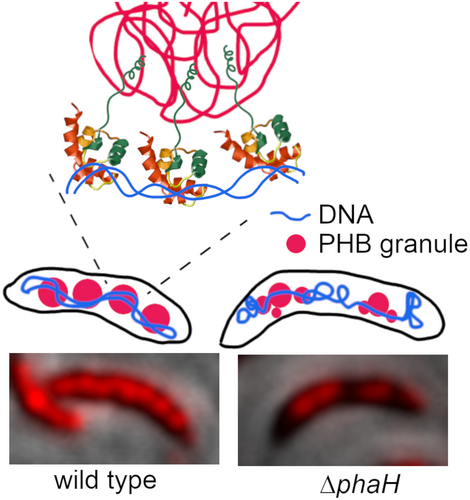
A new type of phasin characterized by the presence of a helix-hairpin-helix domain is required for normal polyhydroxybutyrate accumulation and granule organization in Caulobacter crescentus
Abstract
Bacteria frequently store excess carbon in hydrophobic granules of polyhydroxybutyrate (PHB) that in some growth conditions can occupy most of the cytoplasmic space. Different types of proteins associate to the surface of the granules, mainly enzymes involved in the synthesis and utilization of the reserve polymer and a diverse group of proteins known as phasins. Phasins have different functions, among which are regulating the size and number of the granules, modulating the activity of the granule-associated enzymes and helping in the distribution of the granules inside the cell. Caulobacter crescentus is an oligotrophic bacterium that shows several morphological and regulatory traits that allow it to grow in very nutrient-diluted environments. Under these conditions, storage compounds should be particularly relevant for survival. In this work, we show an initial proteomic characterization of the PHB granules and describe a new type of phasin (PhaH) characterized by the presence of an N-terminal hydrophobic helix followed by a helix-hairpin-helix (HhH) domain. The hydrophobic helix is required for maximal PHB accumulation and maintenance during the stationary phase while the HhH domain is involved in determining the size of the PHB granules and their distribution in the cell.
Graphical Abstract
Granules of the reserve polymer PHB can occupy most of the cytoplasm under some growth conditions. We describe PhaH, a granule-associated protein that in Caulobacter crescentus allows the interaction of the granules with the nucleoid, allowing a better distribution of the granules in the cell. Unlike other similar proteins, PhaH does seems to only have a role in PHB granule distribution but not in segregation.
INTRODUCTION
When bacteria have an available carbon source, but their growth is limited by a low concentration or absence of another nutrient, the excess carbon can be stored in different compounds (Mason-Jones et al., 2022). A frequent type of storage polymer is some type of polyhydroxyalkanoate (PHA), of which one of the most common is poly-3-hydroxybutyrate or PHB. Synthesis of PHB starts with the derivation of acetyl-CoA from the main metabolism by the PhaA and PhaB enzymes that synthesize 3-hydroxybutyryl-CoA, the substrate of the PHB synthase PhaC. This enzyme produces long chains of a hydrophobic polymer that forms a granule even when its recombinantly expressed in bacteria that do not normally synthesize PHB or in vitro (Gerngross & Martin, 1995; Rehm, 2010). The genes that code for these three enzymes are frequently together in an operon (Rehm, 2003). PHB can be used (mobilized) when the available carbon source in the medium is depleted, the PHB depolymerase (PhaZ) breaks down the polymer so that it can be used as an energy or carbon source. Studies on the mobilization of PHB have been done in a few bacteria, this is usually tested by shifting the cells from a PHA-producing media into a media with no carbon source but that otherwise allow normal growth. After this, the PHA concentration starts diminishing and the cells still divide a few times until the PHA storage is reduced to a minimal amount (Handrick et al., 2000; Kadouri et al., 2003). Mutants lacking phaC, the gene coding for the PHB synthase, frequently secret organic acids into the media and show growth defects that can be alleviated by redirecting the metabolic intermediaries into other storage compounds or metabolic pathways (Cevallos & Mora, 1996; Chen et al., 2012; Kadouri et al., 2002). Although PHAs are normally viewed as storage polymers, they are constantly being synthesized and degraded, balancing the cell metabolism (Obruca et al., 2020; Prieto et al., 2016), with the overall direction being determined by the metabolic state of the cell (Mitra et al., 2022) through second messengers and phosphorylation of the PHB related enzymes (Juengert et al., 2018). Besides their metabolic role, PHAs also contribute to the survival of the cells to different stresses by stabilizing proteins, protecting against oxidative, UV damage or cold shock (Müller-Santos et al., 2021; Obruca et al., 2020). The granule formed by the PHA polymer is covered by different PHA granule associated proteins (PGAPs) making it an organized structure that is also known as carbonosome (Jendrossek, 2009). The proteins commonly associated with the granule are one or several PHB synthases and depolymerases, a transcriptional regulator as well as a diverse group of small proteins known as phasins. Different functions have been assigned to phasins, among which are regulating the size and number of carbonosomes, reduce unspecific interactions of other proteins with the granule, activation of the synthases and partitioning of the granules during cell division (Maestro & Sanz, 2017; Mezzina & Pettinari, 2016). Some of these PGAPs directly interact with the hydrophobic granule or can be recruited through interactions with other proteins. The interactions between PGAPs on the surface of the granule are relevant no only to allow the recruitment of proteins but also to regulate the activity and expression of PHB-related proteins (Maestro & Sanz, 2017; Mitra et al., 2022; Tarazona et al., 2020).
Oligotrophic bacteria thrive in environments where nutrients are very diluted and are able to maintain an active metabolic state in these conditions (Morita, 1997). The freshwater oligotrophic bacterium Caulobacter crescentus has been intensively studied for its dimorphic cell cycle that allows this bacterium to generate after each division a motile daughter cell from a sessile mother cell (Curtis & Brun, 2010). The mother cell attaches to solid surfaces by a holdfast that is secreted at the end of the stalk, a thin tubular extension of the cell envelope that is synthesized at the old cell pole. The stalk has been proposed to help in nutrient capture and the best-studied signal for stalk growth is a low phosphate availability (Gonin et al., 2000; Ireland et al., 2002; Klein et al., 2013; Wagner et al., 2006). Low phosphate triggers PHA accumulation in other bacteria, suggesting that PHB synthesis could be an important part of the C. crescentus metabolism under natural growth conditions. Furthermore, it has been shown in Cupriavidus necator that PHB synthesis and mobilization depends on the levels of the second messenger ppGpp (Juengert et al., 2017; Müller-Santos et al., 2021), the concentration of this molecule increases as a response to low levels of different nutrients, and in C. crescentus controls the cell cycle (Hallez et al., 2017).
The only report on PHB synthesis in C. crescentus, besides the initial description on the presence of PHA granules (Poindexter, 1964), characterized the polymer produced by this bacterium, showing that it consisted exclusively of PHB. It was also shown that the PhaC enzyme of this bacterium synthesizes PHB when heterologously expressed in Escherichia coli or C. necator cells (Qi & Rehm, 2001). Production of PHB as a bioplastic is an important biotechnological research area and recently Caulobacter species have been investigated as a possible option for industrial PHB production from whey or seaweed (Bustamante et al., 2019; Leadbeater et al., 2022). Given the relevance that carbon storage could have in the survival of C. crescentus and the known significance of the carbon metabolism in the cell cycle and cell morphology (Berge et al., 2020; Boutte & Crosson, 2013; Irnov et al., 2017; Poindexter, 1981), we decided to identify the PGAPs in this bacterium. Although some of these proteins can be predicted, others have to be identified by genetic or biochemical techniques. As an initial approach to identify the C. crescentus PGAPs, we obtained the proteome from isolated native PHB granules. Fluorescent protein fusions allowed us to identify a phasin (PhaH) with a predicted non-sequence-specific DNA binding domain not previously observed in other phasins. Our results show that PhaH has a role in PHB accumulation and maintenance during the stationary phase. In its absence the cells produce smaller PHB granules and their distribution inside the cell is affected, suggesting that this protein also has a role in carbonosome organization inside the cell.
RESULTS
Proteins with a predicted function related to PHB in C. crescentus
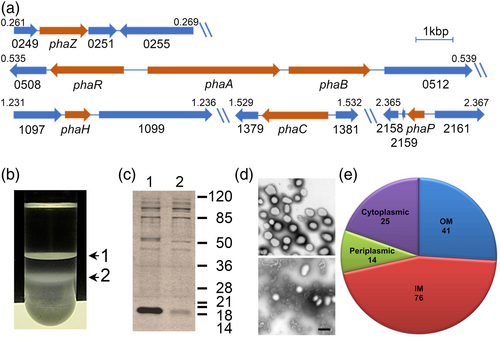
A previous work confirmed the synthase activity of the predicted PhaC protein encoded by CC_1380 in C. crescentus. This study also showed that C. crescentus accumulates PHB (Qi & Rehm, 2001). As previously noted for C. crescentus and other α-proteobacteria (Rehm, 2003), the phaC gene is not in the same genome position as other PHB-related genes (Figure 1a). A bioinformatic analysis of the C. crescentus genome showed the presence of a putative phasin (PhaP, CC_2160) and a depolymerase (PhaZ, CC_0250) encoding genes in independent operons located in different positions of the genome. In a different genome region, the genes coding for the PhaA and PhaB enzymes (CC_0510 and CC_0511 respectively) are located next to a transcriptional regulator predicted to code for PhaR (CC_0509). A previously published list of transcriptional start sites (McGrath et al., 2007) indicates the presence of a transcriptional start site upstream of phaA, suggesting that phaA and phaB are transcribed from the same promoter. The gene downstream of phaB (CC_0512) consists of an uncharacterized conserved domain that is only present in bacteria closely related to C. crescentus, CC_0512 is followed by a putative transposable element. An examination of the genomic synteny (Oberto, 2013) of CC_0512 revealed that it is frequently downstream of phaB, suggesting a possible relation with PHB metabolism. However, this gene is also frequently together with genes involved in different metabolic pathways. Our bioinformatic search did not reveal the presence of any additional phasins or alternative PHB synthases.
Proteomic analysis of isolated native carbonosomes
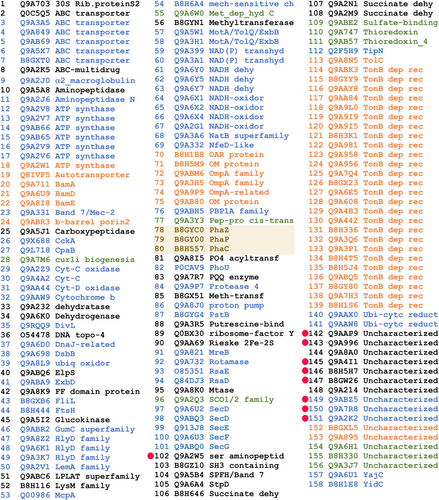
To identify other proteins that could be associated with the C. crescentus PHB granules, we decided to isolate native carbonosomes and to identify the proteins present by proteomic analysis. Staining C. crescentus cells with Nile Red from a culture grown to an OD660 of 0.6 in low phosphate conditions (M5GG medium), indicated the presence of several granules per cell. Fractionation in a sucrose gradient of a cell lysate of cells grown in this condition resulted in two bands (Figure 1b) that were independently recovered. The protein composition of these samples was analyzed by Tris-tricine SDS-PAGE (Figure 1c), the more evident difference between these fractions is the clear enrichment in the top fraction of a small protein with an apparent molecular weight similar to that of the predicted phasin PhaP (15 kDa), suggesting that this fraction contains the PHB granules. Another protein enriched in this fraction had a molecular weight similar to that of the predicted depolymerase PhaZ (47 kDa). Electron microscopy images showed that the better-defined top fraction was mainly composed of granules with an average diameter (shortest axis in case of irregular shape) of 267 ± 46 nm (granule size may change due to sample preparation for electron microscopy) and some membrane contamination, while the more diffuse second fraction consisted mainly of membrane debris (Figure 1d, top and bottom respectively). These results together suggest that the top fraction is enriched with PHB granules. The proteins present in the top fraction were identified by two-dimensional liquid chromatography coupled to mass spectrometry (MudPIT), this resulted in 158 possible carbonosome-associated proteins (Figure 2 and Table S1). Among the proteins identified were PhaC and the predicted PhaZ and PhaP proteins (Figure 2, shaded proteins). Carbonosome-associated proteins are generally predicted as soluble or in some cases have a hydrophobic region that can be predicted as a transmembrane helix. To reduce the number of candidate proteins, we predicted the subcellular location of the identified proteins (Figures 1e and 2). Most of the proteins identified were predicted as integral inner membrane proteins, indicating that the strongest contamination of the sample came from this membrane. This analysis allowed us to discard 55 candidates predicted to be periplasmic or outer membrane proteins. Conserved domain analysis of the identified proteins did not reveal any other protein with a function directly related to PHB metabolism (Figure 2).
PhaH is a new type of carbonosome-associated protein
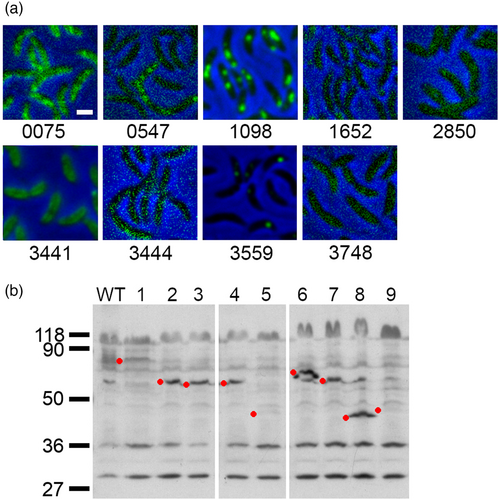
As a first step to identify possible PGAPs, from the remaining list of 103 proteins, we discarded those for which a clear function not related to PHB could be assigned by their conserved domains composition. A putative function could be assigned for the majority of the proteins identified in the proteomic analysis of the purified carbonosomes so we decided to further investigate 9 candidates, 6 predicted cytoplasmic and 3 putative transmembrane proteins (Figure 2, red dots and Table 1). Of these only CC_3441 has a clear enzymatic domain, but was included as a candidate because, similarly to the PhaM and PhaF phasins of C. necator and P. putida (Galan et al., 2011; Pfeiffer et al., 2011), the C-terminal region of CC_3441 is mainly composed of alanine, lysine, and proline residues that probably arrange in a AKP helix. To evaluate if any of the candidate proteins associates with the PHB granules, we examined the localization of fluorescent fusions of these proteins (Figure 3a and Figure S1). For this, C-terminal fusions with the fluorescent YFP protein were generated. All the gene fusions were expressed from their native promoter and as single copy, with the exception of CC_3559 that was expressed as a second copy. Of all the proteins tested, only CC_1098 showed a strong localization in cytoplasmic foci similar to that observed in cells stained with Nile Red to visualize the PHB granules (see Figure 4b WT for an example). Proteins CC_0547 and CC_3559 showed a localization in foci that were not present in all the cells and the fusion of CC_3441, that has a probable AKP helix, showed a possible membrane localization. We tested the localization of all the protein fusions in high phosphate growth conditions but only CC_1098-YFP showed a localization similar to that observed in Nile Red stained cells. In a Western blot against YFP using α-GPF antibodies (Figure 3b), we detected the presence of all the proteins although some of them showed an apparent molecular weight different than expected (see Figure 3 and Figure S2).
| Gene tag | Accession | Description | Localization | MW (kDa) |
|---|---|---|---|---|
| CC_0250 | B8GYC0 | PHA depolymerase, PhaZ | Cyt | 47.3 |
| CC_1380 | B8H557 | PHA synthetase, PhaC | Cyt | 64.9 |
| CC_2160 | B8GY00 | Phasin_2, PhaP | Cyt | 15 |
| CC_0075 | Q9ABZ5 | TPR_hemY_coli superfamily | IM | 54.6 |
| CC_0547 | Q9AAP9 | DUF1353 | Cyt | 18.5 |
| CC_1098 | Q9A996 | HhH_5 superfamily | Cyt | 24 |
| CC_1652 | Q9A7R8 | Conserved protein | IM | 26.5 |
| CC_2850 | Q9A4I1 | Conserved protein | Cyt | 18.8 |
| CC_3441 | Q9A2W5 | Hydrolase-4, AKP helix | Cyt | 44.3 |
| CC_3444 | B8H5H7 | Conserved protein | Cyt | 29.2 |
| CC_3559 | Q9A2K2 | DUF883 | IM | 12 |
| CC_3748 | B8GW26 | DUF3576 | Cyt | 24.7 |
- Note: Candidate PHB granule-associated proteins, the identified domains if present are indicated as well as the subcellular localization and predicted molecular weight. The first three proteins are the PHB-related proteins known or identified by the bioinformatic search.
- Abbreviations: Cyt, cytoplasmic; IM, inner membrane.
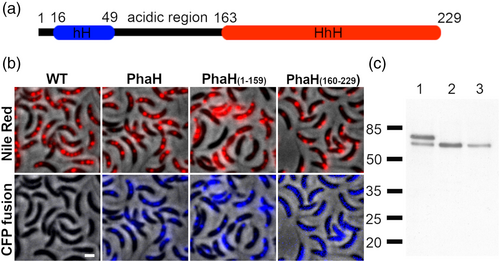
A bioinformatic analysis showed that CC_1098 is a cytoplasmic protein that consists of a C-terminal helix-hairpin-helix domain (HhH) and an N-terminal sequence with no conserved domain (Figure 4a). HhH domains bind DNA in a no-site-specific manner and are mainly present in proteins involved in DNA metabolism (Doherty et al., 1996). This is the first phasin described with this domain and has no other detectable sequence similarity with other phasins. As previously mentioned, phasins PhaM and PhaF have a histone-like domain that binds DNA in the same way. A secondary structure prediction of the N-terminal region of CC_1098 with Ali2D (Gabler et al., 2020) showed that similar to other phasins (Maestro et al., 2013; Maestro & Sanz, 2017; Neumann et al., 2008), it mainly consists of α-helixes. Analysis of the predicted α-helixes with HeliQuest (Gautier et al., 2008) showed the presence of a hydrophobic helix (hH, amino acids 16–49 with hydrophobicity score above 0.5 calculated with a window of 18), that could be involved in PHB binding. A nonstructured region rich in acidic residues connects the hydrophobic helix with the HhH domain. From here on we refer to CC_1098 as PhaH.
To corroborate that PhaH associates with the carbonosomes, we tested if the fluorescence from stained PHB granules colocalizes with that of the fluorescent fusion protein. Since PHB granules stained with Nile Red were fluorescent in the YFP channel, but barely detectable in the CFP channel (Figure 4b, WT panels), we constructed a phaH-CFP gene fusion. As can be observed in Figure 4b, the fluorescence of PhaH-CFP colocalized with the carbonosomes (Pearson's R value of 0.84), supporting that PhaH is a carbonosome-associated protein. To test if the interaction of PhaH with the carbonosome was mediated by its N-terminal hydrophobic domain, we replaced the wild-type phaH allele with fluorescent fusion alleles deleted of the HhH domain (phaHΔHhH-CFP, coding for PhaH1-159-CFP) or of the hH region (phaHΔhH-CFP, coding for PhaH160-229-CFP). While the PhaH1-159-CFP fluorescent fusion showed a localization similar to that of the Nile Red stained granules, PhaH160-229-CFP was mainly dispersed in the cytoplasm although a weak colocalization could be observed in some granules (Pearson's R value of 0.71 and 0.32 respectively). In a Western blot using α-GFP antibody (Figure 4c) we were able to identify a band corresponding to the fluorescent fusion although a minority band of a lower molecular weight was observed for the PhaH-CFP fusion. This result indicates that all the CFP fusions are stable and that the delocalization of PhaH160-229-CFP is not caused by proteolysis of protein fusion. We also observed that the PhaH(160-229)-CFP was in a lower concentration than the other two fusions, since we did not detect any smaller molecular weight bands suggestive of degradation, this could be due to the modified start of the protein resulting from the deletion of the hH coding region. These results indicate that PhaH is a phasin that is recruited to the carbonosome mainly through its hH domain, either by directly binding the polymer or mediating the interaction with another PGAP. The weak localization of PhaH160-229-CFP fusion suggests that this region of PhaH may interact with other PGAPs.
PhaH is necessary for full PHB accumulation and maintenance
To determine the role of PhaH in the carbonosome we obtained a phaH null mutant as well as phaH truncated mutants lacking the regions coding for the hH or the HhH domain. The truncated phaH alleles were expressed from the native promoter and locus. The phaH mutant was complemented by introducing the phaH gene under the control of its native promoter in the chromosomal xylX locus, the same strategy was used to introduce a second copy of phaH in the wild type strain to test the effect of the resulting overexpression of the gene. We then determined the growth characteristics of these strains (maximal optical density, duplication time, and colony forming units) in PHB accumulating growth conditions. These parameters were initially determined in the previously reported M5GG and M6Higg media but after 12 h of growth, the pH of these media started shifting downward and upward respectively. To solve this problem we substituted the buffering agent of M5GG (Tris 20 mM pH 7) with Imidazole 20 mM pH7. This was done after testing different concentrations of Imidazole for pH stability and cell morphology, we refer to this media as M5IGG.
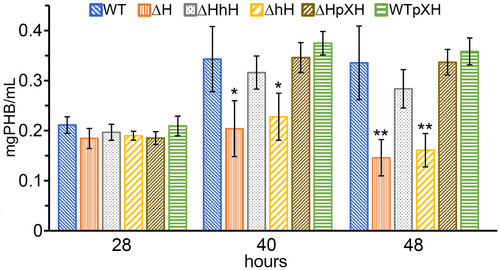
All the strains showed the same duplication time during the exponential growth phase that extended for the first 24 h of incubation (Figure 5a and Figures S3 and S4). After this, the OD660 of all the strains continued to increase at a slower rate and all the strains reached their maximal optical density at around 32 h (Figure 5b, first bar of each strain), only the maximal OD660 of the ΔphaH and the phaHΔhH mutants were significantly different from that of the wild type. While the OD660 of the rest of strains remained stable until the last time point at 48 h, the OD660 of the ΔphaH and the phaHΔhH mutants started to decrease after reaching their maximal OD660 (Figure 5b second bar of each strain). Interestingly the strain that expresses only the N-terminal region of PhaH (ΔHhH) shows a wild-type phenotype, indicating that this region is sufficient to reach and maintain the same OD660 of the wild-type. The phenotype of the phaH mutant was complemented by the expression of phaH from the xylX locus (see ΔphaH/pXH) and the overexpression of PhaH did not have an effect on the maximal optical density (see WT/pXH). It is well known that besides the number of cells, also the accumulation of PHB can modify the optical density of a culture. To determine the cause in the difference in the OD660, we first determined the number of viable cells as the number of colony forming (CFUs) at the early stationary phase (28 h) when all the strains had the same OD660 and at 48 h when the OD660 of the ΔphaH and the phaHΔhH cultures had dropped (Figure 5c). No significant changes in the number of CFUs between early and late stationary phase were observed for any of the strains, indicating that the difference in maximal OD660 is not due to a lower number of cells in the ΔphaH and the phaHΔhH mutants and that the drop in OD660 observed for these two strains during the late stationary phase is not caused by cell death. We then quantified the amount of PHB of cultures in early stationary phase (28 h), after they reached their maximal OD660 (40 h) and in late stationary phase (48 h). All the strains had the same amount of PHB in early stationary phase (Figure 6, 28 h). At 40 h when the strains had reached their maximal OD660, the amount of PHB was significantly lower in the ΔphaH and phaHΔhH mutants than in the rest of the strains and showed a similar amount to that detected at the 28 h time point, while it almost doubled for the rest of the strains. In the late stationary phase, the only strains that had a lower PHB content than in the previous time point were the ΔphaH and the phaHΔhH mutants. In accordance with our CFU results, the protein content was similar for all the strains. As it was the case for the maximal OD660, the ΔphaH mutant was complemented by expression of phaH from the xylX locus and no effect was observed by overexpression of phaH. These results indicate that PhaH is required to achieve wild-type levels of PHB accumulation to maintain the accumulated PHB. The fact that no drop in the OD660 or in the amount of PHB was observed in the phaHΔHhH strain indicates that the PhaH N-terminal hydrophobic helix is sufficient for these activities.

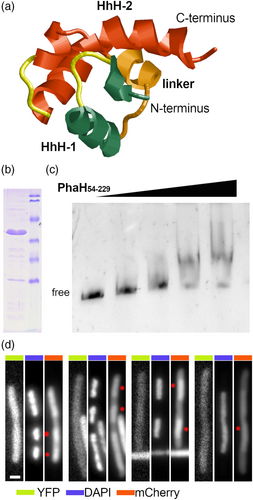
PhaH can bind DNA
Our initial bioinformatic analysis of PhaH revealed only the presence of a single HhH domain, however the majority of the proteins have at least two HhH domains and it has been proposed that two HhH domains (HhH2) is the minimal functional unit (Shao & Grishin, 2000). A closer examination of the primary structure and the alphafold predicted tertiary structure of the C-terminal domain of PhaH showed the presence of an HhH2 domain (Figure 7a), suggesting that this region of PhaH should be able to bind DNA. To test this, the PhaH54-228 protein was expressed as a 6-His fusion and purified by metal affinity chromatography (Figure 6b). Increasing amounts of this protein were incubated with a purified 550bp PCR product containing the sequence of an unrelated gene and run in a native poly-acrylamide gel (Figure 7c). As expected from the structure analysis, 6His-PhaH54-228 was able to bind DNA and retard the migration of the DNA fragment. To further test the ability of the HhH2 domain of PhaH to interact with the DNA, we decided to determine if it was capable of binding to the nucleoid of live cells. For this, we generated a plasmid that expresses in Escherichia coli cells an N-terminal fluorescent fusion of PhaH54-228 with mCherry (mCh-PhaH54-228). To label the cytoplasm of the cells, the same plasmid expresses the fluorescent YFP protein. Cells expressing these two proteins were then filamented with cephalexin and then treated with chloranphenicol, since this antibiotic has been shown to induce the condensation of the nucleoids, creating significant DNA-free regions inside the cell. Cells treated in this way were then stained with DAPI to visualize their nucleoids. In these cells we could observe an accumulation of mCh-PhaH54-228 in the same regions in which the nucleoids were present (Figure 7d, white dots). However the mCh-PhaH54-228 was not only present in the nucleoid regions, indicating an excess of this protein or that the affinity for the nucleoid is not sufficient to maintain all the protein in a bound state. In contrast, the fluorescence of the YFP protein was evenly distributed in the cytoplasm. This result further supports the idea that the HhH2 domain of PhaH is functional, allowing it to interact with the DNA.
The HhH domain of PhaH is required for carbonosome size and organization
Phasins frequently have a role in regulating carbonosome number, size and distribution inside the cell (Maestro & Sanz, 2017; Mezzina & Pettinari, 2016). To investigate the possible role of PhaH on these characteristics, we first stained with Nile Red wild type C. crescentus cells grown to stationary phase (28 h) in M5IGG low phosphate medium (Figure 8a upper panels). These cells showed a uniform distribution of carbonosomes along the cell in what appeared to be a series of granules, frequently giving a beaded string appearance similar to that described for P. putida (Galan et al., 2011). In contrast, the cells carrying the ΔphaH mutant allele showed a less clear definition of the PHB granules, and frequent PHB free spaces (Figure 8a, white dots) that were not caused by cell constriction. The same was observed for the phaHΔhH and phaHΔHhH strains. The PHB free spaces are not due to different amounts of PHB between the strains since at this growth phase all the strains have similar levels of accumulated PHB (Figure 6). Complementation of the mutants restored the wild-type distribution of the granules (Figure 8a, lower panels). Since in the phaHΔhH and phaHΔHhH complemented strains the truncated and full version of the proteins were present, this result shows that the mutant version of the proteins does not interfere with the function of the full protein. To better understand the reason behind these two PHB distribution patterns, we obtained electron microscopy images of cells grown in the same condition and for the same time as those used for the Nile Red staining (Figure 8b), we did not include the phaHΔhH mutant strain since it shows the same phenotype of the ΔphaH strain. Likewise the fluorescent images, the electron micrographs showed that the PHB granules were linearly distributed along the cell in the majority of the WT cells. In contrast, we could frequently observe PHB-free regions and a more frequent clustering of PHB granules of smaller size in the ΔphaH and the phaHΔHhH mutants. The white regions inside the granules are probably caused by an artifact of the staining used and were present in all the strains. To better describe the effect of the absence of the HhH domain of PhaH in the size of the PHB granules, we determined the average granule area and the number of granules per cell length in the wild type and phaHΔHhH strains (Figure 8c). To avoid artifacts, only cells that had been clearly sectioned by the middle longitudinal plane were used in this analysis, this plane can be easily identified in C. crescentus cells due to the polar morphology and the presence of the base of the stalk. This analysis showed that the phaHΔHhH strain had a larger proportion of smaller PHB granules and a wider distribution of granule size than the wild type, making the size distribution between the two strains significantly different (Figure 8c, left panel). The fact that the phaHΔHhH strain has the same amount of PHB as the wild type can likely be explained by the significant increase in the average number of granules per cell length in this strain (Figure 8c, right panel). These results indicate that although the presence of the hydrophobic helix (phaHΔHhH) is sufficient to reach and maintain maximal OD660 and PHB concentration, to maintain the PHB granule size and distribution the HhH region is also required.

Role of PhaH in carbonosome segregation
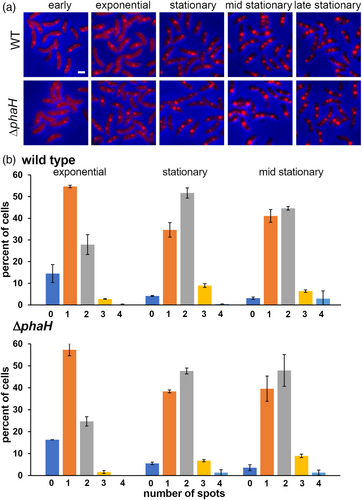
Phasins with a domain that interacts in a non-site specifically with the DNA have been involved in carbonosome segregation between the daughter cells (Galan et al., 2011; Pfeiffer et al., 2011). The HhH domain present in PhaH could interact in the same way with the DNA, suggesting a possible role in this process. Although all the phaH mutant alleles showed a modified carbonosome distribution (Figure 8), no cells without PHB were found, indicating that PhaH is not required for carbonosome segregation during division. To further test this possibility, we compared the PHB granule distribution in wild-type and ΔphaH cells when grown in high phosphate medium. Under this condition the cells synthesize lower amounts of PHB which allows the visualization of single carbonosomes by fluorescent staining. If PhaH was relevant for carbonosome segregation we expected a higher proportion of cells with more and without granules in the ΔphaH mutant than in the wild type. We visualized cells from samples of cultures at different stages of the growth curve to obtain the distribution of foci number per cell when the cells had different number of foci (Figure 9a). Cells from exponential cultures (OD660 0.4–0.75) showed similar distributions although an increase in fluorescent intensity was observed, for this reason we only characterized the distribution of exponential cells (OD660 0.55) to minimize false negatives while still being in the exponential growth phase. Exponential cells have mainly one fluorescent foci, and in both strains, there is a relevant proportion of cells without a detectable focus (Figure 9b, exponential). Cells at the onset of the stationary phase (OD660 1.2) showed an increase in the number of cells with two granules while the percentage of cells without signal decreased (see Figure 9b stationary). In contrast to low phosphate conditions, no change in the granule distribution pattern was observed if the cells were further incubated (24 h total time, Figure 9b, mid stationary). The brightness of the fluorescent foci increased as the cells reached the stationary phase, indicating an increment in the amount of PHB. No significant differences were observed in the carbonosome number distribution between the wild type and ΔphaH mutant strains at any of the time points, supporting the idea that PhaH is not required for carbonosome segregation.
DISCUSSION
In this work, we identified the proteins present in purified carbonosomes obtained from the oligotrophic bacterium C. crescentus. Our results confirmed the presence of the previously described PHB synthase (PhaC), the predicted depolymerase (PhaZ), and phasin (PhaP). Fluorescent fusions of 9 proteins allowed us to identify PhaH as an additional PAGP. PhaH is a new type of phasin characterized by a hydrophobic N-terminal region followed by a helix-hairpin-helix domain. Although proteins with significant sequence similarity can mainly be found in other Caulobacterales, using Delta BLAST, proteins with a similar architecture and size can be found in bacteria belonging to the Sphingomonadales, Hyphomicrobiales, Rhodospirillales, and Rhodobacterales orders of α-proteobacteria (see Table S2 for a list of some examples), it remains to be determined if these proteins have the same function as PhaH. Given that the HhH domain has been reported to non-site-specifically bind DNA (Shao & Grishin, 2000), PhaH is reminiscent of PhaM and PhaF from C. necator and P. putida, respectively. These two phasins have histone-like amino acids signatures that allow them to interact with the DNA (Bresan & Jendrossek, 2017; Galan et al., 2011; Maestro et al., 2013; Pfeiffer et al., 2011) and both are required for normal PHA accumulation, by interacting with transcriptional regulators (Tarazona et al., 2020) or in the case of PhaM by regulating the activity of the synthase and the initiation of granule formation (Bresan & Jendrossek, 2017; Pfeiffer et al., 2011; Pfeiffer & Jendrossek, 2014; Wahl et al., 2012). PhaH does not seem to be required during the initial accumulation of PHB and it only seems to be required when the cell has entered the stationary phase since the absence of PhaH resulted in an early stop of PHB accumulation and in a loss of PHB content in late stationary phase. However PhaH is already present in the exponential growth phase but it only seems to be required in the stationary phase, the mechanism behind this change requires further investigation. Since the mutant expressing the truncated version of PhaH that lacks the HhH domain did not show the PHB accumulation phenotype of the phaH null mutant, it is likely that the N-terminal domain of PhaH besides recruiting the protein to the granule also interacts with other PGAPs. Fluorescent fusions with the two different domains of PhaH showed that, as is the case for PhaF, the interaction of PhaH with the granule depends on the N-terminal region of the protein. Instead of the amphipathic α-helix present in PhaF, the N-terminal region of PhaH consists of a hydrophobic region that is also predicted to form an α-helix.
In P. putida, PhaF phasin is required to distribute the granules along the cell in a “needle” pattern and in its absence the granules tend to cluster, resulting in an uneven distribution of the granules in the cell and between daughter cells after cell division (Galan et al., 2011). In C. necator, PhaM allows the PHB granules to associate with the DNA and it has been proposed that the granules are then segregated with the nucleoid (Bresan & Jendrossek, 2017; Wahl et al., 2012). A segregation mechanism that depends on the segregation of the nucleoid has also been proposed for the polyphosphate granules in C. crescentus (Henry & Crosson, 2013). Although the phaHΔHhH strain did not show a PHB accumulation phenotype, it did have a carbonosome distribution similar to that of the phaH null mutant. Electron microscopy images showed that in the phaH null mutant but more relevantly, in the phaHΔHhH strain, the cells had smaller but more numerous carbonosomes that were frequently cramped together, leaving regions free of granules. The more organized distribution of granules in the wild type could be the result of the larger granule size that combined with the cell width, forces a lineal granule distribution. A different possibility is that the interaction of the HhH domain of PhaH with the DNA allows a better interaction of the granules with the nucleoid allowing the carbonosomes to more evenly distribute inside the cells. The smaller granule size could be the result of a regulatory role of PhaH as it has been shown for PhaF that interacts with PhaR modulating the expression of other PHA-related genes (Tarazona et al., 2020) or as a consequence of the inability of the granules to interact with the nucleoid. The fact that PhaH shares similar functional characteristics with phasins of evolutionary distant bacteria indicates that the interaction of the granules with the nucleoid is a relevant function related to the presence of the carbonosomes. This interaction probably allows a more efficient use of the cytoplasmic space and carbonosome segregation, avoiding problems with the organization of the nucleoid. Comparison of the PHB granules distribution in natural and non-natural producing PHA bacteria also suggests the same idea (Choi et al., 2021). Interestingly PhaH has an HhH domain instead of a histone-like domain like that present in PhaF and PhaM. It is likely that the DNA binding characteristics of these two non-site-specific DNA binding domains are different and that these differences may be tuned for the required properties of these proteins in their respective system.
EXPERIMENTAL PROCEDURES
Bacterial strains and growth conditions
Strains of E. coli were grown in LB medium with the appropriate antibiotic at 37°C (Ausubel, 1987). Plasmids were maintained and purified from E. coli DH5α or TOP10 strains. Strains of C. crescentus were grown at 30°C in PYE rich medium, Tris-based M5GG medium (20 mM Tris-HCl pH 7, NaCl 1 mM, KCl 1 mM, NH4Cl 0.05%, Sodium glutamate 1 mM, MgSO4 0.5 mM, Glucose 0.2%, FeSO4 EDTA chelated 0.01 mM, CaCl2 0.5 mM) supplemented with 0.05 mM Na/K phosphate buffer pH 7 (Domian et al., 1997; Ely, 1991; Jacobs et al., 2001) or in M5IGG, with the same composition as M5IGG but with Imidazole 20 mM pH 7 as buffer instead of Tris-HCl. Antibiotics were used at the following concentrations (μg/mL) for E. coli: gentamycin, 20; kanamycin, 50; spectinomycin, 50; nalidixic acid, 20; chloramphenicol, 30. For C. crescentus, antibiotics were added at the following concentrations (μg/mL) for liquid and solid media, respectively: gentamycin, 2 and 5; kanamycin, 5 and 20; spectinomycin, 15 and 100; chloramphenicol 2 and 5. Strains and plasmids are listed in Table 2, plasmids and primers are listed in Table 3.
| Strains | ||
| CB15N | Synchronizable derivative of CB15 | Evinger and Agabian (1977) |
| SP1273 | CB15N CC0075::p0075YFP-4 | This study |
| SP1274 | CB15N CC0547::p0547YFP-4 | This study |
| SP1275 | CB15N CC1098::p1098YFP-4 | This study |
| SP1276 | CB15N CC1098::p1098CFP-4 | This study |
| SP1278 | CB15N CC1652::p1652YFP-4 | This study |
| SP1279 | CB15N CC2850::p2850YFP-4 | This study |
| SP1281 | CB15N CC3441::p3441YFP-4 | This study |
| SP1282 | CB15N CC3444::p3444YFP-4 | This study |
| SP1284 | CB15N CC3559::p3559YFP-4 | This study |
| SP1285 | CB15N CC3748::p3748YFP-4 | This study |
| SP1287 | CB15N ΔCC1098::ΩSpc | This study |
| SP1289 | CB15N phaH::p1098hHCFP-4 | This study |
| SP1346 | CB15N phaHΔHhH | This study |
| SP1347 | CB15N phaHΔhH | This study |
| SP1385 | CB15N phaHΔhH::p1098CFP-4 | This study |
| TOP10 | E. coli cloning strain | Invitrogen |
| BL21 | E. coli strain for protein expression | Novagen |
| Rosseta DE3 | BL21 derivative for protein expression | Novagen |
| Plasmids | ||
| p0075YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC0075 | This study |
| p0547YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC0547 | This study |
| p1098YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC1098 | This study |
| p1098CFP-4 | pCFPC-4 carrying 3′ terminal fragment of CC1098 | This study |
| p1098hHCFP-4 | pCFPC-4 carrying 5′ terminal fragment of CC1098 | This study |
| p1652YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC1652 | This study |
| p2850YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC2850 | This study |
| p3441YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC3441 | This study |
| p3444YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC3444 | This study |
| p3559YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC3559 | This study |
| p3748YFP-4 | pYFPC-4 carrying 3′ terminal fragment of CC3748 | This study |
| pACYCDuet-1 | Expression vector for two genes | Novagen |
| pCFPC-4 | Used to generate C-terminal fusions with CFP | Thanbichler et al. (2007) |
| pDCHYphaHΔhH/YFP | pACYCDuet-1 carrying mCherry-1098ΔhH and YFP coding fragments | This study |
| pET1098ΔhH | pET28a carrying the 1098ΔhH coding fragment | This study |
| pET28a+ | Protein expression vector | Novagen |
| pNPTΔphaH::Ω | pNPTS138 carrying the ΔCC1098::ΩSpc allele | This study |
| pNPTphaHΔhH | pNPTS138 carrying the CC1098ΔhH allele | This study |
| pNPTphaHΔHhH | pNPTS138 carrying the CC1098ΔHhH allele | This study |
| pNPTS138 | pUC derivative carrying oriT and sacB | M.R.K. Alley |
| pYFPC-4 | Used to generate C-terminal fusions with YFP | Thanbichler et al. (2007) |
| pX1098-5 | Used to introduce a copy of phaH with its promoter | This study |
| pXTCYC-5 | Used to introduce an allele in the xylX locus | Thanbichler et al. (2007) |
| Name | Sequence |
|---|---|
| 1. 0075F1 HindIII | CAAAAGCTTGCCTGATCGAGACCGCCTGGAAGG |
| 2. 0075R1 EcoRI | CAAGAATTCTCTTTAGCGGCGCGCGAGCCGCTC |
| 3. 0547F1 HindIII | CAAAAGCTTGGAGGGGCGGACGTGCGCGATCG |
| 4. 0547R1 EcoRI | CAAGAATTCTCGGGTCGGAAATCCGGGCGCGGCGGAAC |
| 5. 1098Nfus F1 SacI | CAAAGAGCTCGCGAGGCGGTGAACCTGGAAGC |
| 6. 1098Nfus R1 NheI | CAAA GCTAGC GGCTGAGAGCCCCTACCCCTCC |
| 7. 1098F1 HindIII | CAAAAGCTTTCTGTGGCTGATGTTCGGTGG |
| 8. 1098R1 EcoRI | CAAGAATTCCCCTCCGAAGATACGGCGAAG |
| 9. 1098R3 EcoRI | CAAAGAATTCAGAGCCCCTACCCCTCCGAAG |
| 10. 1098R4 SacI | CAAAGAGCTCGGCTGAGAGCCCCTACCCCTCC |
| 11. 1098F3 BamHI | CAAA GGATCC CGCGAGGCGGTGAACCTGGAAG |
| 12. 1098R4 SacI | CAAGGATCCGTTTCGCTTTTCACCTTTCTTCC |
| 13. 1652F1 HindIII | CAAAAGCTTGCAGACGCAAGGGCCGGCCAAGG |
| 14. 1652R1 EcoRI | CAAGAATTCTCTTGGGCGGCGTTCGGGGCGAC |
| 15. 2850F1 HindIII | CAAAAGCTTCGCATCGAGAGCCAGAACACG |
| 16. 2850R1 EcoRI | CAAGAATTCTCAAAATCCTCATCCTGAGCCTG |
| 17. 3441F1 HindIII | CAAAAGCTTTGGACCGCACCACCCCGCTGG |
| 18. 3441R1 EcoRl | CAAGAATTCTCCTTCTTGGCCTTGGGCGAGGATTTGG |
| 19. 3444F1 HindIII | CAAAAGCTTGCCGCCGCTCAGGAACAAACC |
| 20. 3444R1 EcoRI | CAAGAATTCTCGCGGCTGGTCGTGCTGTACAGG |
| 21. 3559F1 HindIII | CAAAAGCTTAGCGCCTTTGGATTCCTCGGTGGTGAAGCC |
| 22. 3559RI EcoRI | CAAGAATTCTCCCGGTTGTGACGGCTGCCGCCGGCGATG |
| 23. 3748F1 HindIII | CAAAAGCTTCGGCGAGACATTGGAGCGAG |
| 24. 3748R1 EcoRI | CAAGAATTCTCGCCCTTGATGTTCGAAAGTCGAAG |
| 25. del1098F1 HindIII | CAAAAGCTTTTCTGGTCTTCGTGTTCGTCGGCTTCGTCG |
| 26. del1098R1 BamHI | CAAGGATCCGAAACCATGGCGCGCTCCTGGCAACC |
| 27. del1098F2 BamHI | CAAGGATCCCTTCGGAGGGGTAGGGGCTCTCAGCCAGC |
| 28. del1098R2 EcoRI | CAAGAATTCTGATCGCCATCGGCAGCACGTTCAGGTCG |
| 29. del1098R3 BamHI | CAAAGGATCCTCATTCCACCACCGCATCCGCGAC |
| 30. del1098F3 BamHI | CAAAGGATCCGCGCGAGGCGGTGAACCTGGAAG |
| 31. mCherry F1 EcoRI | CAAAGAATTCGATGGTGAGCAAGGGCGAGGAGG |
| 32. prom1098 EcoRI | CAAAGAATTCCGCTTGCCCATGCTGCTGATG |
Molecular biology techniques and Western blot
Standard methods were used to purify and analyze chromosomal or plasmid DNA (Ausubel, 1987). Restriction enzymes and T4 DNA ligase were bought from New England Biolabs or Invitrogen. Polymerase chain reactions were done with TaKaRa PrimeSTAR-HS enzyme with high GC buffer. For Western blots, SDS-PAGE resolved proteins were transferred to nitrocellulose membranes that were then incubated with a mouse polyclonal α-RFP antibody raised against 6His tagged mRFP (Ginez et al., 2014) or a commercial polyclonal α-GFP antibody (Clontech). For detection, an alkaline phosphatase-conjugated α-mouse IgG antibody (Sigma) was used together with CDP-Star/Nitro-Block substrate. Samples were collected at OD660 0.6 or 1.2, the cells from 1.5 mL of the culture were harvested and resuspended in 100 μL of lysis buffer containing SDS and β-mercaptoethanol. The samples were then boiled and sonicated, and 10 or 5 μL were loaded depending on the original OD660 of the culture.
Detailed information on plasmid construction and strain selection can be found in supplementary methods in the supplementary information. Briefly, fluorescent protein fusions were obtained by either amplifying with the appropriate primers (Table 3) the 3′ terminal region of the selected gene and the product cloned in the suicide vector pYFPC-4 or pCFPC-4 or by a specific strategy. Suicide plasmid carrying a fluorescent fusion were electroporated into CB15 cells and integration of the plasmid into the gene locus was selected by the appropriate antibiotic resistance. Deletion alleles were obtained by cloning the upstream and downstream regions of the region to be deleted in the pNPTS138 suicide vector, for the SP1287 (ΔphaH::ΩSpc) the ΩSpc cassette was then inserted between the two fragments. The resulting plasmids were then electroporated into CB15N cells and allele replacement was selected by plating on PYE plates containing 3% sucrose and spectinomycin in the case of SP1287. The mutant alleles were confirmed by PCR. To complement the different phaH mutant alleles, plasmid pXphaHprom-5 was constructed by cloning a PCR product containing the phaH coding region and 600bp up-stream of it (primers del1098F1 HindIII and 1098R1 EcoRI) in pXTCYC-5. This plasmid is not replicative in C. crescentus and can integrate in the xylX locus.
Native PHB granule isolation
Isolation of PHB granules was carried by a modification of a previously reported method (Preusting et al., 1993). A 500 mL culture medium was inoculated with an overnight culture grown in M5GG medium to an OD660 0.03 and grown to a final OD660 0.6, the cells were concentrated to a final volume of 20 mL, and mixed with two volumes of sucrose 0.5 M in Tris 10 mM pH8. Lysozyme and EDTA pH8 were added to a final concentration of 170 μg/mL and 4 mM, respectively. After the cells converted to spheroplasts (approximately 5 min), 600 μL of Tris 1 M pH8 was added (10 mM final concentration), and the spheroplasts were lysed by sonication in an ice bath. A discontinuous sucrose gradient (Tris 10 mM, pH8) was prepared (5 layers of 5 mL – 1, 1.3, 1.5, 1.7, and 1.9 M sucrose) and 5 mL of the cell lysate was put on the top. The gradients were centrifuged in a Beckman SW28 rotor at 122,000 g for 15 h. The bands were recovered, washed twice with 30 mL of Tris 10 mM, pH8 and concentrated by centrifugation at 12,000 g in a Beckman JA-20 rotor. The pellets were resuspended in 1 mL of Tris 10 mM, pH8 and stored at −70°C.
Proteomic analysis
The purified native PHB granules were subjected to MudPIT analysis (ITSI Biosciences). Briefly, the sample was precipitated, resuspended, and then reduced and alkylated. 25 μg of protein was precipitated and resuspended in 100 mM triethylammonium bicarbonate buffer and digested with trypsin. For MudPIT, five fractions eluted at 75, 150, 250, 350, and 450 mM of ammonium acetate were collected and individually analyzed by nano-LC/MS/MS. A linear acetonitrile gradient was used in the LC phase to separate the tryptic peptides based on their hydrophobicity prior to MS analysis on a Thermo Scientific LTQ XL mass spectrometer. The total run time was 150 min per sample. For MS, a data-dependent Top 5 method was used where a full MS scan from m/z 350–1700 was followed by MS/MS scans of the five most abundant ions.
Each ion was subjected to CID (Collision Induced Dissociation) for fragmentation and peptide identification. Raw data files from all 5 fractions were searched against the fasta database for C. crescentus from Uniprot using Proteome Discoverer 1.4 (Thermo Scientific) and Sequest HT algorithm. Target Decoy PSM Validator was used for PSM (peptide-spectrum match) validation in the database searches. Trypsin was the selected enzyme allowing for up to two missed cleavages per peptide in protein digestion. High-confidence peptides were used in sorting these search results. Protein filters (minimum number of unique peptide: 1 per protein, only rank 1 peptides, peptides only in top-scored proteins) were also used to refine the search results. A total of 175 proteins were identified using stringent quality parameters, and peptides that were identified with high confidence. Out of these 90 proteins were identified with 2 or more unique peptides.
The 175 identified proteins were manually annotated, domain analysis was carried out using the NCBI CD-Search database (Lu et al., 2020), protein localization was predicted with the SignalP, LipoP, and TMhMM servers (Almagro Armenteros et al., 2019; Juncker et al., 2003).
Microscopy
For fluorescence microscopy, cells were grown and stained (see below), after which a 1.5 μL sample was spotted on a microscope slide covered with a 1.5% agar bed prepared with 10 mM Na/K phosphate buffer pH 7. Epifluorescence images were acquired using a Nikon Eclipse 600 microscope equipped with a Hamamatsu Orca-ER cooled charge-coupled-device (CCD) camera, and an X-Cite 120 as light source or with a Nikon Ti microscope equipped with a SCMOS pco.edge camera, controlled with MicroManager (Edelstein et al., 2014). Fluorescence cubes were acquired from Chroma (EYFP, 49003; ECFP, 49001; mCherry, 49008). Nile Red staining was carried out on concentrated cell samples to an OD660 of 2. A 100 μL sample was incubated with a final concentration of 0.005% of Nile Red for 5 min. Images were processed with ImageJ and quantification was done manually in ImageJ or with MicrobeTracker (Sliusarenko et al., 2011). PHB granule distributions were determined using the spotFinderZ program. Filamented cells were discarded and cells in late divisional cells were split. Granule detection was optimized for each condition. Colocalization was determined using the Coloc2 plugin for ImageJ, and Pearson values without threshold are reported.
Electron microscopy
C. crescentus pellets were processed for standard transmission electron microscopy (Nepomuceno-Mejia et al., 2016). Briefly, samples were fixed in 2.5% glutaraldehyde and 4% paraformaldehyde in PBS for 4 h, postfixed in 1% osmium tetroxide overnight, dehydrated using a series of ascending concentrations of ethanol and embedded in epoxy resin (epon 812, Electron Microscopy Science). Sample integrity was evaluated in semithin sections of about 500 nm stained with toluidine blue and observed with bright-field microscopy (Axiostar, Carl Zeiss). Ultrathin sections (50–60 nm) were mounted on formvar-coated copper grids, contrasted with 4% uranyl acetate and 0.3% lead citrate, examined at 80 kV with a JEM-1010 (JEOL) transmission electron microscope. Images were captured with a CCD camera model Gatan Orius SC600 and a digital micrograph software.
PHB quantification
Quantification of PHB was carried out by the crotonic acid assay (Law & Slepecky, 1961). Briefly, cells from 5 mL of culture were collected in 1.5 mL Eppendorf tubes, washed with 1 mL of MgSO4 10 mM and then digested with 1 mL of 30% sodium hypochlorite at 30°C for 1 h. The samples were then successively washed with 1 mL of deionized water, ethanol and acetone and then air-dried at 50°C. To generate crotonic acid, 0.5 mL of concentrated H2SO4 was added and the samples were incubated at 95°C for 30 min, with occasional mixing. Crotonic acid was quantified by serial dilutions (1:100 to 1:1000) of the samples in 0.045 N H2SO4. The absorbance of these samples was measured at a wavelength of 208 nm in a spectrophotometer and compared to a reference curve of PHB (Karr et al., 1983). PHB concentrations were divided by the amount of protein in the original samples quantified in a Bradford assay (BioRad).
Protein structure analysis
The original PDB file (AF-Q9A996-F1) was downloaded from the AlphaFold database (Varadi et al., 2022) and then manipulated with RasMol.
Protein purification and Electrophoretic Mobility Assay (EMSA)
Plasmid pETphaHΔhH was transformed into Rosseta strain, an exponential (DO600 0.6)100 mL culture in LB media at 30°C was induced with 1 mM IPTG for 3 h. The culture was concentrated and resuspended in 1 mL of 100 mM phosphate buffer pH7 EDTA and lysozyme and sonicated. The lysate was then cleared by centrifugation at 4°C and passed through a 0.5 mL resin bed (cOmplete His-Tag purification resin, Roche), washed with 10 volumes of phosphate buffer and eluted in 4 fraction of 1 mL with 40 mM Imidazole in the same buffer.
For EMSA assays the DNA (550 bp fragment) and protein were incubated for 30 min at 4°C in a final volume of 10 μL in a buffer with final concentration of 10 mM sodium phosphate buffer pH 6, 1 mM MgCl2, 10 μg/mL BSA and 30 mM NaCl. Glycerol was added to the reactions to a final concentration of 10%. The samples were then loaded in a native 5% polyacrylamide gel made with 0.5% TBE and 5% glycerol and run with 0.5% TBE buffer at 1 V/cm2
Nucleoid binding
A 5 mL culture was inoculated with an aliquot of an overnight culture of strain BL21 carrying plasmid pDCHYphaHΔhH/YFP to an OD600 of 0.01 and incubated at 37°C with shaking until an OD600 of 0.1, at this point IPTG and arabinose were added to a final concentration of 1 mM and 0.1%, and incubated for an additional hour. To 1 mL of this culture, cephalexine (3 μg/mL) was added and incubated for 30 min and then chloramphenicol (600 μg/mL) was added. It should be noted that pDCHYphaHΔhH/YFP confers resistance to chloranphenicol, so a higher concentration of this antibiotic was required to induce chromosome condensation. The cells were incubated for 45 min, concentrated to 200 μL and DAPI (0.5 μg/mL) was added. The cells were incubated at room temperature for 15 min and then mounted on 1% agarose pads made with PBS.
AUTHOR CONTRIBUTIONS
Sebastian Poggio: Conceptualization; investigation; funding acquisition; writing – original draft. Ana Laura Salinas: Investigation; methodology. Aurora Osorio: Investigation. Tonatiuh Legorreta-Hissner: Investigation; methodology. Reyna Lara-Martinez: Methodology. Luis Felipe Jimenez-Garcia: Methodology; funding acquisition. Laura Camarena: Conceptualization; funding acquisition.
ACKNOWLEDGEMENTS
Ana Laura Salinas Romero is a doctoral student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and received fellowship 295873 from CONACYT, México. This work was supported by grant UNAM PAPIIT IN218622.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.