Elucidation of the role of microglia-macrophage-based neuroinflammation in neurological diseases
Abstract
Objective
To elucidate the roles of microglia and macrophages in neuroinflammation and their dual impact on the progression of neurological diseases.
Background
Microglia and macrophages are integral components of the central and peripheral nervous systems, where they play important roles in maintaining homeostasis, influencing disease progression, and facilitating repair mechanisms.
Methods
The review integrates recent advancements in single-cell RNA sequencing that highlight the extensive heterogeneity of microglia and macrophages, which helps in understanding their wide-ranging roles in different neurological conditions.
Results
The analysis reveals that microglia and macrophages have a dual nature, capable of both exacerbating and mitigating disease processes across various conditions, including Alzheimer’s disease, multiple sclerosis, stroke, peripheral nerve disorders, and brain tumors.
Conclusion
Modulating the activity of microglia and macrophages offers a promising avenue for therapeutic interventions in neurological disorders. There is a critical need for further research to fully leverage their therapeutic potential in targeting neuroinflammation to enhance patient outcomes.
1 INTRODUCTION
The mononuclear phagocyte system (MPS), including macrophages and microglia, plays a key role in neurological disorders. The landmark discovery of phagocytosis by Elie Metchnikoff in 1883, earning them a Nobel Prize, was pivotal in recognizing the immune system's role in health.1 Pío del Río-Hortega's 20th-century work on microglia distinguished these central nervous system macrophages from their peripheral counterparts.2, 3 Today's research, powered by techniques including single-cell RNA sequencing, has revealed the complexity of these cells and their involvement in diseases like (AD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS).4, 5 This review explores the roles of microglia and macrophages in neurological disorders, aiming to connect historical context with modern insights for a full perspective.
2 ORIGINS AND CLASSIFICATION OF MPS CELLS
2.1 Nomenclature of MPS
The nomenclature for microglia has a complex history that has evolved significantly over the years. Originally, the simplistic M1/M2 classification was adopted to describe the polarized activation states of macrophages, which was subsequently extended to microglia. However, this binary classification fails to adequately represent the multifaceted and dynamic nature of microglia and macrophages across various contexts, including development, disease progression, and aging. The position paper strongly encourages a shift away from the M1/M2 model toward a more precise functional nomenclature. This new approach should incorporate specific functional states, molecular signatures, and contextual interactions that more accurately reflect the complex roles of these phagocytic cells. By adopting descriptors that align with detailed functional and molecular data, researchers can better communicate the diverse functionalities and regulatory mechanisms of microglia and macrophages, thus enhancing the understanding of their roles in health and disease. For example, macrophages can be referred to as “tissue-resident macrophages” to emphasize their role in maintaining tissue homeostasis, “wound-healing macrophages” to highlight their involvement in tissue repair, or “inflammatory macrophages” when describing their activated state in response to infection. Similarly, microglia can be named “surveying microglia” to reflect their role in monitoring brain health, “reactive microglia” when they respond to neuronal damage, or “disease-associated microglia (DAM)” in the context of specific pathologies like AD.6
2.2 Fate mapping and origins
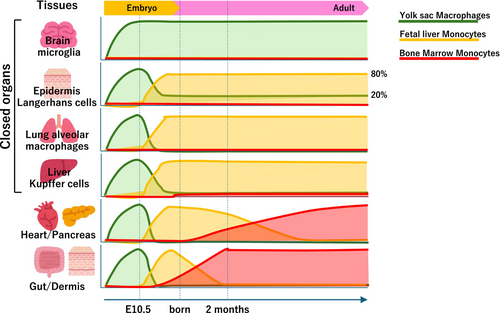
Fate mapping experiments have demonstrated that microglia originate from yolk sac progenitors early in embryogenesis, before the development of the blood–brain barrier, and populate the brain independently of hematopoiesis.7 This early seeding and the unique developmental pathway distinguish microglia from other macrophage populations that derive from later hematopoietic precursors, such as fetal liver and bone marrow. In contrast, tissue-resident macrophages in organs outside the central nervous system (CNS), including liver Kupffer cells, skin Langerhans cells, and lung alveolar macrophages, are derived from a mix of embryonic and postnatal hematopoietic sources, indicating a more complex ontogeny.8 These findings highlight the diversity in the origins of MPS cells, suggesting that their developmental lineage significantly influences their function and behavior in tissues (Figure 1).
2.3 Surface markers for macrophages/microglia
The identification and functional characterization of macrophages and microglia depend on the expression of specific surface markers. These markers not only help distinguish between different cell types but also provide insight into their functional states, especially when distinguishing between the proinflammatory and anti-inflammatory activation states of macrophages (summarized in Table 1).9-14
| Category | Type of Macrophage | Location | Function/Characteristic | Surface Markers | References |
|---|---|---|---|---|---|
| Central Nervous System Macrophages (CNS Macrophages) | Microglia | Throughout the brain and spinal cord | Maintenance of CNS homeostasis, immune defense, synaptic pruning, modulation of inflammatory responses | CD11b, CX3CR1, Iba-1, P2RY12 | Prinz et al., 2019 |
| Homeostatic Microglia | Throughout the brain | Maintenance of homeostasis, environmental monitoring | TMEM119, P2RY12, CX3CR1 | Keren-Shaul et al., 2017 | |
| Inflammatory Microglia | Inflammation sites | Response to injury, production of pro-inflammatory mediators, removal of pathogens and cellular debris | CD16, CD32, MHC-II | Butovsky et al., 2014 | |
| Neuroprotective Microglia | Injury or neurodegenerative sites | Protection of neuronal cells, release of reparative mediators, support regeneration | Arg1, Ym1, CD206 | Butovsky et al., 2014; Ransohoff, 2016 | |
| Disease-associated Microglia (DAM) | Neurodegenerative disease sites | Specific responses according to disease progression, modulation of pathological processes | TREM2, CD68, APOE | Keren-Shaul et al., 2017 | |
| Border-associated Macrophages (BAMs) | Perivascular, meningeal, choroid plexus | Immune surveillance, maintenance of CNS barriers, inflammatory response | CD163, CD206, LYVE1 | Prinz et al., 2019 | |
| Infiltrating Monocyte-derived Macrophages | Infiltrate CNS in injury or disease states | Involvement in inflammation and repair, pathogen clearance | CCR2, CX3CR1, CD45 | Prinz et al., 2019; Sipe et al., 2016 | |
| Peripheral Nervous System Macrophages (PNS Macrophages) |
Endoneurial Macrophages (Relmα−Mgl1−Lyve1−CX3CR1+) |
Innermost layer of peripheral nerves | Maintenance of homeostasis, removal of pathogens and cellular debris, support for nerve regeneration |
CD68, CD206 Iba1 |
Ydens et al., 2020 |
|
Epineurial Macrophages (Relmα+Mgl1+Lyve1+CX3CR1−) |
Outermost connective tissue layer of peripheral nerves | Defense against external injuries and infections, involvement in repair processes following nerve damage |
CD68, CD206 Iba1 |
Ydens et al., 2020 |
- Note: CD11b: A protein that is part of the integrin family involved in processes such as cellular adhesion and migration. CX3CR1: Chemokine (C-X3-C motif) receptor 1, involved in the communication between neurons and microglia, which is critical for microglial survival and function. Iba-1: Ionized calcium-binding adapter molecule 1, a marker of microglial activation. P2RY12: A purinergic receptor important for the microglial sensing of ATP, contributing to microglial chemotaxis and process extension. TMEM119: Transmembrane protein 119, a marker specific for microglia, not upregulated in infiltrating myeloid cells, which is useful for distinguishing resident microglia. CD16/CD32: Fc receptors that bind to IgG, involved in the phagocytosis of opsonized antigens. MHC-II: Major histocompatibility complex class II, essential for presenting antigen to helper T cells. Arg1 (arginase 1), Ym1, and CD206 (mannose receptor): Markers associated with the anti-inflammatory or alternative activation state of macrophages. TREM2: Triggering receptor expressed on myeloid cells 2, involved in microglial activation and phagocytosis, particularly relevant in neurodegenerative diseases. CD68: A lysosomal protein used as a marker for phagocytic activity, found in macrophages and microglia. APOE: Apolipoprotein E, involved in lipid metabolism and implicated in AD pathology and microglial modulation. CD163: A scavenger receptor expressed on the surface of macrophages that binds to and mediates the endocytosis of hemoglobin-haptoglobin complexes. LYVE1: Lymphatic vessel endothelial hyaluronan receptor 1, a marker of lymphatic endothelial cells that is involved in immune surveillance and inflammation. CCR2: CC chemokine receptor 2, a receptor for monocyte chemoattractant protein-1 (MCP-1), important for the recruitment of monocytes to sites of inflammation. CD45: A tyrosine phosphatase, also known as leukocyte common antigen (LCA), expressed on all nucleated hematopoietic cells, and used as a marker to distinguish leukocytes from non-leukocytes.
2.4 Classification via single-cell analysis
Recent advancements in single-cell RNA sequencing (scRNA-seq) have revealed the heterogeneity of CNS macrophages, allowing microglia to be distinguished from CNS-associated macrophages (CAMs) by gene expression. CAMs express genes including Mrc1 and Lyve1, whereas microglia contain high levels of Hexb and P2ry12, indicating their unique roles. To study the origins and functions of CMAs, researchers have developed transgenic mouse models, such as Mrc1CreERT2. These have helped trace CAMs back to erythromyeloid progenitors in the yolk sac, showing that microglia and CAMs share a common ancestor. This challenges the notion of separate lineages and provides insights into CNS macrophage development, which is crucial for understanding their roles in health and disease.15
3 MECHANISMS OF ACTIVATION AND FUNCTIONAL REGULATION
3.1 Activation pathways and molecular signals
In the CNS, microglial activation is typically triggered by damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns, which bind to pattern recognition receptors (PRRs) on the cell surface. This interaction leads to the activation of intracellular signaling cascades, including those mediated by Toll-like receptors and the NOD-like receptor family, culminating in the production of proinflammatory cytokines and chemokines.16 Recent studies have highlighted the importance of the colony stimulating factor 1 receptor (CSF1R) signaling pathway in regulating the survival, proliferation, and differentiation of microglia and macrophages. CSF1R inhibition depletes microglial populations, suggesting a potential therapeutic strategy for conditions characterized by microglial overactivation.17
3.2 Transcriptional regulation and functional states
The transition between different functional states of macrophages and microglia is tightly regulated at the transcriptional level. Key transcription factors, including nuclear factor-kappa B (NF-κB), interferon-regulatory factor (IRF), and signal transducer and activator of transcription (STAT) families, have critical roles in mediating inflammatory responses and are activated downstream of PRR signaling. These transcription factors regulate the expressions of cytokines and chemokines and modulate cell survival, proliferation, and migration.4 A significant advancement in understanding microglial function was provided by the identification of disease-associated microglia (DAM), a unique activation state associated with neurodegenerative diseases like AD. DAMs are characterized by a distinct transcriptional profile, including the downregulation of homeostatic genes and upregulation of neurodegenerative disease-related genes.10
4 PHAGOCYTOSIS-RELATED MOLECULES IN MPS
4.1 Phagocytosis-promoting molecules
4.1.1 Triggering receptor expressed on myeloid cells 2 (TREM2)
TREM2 engagement enhances microglial phagocytosis of cellular debris and amyloid-beta (Aβ), crucial for mitigating neurodegenerative diseases like AD.18
4.1.2 CSF-1R
CSF1R signaling is vital for microglial survival, proliferation, and phagocytic function. Inhibitors of CSF-1R are studied for their potential to modulate microglial activity in disease.17
4.1.3 Fcγ receptors
Recognition of the Fc region of opsonized antibodies by macrophage Fcγ receptors promotes the phagocytosis of pathogens or diseased cells, facilitating adaptive immune responses.19
4.2 Phagocytosis-inhibiting molecules
4.2.1 CD33
CD33 expression on MPS is implicated in reduced phagocytosis of Aβ, contributing to AD pathology. Targeting CD33 to enhance microglial phagocytosis of Aβ is a potential therapeutic strategy.20
4.2.2 SIRPα-CD47 interactions
Signal regulatory protein alpha (SIRPα), the “don't eat me” signal provided by interactions between SIRPα on macrophages and CD47 on healthy cells inhibits phagocytosis, preventing autoimmunity and the destruction of healthy tissues.21
5 INTERACTIONS BETWEEN MICROGLIA/MACROPHAGES AND NEURAL CELLS
5.1 Interactions with neurons
Microglia survey the neuronal environment, responding to changes by altering their morphology and functional state. They phagocytose apoptotic neurons and synaptic debris, a process essential for synaptic pruning during development and for clearing damaged cells following injury.22 Moreover, microglia release factors that can influence neuronal survival, differentiation, and synaptic formation.23
5.2 Interactions with astrocytes
Astrocytes and microglia/macrophages interact to regulate inflammatory responses within the CNS. Astrocytes release cytokines and chemokines that activate microglia/macrophages, enhancing their phagocytic and immune functions. Conversely, activated microglia influence astrocyte reactivity, promoting the formation of a glial scar in response to injury.24
5.3 Interactions with oligodendrocytes
Microglia clear myelin debris and secrete factors that help oligodendrocyte progenitors mature and remyelinate. Although they can release damaging proinflammatory cytokines, they also promote repair through anti-inflammatory agents. Recent research indicated that manipulating microglia/macrophage responses might be therapeutic in demyelinating diseases like MS, particularly through pathways including the fibroblast growth factor (FGF)/FGFR signaling pathway.25
5.4 Modulatory effects on neural cell functions
Recent studies have highlighted the roles of microglia/macrophages in modulating the functions of neural cells. For example, microglial-derived cytokines influenced neuronal gene expression, synaptic transmission, and plasticity, thereby affecting cognitive functions such as learning and memory.26 Similarly, astrocyte–microglia interactions modulated the astrocytic release of neurotrophic factors and uptake of neurotransmitters, further influencing neuronal activity and CNS homeostasis.27
5.5 Microglia and connexins
Connexins form gap junctions between cells, facilitating direct cell-to-cell communication, and form hemichannels, allowing the release and uptake of various molecules in the extracellular space. Connexins, such as Cx43, are abundantly expressed by astrocytes and microglia, where they contribute to the propagation of calcium waves and release of inflammatory mediators, influencing the inflammatory milieu of the CNS.28 Connexin-based communication is crucial for the regulation of microglial activation and subsequent inflammatory responses, suggesting that modulating connexin functionality could mitigate neuroinflammation and its deleterious effects on neural tissues.29 Connexins facilitate the spread of neuroinflammatory signals across glial networks, affecting disease progression and severity. Thus, targeting connexin channels in microglia offers a promising avenue for modulating neuroinflammatory responses in a wide array of CNS disorders.30
6 ROLES IN CNS DISORDERS
In some central and peripheral neurological disorders, macrophages and microglia play central roles in their pathomechanisms, as summarized in Table 2.
| Disease | Role of Microglia/Macrophages | References |
|---|---|---|
| Ischemic Stroke | First responders in stroke, activating for debris clearance but prolonged activation leads to chronic inflammation and damage. |
Liu 2016, Masuda 2023 |
| Alzheimer's Disease | Involved in the initial recognition and clearance of Aβ, modulation of neuroinflammation, with both protective and detrimental effects on disease progression. | Keren-Shaul, 2017, Hansen 2018, Heneka 2015, Sevigny 2016 |
| Multiple Sclerosis | Key players in the immune-mediated attack against myelin and neurons, contributing to inflammation, demyelination, and potential remyelination. | Dutta 2007, Yamasaki 2014, Yamasaki 2019 |
| Amyotrophic Lateral Sclerosis | Participate in both protective and pathological processes, influenced by local environment and disease stage. |
Henkel 2009, Clarke 2020, Beers 2011 |
| Multiple System Atrophy | Key mediators of neuroinflammation, believed to contribute to neuronal and oligodendroglial degeneration. | Yamasaki 2017, Ishizawa 2004 |
| ALSP(HDLS)/Nasu-Hakola Diseases | Central to pathogenesis, with impaired proliferation and altered activation states due to genetic mutations. |
Rademakers 2011, Paloneva 2003 |
| Histiocytosis / Erdheim-Chester Disease | Characterized by the abnormal proliferation of histiocytes, contributing to organ dysfunction including in the CNS. | Diamond 2016, Haroche 2011, Haroche 2012 |
| Brain Tumor | Play a dual role in brain tumor environments as tumor-associated macrophages (TAMs), promoting tumor growth and suppressing immune responses. |
Hambardzumyan 2016, Quail 2016 |
| Guillain-Barré Syndrome/Chronic Inflammatory Demyelinating Polyneuropathy | Involved in the stripping of myelin from axons and the phagocytosis of myelin debris, with treatments aimed at modulating the immune response. |
Hughes 2005, Yuki 2012 |
| Diabetic Polyneuropathy | Play a critical role in both the progression and resolution of nerve damage through metabolic disruption, oxidative stress, and inflammation. | Mathey 2015 |
6.1 Ischemic stroke
Following a stroke, microglia are among the first responders, quickly becoming activated in response to cerebral ischemia. Their activation is a double-edged sword; while initially beneficial for clearing debris and limiting damage, prolonged activation can lead to chronic inflammation, exacerbating neuronal death and impairing recovery.31 Macrophages derived from circulating monocytes infiltrate the ischemic brain, contributing to the inflammatory milieu. Together, microglia and macrophages orchestrate a complex inflammatory response that significantly impacts the progression of ischemic injury and the healing process.
A critical function of microglia and macrophages in stroke involves phagocytosis—the clearance of dead neurons, myelin debris, and blood products. This process is essential for resolving inflammation and initiating repair mechanisms. However, the efficiency and outcome of phagocytosis depend on the balance between proinflammatory and anti-inflammatory phenotypes of these cells. A detailed examination of post-ischemic inflammation by Shichita et al. (2012) revealed that after an initial proinflammatory response characterized by inflammatory microglia/macrophages, there is a critical shift toward an anti-inflammatory phenotype. This shift is mediated by γδT cells that produce interleukin-17 (IL-17) in the delayed phase of ischemic brain injury. IL-17 production by γδT cells was critical for the induction of anti-inflammatory macrophages, which are integral for promoting tissue repair and resolving inflammation. This study underscores the importance of the temporal modulation of microglia and macrophage activities, as delayed IL-17 production by γδT cells facilitates the transition to a reparative state, thereby improving outcomes after stroke. These findings highlight a potential therapeutic window where the modulation of these immune responses could enhance recovery from ischemic injury.32
In the later stages of stroke recovery, microglia and macrophages contribute to neuroprotection and neurogenesis. By releasing growth factors and cytokines, they promote the proliferation and differentiation of neural progenitor cells, aiding tissue repair and functional recovery. The ability of these immune cells to adopt an anti-inflammatory phenotype is critical for supporting regenerative processes, underscoring the therapeutic potential of modulating their activity post-stroke.33 Recent findings introduced a novel strategy for stroke recovery, demonstrating that direct neuronal conversion of microglia/macrophages reinstated neurological functions post-stroke. By transforming microglia/macrophages into neurons, this research offers a new method for neuroregenerative treatments, potentially revolutionizing post-stroke rehabilitation strategies.34
6.2 AD/cerebral amyloid angiopathy (CAA)
Microglia and macrophages have critical roles in AD and CAA, conditions characterized by the accumulation of Aβ plaques within the brain and blood vessels, respectively. Their functions in these disorders span from the initial recognition and clearance of Aβ to the modulation of neuroinflammation, which can have protective and detrimental effects on disease progression. Recent advances, including therapeutic approaches targeting Aβ clearance, have further highlighted the significance of these immune cells in AD and CAA.
Microglia are intimately involved in the response to Aβ accumulation. They possess receptors that recognize Aβ, such as TREM2, which upon activation can initiate the phagocytosis of Aβ aggregates.10 However, the efficiency of Aβ clearance by microglia is influenced by various factors, including their activation state and the presence of proinflammatory signals that inhibit phagocytic functions.35 Similarly, perivascular macrophages play a role in clearing Aβ from cerebral blood vessels, impacting the severity of CAA and associated neurovascular dysfunction.
The chronic activation of microglia and macrophages in response to Aβ deposition is a hallmark of AD and CAA, and contributes to a sustained inflammatory environment that exacerbates neuronal damage and cognitive decline. The production of inflammatory cytokines, reactive oxygen species, and complement factors by activated microglia can further promote Aβ aggregation and tau pathology, highlighting the complex role of these cells in disease pathogenesis.36
Lecanemab, an anti-Aβ antibody, has shown promise in promoting the clearance of Aβ plaques by engaging microglia-mediated phagocytosis. Clinical trials have demonstrated its potential in slowing cognitive decline in AD patients by enhancing the immune-mediated removal of Aβ.37, 38 These findings underscore the potential of therapies that harness the phagocytic capabilities of microglia and macrophages to mitigate AD pathology.
6.3 MS
In MS, a chronic autoimmune disease characterized by demyelination and neurodegeneration in the CNS, the roles of microglia and macrophages are multifaceted, contributing to disease pathology and repair mechanisms; they act as key players in the immune-mediated attack against myelin and neurons. Upon activation, they release proinflammatory cytokines, chemokines, and reactive oxygen species, contributing to the inflammatory environment and demyelination observed in MS lesions.39 These cells also participate in the phagocytosis of myelin debris, a process essential for potential remyelination which can also perpetuate inflammation and autoimmunity by presenting myelin antigens to T cells.40 The destruction of axons and neurons is a hallmark of MS, leading to the progressive neurological decline observed in patients. Microglia and macrophages contribute to this axonal and neuronal damage through the secretion of toxic substances, including nitric oxide and glutamate, which induce axonal injury and neuronal death.39 Furthermore, their aberrant activation disrupts CNS homeostasis and impedes repair processes, exacerbating disease progression.
Although microglia and macrophages are instrumental in mediating inflammation in MS, they also play roles in tissue repair and remyelination. The clearance of myelin debris by these cells is a prerequisite for remyelination; however, their persistent activation and secretion of inflammatory mediators can inhibit the differentiation of oligodendrocyte precursor cells and the remyelination process.41 Thus, the balance between their damaging and reparative functions is critical in determining disease outcomes.
6.4 ALS
In ALS, a devastating neurodegenerative disorder characterized by the progressive loss of motor neurons, the roles of microglia and macrophages are critical to understanding disease mechanisms and identifying potential therapeutic targets. These cells participate in protective and pathological processes and are influenced by their local environment and the stage of disease.42, 43
Microglia and macrophages in ALS exhibit a spectrum of activation states, from neuroprotective phenotypes that promote debris clearance and neurotrophic support to neurotoxic phenotypes that exacerbate neuronal death. This dichotomy is influenced by signaling molecules in the microenvironment, including cytokines, chemokines, and DAMPs.44 The balance between these activation states is critical for disease progression and represents a potential therapeutic target.
In ALS, activated microglia and macrophages contribute to neurodegeneration through several mechanisms, including the secretion of proinflammatory cytokines such as TNF-α and IL-1β, and reactive oxygen species (ROS), which can directly damage neurons or induce astrocyte-mediated toxicity. The mouse model of familial ALS, the mutant SOD1 transgenic mice, also demonstrated microglia-oriented neuroinflammation characterized by an increase in the release of inflammatory cytokines by microglia harboring the SOD1 protein.45 The mutant SOD1 transgenic mice also provide some interesting findings with genetically labeled monocytes/microglia, indicating a toxic conversion of microglia and neurons, which suggests a vicious cycle of inflammatory milieu.46 Furthermore, the failure to efficiently clear apoptotic cells and debris can exacerbate neuroinflammation and hinder regenerative processes.47, 48 Another critical aspect of microglia and macrophage involvement in ALS concerns the clearance of misfolded proteins, which accumulate in motor neurons and contribute to cellular stress and death. We previously demonstrated that infiltrated macrophages in peripheral nerves cleared misfolded mutant proteins, correlating with disease progression in motor neuron disease models.49 This finding underscores the importance of peripheral immune responses in ALS and suggests that enhancing the clearance capacity of macrophages may represent a viable therapeutic approach.
The modulation of microglia and macrophage activity represents a promising therapeutic strategy in ALS. Research into anti-inflammatory agents, regulatory T cell therapy, and CSF1R inhibitors, which can modulate microglial activation, are areas of active investigation.50 By modulating connexin hemichannels, it is possible to control neuroinflammation and prevent neuronal damage, offering a promising direction for developing treatments for such conditions.29
6.5 Multiple system atrophy (MSA)
Microglia and macrophages are key mediators of neuroinflammation, a prominent feature of the disease pathology of MSA. Activated microglia have been identified in various brain regions affected by MSA, where they are thought to contribute to neuronal and oligodendroglial degeneration through the release of proinflammatory cytokines and reactive oxygen species. We have shown evidence of early strong intrathecal inflammation in cerebellar-type MSA, demonstrating elevated levels of proinflammatory cytokines/chemokines in the cerebrospinal fluid, which correlated with disease progression and severity.51
The activation of microglia and macrophages in MSA is a response to the accumulation of aberrant α-synuclein in oligodendrocytes, known as glial cytoplasmic inclusions. This accumulation triggers an immune response, leading to the activation of microglia and the infiltration of macrophages into the CNS. Once activated, these cells exacerbate the pathological process through the production of neurotoxic substances, further contributing to the cycle of neurodegeneration observed in MSA.52
Studies have shown that the extent of microglial activation in MSA correlates with disease progression and the severity of clinical symptoms. Furthermore, microglial activation in MSA is associated with the disruption of the blood–brain barrier, facilitating the entry of peripheral immune cells and compounds that may worsen neuroinflammation.53
6.6 Adult-onset Leukoencephalopathy with Spheroids and Pigmented Glia (ALSP)/Nasu-Hakola Disease
ALSP and Nasu-Hakola disease represent distinct neurological conditions where the roles of microglia and macrophages are increasingly recognized to be central to their pathogenesis. ALSP, previously known as hereditary diffuse leukoencephalopathy with spheroids, is a rare neurodegenerative disorder characterized by mutations in the CSF1R gene, which is critical for microglial development and survival. The pathology of ALSP includes white matter degeneration, axonal spheroids indicating axonal damage, and pigmented glia, reflective of microglial and astrocytic responses to myelin and axonal degeneration. Microglia in ALSP have impaired proliferation and altered activation states related to CSF1R mutations, leading to reduced phagocytic activity and altered cytokine production. These changes result in the insufficient clearance of myelin debris and aberrant inflammatory responses, which exacerbate white matter pathology.54, 55
Nasu-Hakola Disease is distinguished by mutations in the tyrosine kinase binding protein (TYROBP) DNAX activating protein-12 (DAP12) or triggering receptor expressed on myeloid cells 2 (TREM2) genes, which encode signaling molecules important for microglial function. These mutations disrupt microglial signaling pathways, affecting their ability to respond to and clear damaged cells and myelin debris. This leads to abnormal microglial activation, contributing to the neuroinflammation and neurodegeneration seen in Nasu-Hakola patients. The disease's hallmark features include presenile dementia and bone cysts, with neuropathological findings showing extensive microglial activation and inflammatory responses within the CNS. These findings underscore the critical role of microglia in maintaining CNS homeostasis and highlight how their dysfunction can lead to severe neurodegenerative outcomes.56
The involvement of microglia and macrophages in ALSP and Nasu-Hakola disease underscores the potential for therapeutic strategies targeting these cells to modify disease progression. Approaches to enhance microglial phagocytosis, modulate inflammatory responses, or correct genetic mutations causing these diseases could provide avenues for the development of novel treatments. The development of CSF1R agonists, TREM2 agonists, or DAP12 signaling modulators represents potential therapeutic strategies aimed at restoring normal microglial functions in these conditions.57-59
6.7 Histiocytosis/Erdheim-Chester disease (ECD)
Histiocytosis, including ECD, represents a group of rare disorders characterized by the proliferation of histiocytes, which are cells derived from macrophages and dendritic cells. These diseases can affect multiple organs, including the brain, highlighting the significance of understanding the roles of macrophages and microglia in their pathogenesis and progression.
Histiocytosis encompasses a range of conditions characterized by the abnormal proliferation of histiocytes. In these diseases, macrophages play a central role owing to their origin as tissue-resident histiocytes. Their proliferation leads to the formation of lesions that can infiltrate various organs, including the CNS. In the CNS, this proliferation can cause neurological symptoms ranging from cognitive impairment to motor dysfunction, depending on the location and extent of the lesions.60
ECD is a specific form of non-Langerhans cell histiocytosis, marked by the infiltration of lipid-laden macrophages into tissues throughout the body, including the CNS. This infiltration is often accompanied by fibrosis and results in significant organ dysfunction. Within the CNS, ECD lesions typically affect the hypothalamus, pituitary gland, and brainstem, although other areas can also be involved, leading to a wide range of neurological symptoms.61 In ECD, BRAF V600E mutations have been identified in a significant proportion of cases, contributing to the aberrant proliferation of histiocytes. This discovery has opened new avenues for targeted therapy with BRAF inhibitors.62
6.8 Brain tumors
Microglia and macrophages, integral to the brain's immune system, play a dual role in the environment of brain tumors, particularly gliomas. These cells, collectively known as tumor-associated macrophages (TAMs), can be co-opted by tumor cells to promote tumor growth, suppress immune responses, and facilitate tumor invasion. A previous study by Hambardzumyan et al. (2016) highlighted the recruitment and pro-tumor activities of TAMs in glioblastoma, underscoring the therapeutic potential of targeting these cells to combat brain tumors.63 Quail et al. (2016) further detailed how TAMs contribute to the immunosuppressive tumor microenvironment, hindering the effectiveness of immune-based therapies.64 Pyonteck et al. (2013) demonstrated the potential of reprogramming or inhibiting TAMs to block glioma progression, offering hope for new treatment strategies.65 These findings indicate the complex role of microglia and macrophages in brain tumor biology and the promise of targeting these cells by novel therapies.
7 TYPES AND LOCALIZATION OF MACROPHAGES IN THE PERIPHERAL NERVOUS SYSTEM
7.1 Endoneurial macrophages
Located within the endoneurium—the innermost layer of peripheral nerves. Endoneurial macrophages (Relmα−Mgl1−Lyve1−Cx3cr1+ macrophages) are essential for maintaining homeostasis and participating in the immune defense of the peripheral nervous system (PNS). They are more abundant than epineurial macrophages and are involved in clearing cellular debris and pathogens. Furthermore, they play a significant role in nerve regeneration following injury by expressing chemoattractants. Peripheral macrophages are then recruited, rapidly acquiring Arg1, Chil3, and VEGF production, and functioning as scavengers.14, 66
7.2 Epineurial macrophages
Located in the epineurium—the outermost connective tissue layer of peripheral nerves. Epineurial macrophages (Relmα+Mgl1+Lyve1+Cx3cr1− macrophages) are primarily involved in defending against external injuries and infections. Additionally, they contribute to repair processes following nerve damage14, 66 (Table 1).
8 INVOLVEMENT IN PERIPHERAL NERVE DISORDERS
8.1 Guillain-Barré Syndrome (GBS)/chronic inflammatory demyelinating polyneuropathy (CIDP)
GBS is an acute autoimmune disorder often triggered by infections. The immune response in GBS targets the myelin or axons of peripheral nerves, leading to rapid-onset muscle weakness and paralysis. Macrophages are central to this process, where they are involved in the stripping of myelin from axons and the phagocytosis of myelin debris. This macrophage-mediated demyelination is thought to be initiated by molecular mimicry, where antibodies produced in response to infectious agents recognize and cross-react with components of myelin in peripheral nerves.67, 68
CIDP is characterized by a chronic, progressive, or relapsing course of muscle weakness and sensory symptoms, with histological evidence of demyelination and remyelination in peripheral nerves. Similar to GBS, macrophages play a critical role in CIDP pathology by infiltrating nerves and contributing to demyelination by myelin stripping and phagocytosis. Additionally, macrophages secrete proinflammatory cytokines that perpetuate the immune response and damage the nerve tissue. The chronic nature of CIDP involves repeated episodes of immune attack on peripheral nerves, highlighting the role of macrophages in disease progression and periods of remission.69
Treatments including intravenous immunoglobulins (IVIg) and plasmapheresis were designed to modulate the immune response. They aim to reduce the activity of pathogenic macrophages and promote remission. IVIg, for example, was shown to decrease macrophage infiltration into lesions.70 Similarly, steroids reduce macrophage infiltration without affecting C3 deposition.71 However, one reason why steroids may be detrimental to GBS is because of their reducing effect on macrophages. This reduction can delay the clearance of debris, resulting in slower remyelination processes and axonal repair.67
8.2 Diabetic polyneuropathy (DPN)
Diabetic polyneuropathy represents one of the most common complications of diabetes, characterized by a progressive loss of peripheral nerve function. The pathology of DPN involves a complex interplay between metabolic disruption, oxidative stress, and inflammation, with macrophages playing a critical role in the progression and resolution of nerve damage.
In the context of diabetes, chronic hyperglycemia triggers a cascade of metabolic and inflammatory processes that contribute to peripheral nerve damage. In the early stages of DPN, macrophages are recruited to sites of nerve injury, where they contribute to inflammation through the release of proinflammatory cytokines including TNF-α, IL-1β, and IL-6. These cytokines exacerbate neural damage by promoting further immune cell infiltration, increasing oxidative stress, and impairing Schwann cell function, essential for nerve regeneration and myelination.72
Oxidative stress is a critical factor in the development of DPN. Macrophages contribute to this process by generating ROS and nitrogen species in response to hyperglycemia-induced metabolic distress. The excessive production of ROS by activated macrophages leads to direct nerve damage and promotes the further activation of inflammatory pathways, exacerbating the cycle of injury and inflammation.73
In DPN, the collaboration between macrophages and Schwann cells is critical for nerve regeneration. Macrophages, which are divided into proinflammatory and anti-inflammatory types, invade injured sites to aid debris clearance and contribute to the regeneration process. Their interaction with Schwann cells facilitates the remodeling of the nerve environment, which is essential for axonal regrowth.74
9 CONCLUSION
This review underscores the critical roles of microglia and macrophages in the neuroinflammatory landscape across a broad spectrum of neurological disorders. Their dual capabilities as facilitators of damage and repair within the nervous system highlight their significance in health and disease. The exploration into their diverse functions, from maintaining CNS homeostasis to contributing to the pathogenesis of diseases like AD, ALS, and others, opens up potential therapeutic avenues that target neuroinflammation. Advances in understanding their complex biology, particularly through techniques like single-cell RNA sequencing, offer promising strategies for modulating their activity to combat neurological diseases. Continued research into microglia and macrophages is a beacon of hope for developing more effective interventions against these conditions, emphasizing the necessity of integrating insights from various studies to forge comprehensive treatment approaches.
ACKNOWLEDGMENTS
The publication of this manuscript is not related to any funding bodies. We thank J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest related to this manuscript.




