Posaconazole for Prevention of COVID-19-Associated Pulmonary Aspergillosis in Mechanically Ventilated Patients: A European Multicentre Case–Control Study (POSACOVID)
Funding: This study was funded by an investigator-initiated-trial funding from Merck Sharp & Dohme Corp (IIS 60879).
Juergen Prattes and Daniele R. Giacobbe contributed equally to this study, shared first authors.
Matteo Bassetti, Jean-Pierre Gangneux and Martin Hoenigl contributed equally to this study, shared senior authors.
ABSTRACT
Background
This study investigated the impact of posaconazole (POSA) prophylaxis in COVID-19 patients with acute respiratory failure receiving systemic corticosteroids on the risk for the development of COVID-19-associated pulmonary aspergillosis (CAPA).
Methods
The primary aim of this prospective, multicentre, case–control study was to assess whether application of POSA prophylaxis in mechanically ventilated COVID-19 patients reduces the risk for CAPA development. All consecutive patients from centre 1 (cases) who received POSA prophylaxis as standard-of-care were matched to one subject from centre 2 and centre 3 who did not receive any antifungal prophylaxis, using propensity score matching for the following variables: (i) age, (ii) sex, (iii) treatment with tocilizumab and (iv) time at risk.
Results
Eighty-three consecutive patients receiving POSA prophylaxis were identified at centre 1 and matched to 166 controls. In the matched cohort, incidence rates of CAPA were 1.69 (centre 1), 0.84 (centre 2) and 7.18 (centre 3) events per 1000 ICU days. In multivariable logistic regression analysis, the presence of an EORTC/MSGERC risk factor at ICU admission (OR 4.35) and centre 3 versus centre 1 (OR 6.07; 95% CI 1.76–20.91; p = 0.004) were associated with an increased risk of CAPA. No increased risk of CAPA was registered for centre 2 versus centre 1.
Conclusions
The impact of POSA prophylaxis depends on the baseline CAPA incidence rate, which varies widely between centres. Future trials should therefore investigate targeted antifungal prophylaxis (e.g., stratified for high-prevalence centres or high-risk patients) in COVID-19 patients.
Trial Registration: NCT05065658
Abbreviations
-
- ARF
-
- acute respiratory failure
-
- BALF
-
- bronchoalveolar lavage fluid
-
- CAPA
-
- COVID-19-associated pulmonary aspergillosis
-
- ECMM/ISHAM
-
- European Confederation of Medical Mycology/ International Society for Human & Animal Mycology
-
- EORTC/MSGERC
-
- The European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium
-
- GM
-
- galactomannan
-
- ICU
-
- intensive care unit
-
- IMV
-
- invasive mechanical ventilation
-
- OR
-
- odds ratio
-
- POSA
-
- posaconazole
-
- PS
-
- propensity score
1 Background
COVID-19-associated pulmonary aspergillosis (CAPA) has been reported throughout the COVID-19 pandemic. Based on the multicenere cohort studies, it is estimated that approximately 10% to 15% of patients who develop critical COVID-19 and are treated on intensive care units (ICUs) are diagnosed with CAPA [1-4] and up to 35% of those who receive invasive mechanical ventilation (IMV) [5]. However, incidence rates vary widely by centre, as shown before for the three centres participating in this study [6]. Several characteristics of a SARS-CoV-2 infection are increasing the risk for CAPA development including viral cell tropism, respiratory epithelial damage and the release of pro-inflammatory cytokines as a consequence of release of danger-associated molecular patterns [7, 8]. The outcome is poor with mortality rates greater than 40% and CAPA being an independent risk factor for death in most studies [2, 3, 9, 10].
As invasive procedures for tissue sampling (e.g., lung biopsy) and consecutive histological work-up are rarely feasible in patients with acute respiratory failure (ARF), CAPA diagnosis is usually based on work up of respiratory samples obtained by bronchoscopy including fungal culture, polymerase chain reaction and biomarker testing as well as imaging (although often unspecific) [1]. The majority of CAPA patients present an impeded fungal growth in histopathology from lung tissue and usually lack angio-invasion [11] – the hallmark of invasive aspergillosis in neutropenic patients. Consequently, testing for fungal blood-biomarkers comes along with limited sensitivity [1] and suspicion for CAPA should trigger sampling and testing of lower respiratory tract specimen. Nevertheless, diagnosis remains challenging andrates of CAPA among patients with COVID-19 requiring ICU admission are increasing since the introduction of COVID-19 vaccination programs [12]. A recently published prospective observational study highlighted, that CAPA rates more than doubled (59% vs. 24%) since the population-wide roll out of COVID-19 vaccination programs [13]. This finding is at least partly explained by the fact that patients who have underlying immunocompromising condition are less likely to adequately immunologically response to COVID-19 vaccination [14] and are therefore more likely to develop critical disease requiring treatment on ICU. Consequently, a higher percentage of patients with critical COVID-19 will have underlying conditions that are associated with increased risk for invasive fungal infections and increased mortality.
Given the high burden of CAPA in patients with COVID-19-associated ARF, the high CAPA mortality rate and the challenges in rapid and reliable diagnosis, antifungal prophylaxis was introduced in some centres [15-18]. However, the only randomised controlled trial evaluating the impact of prophylaxis had to be terminated before significant enrolment [19]. Several case reports and case series have reported on inhaled amphotericin B (lipid formulations and conventional) prophylaxis and most found clinically relevant reduction of CAPA rates [15, 16, 18]. One single-centre observational study including non-ventilated patients reported on posaconazole (POSA) prophylaxis in COVID-19 patients on ICU and observed that there was clear reduction of CAPA cases [17].
Here, we report the results of our matched case–control analysis from a prospective multicentre study investigating the potential effect of POSA prophylaxis on the risk of CAPA in mechanically ventilated COVID-19 patients who received corticosteroids.
2 Methods
2.1 Study Design
The POSACOVID Study (NCT05065658) is a prospective observational study aiming to assess the impact of POSA prophylaxis applied to patients with COVID-19-associated ARF on the risk of CAPA development [6]. The study was conducted in three centres in Europe [Medical University of Graz/Austria (centre 1), University of Genoa/Italy (centre 2) and Rennes University Hospital/France (centre 3)]. The main study protocol was approved by the local ethic committee at the Medical University of Graz, Austria (32–296 ex 19/20). Centres 2 and 3 provided data for the control group. Both centres obtained ethic committee approval by their local ethic committees [163/2020 with an amendment approved at May 5th 2022 (Genoa) and approval number 20.56 (Rennes)].
The authors designed the study, collected the study relevant data and analysed the data. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The main objective of the study was to perform a matched case–control analysis, while incidence rates of CAPA in the three participating centres (without any matching for baseline characteristics) were published before [6]. In detail, unadjusted incidence rates of CAPA were 1.69 (centre 1), 1.42 (centre 2) and 9.53 (centre 3) events per 1000 ICU days [6].
2.2 Study Cohort
Patients with COVID-19-associated ARF requiring IMV on ICU who received POSA prophylaxis (cases), were matched to patients with COVID-19-associated ARF who received standard-of-care treatment but no antifungal prophylaxis (controls).
All cases were recruited from centre 1. Centres 2 and 3 provided the matched controls. Each case was matched with one control from centre 2 and one control from centre 3 (see below). At centre 1, systemic antifungal prophylaxis with intravenous (i.v.) or oral (tablet) POSA 300 mg twice daily at day 1, followed by 300 mg once daily from day 2 onwards, was recommended for all patients with COVID-19-associated ARF by local COVID-19 management guideline starting September 2020. All consecutive patients admitted to ICU at centre 1 from September 2020 to April 2022 with COVID-19-associated ARF, requiring IMV and who received POSA prophylaxis were considered eligible for study inclusion. Exclusion criteria were (i) age under 18 years, (ii) ICU admission due to COVID-19 ARF but no need for IMV and (iii) ICU admission of COVID-19 (with or without IMV) due to other reason than COVID-19-associated ARF.
All patients were classified regarding presence of CAPA based on the 2020 ECMM/ISHAM consensus definitions [20].
Parts of the cohort reported here have already been published in previous analyses [1, 9, 17, 21].
2.3 Matching Procedure
Each case from centre 1 was matched as a case with one control from centre 2 and one control from centre 3, using two separate 1:1 propensity score (PS) matching procedures (one for identifying the control from centre 2 and one for identifying the control from centre 3) [22, 23]. The following variables were considered for matching cases with controls: (i) age, (ii) sex, (iii) treatment with tocilizumab and (iv) time at risk. We did not match for systemic corticosteroid treatment since all cases from centre 1 received systemic corticosteroids, thus we only considered controls for possible match in case they also received corticosteroids.
Time at risk was defined as follows: (i) for cases: days on POSA prophylaxis plus, if applicable, days in ICU before POSA initiation and (ii) for controls: days on ICU. To guarantee equal time at risk in cases and respective controls, we started by matching the case with the longest time at risk (53 days) to possible controls with time at risk equal or longer than 53 days. Then, we selected the case with the second longest time at risk and possible remaining controls with equal or longer time at risk and so on. Eventually, for the study analyses, we considered only the period of time at risk in controls (starting from ICU admission) that was equal to the time at risk in the respective case (to have an exactly equal time at risk in cases and their respective controls).
As sensitivity analysis, we conducted a multinomial logistic regression on all patients with observation time greater than or equal to the longest time at risk for the cases (thus, one case and all potential controls with equal or longer time at risk) to find for each subject the probability of belonging to the centre 1 (p1), the probability of belonging to the centre 2 (p2) and the probability of belonging to the centre 3 (p3). These probabilities sum up to 1, so we considered only the first two probabilities to figure each subject in a Cartesian plane with x-axis p1 and y-axis p2. Then, we calculated the perimeter of the triangle resulting from each combination of the case with two controls and chose the pair of controls for which that perimeter was the smallest. Subsequently, we repeated the procedure for the case with the second longest time at risk and all remaining potential controls with equal or longer time at risk. The procedure was then repeated until all cases were matched to one control from each centre (1:1:1 matching).
2.4 Statistical Analyses
The primary aim of the study was to investigate the impact of POSA prophylaxis on the risk of CAPA development. Characteristics of cases and controls after the matching procedure were compared through the Friedman test and the Cochran's Q test. The risk of CAPA development was compared between centre 1 and the other centres by means of multivariable logistic regression. Besides centre, other variables included in the multivariable logistic regression model were: (i) European Organization for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) risk factor present at ICU admission and (ii) presence of extracorporeal membrane oxygenation (ECMO). Of note, no mixed effect models with centre as random effect were employed in this study due to the lack of generalisation potential for between-centre variability connected to the low number of centres [24].
Baseline characteristics were compared between centres through the Wilcoxon rank-sum test and the chi-squared test for continuous and categorical variables, respectively.
A p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Study Population
Between September 2020 and April 2021, 83 consecutive cases were identified at centre 1 who received POSA prophylaxis (median 13 days, range: 1 to 48 days) and fulfilled the inclusion criteria. Centre 2 provided 239 potential controls and centre 3 provided data from 192 potential controls (Figure 1). All cases (centre 1) were treated with corticosteroids. The total of 83 patients receiving POSA prophylaxis from centre 1 was matched to 83 patients from centres 2 and 3 each.
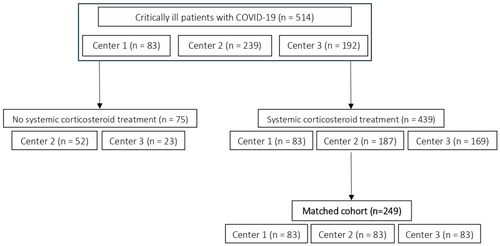
Demographic data for the matched study cohort are displayed in Table 1. Of note, the proportion of patients on ECMO differed significantly between centres (16% in centre 1 vs. 2% and 4% in centre 2 and centre 3, respectively, p < 0.001).
| Variablesa | Centre 1 (n = 83) | Centre 2 (n = 83) | Centre 3 (n = 83) | p | SD (Centre 1 vs. 2) | SD (Centre 1 vs. 3) |
|---|---|---|---|---|---|---|
| (A) | ||||||
| Posaconazole prophylaxis | 83 (100) | 0 (0) | 0 (0) | < 0.001 | Inf. | Inf. |
| Age at ICU admission in years, median (IQR)b | 65 (58–71) | 66 (61–71) | 65 (57–72) | 0.949 | 0.016 | −0.122 |
| Male sexb | 56 (68) | 54 (65) | 55 (66) | 0.948 | 0.043 | 0.064 |
| EORTC/MSGERC risk factor present at ICU admissionc | 8 (10) | 5 (6) | 5 (6) | 0.593 | 0.148 | 0.148 |
| ECMO | 13 (16) | 2 (2) | 3 (4) | 0.001 | 0.408 | 0.504 |
| Treatment with tocilizumabb | 2 (2) | 1 (1) | 3 (4) | 0.600 | −0.117 | 0.082 |
| Systemic corticosteroid treatment | 83 (100) | 83 (100) | 83 (100) | — | 0 | 0 |
| Time at risk in days, median (IQR)b | 15 (10–23) | 15 (10–23) | 15 (10–23) | — | 0 | 0 |
| Variablesa | Centre 1 (n = 83) | Centre 2 (n = 83) | Centre 3 (n = 83) | p | SD (cases vs. controls) |
|---|---|---|---|---|---|
| (B) | |||||
| Posaconazole prophylaxis | 83 (100) | 0 (0) | 0 (0) | < 0.001 | Inf. |
| Age at ICU admission in years, median (IQR)b | 65 (58–71) | 65 (58–71) | 65 (58–72) | 0.988 | −0.041 |
| Male sexb | 56 (68) | 53 (64) | 59 (71) | 0.610 | 0 |
| EORTC/MSGERC risk factor present at ICU admissionc | 8 (10) | 7 (8) | 4 (5) | 0.490 | 0.108 |
| ECMO | 13 (16) | 2 (2) | 4 (5) | 0.003 | 0.408 |
| Treatment with tocilizumabb | 2 (2) | 1 (1) | 2 (2) | 0.815 | 0.042 |
| Systemic corticosteroid treatment | 83 (100) | 83 (100) | 83 (100) | — | 0 |
| Time at risk in days, median (IQR)b | 15 (10–23) | 15 (10–23) | 15 (10–23) | — | 0 |
- Abbreviations: ECMO, extracorporeal membrane oxygenation; EORCT, European Organization for Research and Treatment of Cancer; ICU, intensive care unit; IQR, interquartile range; MSGERC, Mycoses Study Group Education and Research Consortium; SD, standardised difference in means or proportions divided by standard error; imbalance defined as absolute value greater than 0.20 (small effect size).
- a Expressed as n (%), unless otherwise indicated.
- b Matching variables.
- c Presence of missing values (0/83 for centre 1, 0/83 for centre 2, 1/83 for centre 3).
3.2 Diagnostic Procedures
In centre 1, 78 of the 83 (94%) patients had at least one respiratory sample sent in for microbiological work-up; in 62 of those 78 patients ≥ 1 bronchoalveolar lavage fluid (BALF) samples had been tested (all had culture, 38 had also BALF-GM tested), while in the remaining 16 patients ≥ 1 tracheal aspirate had been tested. In centre 2, in all patients at least one BALF sample had been obtained for microbiological work-up (culture and BALF-GM in all cases). In centre 3, all patients were screened once weekly by tracheal aspirates for mycological work-up (mycological cultures and GM determination). In case of positive mycological screening or in case of suspicion of a bacterial or viral superinfection, BALF sample was obtained for microbiological work-up (91% of the patients).
3.3 CAPA Incidence Rate and Incidence Rate Ratio
After 1:1 matching, 23 CAPA cases occurred within the study cohort. Four in centre 1 (3 probable and 1 possible), two in centre 2 (2 probable) and 17 in centre 3 (10 probable and 7 possible).
The incidence rates for CAPA among the centres after 1:1 matching were as following: 1.69 CAPA/1000 ICU days in centre 1, 0.84 CAPA/1000 ICU days in centre 2 and 7.18 CAPA/1000 ICU days in centre 3, respectively. The incidence rate ratio for CAPA development in cases versus controls was 2.38 (95% CI0.79 to 9.60 according to exact Poisson method; p = 0.102 by exact mid-p test).
Median time from ICU admission to CAPA onset was 5 days (IQR 3–6.5) in centre 1, 10 days (IQR 2–26) in centre 2 and 4.5 days (IQR 1–5) in centre 3.
3.4 Factors Associated With CAPA Development
In the multivariable logistic regression model, the presence of an EORTC/MSGERC risk factor at ICU admission (OR 4.35; 95% CI 1.15–16.49; p = 0.031) and the centre in which the patients were treated were associated with increased odds of CAPA development (p = 0.007). However, centre odds for CAPA development were only elevated significantly when comparing centre 3 (controls) to centre 1 (cases) (OR 6.07; 95% CI 1.76–20.91; p = 0.004). There was no difference in the odds regarding CAPA development when comparing centre 2 (controls) to centre 1 (cases) (OR 0.59; 95% CI 0.1–3.53; p = 0.566) (Table 2A).
| Variables | OR (95% CI) | p |
|---|---|---|
| (A) | ||
| EORTC/MSGERC risk factor present at ICU admission | 4.35 (1.15–16.49) | 0.031 |
| ECMO | 1.85 (0.34–9.99) | 0.475 |
| Centre | 0.007 | |
| Centre 3 (vs. centre 1 as reference) | 6.07 (1.76–20.91) | 0.004a |
| Centre 2 (vs. centre 1 as reference) | 0.59 (0.10–3.53) | 0.566a |
| (B) | ||
| EORTC/MSGERC risk factor present at ICU admission | 2.84 (0.70–11.63) | 0.146 |
| ECMO | 1.48 (0.29–7.50) | 0.639 |
| Centre | 0.002 | |
| Centre 3 (vs. centre 1 as reference) | 5.10 (1.54–16.90) | 0.008 |
| Centre 2 (vs. centre 1 as reference) | 0.80 (0.17–3.80) | 0.776 |
- Note: (A) Overall, 4, 2 and 17 cases of CAPA were registered in centre 1, centre 2 and centre 3, respectively, after 1:1 matching. The distribution of proven, probable and possible CAPA in the different centres was as follows: centre 1 (n = 0 proven, n = 3 probable, n = 1 possible), centre 2 (n = 0 proven, n = 2 probable, n = 0 possible) and centre 3 (n = 0 proven, n = 10 probable, n = 7 possible). (B) Overall, 4, 3 and 16 cases of CAPA were registered in centre 1, centre 2 and centre 3, respectively, after 1:1:1 matching. The distribution of proven, probable and possible CAPA in the different centres was as follows: Graz (n = 0 proven, n = 3 probable, n = 1 possible), Rennes (n = 0 proven, n = 9 probable, n = 7 possible) and Genoa (n = 0 proven, n = 3 probable, n = 0 possible). The total numbers of CAPA cases varies slightly between the 1:1 and 1:1:1 (sensitivity analysis) cohort due to the matching procedure. For details refer Section 2.
- Abbreviations: CAPA, coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; EORCT, European Organization for Research and Treatment of Cancer; ICU, intensive care unit; IQR, interquartile range; MSGERC, Mycoses Study Group Education and Research Consortium; OR, odds ratio.
- a p-Values from subgroup analysis.
In the sensitivity multivariable logistic regression analysis (1:1:1 matching), only centre was significantly associated with CAPA development (p = 0.002). Similar to the 1:1 matched cohort analysis, centre odds for CAPA development were only significantly increased when comparing centre 3 to centre 1 (OR 5.1; 95% CI 1.54–16.90) but not for comparing centre 2 to centre 1 (OR 0.8; 95% CI 0.17–3.8). The presence of an EORTC/MSGERC risk factor was associated with an OR of 2.84 (95% CI; 0.7–11.63) in the sensitivity analysis, thereby showing the same direction of effect than in the primary study population (1:1 matching); however, this association did not reach statistical significance in the sensitivity analysis (p = 0.146) (Table 2B).
Need for ECMO treatment was neither associated with CAPA development in the 1:1 nor in the 1:1:1 matched cohort.
4 Discussion
This case–control study investigated the impact of POSA prophylaxis on risk of CAPA development in mechanically ventilated COVID-19 patients receiving corticosteroids. We observed a high inter-centre variability of CAPA incidence rates among the centres; particularly among the two control centres not using prophylaxis. These findings highlight the importance of a nuanced and centre specific management strategy for CAPA.
CAPA is a well-known potential life-threatening complication primarily affecting COVID-19 patients with ARF who require invasive mechanical support [1-3]. As reliable diagnostic methods in non-invasive specimen (e.g., blood) lacks satisfactory clinical and diagnostic performance and imaging patterns are often unspecific, antifungal prophylaxis may contribute to successful management of such patients. Posaconazole is a widely used antifungal agent for mould-active prophylaxis. It has proven efficacy and safety in the haematological malignancy setting [25-27] and is therefore considered standard of care in high-risk patients [28]. Based on its favourable pharmacokinetics, the generally good safety profile and the high concentration of POSA in the cell membrane of leucocytes [29, 30] POSA may also be a favourable agent for CAPA prophylaxis on ICU. Thus, POSA prophylaxis has been established as part of management in COVID-19 ARF patients at centre 1. Prior investigations at centre 1 have shown that CAPA rate dropped locally after implementation of POSA prophylaxis from approximately15%–25% to < 1% [17, 31]. These results indicate that the baseline CAPA rates in centre 1 would have been comparable to that of centre 3 in case there would have been not implementation of POSA prophylaxis. Centre 2, however, turned out to have a low CAPA incidence rate, even lower than the incidence rate in centre 1, despite the fact that centre 2 has not implemented antifungal prophylaxis as standard of care for COVID-19 ARF patients. All study patients received systemic corticosteroid treatment for COVID-19 ARF, which is maybe the most important risk factor [32], but only very few patients in centre 1 (2%), and consequently also among matched controls form centres 2 and 3, received additional tocilizumab treatment. Thus, as anti-IL-6 treatment is a relevant risk factor for CAPA development [2, 33] one may speculate that the study cohort investigated had a low baseline risk for CAPA development. This observation may in fact be underlined by previously reported data from centre 2, where CAPA incidence rate was higher in the sub-cohort receiving tocilizumab versus those not receiving tocilizumab (2.63 versus 1.16 CAPA/1000 ICU days) [6].
In addition, the high variation in CAPA incidence rate among the two control centres may be explained by factors like variation in diagnostic approaches (e.g., biomarker availability [34-36], bronchoscopy strategies [37, 38], etc.) which were not standardised among the centres. Variations in diagnostic strategies may also be a relevant reason why there was no possible CAPA case in centre 2 versus 19 possible CAPA cases in centre 3 before matching. Particularly rate of BALF sampling has been shown to be a very strong driver of CAPA incidence [37], however, in this study rate of BALF sampling were high in all three participating centres, while rate of BALF-GM testing varied between centres. Besides the variations in diagnostic strategies, variations in genetically determined predisposing factors, like aberrations in pathogen-recognition receptors [39], and variations in environmental fungal exposure contribute to the net-state of CAPA risk development in critically ill COVID-19 patients. Besides all the factors mentioned, a standardised diagnostic approach using standardised diagnostic criteria like the recently published FUNDICU consensus definitions are considered an important step for future standardisation and furthermore comparability of study results [40].
In addition, the proportion of patients on ICU with COVID-19 ARF who have an underlying immunosuppressive disease has been increasing during the COVID-19 vaccination era [13]. We therefore investigated the impact of presence of EORTC/MSGERC risk factors on the risk for CAPA development and observed a more than 4-fold increase in odds to develop CAPA for those with EORTC/MSGERC risk factors versus those without. These finding indicate that stratifying antifungal prophylaxis by presence of EORTC/MSGERC risk factors may be a viable option for centres with low CAPA prevalence rates that fall below the threshold to justify universal antifungal prophylaxis for all ventilated patients with COVID-19.
Most of the currently available evidence on antifungal prophylaxis in critically ill COVID-19 patients is showing a reduction of CAPA rates (generally prevalence rates are given) but no significant difference in overall survival rates. While lack of statistical power and small sample sizes may be the most important of this lack of association, there may be other factors that play a role as well. First, most of the studies currently available were performed in centres with a lot of experience and a high awareness for invasive fungal infections. The high awareness and in house available diagnostic tests (e.g., CT-scans, fungal biomarkers, etc.) will usually facilitate a rapid diagnosis, limiting treatment delay and mortality. Autopsy studies in patients with viral-associated pulmonary aspergillosis highlighted, that in the absence of classical risk factors like prolonged neutropenia, Aspergillus spp. usually shows an impeded fungal growth pattern in patients with CAPA [11]. This in some extent complicates early diagnosis as positive serum biomarkers are less likely and further diagnostic procedures are warranted. However, in case of delayed antifungal treatment or missed diagnosis, CAPA is a life-threatening and a deadly disease. Second, the recent studies investigated all COVID-19 patients on ICU, regardless of the required underlying respiratory support. This is a valid approach; however, we have learned that the extent of lung tissue damage and consequently need for respiratory support impacts the risk for the development of CAPA [9]. This study therefore focused on ventilated patients only. Future multicentre studies, optimally randomised controlled trials, are therefore needed to investigate the impact of antifungal prophylaxis on mortality in a wider variation of centres, including centres with less in house availability and longer turnaround times of fungal diagnostics. Finally, the studies are needed for centres with low CAPA prevalence, that focus prophylactic efforts on subpopulations at higher risk, like patients with EORTC/MSGERC host factors.
This study is not exempt from limitations. For example, by employing a case–control design the number of CAPA cases was reduced compared with the numbers originally observed in centre 2 and centre 3. Thus patients included from centre 2 and centre 3 may be not representative of the local epidemiology. However, the case–control design was eventually preferred in order to reliably assess any possible impact of POSA prophylaxis in our cohort. Indeed, this has allowed to avoid a selection bias related to the high baseline proportion of patients treated with tocilizumab (an already recognised risk factor for CAPA) from centre 2 (no prophylaxis), that could have biased results towards a favourable effect of prophylaxis. Of note, without matching, this bias could have not been adjusted/reduced in multivariable analysis due to collinearity between centre (i.e., prophylaxis present versus absent) and tocilizumab use. At the same time, assuming that tocilizumab was given to patients with more severe disease in centre 2, we cannot exclude that our approach resulted in a selection of a lower risk cohort from centre 2 and that the higher CAPA rate in those with tocilizumab was a result of other factors than IL-6 inhibitor treatment. Overall, the potential role of POSA prophylaxis in mechanically ventilated COVID-19 patients treated with tocilizumab may merit a dedicated investigation in this specific subgroup, which was unfeasible in the present study due the very low number of tocilizumab-treated patients receiving POSA prophylaxis. In addition we observed a high variation of ECMO cases among the centres may influencing the baseline risk for CAPA development. It should be noted that we had no influence over the local CAPA screening strategies implemented at each centre. While the number of lower respiratory tract samples sent for mycological testing was comparable across centres, variations in the timing and intensity of screening may have occurred. These differences could have influenced the detection rates of CAPA cases during routine clinical work-up. Lastly, the final analyses reported here differed slightly from the original analyses plan (available at clinicaltrials.gov) based on missing data in the final data set.
5 Conclusions
POSA prophylaxis may be a suitable approach to reduce CAPA rates among COVID-19 patients with ARF depending on the local characteristics of COVID-19 patients on ICU and the local epidemiology. Further investigational trials are needed that investigate the efficacy of POSA prophylaxis in pre-selected patients with certain baseline characteristics that come with increased risk for the development of invasive fungal infections.
Author Contributions
Juergen Prattes: conceptualization, methodology, writing – original draft, funding acquisition, project administration. Daniele R. Giacobbe: methodology, formal analysis, writing – original draft, project administration. Cristina Marelli: formal analysis. Alessio Signori: formal analysis. Silvia Dettori: data curation. Greta Cattardico: data curation. Stefan Hatzl: data curation. Alexander C. Reisinger: data curation. Philipp Eller: data curation. Robert Krause: data curation. Florian Reizine: data curation. Matteo Bassetti: methodology, writing – review and editing, supervision. Jean-Pierre Gangneux: methodology, writing – review and editing, supervision. Martin Hoenigl: conceptualization, methodology, writing – review and editing, funding acquisition, project administration, supervision.
Acknowledgements
This study was funded by an investigator-initiated-trial funding from Merck Sharp& Dohme Corp (IIS 60879). The funder was not involved in the study design, the conduct of the study, data analysis or manuscript preparation.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.