl transpeptidase were similar among all the groups, but the serum glutamic-pyruvic transaminase was greater in the ALS and the dyslipidemic groups than in the disease controls (Table 2).
| ALS | Disease control | Dyslipidemic control | anova P-value | Post-hoc | |||
|---|---|---|---|---|---|---|---|
| (n = 54) (group A) | (n = 37) (group B) | (n = 32) (group C) | A vs B | B vs C | A vs C | ||
| Age (years) | 60.0 ± 11.9 (34–88) | 75.8 ± 8.3 (57–89) | 51.5 ± 9.1 (35–73) | <0.001 | <0.001 | <0.001 | <0.001 |
| Sex (female/male) | 24/30 | 17/20 | 14/18 | 0.9 (χ2) | 0.9 | 0.8 | 0.9 |
| Height (cm) | 161.9 ± 9.0 (142–187) | 156 ± 9.0 (135–172) | 162.4 ± 8.8 (145–182) | <0.01 | <0.02 | <0.02 | 0.9 |
| Weight (kg) | 55.3 ± 13.2 (37–93) | 50.0 ± 10.3 (32–70) | 63.3 ± 13.2 (40–87) | <0.001 | 0.1 | <0.001 | <0.05 |
| Body mass index (reference: 18.5–25) | 20.9 ± 3.6 (14.8–29.2) | 20.4 ± 3.7 (11.8–27.4) | 23.8 ± 3.6 (18.6–32.0) | <0.001 | 0.8 | <0.01 | <0.01 |
| Underweight (%) | 16 (30%) | 11 (30%) | 0 (0%) | ||||
| Overweight (%) | 7 (13%) | 5 (14%) | 11 (34%) | ||||
- ALS, amyotrophic lateral sclerosis.
| Reference | ALS (A) | Disease control (B) | Dyslipidemic control (C) | anova P-value | A vs B | B vs C | A vs C | |
|---|---|---|---|---|---|---|---|---|
| LDL (mg/dL) | 60–139 | 118.5 ± 30.8 (57–181) | 102.3 ± 28.4 (28–165) | 149.2 ± 33.5 (74.4–211) | <0.001 | <0.05 | <0.001 | <0.001 |
| High (%) | 13 (24%) | 3 (8%) | 19 (59%) | |||||
| Low (%) | 1 (2%) | 2 (5%) | 0 (0%) | |||||
| HDL (mg/dL) | 40–74 | 54.3 ± 13.5 (31–89) | 61.9 ± 19.8 (34–119) | 59.8 ± 17.2 (34–101) | 0.09 | 0.1 | 0.9 | 0.3 |
| High (%) | 4 (7%) | 11 (30%) | 7 (22%) | |||||
| Low (%) | 7 (13%) | 4 (11%) | 5 (16%) | |||||
| LDL/HDL | <2.3 | 2.34 ± 0.9 (1.0-4.8) | 1.78 ± 0.7 (0.8-3.4) | 2.72 ± 1.0 (0.74-5.37) | <0.001 | <0.01 | <0.001 | 0.2 |
| High (%) | 26 (48%) | 9 (24%) | 21 (65%) | |||||
| Triglyceride (mg/dL) | 45–150 | 150.3 ± 70.7 (39–360) | 94.9 ± 54.4 (30–263) | 186.3 ± 139.8 (57–909) | <0.001 | <0.001 | <0.01 | 0.4 |
| High (%) | 22 (41%) | 5 (14%) | 22 (68%) | |||||
| Low (%) | 2 (4%) | 6 (16%) | 0 (0%) | |||||
| SGOT (IU/L) | 8–40 | 29.0 ± 15.4 (14–103) | 24.1 ± 10.5 (10–61) | 27.1 ± 13.5 (10–58) | 0.3 | 0.2 | 0.6 | 0.8 |
| High (%) | 8 (15%) | 4 (11%) | 5 (16%) | |||||
| SGPT (IU/L) | 5–45 | 29.0 ± 19.4 (8–101) | 16.5 ± 12.1 (4–70) | 26.9 ± 10.7 (15–60) | <0.01 | <0.01 | <0.01 | 0.8 |
| High (%) | 8 (15%) | 1 (3%) | 2 (6%) | |||||
| γGTP (IU/L) | 0–60 | 37.2 ± 34.8 (10–166) | 37.9 ± 61.0 (7–367) | 59.8 ± 61.2 (14–285) | 0.2 | 0.9 | 0.4 | 0.2 |
| High (%) | 7 (13%) | 4 (11%) | 8 (25%) |
- γGTP, gamma-glutamyl transpeptidase; ALS, amyotrophic lateral sclerosis; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDL/HDL, low-density lipoprotein-to-high-density lipoprotein ratio; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic-pyruvic transaminase.
| ALS | Disease control | Dyslipidemic control | Post-hoc | |||
|---|---|---|---|---|---|---|
| (n = 54) (group A) | (n = 37) (group B) | (n = 32) (group C) | A vs B | B vs C | A vs C | |
| Sonography | ||||||
| 0 (normal) | 13 (24%) | 30 (81%) | 20 (62%) | <0.0001 | <0.01 (Fisher's exact test) | <0.001 |
| 1 | 26 (48%) | 7 (19%) | 6 (19%) | |||
| 2 | 12 (22%) | 0 (0%) | 5 (16%) | |||
| 3 | 3 (6%) | 0 (0%) | 1 (3%) | |||
| Normal (0) | 13 (24%) | 30 (81%) | 20 (62%) | <0.001 | <0.05 | <0.001 |
| Abnormal (1,2,3) | 41 (76%) | 7 (19%) | 12 (38%) | (χ2-test) | ||
| Non-PEG/ventilator | n = 45 |
|---|---|
| 0 (normal) | 11 (24%) |
| 1 | 20 (45%) |
| 2 | 11 (24%) |
| 3 | 3 (7%) |
- ALS, amyotrophic lateral sclerosis; PEG, percutaneous endoscopic gastrostomy.
Sonography showed that the ALS group had the greatest mean steatosis grade and the percentage of abnormality (Fig. 1, Table 3). The high frequency of hepatic steatosis was also present after excluding the ALS patients requiring percutaneous endoscopic gastrostomy placement or ventilator support. None had cirrhosis or liver cancer in any group. The frequencies of steatosis in ALS were similar between the sexes. The disease duration of ALS and ALS Functional Rating Scale-Revised did not correlate with the sonographic grade (P-values of 0.3 and 0.6, respectively by Spearman's rank-order correlation). To assess a possible cofounding factor among the groups, multiple linear regression analysis was carried out. Only the patient group was significantly correlated with the sonographic grade for steatosis (Table 4), which argues against a possible confounding factor to explain the different distribution of the sonographic grades among the three groups.
| Variable | Β (SEM) | P-value |
|---|---|---|
| Height | −0.019 (0.04) | 0.6 |
| Weight | 0.047 (0.06) | 0.4 |
| BMI | −0.048 (0.14) | 0.7 |
| Age | −0.004 (0.006) | 0.5 |
| Patient group | −0.433 (0.08) | < 0.001 |
| HDL | −0.002 (0.005) | 0.7 |
| LDL | 0.002 (0.003) | 0.5 |
| LDL/HDL | 0.062 (0.16) | 0.7 |
| Triglyceride | 0.001 (0.001) | 0.9 |
| SGOT | −0.005 (0.007) | 0.5 |
| SGPT | 0.013 (0.007) | 0.07 |
| γGTP | −0.002 (0.001) | 0.2 |
- γGTP, gamma-glutamyl transpeptidase; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDL/HDL, low-density lipoprotein-to-high-density lipoprotein ratio; SGOT, serum glutamic oxaloacetic transaminase; SGPY, serum glutamic-pyruvic transaminase.
Discussion
It has been recently recognized that ALS is a multi-organ disease. Hypermetabolism and abnormal lipid metabolism out of proportion to nutritional status have been identified in ALS.5, 8 In line with these, a post-mortem pathological study showed frequent steatosis in ALS patients.3 The present study showed that sonographic evidence of steatosis was frequently present, in three-quarters of the present series. Sonography has high sensitivity and specificity in detecting steatosis,11, 12 which detects steatosis in 20–30% of the general population in Japan and in the Western world.11, 13 In the present series, the ALS patients had a higher prevalence of steatosis than the dyslipidemic controls and other neurodegenerative patients. Given the data that the dyslipidemic subjects who had greater body mass index and low-density lipoprotein, but less frequent steatosis than ALS, it is unlikely that the frequent steatosis in ALS patients is a mere consequence of dyslipidemia; instead, steatosis likely reflects extraneuronal involvement in ALS. The present data also suggest that liver sonography can detect dysmetabolism in ALS patients more sensitively than the blood lipid profiles. Although it is possible that hypoxia causes derangement of metabolic adaptation, the sonographic grade did not correlate with ALS Functional Rating Scale-Revised that contains scoring of respiratory status.14 However, because the lack of data on oxygenation, such as arterial blood gas, limits further assessment, future study should elucidate this potential relationship.
A question remains open regarding the pathophysiological and prognostic values of steatosis in ALS. It has been known that mutations associated with ALS have extraneuronal effects.5, 15 For example, SOD1 plays an important role in regulating fatty acid synthesis and its metabolism in the liver.16 Mutated SOD1 model mice showed prominent inflammatory responses in the liver such as natural killer-T cells and abnormal insulin-like growth factor-1 axis along with lipid redistribution.9 Genetic deletion of Tardbp (encoding TDP-43) also led to dysregulated lipid homeostasis.17 It should be recognized, however, that animal models for ALS might not properly reflect the molecular and metabolic dysfunction in patients with ALS. Beside energetic metabolism, lipids also play a critical role in the central nervous system and peripheral nervous system by controlling membrane fluidity, improving transmission of electrical signals and stabilizing synapses.8 In line with the previous reports that generally support favorable prognosis in ALS patients who had high body mass index and high levels of blood lipids, the presence of steatosis might be associated with a favorable outcome.8 Further longitudinal study correlating the outcome and the degree of steatosis would elucidate the significance of sonography in the liver in ALS. Such studies should also elucidate whether steatosis is uniquely present at some particular disease stages and if there were potential confounding factors, such as disease duration and stage.
Acknowledgment
The authors declare no conflict of interest.




