Bony labyrinths of the blackfish (Delphinidae: Globicephalinae)
Abstract
Bony labyrinth morphology varies across marine mammals and contains key information regarding hearing sensitivity and ecology. The hearing ranges of globicephaline (Delphinidae: Globicephalinae) or melon-headed dolphins, known as “Blackfish,” have been extensively studied using acoustic technologies, but clade-wide morphological analysis of the bony labyrinth is lacking. In this study, we investigate the variation in hearing-relevant bony labyrinth morphology within globicephalines using μCT scans of isolated petrosals and digitally isolating the bony labyrinth of all species. Principal components analysis (PCA) of nine hearing-relevant measurements of the cochlea alongside a broader sampling of terrestrial and aquatic artiodactyls shows Orcaella brevirostris and other globicephalines with higher levels of facial asymmetry and potentially more specialized echolocation abilities plotting near Monodon monoceros, Delphinapterus leucas, and Orcinus orca. The remaining globicephalines, which have more symmetrical skulls and other unique and acoustically relevant attributes, plotted towards the middle of the echolocating odontocete portion of the PCA. Our analysis thus reveals that inner ear morphology may correlate with both facial skull morphology and echolocation specializations, as these are intertwined. Furthermore, this study illustrates how morphological analyses, especially those centered on hearing, may provide critical conservation-relevant information as direct access to audiograms becomes less tenable.
1 INTRODUCTION
Understanding the hearing sensitivities of cetaceans is a central goal in marine mammal science, as these data provide crucial information regarding ecology, including interspecies interactions, habitat suitability, and evolution. In the absence of existing audiograms, the geometry of the inner ear structure, and establishing correlations between ear geometry and hearing sensitivities, offers not only a means for studying hearing in rare extant species (e.g., Park, Marx, et al., 2017), but also for reconstructing the evolutionary history of hearing within this charismatic group (Churchill et al., 2016; Ekdale & Racicot, 2015; Galatius et al., 2019; Mourlam & Orliac, 2017; Park et al., 2016; Park, Evans, et al., 2017; Park et al., 2019; Racicot et al., 2016, 2018, 2019). In this regard, studying the morphology of the bony labyrinth within well-understood groups is vital, and allows us to calibrate the effectiveness of existing methods for inferring hearing sensitivities from morphological data. In this study, we describe the inner ear morphology of the globicephalines (Delphinidae: Globicephalinae)—a subfamily of blunt-headed dolphins with relatively well understood hearing sensitivities, echolocation abilities, and ecologies. We focus on the relationship between the bony labyrinth, the part of the inner ear commonly preserved in bone and fossils, and echolocation signals, with the goal of providing a baseline for further understanding the evolutionary morphology of cetacean sensory systems.
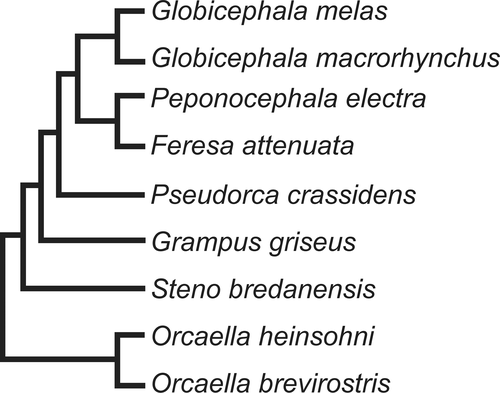
Globicephalines or “Blackfish” currently comprise Orcaella brevirostris, Orcaella heinsohni, Steno bredanensis, Grampus griseus, Pseudorca crassidens, Feresa attenuata, Peponocephala electra, Globicephala macrorhynchus, and Globicephala melas. Recent phylogenetic hypotheses have reconstructed members of the Orcaella genus as sister to the clade including Steno bredanensis + traditional “Globicephalinae” (Figure 1), supporting their inclusion in the subfamily (McGowen et al., 2020).
The bony labyrinth comprises the outer wall of the inner ear cavities and consists of three parts: the cochlea, semicircular canals, and vestibule. While the soft tissues of the cochlea, semicircular canals, and vestibule structures degrade after death, the bony labyrinth can preserve the shape and size of these cavities in bone and fossils (Ekdale, 2016). Furthermore, the morphology of the cochlear portion of the bony labyrinth correlates with hearing sensitivity in mammals. The apex of the cochlea detects low frequency sound waves, whereas the base region detects high frequency sound waves, including ultrasonic sound waves 20 kHz and up (Ketten, 1997). Cochlear traits in cetaceans are greatly influenced by habitat (Costeur et al., 2018; Gutstein et al., 2014). Morphological differences can therefore indicate if the specimen inhabits estuaries or more pelagic zones, for example, because of differences in requirements for hearing in environments of different complexities.
In this study, we build upon previous work describing the relationship between bony labyrinth morphology and hearing sensitivities using principal components analysis (PCA; Churchill et al., 2016; Galatius et al., 2019; Mourlam & Orliac, 2017; Racicot et al., 2018, 2019). Existing knowledge surrounding the ecology of globicephaline species allows us to make specific predictions about where species should plot in the morphospace. First, we expect that hearing and echolocation abilities may correlate with skull and head morphology, because species must be able to hear the echolocation signals they produce. Recent work on odontocete skull morphology (Coombs et al., 2020) has shown that species with more specialized echolocation abilities, including several globicephalines, possess relatively more asymmetrical skulls. We therefore hypothesize that species with highly asymmetrical skulls (such as Globicephala spp., Pseudorca crassidens, Monodon monoceros, and Delphinapterus leucas) will have bony labyrinth morphometric similarities, reflecting their need to hear the more complex echolocation signals within more acoustically complex environments. Second, because of habitat correspondence, we expect Orcaella brevirostris to plot together with other dolphins that inhabit riverine environments. Testing these hypotheses will shed light on the evolution of hearing in cetaceans, particularly this relatively well-studied group, and provide links between morphology and direct audiogram or passive acoustic monitoring studies of hearing and echolocation in live animals. Morphological correspondences with known hearing ranges have conservation implications for lesser-known species that are seldom or logistically difficult to directly study, providing data useful for mitigation strategies for anthropogenic impacts.
2 METHODS
2.1 Specimens and CT scanning
We examined representatives of nearly all species of the monophyletic Globicephalinae (per McGowen et al., 2020), excluding only Orcaella heinsohni (Australian snubfin dolphin) due to lack of availability. Eleven specimens including the left and right petrosals of Orcaella brevirostris and one individual petrosal from each remaining species, i.e., Feresa attenuata, Globicephala macrorhynchus, Globicephala melas, Grampus griseus, Peponocephala electra, Pseudorca crassidens, and Steno bredanensis, were included in our analyses (see Figure 1). Intraspecific variation should not be a concern for our analyses because odontocetes possess low levels of individual variation (Martins et al., 2020). Two isolated left petrosals presumed to be from the same extinct (fossil) globicephaline taxon (UCMP 219487 and UCMP 219488; Boessenecker et al., 2015) were also included to provide additional evolutionary perspective. Museum abbreviations used: LACM, Natural History Museum of Los Angeles County, Los Angeles, CA; UCMP, University of California Museum of Paleontology, Berkeley, CA; USNM, National Museum of Natural History, Washington, DC. MicroCT scans of the petrosals were performed at Vanderbilt University using the North Star Imaging micro-CT scanner in the Department of Earth and Environmental Sciences (see Table S1 for specimen and scan resolution information).
2.2 Segmentation and measurement methods
Digital endocasts of inner ear labyrinths were generated in VGStudioMax v. 2.2 using the region of interest (ROI) tools at varying grayscale thresholds. Threshold limits were adjusted to exclude interferences such as pebbles, dermestid beetle debris, or matrix in the case of the fossil (Balanoff et al., 2016; Racicot, 2017). Endocasts comprised cochleae, semicircular canals, vestibuli, canaliculus cochleae, fenestrae cochleae, and aqueductus vestibuli. Using the polyline 3D tool, all canals leading to inner ear labyrinths were cut off as close to their apertures as possible. Curved linear measurements were obtained using the polyline length tool in VGStudioMax v. 2.2. Straight-line measurements and the numbers of turns were obtained using angle and line tools in the measurement module in Avizo v. 6 from oblique slices following importation of high-resolution image stacks of the segmented cochleae and full scans following previous work (Racicot et al., 2016, 2018, 2019).
2.3 Correlates for hearing sensitivity
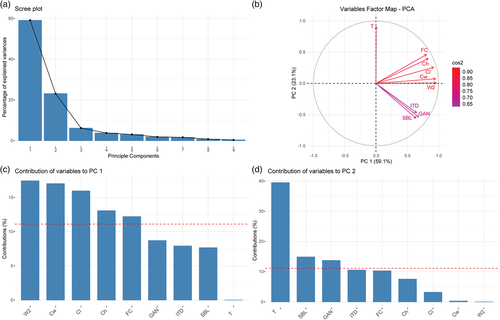
Previous studies have established that nine measurements that correspond to hearing sensitivity in artiodactyls, including cetaceans (Churchill et al., 2016; Galatius et al., 2019; Mourlam & Orliac, 2017; Racicot et al., 2018, 2019), can be used to distinguish different hearing types in a principal components analysis (PCA). We took the same measurements and added them to the latest published data set provided by Racicot et al. (2019). The measurements consisted of cochlear length (Cl), cochlear height (Ch), cochlear width at its widest point (Cw), cochlear width perpendicular to Cw (W2), secondary bony lamina length (SBL), interturn distance (ITD), spiral ganglion canal width at the first quarter turn (GAN), surface area of the fenestra cochleae (FC), and the number of turns (T). Number of turns was calculated using previously established methods (e.g., Ekdale & Rowe, 2011; Geisler & Luo, 1996; Racicot et al., 2018). A line was created starting from the beginning of the laminar gap through the axis of the cochlea and the number of times the cochlea crossed the line was counted. This value is multiplied by 180 and added to the angle between the remaining end of the apex of the cochlea and the initial line through the laminar gap and cochlear axis. The sum of that is divided by 360 to yield the number of turns. Principal components analysis was performed according to the methods used by Mourlam and Orliac (2017) and Racicot et al. (2018, 2019) and visually represents hearing sensitivities of the specimens used in this study in comparison to other species by showing clear separation among phylogenetic and hearing groups. The PCA was performed in R using the FactoMineR and FactoExtra packages (Le et al., 2008; R Development Core Team, 2014), and analysis of contributions of data to the PCA was done in FactoExtra. Missing data were imputed using the missMDA package (Josse & Husson, 2016).
3 RESULTS
3.1 Cochlear morphospace distribution
PCA placed all specimens from our study within non-narrow-band high-frequency specialist (NBHF) odontocetes (Figure 2a), and in two distinct clusters (Figure 2b). The cluster including Feresa attenuata, Grampus griseus, Peponocephala electra, Steno bredanensis, and the two fossils is located more to the left of the morphospace, in the center of the whole echolocating odontocete grouping (Figure 2b). Other species within or near this morphospace include Echovenator sandersi, an extinct odontocete from the late Oligocene (Churchill et al., 2016), and Lipotes vexillifer, a functionally extinct river dolphin from China. Orcaella brevirostris is located in the cluster more to the right of the morphospace along with Globicephala macrorhynchus, Globicephala melas, and Pseudorca crassidens (Figure 2b). This grouping is situated close to or within members of extant Monodontidae (Delphinapterus leucas and Monodon monoceros) and Orcinus orca. Principal component 1 (PC1) accounted for 59.12% of the variation and principal component 2 (PC2) accounted for 23.14% of the variation, indicating that the first two principal components explain >80% of the variation in the data set, and thus that the plot is a good representation of the morphological data.
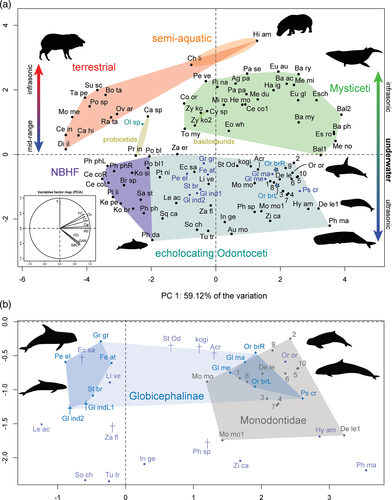
The most significant variables leading to the morphospace grouping of the artiodactyls sampled include cochlear width, length, and the number of turns (Figure 3). Cochlear width and cochlear length contribute the most to PC1, with cochlear height and the surface area of fenestrae cochleae also contributing more than average. The number of turns is the most significant contributor to PC2 (Figure 3).
3.2 Morphology of the cochleae
The basal portion of the cochlear coil, just past the cochlear hook, of Orcaella brevirostris (Figure 4) and all other specimens examined begins in a near straight line, and then forms a pronounced curve that leads into the spiral shape of the cochlea (Figure 5, vestibular view). The latter part of the coil begins as a loose spiral and becomes progressively tighter towards the apex. A bulge excavates dorsally along the length of the first basal turn extending until just after the end of the first full turn, narrowing to approximately half the width of the basal turn in Orcaella brevirostris (Figures 4e–f and 5, nonorthogonal dorsal view). Although most prominent in Orcaella, this bulge is also visible to lesser degrees in other species examined. Steno bredanensis and the fossils (Figure S1) have roughened bulges reaching to just past the first full turn along the basal turn, whereas remaining specimens have less obvious or more flattened dorsal excavations. This feature is also present, although not previously commented upon, in monodontid bony labyrinth endocasts (Racicot et al., 2018), and in histological sections of Delphinapterus leucas specimens (Sensor et al., 2015).
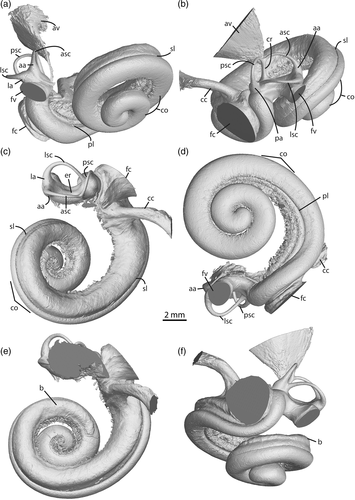

At 43.4–44.36 mm, Orcaella brevirostris has some of the longest cochlear lengths of the specimens examined, similar in size only to that of Pseudorca crassidens, which has a cochlear length of 44.69 mm (Table S2). Measurements of secondary bony lamina length, cochlear width, cochlear height, spiral ganglion width, fenestra cochleae area, interturn distance, and the number of turns is correspondingly large. Species on the left/middle of the PCA possess smaller fenestra cochleae areas, between 4.71 and 9.77 mm2, whereas those to the right have areas between 12.35 and 15.89 mm2 (Table S2). Scaled measurements show the largest fraction of the SBL belongs to Grampus griseus, Peponocephala electra, and Globicephala macrorhynchus, whereas the largest cochlear heights are overwhelmingly in the globicephaline fossils (Table S3). The spiral ganglion canal of Orcaella brevirostris is relatively small when scaled to the size of the fenestrae cochleae at 5.8, whereas the largest spiral ganglion canal width belongs to Steno bredanensis at 14.4 (Table S3).
3.3 Morphology of the vestibular region
Although this study centers on hearing and thus primarily the cochlea, some comments may be made regarding the vestibular complex. In both Orcaella specimens, a thin groove runs just ventral of the crus communis to ventral of the posterior ampulla, the external representation of the crista vestibuli (Figure S2). The groove indicating the crista vestibuli represents a distinct separation of the elliptical and spherical recesses (Figure S2). This groove continues around the vestibule to a lesser degree, further indicating the separation between the different recesses.
The aqueductus vestibuli begin as thread-like extensions from the vestibule and expand precipitously towards their apices. The aqueductus vestibuli of Orcaella brevirostris are broad and cone-shaped, whereas in Steno bredanensis and the remaining specimens they are much narrower.
4 DISCUSSION
Our PCA provides detailed correspondence of cochlear morphology, and thus hearing sensitivity, to echolocation signals used by cetaceans. In general, globicephalines that echolocate in acoustically complex habitats and have higher levels of skull asymmetry (Coombs et al., 2020) grouped together with monodontids and other species with highly asymmetrical skulls, supporting the hypothesis that there may be correlation with specialized (for acoustically complex environments) echolocation abilities and the inner ear. The river-inhabiting Orcaella brevirostris did not cluster with other river dolphins in the data set, but instead with these specialized echolocators on the right side of the odontocete morphospace. Although the measurements used to perform this PCA have been collected by different researchers (and thus there may be inconsistencies among researchers in the way in which these measurements were taken, see, e.g., Racicot et al. 2018), the clear separation between known groups of animals suggests that these measurement differences have had minimal effect on the results. Furthermore, we note that the first two principal components together explain 82.26% of the variation, and thus these two axes comprise an excellent 2D representation of the overall PCA. In this section we first discuss possible explanations for the two observed clusters of globicephalines based on the biology, cochlear morphology, skull asymmetry, and echolocation abilities of the relevant species. Second, we discuss the position of Orcaella brevirostris among the globicephalines grouping with specialized echolocators of the PCA. Finally, potential complicating factors are discussed, such as why a specialized echolocator like Grampus griseus was recovered towards the middle of the morphospace (between NBHF and specialized echolocators).
The cluster of globicephalines on the right side of the echolocating odontocete morphospace (overlapping with monodontids) in the PCA may be related to unique or specialized echolocation abilities associated with inhabiting and/or seeking prey in acoustically complex environments. For example, Pseudorca, Globicephala, and monodontids were recently found to have some of the most asymmetric skulls amongst cetaceans, suggested to be potentially driven by unique ecological niche occupation and resulting requirements for producing more complex echolocation signals (Coombs et al., 2020). Pseudorca also shares a convergence in skull morphology with Orcinus orca (Galatius et al., 2020), which is recovered in the morphospace near globicephalines and monodontids. Because these animals must hear their echolocation signals and their bony labyrinth morphology groups them together in our PCA, our results further support ecological signal of specialized echolocation abilities in the cochlea. Further, skull morphology may in these instances correlate well with inner ear morphology and would be an interesting avenue for further research.
In terms of cochlear morphology, the grouping of globicephalines with more specialized echolocators on the right-hand side of the PCA morphospace is due in part to relatively larger fenestra cochleae than globicephalines that group towards the middle of the morphospace. The species in the specialized region all have larger overall cochleae both visually and in measurements, despite dissimilar body sizes, e.g., Orcaella brevirostris is 2.1–2.75 m in average adult length, Psuedorca crassidens is 3.5–6 m (Thewissen et al., 2019). Larger fenestra cochleae may allow for a broader range of lower frequency hearing than species that group towards the middle of the morphospace. Another factor influencing the different groupings may be that species with generally larger body size group to the right of the morphospace, while smaller-bodied species cluster towards the middle and left (NBHF species are the extreme, on the far left of the echolocating odontocete morphospace). The exception in our study is Orcaella brevirostris, suggesting that the species may have evolved from a larger ancestor, retaining the larger overall size of the cochlea. Bulges are present on the dorsal surface of the cochleae of globicephalines to varying degrees, the function of which is unclear but nonetheless may impart some additional hearing sensitivity. The cochlea of Globicephala melas had also previously been identified as unusual in morphology in a PCA using highly dimensional 3D geometric morphometric data (Park et al., 2019). The combination of cochlear morphological features in both globicephaline groupings may provide additional possibilities for teasing apart the subtleties of hearing abilities in cetaceans.
The extant globicephalines that plotted more to the middle of the echolocating odontocete morphospace are all generally species with lower cranial asymmetry than those that plot with specialized echolocators on the right, particularly Steno bredanensis (Coombs et al. 2020). The presence of the globicephaline fossils in the middle region of the PCA suggests that they may have used echolocation and communication signals similar to the extant globicephalines in this region of the morphospace. Interestingly, the external morphology of the petrosals of the fossils was suggested to be most like that of Globicephala (Boessenecker et al., 2015), species that in our analyses have generally larger bony labyrinths and group to the right of the PCA with monodontids. Further research on possible correlations between external and internal ear morphology could be helpful for understanding differences in echolocation and hearing abilities.
Orcaella brevirostris was recovered in the same region of the morphospace as those species with highly asymmetrical skulls and unique echolocation abilities. The skull shape of this species ranks relatively high (18 out of 172) in the sum radii of skull asymmetry (Coombs et al. 2020), suggesting that skull asymmetry, echolocation abilities, and hearing as reflected in our PCA are correlated. The habitat of Orcaella brevirostris is a highly complex ecological niche of euryhaline river environments and the echolocation abilities have been shown to be unusual (broad band low frequency, BBLF) compared to marine dolphins of similar size (Jensen et al., 2013). We initially hypothesized that the labyrinth morphology of Orcaella brevirostris would be similar to that of river dolphins because of the effect of environmental factors on hearing sensitivity (e.g., Costeur et al. 2018; Gutstein et al. 2014). Three species of extant river-inhabiting dolphins are represented in this PCA, Pontoporia blainvillei, Inia geoffrensis, and Lipotes vexillifer, which are distributed variably across the morphospace. Orcaella brevirostris does not plot near any of these species, and their distribution across the morphospace suggests that hearing and/or echolocation specializations related to riverine environments may be decoupled from some aspects of ecological signal using the specific measurements from this data set. A way to test this would be to include Platanista gangetica in future analyses, a species that lives in the same river systems as Orcaella brevirostris, similarly has a highly asymmetrical skull (Coombs et al., 2020), and unusual echolocation signals (Jensen et al., 2013).
Interestingly, Grampus griseus possesses highly specialized echolocation signals, as the only globicephaline known to have a specialized diet of cephalopods, requiring deep dives near continental shelves and submarine canyons (Hartman, 2019), but this species plots in the middle of the morphospace, not near the other species with more specialized echolocation signals like Pseudorca crassidens at the right of the morphospace. Grampus griseus use highly directional echolocation clicks, a downward directed beam (10°–20° compared to other max center frequencies) with potentially related good ventral hearing sensitivity, and possess a unique, large indentation along the midline of the anterior portion of the head (Mooney et al., 2015; Nachtigall et al., 2005; Phillips et al., 2003; Smith, 2016). The species produce echolocation clicks with mean centroid frequencies almost an octave lower than those of Delphinapterus leucas, use beams of similar narrow proportions to Pseudorca crassidens, and the highly directional echolocation signals employed may be useful for its primarily cephalopod diet in deeper oceans (Madsen et al., 2004). Grampus griseus may thus compensate for less specialized hearing sensitivity as reflected by the bony labyrinth morphology with a combination of click repertoire and unique internal and external head morphology. Steno bredanensis also use highly directional echolocation clicks that can reach up to 200 kHz (Jefferson, 2019). The species that plot in the middle of the morphospace thus are not necessarily less specialized echolocators, but may achieve similar results to their specialized counterparts on the right side of the morphospace with other aspects of their overall biosonar apparatus.
4.1 Conclusion
This study provides insights into the relationship between bony labyrinth morphology and hearing ranges as well as the ecological and evolutionary impacts on hearing in the blackfish, or Globicephalinae. Principal components analyses of measurements from the cochleae resulted in two groupings of globicephalines in the odontocete morphospace, one on the right-hand side with monodontids and other specialized echolocators, and one towards the middle of the morphospace. The globicephaline grouping on the right can be considered to share high levels of skull asymmetry with other species that plot in this region (such as monodontids), and share specializations in echolocation abilities, including Orcaella brevirostris, which inhabits complex riverine environments. Globicephaline species that plot in this region have larger fenestra cochleae than species that plot toward the middle of the morphospace, suggesting a larger range of lower frequency hearing. Species that plot towards the middle of the morphospace do not necessarily have less specialized echolocation abilities, as evidenced by the presence of Grampus griseus in this region. In this case, unique external and internal head characteristics and higher directionality of echolocation signals may compensate for perhaps a smaller range of lower frequency hearing. The detection of subtleties between hearing and echolocation abilities using inner ear morphology allows for better-informed decisions regarding anthropogenic noise without the need to directly track, record, and measure hearing in wild individuals. Nonintrusive morphological analyses will aid in the conservation of blackfish along with other marine mammals, many of which are endangered or vulnerable to extinction.
ACKNOWLEDGMENTS
We thank the curators and collections managers for facilitating loans of specimens used in our analyses: Drs. Michael McGowen (USNM), Patricia Holroyd (UCMP), Jim Dines (formerly LACM), and Mr. Charley Potter and Mr. John Ososky (USNM). Dr. Simon Darroch is thanked for μCT scanning the specimens and for guidance and suggestions for coding with R. The editor, Dr. Daryl Boness, and Drs. Eric Ekdale, Jonathan Geisler, and Travis Park are gratefully acknowledged for constructive reviews that improved an earlier draft of the manuscript. This research was supported by grants to V.E.P. from Pitzer Student Senate and Pitzer College to attend a conference to present earlier versions of this work, and both authors were supported by the W.M. Keck Science Department of the Claremont Colleges while performing the research in partial fulfillment of V.E.P.’s senior thesis. RAR was supported by Vanderbilt University during data acquisition stages of the research, and is currently supported by the Alexander von Humboldt Foundation, which is sponsored by the Federal Ministry for Education and Research (Germany). Open Access funding enabled and organized by Projekt DEAL.
AUTHOR CONTRIBUTIONS
Rachel Racicot: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing-original draft; writing-review & editing. V. Preucil: Formal analysis; funding acquisition; investigation; software; visualization; writing-original draft.




