Whale killers: Prevalence and ecological implications of killer whale predation on humpback whale calves off Western Australia
Abstract
Reports of killer whales (Orcinus orca) preying on large whales have been relatively rare, and the ecological significance of these attacks is controversial. Here we report on numerous observations of killer whales preying on neonate humpback whales (Megaptera novaeangliae) off Western Australia (WA) based on reports we compiled and our own observations. Attacking killer whales included at least 19 individuals from three stable social groupings in a highly connected local population; 22 separate attacks with known outcomes resulted in at least 14 (64%) kills of humpback calves. We satellite-tagged an adult female killer whale and followed her group on the water for 20.3 h over six separate days. During that time, they attacked eight humpback calves, and from the seven known outcomes, at least three calves (43%) were killed. Overall, our observations suggest that humpback calves are a predictable, plentiful, and readily taken prey source for killer whales and scavenging sharks off WA for at least 5 mo/yr. Humpback “escorts” vigorously assisted mothers in protecting their calves from attacking killer whales (and a white shark, Carcharodon carcharias). This expands the purported role of escorts in humpback whale social interactions, although it is not clear how this behavior is adaptive for the escorts.
Killer whales (Orcinus orca) as apex predators are large, active, long-lived, intelligent, and highly social—these attributes create high energetic demands and a clear potential to shape marine communities through trophic interactions (Estes et al. 1998, Corkeron and Connor 1999, Connor and Corkeron 2001, Springer et al. 2003, Williams 2006, Estes et al. 2011). However, an understanding of the overall ecosystem impact of these mega-predators remains elusive and contentious (e.g., Williams et al. 2004, Mizroch and Rice 2006, Trites et al. 2007, Wade et al. 2007, Springer et al. 2008).
Although, historically, killer whales were considered to be opportunistic predators, it is now clear that at least in some high-latitude areas, instead of being “top omnivores”, killer whale communities comprise divergent lineages of prey specialists. In the North Pacific and in Antarctica, for example, there are sympatric, noninterbreeding killer whale “ecotypes” that feed exclusively on either fish or marine mammals (Berzin and Vladimirov 1983, Bigg et al. 1987, Ford et al. 2000, Pitman and Ensor 2003, Morin et al. 2010). In lower latitudes, however, and perhaps due to a lack of abundance or predictability of any specific prey types, killer whales appear to be more opportunistic, and catholic, in their diets although those communities are relatively less-well studied (Baird et al. 2006, Alava and Merlen 2009).
Worldwide, killer whales have been reported to prey on nearly every species of “great whale” (i.e., baleen whales and the sperm whale, Physeter macrocephalus; Jefferson et al. 1991, Pitman et al. 2001, Reeves et al. 2006, Ford and Reeves 2008), but the extent to which they actually do prey on large whales (or did, see below), and the impact of that predation, is controversial (Springer et al. 2003, Whitehead and Reeves 2005, Reeves et al. 2006, Doak et al. 2006, Mizroch and Rice 2006, Mehta et al. 2007, Wade et al. 2007).
This uncertainty about the prevalence and significance of killer whale predation on large whales has been due in large part to the fact that, apart from a few notable exceptions (e.g., Tarpy 1979, Silber et al. 1990, Flórez-González et al. 1994, Pitman et al. 2001, Barrett-Lennard et al. 2011), successful attacks have rarely been documented (Jefferson et al. 1991, Reeves et al. 2006, Ford and Reeves 2008, Springer et al. 2008). These events are still so infrequently observed that documentation of even individual attacks continue to appear in the scientific literature (e.g., Alava et al. 2013). On the other hand, the prevalence of presumed killer whale tooth rake marks on the flukes and flippers of nearly every large whale species has been cited as evidence that attacks might, in fact, be fairly common (Shevchenko 1975, George et al. 1994, Clapham 2000, Mehta et al. 2007, Rhinehart et al. 2013).
This paradox is well illustrated by the most widely studied species of great whale—the humpback whale (Megaptera novaeangliae; Clapham 2000). Branch and Williams (2006) reviewed the available data for killer whale predation on humpbacks and concluded that humpbacks were rarely eaten by killer whales. But more so than any of the other great whales, humpbacks have long been known to show a high incidence of presumed killer whale rake marks on their flukes (Katona et al. 1980), leading many to conclude that they probably are regularly attacked (Clapham 2000, 2001; Naessig and Lanyon 2004; Mehta et al. 2007; Steiger et al. 2008). When, where, and how these attacks might occur, however, are largely unknown because, despite the countless hours that researchers have spent on the water studying both humpbacks and killer whales, only a handful of attacks have been reported (Chittleborough 1953, Whitehead and Glass 1985, D'Vincent et al. 1989, Jefferson et al. 1991, Flórez-González et al. 1994, Naessig and Lanyon 2004, Ford and Reeves 2008). Furthermore, although two or three of these attacks were purportedly lethal, to date there have been no published firsthand observations of predation on humpbacks (Jefferson et al. 1991, Naessig and Lanyon 2004, Springer et al. 2008). These few, conflicting reports mean that the significance of killer whale predation on humpbacks, for either species, remains unresolved (Springer et al. 2003, Wade et al. 2007, Kuker and Barrett-Lennard 2010).
In a further effort to understand the prevalence of rake marks on humpback flukes on a global scale, Mehta et al. (2007) reviewed humpback photo-identification catalogs from various populations around the world. They found that rake-marked individuals were globally widespread and fairly common, with a rate of occurrence of rake-marking within individual populations ranging from 0% to >40%. They also found that humpbacks rarely acquired new marks after they were first photographed, supporting previous suggestions that killer whales were targeting mainly calves (Clapham 2000, Naessig and Lanyon 2004).
Furthermore, Clapham (2000, 2001) suggested that calves were attacked mainly during their first migration to the feeding grounds based on the fact that researchers in the western North Atlantic had not encountered any killer whales during 16 field seasons on the humpback breeding grounds in the West Indies, and had seen few killer whales, and no attacks, on humpbacks during 20 seasons of observations on the feeding grounds off New England. This idea of calf predation during migration was also supported by Mehta et al. (2007), but when Steiger et al. (2008) surveyed rake mark incidence on humpbacks, they concluded that, at least in the eastern North Pacific, calves were attacked primarily on the winter breeding grounds.
A further impediment to understanding predatory interactions between killer whales and large whales has been the ongoing legacy of 20th Century industrial whaling, which decimated whale stocks worldwide (Tønnessen and Johnsen 1982, Baker and Clapham 2002). Although commercial whalers generally did not target killer whales (but see Ivashin 1981), if there were populations of mammal-eating killer whales that preyed primarily on large whales, then they probably would have been indirectly impacted by whaling (Springer et al. 2003, Branch and Williams 2006), even if whaling operations themselves provided a temporary glut of carcasses for killer whales to scavenge (Whitehead and Reeves 2005). This debate can be narrowed down to two important questions: Were there killer whales that depended on large whale prey prior to whaling? And if so, how did they respond to the precipitous decline in large whale populations?
The answers to these questions have potentially important implications for marine ecosystem structure (Estes et al. 2009), especially in high latitudes, where killer whales occur most commonly (Forney and Wade 2006), and documenting killer whale response to recovering large whale populations should provide some insight into these interactions (Wade et al. 2007), keeping in mind that those killer whale populations might also be recovering. Additionally, if killer whales were important predators of large whales, then they could now also be a significant impediment to the recovery of some endangered whale populations (Mitchell and Reeves 1982, Finley 1990, Shelden et al. 2003, Barrett-Lennard et al. 2011, Rhinehart et al. 2013).
Each year, during the austral winter, humpback whales migrate in large numbers along the coast of Western Australia (WA). They have been designated Southern Hemisphere Breeding Stock “D” by the International Whaling Commission (IWC), and their summer feeding grounds in Antarctic waters are assumed to lie mainly within IWC Management Area IV (70°–130°E; IWC 1980). During the breeding season, they travel northward along the continental shelf waters of WA, to calving grounds off the Kimberly Region (15°–18°S; Chittleborough 1953, 1965; Jenner et al. 2001). The timing of this migration extends from at least June until November, and calving occurs throughout that period with a peak in August (Chittleborough 1953, 1965; Jenner et al. 2001).
The WA humpback population was severely depleted during the whaling era, and although legal whaling ceased off WA in 1963, substantial illegal and unreported exploitation on the Antarctic feeding grounds continued on through at least 1968 (Chittleborough 1965, Yablokov 1994, Bannister and Hedley 2001, Clapham et al. 2009). Under protection, however, Stock D humpbacks have increased from the mid- to low-hundreds during the 1960s (Bannister 1964, Chittleborough 1965, Bannister and Hedley 2001), to current estimates ranging from approximately 20,000 to 30,000, making it the largest humpback population known (Branch 2011, Salgado Kent et al. 2012, IWC 2014).
Despite the general lack of empirical evidence for successful killer whale predation on humpback whales globally, published reports of attacks and opportunistic observations of successful predation off WA, coupled with our own observations, suggest that attacks have been occurring in that area for some time and continue to this day. We compiled accounts of killer whale attacks on humpback whales off central WA, including our own observations made during July and August, 2013. Based on these accounts, we describe the nature and scale of predatory interactions between killer whales and humpbacks off WA, and discuss some of the broader implications of these interactions.
Methods
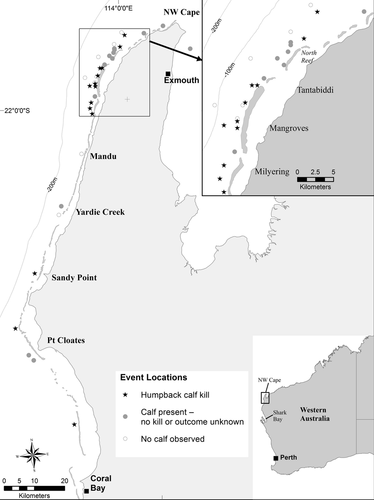
We conducted boat surveys aboard a 6 m launch out of Tantabiddi Boat Landing (15 km west of Exmouth, WA; Fig. 1) from 5 July to 9 August 2013, with the goal of locating, observing, photographing, and deploying satellite tags on killer whales. During 35 d and approximately 330 h on the water, we surveyed approximately 1,600 km of trackline, including 120 linear kilometers of coastline, from the tip of Northwest Cape, to just south of Yardie Creek, and from the edge of Ningaloo Reef out to approximately 10 km offshore (Fig. 1). We used 8× handheld binoculars and unaided eye to search for whales.
For each encounter, we attempted to photograph all of the killer whales present using 35 mm digital cameras with telephoto lenses, following established protocol for killer whale photo-identification studies (e.g., Bigg et al. 1987, Barrett-Lennard et al. 2011). Using these photographs, we identified each individual based on a combination of features on the dorsal fin and adjacent saddle patch following Durban et al. (2010), as well as eye patch pigmentation patterning. Individuals were considered to be in the same group if they were sighted within 500 m of one another and were engaged in same behavior, and were classified to age/sex classes following Olesiuk et al. (1990). For group size/composition analysis, we only used results that were confirmed by photographs or videos.
For satellite tagging, we used a crossbow to deploy a 49 gm transmitter (model SPOT 240C, Wildlife Computers, Redmond, Washington) equipped with a pair of barbed, 6.5 cm long titanium shafts to attach it to the dorsal fin of the killer whale (additional method details in Durban and Pitman 2012). The tag was programmed to transmit up to 700 times during 18 h each day, and locations with error radii were estimated by the Argos satellite system (Lopez and Malardé 2011). To make inference about the movement track and speeds, we fit a continuous time-correlated random walk model (Johnson et al. 2008), assuming the estimated error radii represented the standard deviations of normally distributed errors about each location (e.g., Ford et al. 2013). In addition to tracking killer whale movements over time, we also used the tag transmissions to relocate animals and extend our focal-follow capability (e.g., Pitman and Durban 2012).
In addition to our own observations, we also compiled records of killer whale interactions with humpbacks along the coast of Western Australia from published and unpublished accounts, photographs, video footage, and verbal accounts. Unpublished observations were solicited mainly from tour boat operators that were looking for whale sharks (Rhincodon typus), pilots of whale shark spotter planes, and recreational boaters, all mostly operating out of Exmouth, WA.
Results
Humpback/Killer Whale Interactions
We compiled a total of 35 separate interactions between killer whales and humpback whales that occurred along the coast of central WA, including 20 (57%) witnessed by one or more of the authors and 15 (43%) from other sources (Fig. 1). These comprised 24 (69%) attacks, 5 (14%) approaches (i.e., the humpback[s] responded to the presence of killer whales, but there was no evident attack), and 6 (17%) interactions of indeterminate nature (i.e., we were unable to determine if an approach resulted in an attack; Table 1 and Supplemental material). Of the 24 attacks, 14 (58%) resulted in kills, 8 (33%) were unsuccessful and 2 (8%) had unknown outcomes (Table 1). Overall, when attack outcomes were known (n = 22), 64% resulted in kills and 36% were unsuccessful.
| Event # | Date | Interaction and outcome | Interaction duration (min)a | Number, age, and sex of killer whalesb | Focal-follow time (min) | Number and age of humpbacksb |
|---|---|---|---|---|---|---|
| 1 | 1 October 1951 | Attack; unsuccessful | 4–5: no photos | 2 ad, 1 c | ||
| 2 | 1952 | Attack; unsuccessful | ?: no photos | ? | ||
| 3 | 10 August 2006 | Attack; unsuccessful | 20 | 5: 4 adf, 1 juv | 1 ad, 1 c | |
| 4 | 17 July 2007 | Attack; calf killed | 55 | 4: 3 adf, 1 juv | 2 ad, 1 c | |
| 5 | Mid-July 2009 | Attack; unsuccessful | 4–5: no photos | 1 ad, 1c | ||
| 6 | 26 June 2010 | Attack; calf killed | 3: 1 adf, 1 sam/adf, 1 juv | 1 ad, 1c | ||
| 7 | 10 July 2010 | Attack; calf killed | 6: 1 adm, 1 adf, 3 sam/adf, 1 juv | 1 ad, 1c | ||
| 8 | 20 July 2011 | Attack; calf killed | <1 | 6: 3 adf, 1 sam,1 sam/adf, 1 juv | 1 ad, 1 c | |
| 9 | 20 July 2011 | Attack; calf killed | 20 | 6: 3 adf, 1 sam, 1 sam/adf, 1 juv | 1 ad, 1 c | |
| 10 | 23 July 2012 | Attack; calf killed | ca. 60 | 7: 4 attacking included 2 adf, 1 sam, 1 juv | 1 ad, 1 c | |
| 11 | 26 July 2012 | Attack; calf killed | 5: 3 adf, 1 sam/adf, 1 juv | 1 ad, 1 c | ||
| 12 | 26 July 2012 | Approach only | ca. 1.5 | 5: 3 adf, 1 sam/adf, 1 juv | 2 sa | |
| 13 | 26 July 2012 | Attack; outcome unknown | 5: 3 adf, 1 sam/adf, 1 juv | 2ad, 1 c | ||
| 14 | 8 August 2012 | Attack; calf killed | 10–12: no photos | 2ad, 1 c | ||
| 15 | 11 August 2012 | Attack; calf killed | 30+ | 5: no photos | 1ad, 1 c | |
| 16 | 1 July 2013 | Attack; calf killed | 7: 2 adf, 1 sam, 1 sam/adf, 1 sa unk, 2 juv | 2 ad, 1 c | ||
| 17 | 2 July 2013 | Undetermined | 6: 1 adf, 4 unknown, 1 juv | 2 ad | ||
| 18 | 10 July 2013 | Undetermined | 3: 1 adf, 1 sam/adf, 1 juv | 150 | 2ad, 1 c/sa | |
| 19 | 20 July 2013 | Attack; calf killed | 4: 1 adf, 2 sam, 1 juv | 52 | 2 ad, 1 c | |
| 20 | 26 July 2013 | Attack; unsuccessful | 10 | 4: 2 adf, 1 sam/adf, 1 juv | 130 | 2 ad, 1 c |
| 21 | 26 July 2013 | Approach only | <1 | 4: 2 adf, 1 sam/adf, 1 juv | c | 1 ad |
| 22 | 26 July 2013 | Approach only | <1 | 4: 2 adf, 1 sam, 1 juv | c | 2 ad |
| 23 | 1 August 2013 | Undetermined | 6: 2 adf, 2 sam, 2 juv | 110 | ? | |
| 24 | 2 August 2013 | Approach only | “a few” | 8: 2 adf, 3 sam, 1 sa unk, 2 juv | 45 | 2 ad |
| 25 | 2 August 2013 | Approach only | <1 | 8: 2 adf, 3 sam, 1 sa unk, 2 juv | c | 1 ad |
| 26 | 3 August 2013 | Attack; unsuccessful | 8: 2 adf, 3 sam, 1 sa unk, 2 juv | 136 | 1ad, 1 c | |
| 27 | 3 August 2013 | Attack; calf killed | 8: 2 adf, 3 sam, 1 sa unk, 2 juv | 90 | 2 ad, 1 c | |
| 28 | 3 August 2013 | Undetermined | ?: part of #27 | c | 1 ad, 1 c | |
| 29 | 3 August 2013 | Undetermined | ?: another part of #27 | c | 2 ad, 1c? | |
| 30 | 6 August 2013 | Attack; calf killed | 6 | 8: 2 adf, 3 sam, 1 sa unk, 2 juv | 315 | 2 ad, 1 c |
| 31 | 8 August 2013 | Attack; unsuccessful | “a few” | 11: 2 adf, 1 adm, 4 sam, 2 sam/adf, 2 juv | 360 | 3 ad, 1c |
| 32 | 8 August 2013 | Undetermined | 11: 2 adf, 1 adm, 4 sam, 2 sam/adf, 2 juv | c | At least 2 ad, 1 c? | |
| 33 | 8 August 2013 | Attack; unsuccessful | “several” | 11: 2 adf, 1 adm, 4 sam, 2 sam/adf, 2 juv | c | 2 ad, 1 c |
| 34 | 8 August 2013 | Attack; outcome unknown | ca. 60 | 11: 2 adf, 1 adm, 4 sam, 2 sam/adf, 2 juv | c | 2 ad, 1 c |
| 35 | 15 August 2013 | Attack; calf killed | 5: 1 adf, 2 sam, 1 sa unk, 1 juv | 1 ad, 1 c |
- a Time from first approach of killer whales until either the calf was killed or the killer whales moved on.
- b Obtained from photographs: ad = adult; adm = adult male; adf = adult female; adf/sam = adult female or subadult male; sa = subadult, sex unk; juv = juvenile, c = calf.
- c Focal-follow time included in total above (Total FFT: 1,500 min; FFT of tagged animal: 1,217 min).
When killer whales attacked humpbacks, they targeted only calves; there were no observations of attacks on adults or smaller animals traveling alone (presumably yearlings). When killer whales approached humpbacks and no calf was present (n = 6), the killer whales left almost immediately and did not attack; recorded interaction durations in these instances included 1–2 min (event #12), <1 min (# 21), <1 min (#22), “within a few minutes” (#24), and <1 min (#25). All of the 10 calves that we saw attacked in 2013 were neonates born during the northbound migration: all were very small (ca. 5 m; Fig. 3-6), pale gray, and at least two still had folded-over dorsal fins.
Killer whales appeared to target the throat and lower jaw of calves during at least four of the attacks (#4, 11, 19, 30), as evidenced by broken or dislocated jaws (Fig. 3). It was not clear if jaw injuries were the result of killer whales deliberately ramming the throat in an attempt to kill or injure the calf, or if the killer whales were attempting to feed on the tongue and lips (Fig. 6; see also Discussion).
The median group size for killer whales that attacked humpbacks (n = 17) was 6 (range 3–11). Median group size for successful attacks (6, range 3–8, n = 12) was not significantly different compared to unsuccessful attacks (8, range 5–11, n = 5; Wilcoxon-Mann-Whitney rank sum test, P = 0.09).
Although adult male killer whales are substantially larger than females (Barrett-Lennard and Heise 2006, Fearnbach et al. 2011), their presence did not appear to affect the outcome of attacks. Of 17 known-outcome attacks where exact group composition was known, a single adult male was present during 2 of 5 (40%) unsuccessful attacks and only 1 of 12 (8%) successful attacks. Killer whale groups without adult males were also successful in attacking humpback cow/calf pairs even when they were accompanied and defended by an “escort” (defined as one or more humpback adults accompanying a humpback mother and calf; see below). Of the 11 successful attacks by groups without an adult male killer whale present, at least 6 (46%) were on humpbacks with escorts present (#4, 14, 16, 19, 27, 30).
We observed two specific humpback defensive behaviors in response to killer whale attacks. The first was movement toward shallow water, reef edges, or nearby boats, which appeared to be an effective deterrence on at least five occasions. One attack ended after 70 min when the killer whales left after the mother moved her calf through a gap in the reef and onto the flats (#26). Another attack ended unsuccessfully after only “several minutes” when a mother, calf, and escort moved into shallow waters near the reef edge (#33). Event #11 also ended abruptly after a humpback mother led her calf into shallow reef waters, even though the calf appeared to be mortally wounded (Fig. 3). Another unsuccessful attack lasted 20 min, during which time the mother and calf humpback used a nearby boat for cover (#3). Finally, in event #20, the killer whales broke off an attack just as the mother, calf, and escort passed within a few m of our boat, although the aggressive defense of the escort may also have been a factor (see below).
The second humpback defensive behavior was direct interference or aggression towards killer whales, by the mother and/or an accompanying escort. When killer whales approached a humpback mother and calf, the mother typically moved her calf close by her side (#1, 4, 20), and sometimes lifted the calf out of the water using her head or upper back (#9, 11, 20; Figs. 4, 5). Other aggressive behaviors by the mother included loud and forceful exhalations (“trumpeting”; #8, 19, 27), lunging or charging at the surface (#8, 16, 19, 27), and slashing or slapping of flukes and flippers (#19).
Interestingly, escorts also vigorously defended the calf. During 6 of the 12 attacks when escorts were present, they were described as actively defending the calf (#1, 16, 19, 20, 27, 30); during 3 other attacks, the behavior of the escort was unrecorded (#14, 31, 33), and during 1 (#4), the escort apparently left as the killer whales approached the mother and calf. The defensive behaviors of the escorts were similar to that of the mothers, including flanking the calf opposite the mother (#20), flailing its flukes or flippers (#1, 19, 20, 27), charging toward the attacking killer whales (#1, 16, 19, 27), and trumpeting (#19, 20, 27).
Despite the efforts of the humpback mothers and escorts, killer whales were usually successful in their attacks, but less so when escorts were present. Of the 21 attacks where the outcome was recorded and the presence/absence of escorts noted, escorts were present during 10 (48%) and not present during 11 (52%; Table 1). Overall, calf kills recorded during 14 of the 21 (67%) attacks included 6 attacks (43%) when an escort was present and 8 attacks (57%) when there was no escort. Escorts may have been instrumental in driving off killer whales during at least five attacks (#1, 20, 31, 33, 34), although the calf was killed during at least six attacks when an escort was present (#4, 14, 16, 19, 27, 30).
Other Prey
The only prey other than humpbacks that we observed killer whales taking was spinner dolphins (Stenella longirostris), on one occasion. On 6 August 2013, we followed a group of 7–8 killer whales (including the tagged female, see below) as it moved slowly northward, 1 km out from the main channel at Coral Bay. At 1205, after encountering a small school of spinner dolphins, the killer whales split up and after some spirited chase lasting only a few minutes, they killed and ate at least two of the dolphins. By 1215 the group was heading northeast again and angling in towards the reef edge. Two hours later, the same group killed and ate a humpback calf (#30).
During our 2013 surveys, we encountered schools of spinner dolphins on four survey days. The schools typically numbered in the hundreds of individuals, with one group estimated to be on the order of 1,000 individuals; they were milling in waters 50–100 m deep during the daytime and appeared to be resting. This was by far the most numerous cetacean species that we encountered on the continental shelf adjacent to Ningaloo Reef and was potentially a readily available prey source for killer whales.
Satellite Tracking
On 20 July 2013, we deployed a satellite tag on an adult female killer whale (WA14) just seaward of Ningaloo Reef; her group killed a humpback calf just as we were approaching them (#19). The most detailed subset of our killer whale/humpback interaction data (included in the behavioral summary section above) came from the recorded movements and behavioral observations of this female and her associates, during the time her tag was operational.
The tag transmitted for 22 consecutive days, providing a total of 452 location estimates with a mean error radius of 1,558 m (minimum = 168 m, maximum = 31,060 m). Over this period, the tagged whale (and presumably her group) traveled an estimated 1,964 km, at an average speed of 4 km/h (maximum 12 km/h). Estimated displacement velocities identified two regions along the coast where the group slowed its movement and spent significant time (Fig. 2). On 21 July, the day after being tagged, the whale left our survey area and traveled 400 km southward to an area north of Shark Bay, where she spent 3 d close to shore near Carnarvon, WA (Fig. 2). By 29 July, the group was heading north again, and was back near Tantabiddi, where we tagged her, by 1 August; they remained in that area until at least 10 August when the tag quit transmitting.
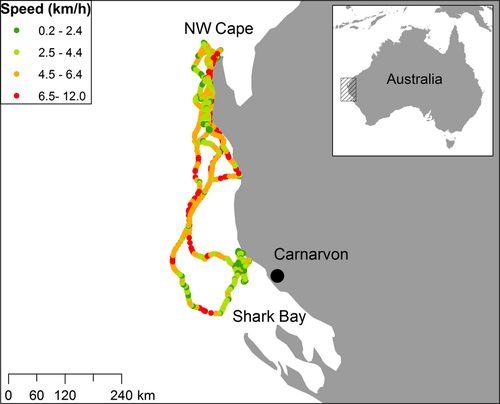

While the tag was transmitting, we located the tagged female and associated whales at sea on 6 separate days (20 July; 1, 2, 3, 6, and 8 August, 2013) and followed them for a total of 20.3 h. During these focal follows, the group had 12 interactions with humpbacks (#23, 24, 26–35) and attacked 8 different calves: 4 attacks were unsuccessful, 3 resulted in kills, and 1 had an uncertain outcome—a 43% success rate for attacks with known outcomes.
Shark Interactions
Despite the fact that most observers usually had limited underwater viewing opportunities, large numbers of sharks were reported among killer whales that were attacking humpbacks, especially after kills. For example, on one occasion killer whales with a freshly killed humpback calf were attended by approximately 30 sharks (Carcharhinus spp.), estimated to be between 1.5 m and 2.5 m in length, and a 3 m tiger shark (Galeocerdo cuvier); as one of the killer whales was swimming around with the humpback calf in its mouth, one shark was filmed tearing flesh off the carcass (#16). Reported numbers of sharks at other kill sites included an estimated 12 (#15), an unspecified number present (#19), 8–12 (#27), and “numerous” sharks (#35).
In addition to scavenging, a white shark (Carcharodon carcharias) reportedly attempted to attack a humpback calf. On 24 July 2013, we interviewed Corey Hann who earlier that day had witnessed an apparent attack by a white shark on a humpback whale calf off Coral Bay, WA. He and a colleague were underwater and filming three humpbacks (mother, calf, and escort), when a 4–5 m white shark approached the whales. The divers immediately got out of the water but continued filming above water, from their boat. The escort began spyhopping when the shark approached from directly behind the whales. The mother humpback, apparently recognizing the danger, lifted the calf out of the water on her head. A few minutes later the calf breached several times. The escort remained behind the mother/calf pair, and began slapping its flukes vigorously when the shark approached, while the mother moved away from the calf. The shark disappeared and all three whales joined up again shortly afterward. According to Hann, the escort was instrumental in driving off the white shark.
Discussion
Humpback Behavioral Responses to Killer Whale Predation
Predators can profoundly influence the behavior of their prey, even if they rarely kill them (Ripple and Beschta 2005, Wirsing et al. 2008). For example, Corkeron and Connor (1999, Connor and Corkeron 2001) suggested that the threat of killer whale predation alone could provide the impetus for large whales to migrate to low latitudes for calving. Clapham (2001) argued that this was not likely because predation on large whale calves had rarely been documented, but that could also be cited as further evidence that migration is an effective antipredator strategy.
It appears that killer whales now occur annually along coastal areas of Western Australia to prey on a flush of seasonally available humpback whale calves—an abundant, predictable, easily taken prey source that could represent a significant contribution to their annual energy intake and perhaps a growing threat to breeding humpbacks. We do not have enough data yet to offer more than a crude estimate of number of calves taken annually, but the group with the tagged female that we followed took three humpbacks during approximately 1 d of foraging (i.e., 20.3 h over 6 d). If this is extrapolated over the larger killer whale population that occurs off Ningaloo, for the 5 mo of humpback whale presence off the coast there, it seems likely that as a minimum, dozens of calves are taken there annually.
Stone et al. (1987) pointed out that humpbacks in the Northern Hemisphere migrate mainly through open oceans, while those in the Southern Hemisphere tend to travel along continental coastlines. Following coastlines in areas like in WA should make the timing and routes of humpback migration even more predictable to predators and increase their vulnerability. Humpbacks produce, on average, only one calf every 2 yr (Clapham 2000), so that each one is a substantial reproductive investment. Killer whales took those calves off the coast of WA with relatively little effort, and not surprisingly, humpbacks appear to have developed behavioral responses to counter that threat. We discuss two of those below.
Migratory routes
During humpback migration off WA, mothers with calves travel closer to the coast than the rest of the population (Jenner et al. 2001; but see Chittleborough 1965). A similar segregation occurs among migrating gray whales (Eschrichtius robustus) in the eastern North Pacific, where 90% of mothers with calves travel within 200 m of the shore (Jones and Swartz 2009). For both humpbacks and gray whales, these differences in migration routes are probably examples of prey behavior being influenced by predator intimidation (i.e., a nonconsumptive predator impact; Wirsing et al. 2008).
Humpback mothers with calves are also known to preferentially occupy nearshore waters on the breeding grounds, and possible reasons cited for this have included: the presence of calmer waters, resting, to avoid harassment from sexually active males, and protection from sharks and killer whales (Whitehead and Moore 1982, Glockner and Venus 1983, Mattila and Clapham 1989, Smultea 1994, Clapham 2000, Martins et al. 2001, Bruce et al. 2014, Craig et al. 2014). Humpbacks off WA generally occur further offshore during the northbound (breeding) migration than on the southbound migration (Jenner et al. 2001), and it has been suggested that this is because the southbound migration includes a higher proportion of mother and calf pairs traveling closer to the coast for protection from sharks and killer whales.1
The narratives in Appendix S1 clearly show how humpback mothers and calves use the reef as protection from attacking killer whales, including mothers with calves born on the northbound migration. During attacks, mothers immediately tried to move their calves toward shallower waters, which at times proved to be effective in deterring attacking killer whales (#5, 11, 26, 33). Other whale species that have been documented retreating to shallow waters when being attacked by killer whales include gray whales, southern right whales (Eubalaena australis), bowhead whales (Balaena mysticetus), minke whales (Balaenoptera acutorostrata and B. bonaerensis), and sei whales (B. borealis; Finley 1990, Ford et al. 2005, Reeves et al. 2006, Goodall et al. 2007, Barrett-Lennard et al. 2011, Haussermann et al. 2013). When they do not have calves, humpbacks and gray whales both take more direct, offshore routes during migration.
Role of the escort
Another specific antipredator behavior employed by WA humpbacks involved escorts assisting in calf defense. A humpback escort has historically been defined as an adult male that swims in close company with an adult female, with or without a calf, on the breeding grounds, in anticipation of a mating opportunity should the female become receptive (Herman and Antinoja 1977, Glockner 1983, Mobley and Herman 1985, Clapham 1996, 2000), although postcopuatory mate guarding has also been suggested as a possible driver (Mobley and Herman 1985, Brown and Corkeron 1995, Clapham 1996). In the Northern Hemisphere, humpbacks have been documented singing along migratory routes as well as on their feeding grounds (Mattila et al. 1987, McSweeney et al. 1989, Charif et al. 2001, Clark and Clapham 2004), and recently Stimpert et al. (2012) documented Southern Hemisphere humpbacks singing on their Antarctic feeding grounds. It is clear that humpback breeding displays and behaviors are not limited exclusively to the breeding grounds and this may also apply to escort behavior.
On the Hawaiian breeding grounds, the “principal escort” (the male closest to the female) aggressively engages other prospective suitors (“challengers”), and will attempt to drive them off (Tyack and Whitehead 1983, Baker and Herman 1984). The focal female in these competitive groups is usually (but not always) without a calf, possibly due to the fact that females with calves-of-the-year are not likely to come into estrus for at least another year (Craig et al. 2002, Spitz et al. 2002). This decreased mating potential could explain why males that associate with females with calves are smaller than males in competitive groups and therefore possibly sexually immature (Spitz et al. 2002). Craig et al. (2002) further suggested that if female humpbacks with neonates were not receptive, males might avoid associating with them altogether, but we regularly saw (presumably male) escorts associated with mothers and neonates at Ningaloo (Table 1).
The behavior of escorts on the breeding grounds, especially the male competitive groups, has captured much of the attention of field researchers, but there have also been observations to suggest that escorts may also have a role in calf defense, both on and away from the breeding grounds. In 1951 a pilot from a whale spotter plane off WA reported that when killer whales attacked a humpback whale group, which included two adults and one calf, the presumed mother stayed close by the calf while the escort “(possibly a bull), charged the killer whales, beating them off with its flukes” (Chittleborough 1953). Similarly, in Hawaii, when a survey aircraft circled over a mother and calf pair and an escort was present, the adult humpbacks responded by moving the calf into a central position and tightening their formation around it—“a clearly protective behavior” (Herman and Antinoja 1977). They further stated that humpback groups without calves dispersed when the aircraft approached. Additional observations of escorts defending calves from attacking killer whales have been reported on breeding grounds off eastern Australia (Naessig and Lanyon 2004) and Colombia (Flórez-González et al. 1994), and on feeding grounds in Alaska (Dolphin 1987, D'Vincent et al. 1989). Brown and Corkeron (1995) suggested that escorts might be useful for defense against predators, although they discounted the threat of killer whale predation in their eastern Australian study area where they considered sharks to be the main predation risk.
Although killer whales are generally considered to be the most important natural predators of humpbacks (Jefferson et al. 1991, Paterson and Paterson 2001, Ford and Reeves 2008), large sharks also pose a threat, especially to young calves (Paterson and van Dyck 1991, Paterson et al. 1993, Mazzuca et al. 1998, Taylor et al. 2013, this study). White sharks, for example, grow to over 6 m (Castro 2011), while humpbacks are only 4–4.6 m at birth (Clapham et al. 1999). Although the relative threat to large whales posed by sharks vs. killer whales has yet to be assessed anywhere, our observations indicate that humpback escorts will defend against both. The question that remains is how do escorts benefit from this apparent alloparental behavior? To answer this, future research will need to determine if the escort is related to the mother or the calf (kin selection), or if the escort's behavior increases his chances for breeding with the mother once she comes back into estrus (reciprocity).
Comparisons with Gray Whales
Gray whales feed in the Bering and Chukchi Seas during the northern summer; then during the winter they undertake a round-trip journey of up to 20,000 km, to the lagoons of Baja California, Mexico, where they calve and mate (Rice and Wolman 1971). Gray whale calves migrating with their mothers to their northern feeding grounds for the first time are subject to intense killer whale predation pressure. For example, during May and June each year from 2003 to 2006, over 170 individual mammal-eating killer whales have been documented aggregating near Unimak Pass, in the eastern Aleutian Islands, Alaska, where they preyed upon northbound gray whale calves (Barrett-Lennard et al. 2011, Matkin and Durban 2013). This is the largest assemblage of mammal-eating killer whales recorded anywhere in the world and is an important source of gray whale calf mortality (Matkin and Durban 2013).
Barrett-Lennard et al. (2011) summarized their observations of killer whale attacks on gray whale calves in the eastern Aleutians and cited seven “key observations.” Our observations at Ningaloo reveal some striking similarities and important differences in the way that gray whales and humpbacks respond to the threat of killer whale attack on their calves. Below we list and address each of the key points presented by Barrett-Lennard et al. (2011):
- “Killer whales sometimes terminated encounters with gray whales after briefly harassing them.” We also witnessed brief encounters (#12, 21, 22, 24, 25), although these occurred only when killer whales approached humpbacks that did not have calves.
- “Killer whales selectively attacked young-of-the-year calves or apparent yearlings.” Ningaloo killer whales attacked only neonate calves. We also saw humpback mothers with what appeared to be yearling calves, but they were not attacked.
- “Female gray whales defended their young by interposing their bodies between the killer whales and the calf and/or by vigorous tail thrashing.” Humpbacks at Ningaloo did the same, but the mothers also regularly lifted their calves out of the water using their head or anterior back (Fig. 4, 5), a behavior that has also been observed in gray whales in Monterey Bay, California (Flood 2000). An important difference was that humpbacks often had an escort present that almost always helped defend the calf (see below), and gray whales are not known to have escorts.2 ,3
 Figure 4A humpback mother lifts her calf out of the water on her back shortly before it was killed by attacking killer whales (#9). The killer whale on the far left is carrying the carcass of another humpback calf taken several minutes earlier (#8). Photo: S. Wenngren.
Figure 4A humpback mother lifts her calf out of the water on her back shortly before it was killed by attacking killer whales (#9). The killer whale on the far left is carrying the carcass of another humpback calf taken several minutes earlier (#8). Photo: S. Wenngren. Figure 5A young humpback calf is lifted out of the water on its mother's back while an escort (above) moves in to assist in driving off a group of attacking killer whales adjacent to Ningaloo Reef in July 2013 (#20); the attack was unsuccessful and the calf was not harmed. Photo: L. Ballance.
Figure 5A young humpback calf is lifted out of the water on its mother's back while an escort (above) moves in to assist in driving off a group of attacking killer whales adjacent to Ningaloo Reef in July 2013 (#20); the attack was unsuccessful and the calf was not harmed. Photo: L. Ballance. - “Killing was accomplished by restricting the movements of a gray whale by holding its flippers or snout and drowning it.” Another important difference here is that the by the time gray whale calves had traveled to the Aleutian Islands from their Baja breeding grounds, they were already several months old and considerably larger than the sometimes only days-old neonate humpbacks off Ningaloo. Due to their small size, Ningaloo humpback calves were much more vulnerable to attack and sometimes killed in <1 min.
- “Gray whales sought refuge in shallow water (3 m deep) close to shore during attacks, and if they succeeded in reaching such an area, the attacks were abandoned” (see also Matkin and Durban 2013). Similarly, when killer whales attacked at Ningaloo, if humpback mothers were able to move their calves in close to the reef or onto the reef flats, the attacks were immediately terminated (#11, 26, 33).
- “Killer whales actively worked to try to prevent passage to shore by ramming, biting, and dragging the gray whale by the flukes and pectoral flippers.” This behavior was not specifically noted off WA, but may have occurred. Again, the small size of the humpback calves may have made this unnecessary.
- “In some cases, one or more groups of killer whales attended but did not participate in an attack pressed by another group nor did they feed on the freshly killed carcass.” Killer whale groups at Ningaloo appeared to be fluid and separate groups readily merged when attacking and feeding on calves (e.g., #26, 27, 31–34).
Scavenger Hunt
Scavenging is an often-underrated energy pathway in food webs (Wilson and Wolkovich 2011). During our survey, not only were humpback calves regular prey for killer whales off WA, but their carcasses appeared to be a major food source for scavengers, especially sharks, which readily feed on whale carrion (Dudley et al. 2000, Curtis et al. 2006, Dicken 2008, Fallows et al. 2013). In Alaska, Barrett-Lennard et al. (2011) suggested that gray whale calf remains left by killer whales were potentially an important food source for sleeper sharks (Somniosus pacificus), and brown bears (Ursus arctos), which fed on carcasses that washed ashore. Red foxes (Vulpes vulpes) and various species of scavenging birds also benefitted.
The shark population of Ningaloo Reef is large and diverse. Carcharinid sharks are the most abundant and diverse forms, but tiger sharks and hammerheads (Sphyrna spp.) are also common, and white sharks occur regularly (Last and Stevens 1994; authors’ personal observations). During and after at least 5 of the 14 (36%) humpback calf kills that we documented off WA, numerous large (2–4+ m) sharks gathered within minutes at the attack site to feed on the carcass (#15, 16, 19, 27, 35).
Another important factor for scavengers off WA is the consumption efficiency of feeding killer whales and the possibility of “surplus killing” (Kruuk 1972, Jefferson et al. 1991). Although surplus killing typically refers to killing more individual potential prey animals than a predator will actually eat (e.g., Stacey et al. 1990, Williams et al. 1990, Gaydos et al. 2005, Barrett-Lennard and Heise 2006), it can refer to any kind of wasteful killing. For example, when prey is abundant, killer whales tend to selectively feed on certain body parts, e.g., the tongues, lips, and ventral grooves of baleen whales or just the breast muscles of penguins (Lowry et al. 1987; Flood 2000; Whitehead and Reeves 2005; Pitman and Durban 2010, 2012; Ferguson et al. 2012), and discard the rest of the carcass, leaving more for the scavengers. Evidence that Ningaloo killer whales may at times selectively feed on the tongue and throat of humpback calves is shown in Figure 6 and in a report of a freshly-dead humpback calf found on the beach near Yardie Creek on 17 September 2013, which was missing its lower jaw, throat, and some of its underside,4 presumably from a successful killer whale attack. A 10 m humpback whale found on the beach at Warnbro Sound, just south of Perth, WA, in June 2008, also had its tongue missing and damage to its lower jaw and throat.5

Killer whales in Alaska often returned to gray whale calf kill sites to feed on submerged carcasses for up to at least 5 d, a behavior that Barrett-Lennard et al. (2011) referred to as “prey caching.” Off WA, however, prey caching is unlikely because a carcass there would be consumed (or carried off) very quickly due to the large number of large sharks present and the fact that WA humpback calves are much younger and smaller than gray whale calves taken off Alaska. As an indication of this scavenger efficiency off central WA, Chittleborough (2003: fig. 1) showed a photograph of a 12.8 m humpback that had been harpooned off Pt. Cloates, Australia, in 1952; by the time the carcass was pulled ashore 4 h later, little more than a skeleton remained. More recently, on 20 May 2014, a live humpback, estimated to be 8–9 m in length (possibly a yearling), was seen being attacked by “dozens” of sharks off Coral Bay, WA. Identified sharks included “mostly tigers, though also bull [Carcharhinus leucas] and the ever-present whalers.” Less than 60 h later, this shallow “whale fall” (Smith 2006) was little more than a skeleton on the ocean floor (Fig. 7).

Fallows et al. (2013) recently suggested that scavenging on dead large whales could be a critical, if underappreciated, aspect of white shark foraging ecology, especially for mature sharks. Although they considered a whale carcass to be a temporary “pulse” resource, a prolonged, seasonal occurrence of large numbers of humpback calf carcasses provided by killer whales along the coast of WA each year could offer a more predictable food source, with possible implications for the abundance and movements of several species of sharks, including whites (Jorgensen et al. 2010).
Whaling–Impact and Recovery
Humpback whale numbers have increased rapidly off WA since whaling ended there in 1963. Bannister (1964) estimated that the population had been reduced to 568 individuals by the end of 1963, and this did not even take into account the illegal whaling that continued on this population in Antarctica until at least 1968 (Yablokov 1994, Clapham et al. 2009). By 2008, however, population estimates for WA humpbacks ranged from 21,750 (95% CI = 17,550–43,000, Hedley et al. 2011) to 26,100 (95% CI = 20,152–33,272, Salgado Kent et al. 2012). Subsequently, Branch (2011) estimated that the population was probably >30,000, and Salgado Kent et al. (2012) further suggested that it could was as high as 33,300. More recently, the IWC (2014) estimated that the population in 2012 was 19,264.
The preexploitation population size of WA humpbacks was estimated by Chittleborough (1965) to be 12,000–17,000, although he was also unaware of the huge illegal Soviet catches. That estimate was revised to 21,686 by the IWC (2014), which when compared with the population estimate of 19,264 cited above would indicate that the WA humpback population could now be at or above 90% of the estimated carrying capacity (probability interval: 74%–98%).
Regardless of the exact figures, WA is now home to the largest humpback breeding population known, having increased annually at an estimated 10.15% (Bannister and Hedley 2001) or 13% (Salgado Kent et al. 2012), which are just below and above, respectively, the maximum plausible rate posited for this species (i.e., 11.8%, Zerbini et al. 2010). Clearly, killer whale predation has not been a significant factor in the recovery and survival of WA humpbacks—at least until recently.
If there had been a sizeable population of killer whales that preyed extensively on WA humpback whale calves prior to commercial whaling, then it is possible or even likely that when the humpback population collapsed in the 1960s, the local killer whale population could have collapsed along with it. In fact, there is some evidence that, historically, the killer whale population off WA was larger than it is today, that it declined after the whaling era, and that it might be increasing today. Chittleborough (1953) reported five sightings of killer whales during aerial surveys for humpbacks off Pt. Cloates, WA, conducted during just two months (August/September) in 1952; his group size estimate for three of the sightings was >30, ca. 40, and ca. 50–60 (the other two were 5 and “several”). Also in September 1952, the pilot for the same spotter plane reported a sighting of “at least 150” killer whales in Exmouth Gulf. By contrast, the largest killer whale group size in our survey of recent records was 11 (Table 1).
After the whaling ended and up until fairly recently, there had been very few reported sightings of killer whales off Ningaloo (Table 1). For example, Sleeman et al. (2007) conducted a series of systematic aerial surveys for whales and dolphins off Ninglaoo Reef (as far south as Pt. Cloates), during every month except December, from June 2000 to April 2002, and they did not record a single sighting of killer whales. The first record of an attack on a humpback calf since the end of the whaling era that we are aware of was in 2006. Craig Kitson, captain of an Exmouth whale shark tour boat, told us: “I moved to Exmouth in 2001 and the first time I saw an attempted kill by the orca pod was on the 10th of August 2006 in Lighthouse Bay [#3]. Up until this time, orcas were not seen that regularly. We did see the odd one occasionally, however.”
One plausible interpretation of these events is that after whaling depleted WA humpbacks, killer whales that preyed on them either declined in numbers or were extirpated. Furthermore, any subsequent recovery of those killer whales would be expected to lag far behind the humpbacks. For example, the maximum population growth rate for killer whales is considerably lower than for humpbacks (3.5% vs. 11.8%, respectively; Matkin et al. 2014, Zerbini et al. 2010), and once killer whales resumed hunting humpbacks off WA, it would be many years, perhaps decades, before they recovered their former population size and realized their full predatory potential on the local humpbacks. This could also apply to populations of killer whales that prey on large whales elsewhere.
Killer Whales as Predators of Large Whales
The debate about whether killer whales were important predators of large whales prior to the advent of industrial whaling, and, if they were, how they responded to the worldwide depletion of whale stocks, could have important implications for marine food web dynamics. For example, Springer et al. (2003) argued that mammal-eating killer whales were important predators of large whales, and that after whale stocks were decimated in the North Pacific, killer whales targeted and serially depleted populations of other, increasingly less-profitable species of marine mammals—a trophic cascade that they referred to as the “sequential megafaunal collapse” hypothesis (SMC; Springer et al. 2003, 2008; Williams et al. 2004, Estes et al. 2009). Since it was published, the SMC has met with numerous criticisms about the assumptions and data used for the analyses (DeMaster et al. 2006; Trites et al. 2007; Wade et al. 2007, 2009), with some authors further contending that large whales are not now and probably never were important prey for killer whales (Mizroch and Rice 2006, Mehta et al. 2007, but see Reeves et al. 2006).
The seminal assumption of the SMC hypothesis—that large whales were important prey for at least some populations of killer whales—has received some support recently from evidence that, in addition to WA humpbacks, another recovered large whale population may also be experiencing increased predation pressure from killer whales. Commercial whaling had depleted eastern Pacific gray whales by 1939, but under international protection their numbers rebounded, and by 1994 they were removed from the US Endangered Species List (Jones and Swartz 2009, Durban et al. 2013). Although, historically, few attacks on gray whales were ever reported (Jefferson et al. 1991, Barrett-Lennard et al. 2011), it has recently been suggested that killer whales could now be taking as much as half of the gray whale calf production in some years (Matkin and Durban 2013).
Research on killer whales only really began after many large whale populations had already been pushed to the brink of extinction by the 1960s and early 1970s (Baird 2002, Roman et al. 2014). By then, any killer whales that had preyed extensively on large whale calves would already have been forced to either starve and die, or switch to other, presumably smaller prey (the SMC hypothesis; Estes et al. 2009). Either way, it is likely that those killer whale populations would have declined or perhaps even became extirpated in some areas. In Alaska, for example, one wonders what would happen to the 170+ mammal-eating killer whales that currently prey on gray whale calves during spring and summer months if those gray whales became depleted, as they were by 1939 (Jones and Swartz 2009).
It is now well established that when killer whales attack large whales they target the calves (Reeves et al. 2006, Pitman et al. 2007, Barrett-Lennard et al. 2011, Higdon et al. 2012, this study). Mehta et al. (2007) and Steiger et al. (2008) both concluded that when killer whales attacked humpbacks, because they targeted mainly calves and in lower latitudes, that this somehow invalidated the SMC. But Springer et al. (2003) never specifically excluded calves as prey in their hypothesis (Springer et al. 2008) or ruled out the possibility of predation in lower latitudes. Furthermore, recent satellite-tracking studies in the North Atlantic (Matthews et al. 2011), North Pacific (Durban et al., unpublished data), and Southern Ocean (Durban and Pitman 2012, unpublished data) have shown that mammal-eating killer whales from high latitudes are capable of rapid, long distance movements (1,000s of km) into mid-latitudes, which means they could be capable of intercepting whales during migration when the calves are smaller and more vulnerable. Perhaps the best test of the Springer et al. (2003) hypothesis will come from monitoring killer whale responses to recovering large whale populations (Kareiva et al. 2006, Wade et al. 2007), an experiment that may already be unfolding off Western Australia, in the eastern Canadian Arctic, in the eastern Aleutian Islands of Alaska, and possibly elsewhere.
Migration and Predation—the Moveable Feast
Large whales are important components of marine ecosystems not only as in situ mega-consumers (Laws 1985, Kareiva et al. 2006, Croll et al. 2006), but also for the role they play in packaging and redistributing nutrients in the ocean (Roman and McCarthy 2010), which their extensive migrations can extend to an ocean-basin scale (Roman et al. 2014). We suggest that further study of the continental shelf waters along WA will show that the vast majority of the cetacean biomass that occurs there on an annual basis is represented by migrating humpback whales (Sleeman et al. 2007), which extract little and contribute much to the local ecosystem. Although they do essentially no feeding during migration (Chittleborough 1965, Dawbin 1966, Baraff et al. 1991), during almost 6 mo every year, 20,000–30,000 humpbacks may visit the nearshore waters of WA, where they slough skin, shed placentae, pass metabolic waste, and sometimes die; killer whales and white sharks prey on their calves, and scavenging sharks and benthic detritivores feed on their carcasses (Smith 2006). All of this largesse ultimately derives from the Southern Ocean where Antarctic krill (Euphausia superba) is shape-shifted into Area IV humpbacks and injected annually into the relatively oligotrophic, littoral-marine ecosystem of Western Australia, where killer whales their do their part in making it available to local consumers. The full impact of this Antarctic subsidy to the WA marine economy will not be realized, however, until that system has recovered, to the extent possible, from the ongoing legacy of commercial whaling.
Acknowledgments
We acknowledge the support and cooperation from DPaW, in particular the Exmouth office, and from regional manager Arvid Hogstrom and marine manager Peter Barnes. For assistance in the field, we thank: Grace Totterdell, Tony Howard, Tanja Ryhanen, Dave Bond, Louise Smith, Lyn Totterdell, Krystal Keynes, Jana McGeachy, Violetta Brosig, and Esther McDonald. The following people shared their observations and images with us: Red Blakely, Violetta Brosig, Natalie Chilvers, Huw Dilley, Corey Hann, Tony Howard, Leith Holtzman, Ian Huxter, Kurt Jenner, Emma Johnson, Felicity Kelly, Krystal Keynes, Craig Kitson, Lou Mackenzie, Esther McDonald, Catherine McKelvie, Tamar Melon, Shane and Danielle Middleton, Rex Morey, Mark Panhuyzen, Jono Shales, James Small, Jessica Spencer, Ant Warner, Serren Wenngren, and Cindy White. Additional thanks to the Ningaloo whale shark tour boat operators, skippers, and crew; Eric Roulston and his pilots at Norwest Air Work, pilot Tiffany Klein, and Doug Anderson, Luke Barnett, and Ellen Husain from Silverback Films for sharing their video footage with us and providing financial support for this project. An earlier version of the manuscript was improved by comments from J. L. Bannister, D. J. Boness, P. J. Clapham, P. J. Corkeron, and an anonymous reviewer. Research was conducted under permits from: The Commonwealth Department of Environment Cetacean Permit #C2013-0003; Marine Parks Reserves Authority Permit # 011-ACTWN-22032013-01; Access to Biological Resources Permit # AU-COM2014-234; Department of Parks and Wildlife (DPaW) Regulation 17 License # SF009299, Regulation 04 License # CE 003998, Regulation 15 License # TF005996, and from Flinders University Animal Welfare Committee, Project #E387, issued to the Marine Information and Research Group–Australia (MIRG). The authors have no conflicts of interest to declare.




