Genetic identification of factors for extracellular cellulose accumulation in the thermophilic cyanobacterium Thermosynechococcus vulcanus: proposal of a novel tripartite secretion system
Summary
Cells of the thermophilic cyanobacterium Thermosynechococcus vulcanus strain RKN (NIES-2134) aggregate and produce extracellular cellulose under induced conditions of blue light and low temperature, and both aggregation and cellulose production require the cellulose synthase Tll0007 (XcsA) and photosensory diguanylate cyclases. However, overexpression of both the cellulose synthase and a constitutively active diguanylate cyclase was not sufficient to induce cellulose-mediated cell aggregation under normal growth conditions. Synteny analysis and gene knockout revealed that two putative genes, hlyD-like tlr0903 (xcsB) and endoglucanase-like tlr1902 (xcsC), are linked to tll0007, although they are located apart from tll0007 in the T. vulcanus genome. Gene knockdown revealed that tlr1605 (tolC) was essential for the cellulose-mediated cell aggregation. Low temperature induced marked upregulation of tlr0903, and overexpression of both tlr0903 (but not tlr1902) and diguanylate cyclase resulted in the strong cell aggregation and cellulose accumulation under normal conditions. Based on these and phylogenetic analysis, we propose that the cyanobacterial extracellular cellulose production is due to a novel variant of the bacterial tripartite secretion system.
Graphical Abstract
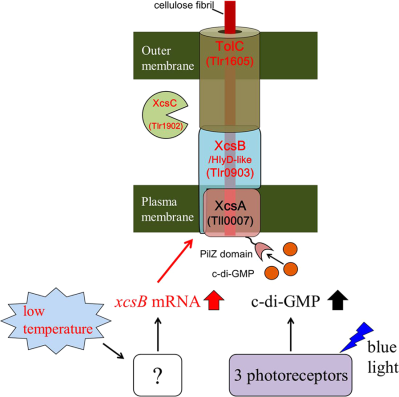
Cell aggregation and cellulose accumulation are induced in the thermophilic cyanobacterium, Thermosynechococcus vulcanus. The discovery of factors (HlyD-like protein XcsB, endoglucanase-like XcsC and TolC) for the cellulose biosynthesis and secretion revealed a novel variant of bacterial tripartite secretion systems and the low temperature induction of xcsB gene.
Introduction
Sessility and motility are two central bacterial lifestyles. The molecular mechanisms underlying these lifestyles have been extensively studied in many pathogenic and heterotrophic bacteria (Mika and Hengge, 2014). The lifestyles can be altered by stress, nutrients (including light for phototrophs), cell density, among other factors. These environmental stimuli (biotic or abiotic signals) are transduced into intracellular signalling that eventually regulates complex cellular functions involved in biofilm formation, pathogenesis, cell division and other processes. (Wolfgang et al., 2003; Lori et al., 2015). A biofilm forms by the deposition of extracellular polymeric substances such as polysaccharides, DNA and proteins around cells (Flemming and Wingender, 2010). In particular, extracellular polysaccharide biosynthesis and regulation have been extensively studied. Examples include cellulose in Acetobacter, Salmonella and other bacteria (Ross et al., 1987; McNamara et al., 2015), alginate in Pseudomonas (Linker and Jones, 1966; Ramsey and Wozniak, 2005) and xanthan in Xanthomonas (Jansson et al., 1975; Becker et al., 1998). Many types of synthase complexes consist of a membrane-spanning glycosyltransferase and several auxiliary subunits that export the nascent sugar chain across the plasma membrane. In general, activation of diguanylate cyclases initiates intracellular second-messenger signalling by cyclic bis-(3′-5′)-diguanylic acid (c-di-GMP) (Römling et al., 2013), and c-di-GMP specifically binds to a protein subunit or a domain of the synthase complex.
Cellulose, the most abundant biopolymer (McNamara et al., 2015), is produced by a wide range of organisms including plants, animals and bacteria that excrete it to the cell wall or extracellular matrix. In pioneering research, Wong et al. (1990) first identified the cellulose synthase four-gene operon, bcsABCD, in Komagataeibacter (Acetobacter) xylinus and indeed c-di-GMP was found to activate the cellulose synthase (Ross et al., 1987). Subsequently, c-di-GMP synthesis and degradation enzymes (Tal et al., 1998), and a c-di-GMP-binding PilZ domain in the catalytic subunit BcsA (Amikam and Galperin, 2006; Morgan et al., 2014) were found. The typical bacterial cellulose synthase complex consists of plasma membrane spanning BcsA and a cocatalytic subunit BcsB and the crystal structure of the BcsA-BcsB complex from Rhodobacter sphaeroides revealed not only the catalytic site that forms a β-1,4-glycosidic linkage on the cytoplasmic side but also that BcsA mediates the translocation of the polysaccharide chain from the catalytic site to the periplasmic space (Morgan et al., 2013; Omadjela et al., 2013). BcsC, an outer membrane porin with periplasmic scaffold motifs, likely forms a pore that is essential for cellulose secretion (McNamara et al., 2015; Nojima et al., 2017). The endoglucanase BcsZ may be required for proper packing of the cellulose fibrils and their overall production (Nakai et al., 2013). These components are conserved in many bacteria, a finding that supports a general structural model for these cellulose synthase complexes (McNamara et al., 2015; Römling and Galperin, 2015).
Cyanobacteria show both sessile and motile lifestyles likely to optimize phototrophic performance. Many cyanobacteria excepting oceanic Prochlorococcus and related Synechococcus harbour numerous c-di-GMP metabolic genes, and c-di-GMP depending extracellular polysaccharide production is observed in Synechocystis sp. PCC 6803 (Agostoni et al., 2016) and Thermosynechococcus (Enomoto et al., 2014). Also, many species in nature accumulate different varieties of extracellular polysaccharides such as those incorporated in biofilms, capsules or slime (De Philippis and Vincenzini, 1998). However, there has been very little characterization of the extracellular polysaccharide-producing enzymes in cyanobacteria except for the cellulose synthase of Thermosynechococcus (Kawano et al., 2011). It is, thus, important to identify the entire set of genes required for the production of each extracellular polysaccharide in the natural environment and for practical applications of phototrophic organisms.
The thermophilic cyanobacterium Thermosynechococcus vulcanus strain RKN (equivalent to NIES-2134) exhibits cellulose-mediated cell aggregation that is induced by light and physiologically low temperature; that is, approximately 30–35°C for aggregation versus approximately 45–57°C for proliferation (Hirano et al., 1997). One of the putative cellulose synthase genes, tll0007, is responsible for cellulose production and cell aggregation in the induced condition (Kawano et al., 2011). Light-induced cell aggregation is mediated by the principal blue-light receptor, SesA, and two accessory photoreceptors, SesB and SesC (Enomoto et al., 2014; Enomoto et al., 2015; Enomoto et al., 2018). These photoreceptors regulate the cellular level of c-di-GMP, and in turn, c-di-GMP activates the cellulose synthase catalytic subunit, Tll0007, via the binding of c-di-GMP to its PilZ domain, leading to accumulation of extracellular cellulose. In contrast with the comprehensive description of the light-induced c-di-GMP signalling in cellulose-assisted cell aggregation, except for the cellulose synthase catalytic gene tll0007, which is homologous to bacterial bcsA, details concerning the cellulose synthase complex and its low-temperature induction remain unclear. Specifically, no apparent homologs for bcsB, bcsC or bcsZ have been found in the Thermosynechococcus genome (Nakamura et al., 2002).
In this study, we surveyed factors involved in production of cellulose and cell aggregation in T. vulcanus. We picked up candidate genes by gene synteny analysis. Their disruption showed us whether they are necessary or not for cellulose-mediated cell aggregation under induced conditions. Overexpression analysis showed us whether they are limiting or not under normal uninduced conditions. The elements we characterized are likely functional homologs of the bacterial cellulose synthase complex. As such, they will undoubtedly expand our understanding of exopolysaccharide production in phototrophic organisms.
Results
Tlr1210 has high diguanylate cyclase activity
To distinguish the cellulose synthase system from its regulation by c-di-GMP, we constructed a strain that constitutively overexpressed diguanylate cyclase. In T. vulcanus, nine genes encode GGDEF domains and thus represent potential diguanylate cyclases (Supporting Information Fig. S1). Of these, we chose tlr1210 that encodes a putative active GGDEF domain but no known sensory or signalling domains (Supporting Information Fig. S2 and Fig. 1A). First, we expressed tlr1210 in E. coli and isolated a protein of 42 kDa, which is consistent with the full-length sequence (Fig. 1B). We confirmed its diguanylate cyclase activity by measuring the side product, pyrophosphate (PPi) (Maeda et al., 2014). The specific activity of Tlr1210 was approximately 0.025 μmol c-di-GMP mg protein−1 min−1 (Fig. 1C and D) or approximately 2.26 μmol PPi μmol protein−1 min−1, which is comparable with that reported for the activated form of SesA (1.99 μmol PPi min−1 μmol protein−1 (Enomoto et al., 2014). Moreover, we did not see any product inhibition for Tlr1210, in contrast with the strong product inhibition for SesA, although many residues critical for catalytic and regulatory activities are conserved (Supporting Information Fig. S2). Then, we disrupted tlr1210 by insertional mutation and confirmed the complete segregation of the mutant chromosome by PCR analysis (Supporting Information Fig. S3). The mutant tlr1210 strain had no apparent phenotype such as cell aggregation when compared with the wild type under normal or cell aggregation-inducing conditions (blue light and 31°C) (Supporting Information Fig. S4). These findings suggested that tlr1210 is cryptic but encodes the active enzyme and validated that tlr1210 overexpression may be used to distinguish the cellulose biosynthetic process from its regulation by c-di-GMP.
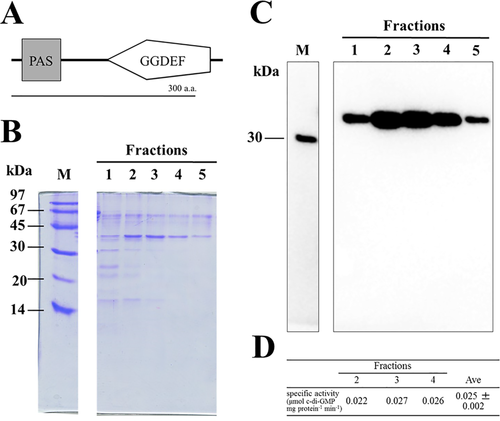
Purification and diguanylate cyclase activity of recombinant Tlr1210.
A. Domain configuration for Tlr1210.
B. SDS-PAGE of the recombinant Tlr1210 prepared by His-tag column chromatography. M: molecular size markers. The fraction numbers indicate the imidazole gradient, from low to high. The molecular mass of Tlr1210 is 42 kDa.
C. Western blotting of the gel shown in panel B to detect His-tag, which confirmed the identity of the 42 kDa protein as Tlr1210.
D. Specific activity of diguanylate cyclase for the indicated chromatography fractions. The error is based on SD.
Effects of overexpression of Tlr1210 and Tll0007
Next, we introduced a construct of tlr1210 with the strong psaA promoter into a neutral region of the genome (Supporting Information Fig. S3). We established the resulting tlr1210 overexpression strain, OX-tlr1210, after complete segregation. In contrast to the wild-type strain, OX-tlr1210 exhibited a stable phenotype of strong cell aggregation at 31°C, which was essentially insensitive to blue-light irradiation (Fig. 2). In the uninduced condition, however, OX-tlr1210 showed only slightly enhanced cell aggregation compared with wild type. These findings suggested that a yet unknown factor(s) is critical for cellulose synthase function under the induced conditions in addition to c-di-GMP. Finally, we introduced the cellulose synthase tll0007 (with a strong promoter) into the OX-tlr1210 strain (Supporting Information Fig. S3) to generate strain OX-tll0007/tlr1210. After complete segregation, OX-tll0007/tlr1210 exhibited lower cell aggregation than did OX-tlr1210.
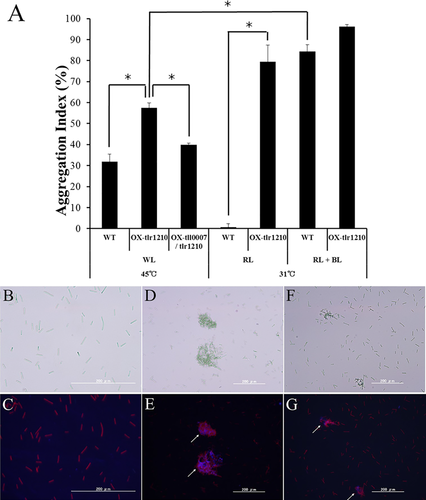
Cell aggregation in the wild type and overexpression strains OX-tlr1210 and OX-tlr1210/tll0007.
A. Cell aggregation indexes of wild type (WT), the tlr1210 overexpression strain OX-tlr1210 and the tll0007/tlr1210 double overexpression strain OX-tll0007/tlr1210. Cells were grown for 48 h under the indicated conditions of temperature and light. WL: white light; RL: red light; BL: blue light. The error bars are based on SE (n ≥ 3). The asterisks represent significant differences based on Welch's t test (p < 0.05).
B–G: fluorescence microscopy of cells grown for 48 h at 45°C with light and stained with calcofluor (white arrows, blue fluorescence). Left: bright-field images; right: fluorescence images. B,C: wild type; D,E: OX-tlr1210; F,G: OX-tlr1210/tll0007. [Color figure can be viewed at wileyonlinelibrary.com]
We also examined the accumulation of extracellular cellulose using fluorescence staining of β-glucan with calcofluor. Under normal growth conditions, the non-aggregated wild-type cells showed very little staining (Fig. 2B and C) and the aggregated cells of OX-tlr1210 showed only weak staining (Fig. 2D and E). Our previous study revealed that the key substance in T. vulcanus cell aggregation is cellulose, which is produced by Tll0007. The extraction method we used therein was complicated and time-consuming (Kawano et al., 2011). In this study, we developed a simpler protocol using ionic liquid extraction of cellulose, which allowed better sample accessibility and more efficient glucose liberation. Indeed, we obtained cellulose yields similar to those generated by our previous laborious method.
When wild-type and OX-tlr1210 cells were grown under normal conditions, OX-tlr1210 cells had a slightly higher cellulose content; however, this amount was significantly less than that generated by the wild-type aggregated cells after induction (31°C with blue light; Fig. 3). The additional overexpression of tll0007 in the OX-tlr1210 mutant did not enhance the cellulose level. These findings suggested that, in addition to cellulose synthase Tll0007 and c-di-GMP, induction generates another unidentified factor(s) that boosts cellulose production.
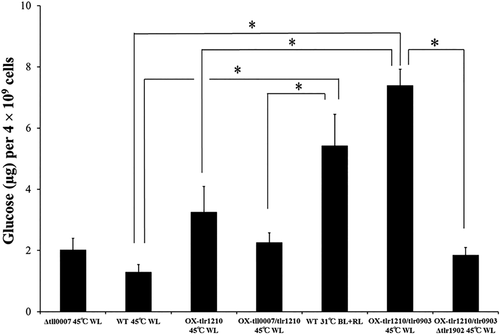
Cellulose content of wild type, deletion strains and overexpression strains with single, double and triple mutations grown for 48 h.
The wild type was grown at 45°C with white light (no induction control) or at 31°C, with both red and blue light (induction control). The deletion and overexpression strains were grown under normal conditions at 45°C with white light. WL: white light; RL: red light; BL: blue light. Error bar = SE, n ≥ 6. The asterisks represent significant differences based on Welch's t test (p < 0.05).
Synteny analysis of tll0007 homologs in cyanobacterial genomes
In general, the bacterial cellulose synthase gene bcsA is accompanied by related bcs genes. To identify tll0007-linked genes, we analysed synteny among cellulose synthase genes in other cyanobacterial genomes, although only certain species possess these genes. In this case, it is important to recognize orthologs and paralogs, because tlr1795 (cellulose synthase-like gene) is a paralog of tll0007 in the T. vulcanus genome (Kawano et al., 2011). Orthologs of both tll0007 and/or tlr1795 were detected for Nostoc punctiforme ATCC 29133 and Synechococcus elongatus PCC 7942 (S. 7942) (see Supporting Information Table S1). Except for tll0007, they are accompanied by an hlyD-like gene in their neighbourhood (Fig. 4A). Cluster analysis (Fig. 4B) revealed that tll0007 and its orthologs (Npun_F6500 and Synpcc7942_1398) are clustered together, as are the hlyD-like genes (Npun_F6499 and Synpcc7942_1399) for these tll0007 orthologs. Conversely, tlr1795 and its orthologs are separately clustered as distinct groups, as are the corresponding hlyD-like genes. This analysis also revealed that an hlyD-like gene tlr0903 is closely linked to tll0007. Moreover, only the tll0007 orthologs, but not those of tlr1795, are accompanied by endoglucanase-like genes (Npun_F6501 and Synpcc7942_1400). Finally, we found that tlr1902 is closely linked to tll0007. A summary of the cluster analysis results is given in Supporting Information Table S1.
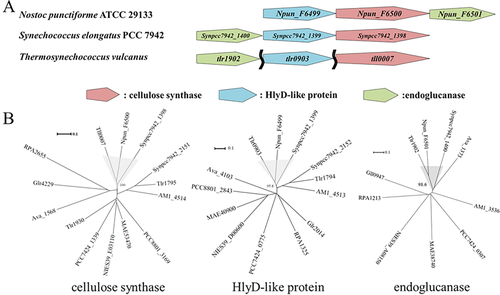
Synteny analysis of tll0007 homologs in cyanobacteria.
A. Gene synteny of tll0007 homologs. The two wavy lines indicate that the three genes are located far apart from each other in the genome.
B. The phylogenic trees for the three homologs in amino acid sequence in panel B based on the neighbour-joining method. The numbers near the junctions (%) represent reliability based on the bootstrap method (n = 1000). Species: ava, Anabaena variabilis ATCC 29413; NIES39, Arthrospira platensis NIES-39; MAE, Microcystis aeruginosa NIES-843; Synpcc7942, Synechococcus elongatus PCC 7942; AM1, Acaryochloris marina MBIC11017; PCC7424, Cyanothece sp. PCC 7424; PCC8801, Cyanothece sp. PCC 8801; Thermosynechococcus vulcanus (orthologs of Tll0007, etc. of T. elongatus BP-1); Npun, Nostoc punctiforme ATCC 29133; Gloeobacter violaceus PCC 7421 (Glr4229, etc.); RPA, Rhodopseudomonas palustris CGA009.
The HlyD domain (Pfam PF16576) is found in HlyD and related proteins called periplasmic adaptors in the tripartite efflux systems of bacteria that include the type I secretion system (Nicaud et al., 1985; Hinchliffe et al., 2013; Kanonenberg et al., 2013). Cellulose fibrils produced by the cellulose synthase on the plasma membrane may be guided through the periplasmic space via an HlyD-like protein in cyanobacteria. Endoglucanase is often an essential factor for cellulose biosynthesis in bacteria and plants, although its precise role is unknown (Nakai et al., 2013). The synteny analysis of the tll0007 orthologs suggested that tlr0903 and tlr1902 may be involved in the biosynthesis and secretion of cellulose that lead to aggregation of T. vulcanus cells.
Physiological roles of tlr0903 and tlr1902
The physiological roles of tlr0903 and tlr1902 were studied by gene inactivation. The deletion mutant genes, Δtlr0903 and Δtlr1902, were segregated from wild-type copies. The cell aggregation phenotypes of the derivative mutant strains were evaluated under the induced conditions of 31°C and blue light (Fig. 5). As expected, the deletion mutations markedly suppressed cell aggregation. These results suggested that tlr0903 and tlr1902 play key roles in cellulose biosynthesis in T. vulcanus.
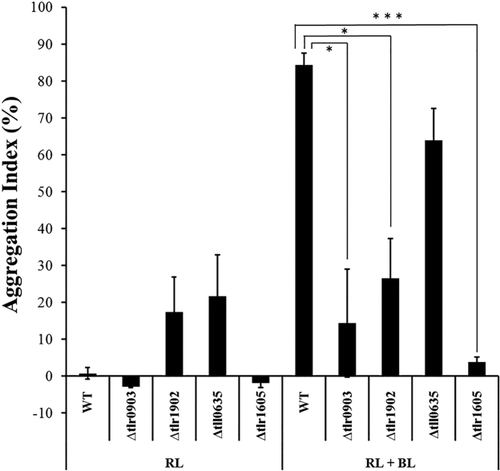
Cell aggregation in wild-type strain and deletion mutant strains (Δtlr0903, Δtlr1902, Δtll0635 and Δtlr1605).
Cells were grown for 48 h at 31°C under red light (uninduced control) or red plus blue light (blue light and low-temperature induction). The error bars represent SE (n ≥ 3). Differences were significant as tested by Welch's t test (*: p < 0.05, ***: p < 0.005).
We then examined the low-temperature induction of these genes by quantitative PCR (qPCR). Total RNA was extracted from T. vulcanus cells at two time points after induction, namely 2 h and 48 h, and transcripts of tll0007, tlr0903 and tlr1902 at 31°C and 45°C were compared (Fig. 6). At both time points, the transcript levels were upregulated at 31°C compared with 45°C. In particular, tlr0903 was markedly upregulated: fivefold at 2 h, and eightfold at 48 h. These results suggested that the transcriptional upregulation of tlr0903 may be another critical event in the induction of cell aggregation and cellulose accumulation in T. vulcanus.
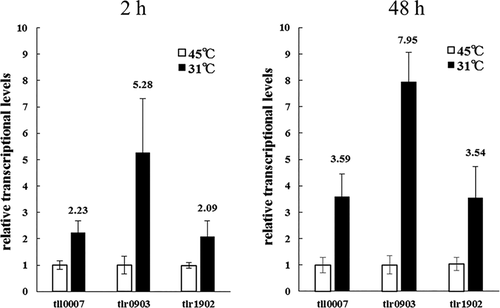
Real-time qPCR assay of tll0007, tlr0903 and tlr1902.
Low temperature induction was shown as relative transcript levels (31°C vs. 45°C). RNA was prepared from wild-type cells grown at given time points (2 h and 48 h). The error bars represent SE (three biological replicates).
Analysis of tlr0903 and tlr1902 overexpression
We generated various overexpression strains for tlr0903 and tlr1902, the hlyD-like and endoglucanase-like genes respectively. The double overexpression strain, OX-tlr1210/tlr0903, exhibited strong cell aggregation under normal conditions with an aggregation index of 93.3 ± 3.1%, which was much higher than that of the parent strain OX-tlr1210 (57.5 ± 4.1%; Fig. 7A and B). This aggregation level was greater than that of the wild type under induced conditions, that is, 31°C and blue light (aggregation index = 84.3 ± 5.7%). Further deletion of cellulose synthase components (OX-tlr1210/tlr0903 Δtll0007, Supporting Information Fig. S9) suppressed this strong aggregation. The calcofluor staining of β-glucan showed that the aggregated cells of OX-tlr1210/tlr0903 were surrounded with bright blue fluorescence, whereas the non-aggregated cells of the wild type and the additional deletion mutant (OX-tlr1210/tlr0903 Δtll0007) were hardly fluorescent except for a weak red fluorescence that emanated from photosynthetic pigments (Fig. 7C). Treatment with cellulase efficiently dispersed the aggregated cells of OX-tlr1210/tlr0903 (Supporting Information Fig. S7). After extraction with an ionic liquid, we determined that the amount of accumulated cellulose in the aggregated cells of OX-tlr1210/tlr0903 was 7.40 ± 0.52 μg for 4 × 109 cells, which was significantly greater than that determined for the single OX-tlr1210 strain under normal growth conditions of white light at 45°C (Fig. 3). Specifically, the accumulation of cellulose in OX-tlr1210/tlr0903 without induction was nearly equal to, or slightly greater than, that of wild-type cells after induction. Similarly, a single overexpression of tlr0903 (OX-tlr0903) showed stronger cell aggregation than the wild type under blue light at 45°C (Supporting Information Fig. S8). Taking these results and the gene expression analysis into consideration, we concluded that enhanced expression of tlr0903 is a critical event in low temperature-induced cell aggregation. Conversely, OX-tlr0903 showed decreased cell aggregation even compared with that of wild type under white light at 45°C (Fig. 7B). This finding suggested that appropriate balance among the factors might be important for partial cell aggregation.
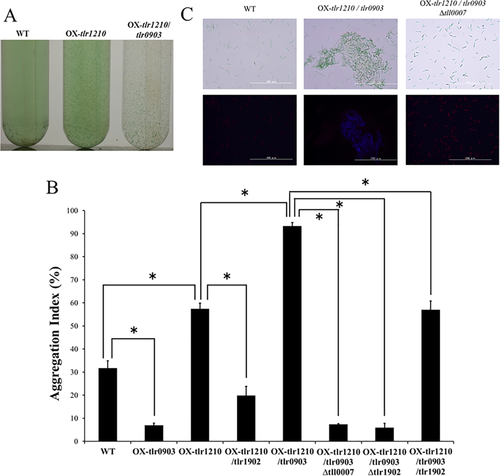
Cellulose mediated cell aggregation of overexpression strains of tlr0903 (hlyD-like) and tlr1902 (endoglucanase) under noninduced condition.
Cells were grown for 48 h at 45°C under white light.
A. Images of cell aggregation observed in test tubes. Wild-type cells showed low aggregated phenotype (left), the overexpression strain of tlr1210 (OX-tlr1210) showed partially aggregated phenotype (centre) and double overexpression strain OX-tlr1210/tlr0903 showed highly aggregated phenotype (right).
B. Aggregation indexes for the overexpression strains (OX-tlr0903) on the background of wild type, OX-tlr1210, and OX-tlr1210/OX-tlr1902 (n ≥ 3, error bar = SE). Some overexpression strains were combined with Δtll0007 or Δtlr1902 strains. Significant differences were tested with Welch's t test (*p < 0.05).
C. Fluorescence microscopy of cellulose stained with calcofluor. Bright-field images (upper) and fluorescence images (lower).
We also introduced an overexpression construct of tlr1902, which encodes an endoglucanase, in wild type and overexpression strains of tlr1210 and/or tlr0903 to potentially deliver different expression levels of the cellulose synthase system (OX-tlr1902, OX-tlr1210/tlr1902 and OX-tlr1210/tlr0903/tlr1902, respectively). In any case, the cell aggregation or cellulose accumulation was not enhanced, but even suppressed compared with their parent strains (Fig. 7). Conversely, deletion of tlr1902 in the OX-tlr1210/tlr0903 background (OX-tlr1210/tlr0903 Δtlr1902) caused almost no cell aggregation (Fig. 7), a phenotype similar to that exhibited by the single Δtlr1902 mutant after induction (compare to Fig. 5). The cellulose accumulation was also very low in the OX-tlr1210/tlr0903 Δtlr1902 strain (Fig. 3). These results suggested that tlr1902 is indeed one of the key factors for cellulose biosynthesis but that the notion ‘the more, the better’ does not apply to tlr1902. We conclude that the appropriate balance of both the levels and activities of enzymatic components is needed for cellulose biosynthesis.
TolC is coupled with HlyD-like protein
Periplasmic HlyD-like protein is typically linked with an outer membrane efflux protein, TolC, in Gram-negative bacteria (Hinchliffe et al., 2013). A TolC trimer forms a β-barrel pore in the outer membrane and an α-helical domain inward, which is associated with periplasmic adaptor proteins consisting of HlyD domain. There are two tolC homologs (tlr1605 and tll0635) in the Thermosynechococcus genome. Gene inactivation revealed that tlr1605 was only partially inactivated but the cellulose-mediated cell aggregation was totally lost, whereas tll0635 was dispensable for survival and the cell aggregation (Fig. 5 and Supporting Information Fig. S10). This finding supported the idea that TolC family protein Tlr1605 may directly interact with HlyD-like protein Tlr0903 to enable secretion of the nascent cellulose chain across the outer membrane.
Discussion
The results presented herein and in our previous reports (Kawano et al., 2011; Enomoto et al., 2015) strongly support the idea that Tll0007 (putative cellulose synthase), Tlr0903 (HlyD-like protein), Tlr1902 (putative endoglucanase) and Tlr1605 (TolC) are responsible for the biosynthesis and accumulation of the extracellular cellulose that is essential for cell aggregation. We propose their designations in T. vulcanus as extracellular cellulose synthase A (XcsA)/Tll0007, XcsB/Tlr0903 and XcsC/Tlr1902 respectively. We also propose a structural model for the machinery likely consisting of these proteins according to their sequence features (Fig. 8). We performed homology modeling of XcsB/Tlr0903 and TolC/Tlr1605 at SWISS-MODEL (Biasini et al., 2014). XcsB/Tlr0903 (Supporting Information Fig. S11) was successfully modeled with a template EmrA, which serve as a periplasmic adaptor for EmrB of ‘major facilitator superfamily’ (MFS) in a tripartite drug transporter in E. coli and other Gram-negative bacteria (Song et al., 2015). The EmrAB-TolC exports several hydrophobic drugs. The crystal structure of EmrA of Aquifex aeolicus is similar to many other periplasmic adaptors except that EmrA lacks a membrane proximal domain in the N-terminal region and instead its α-hairpin domain is much longer than the others [(Hinchliffe et al., 2014), see Supporting Information Fig. S11]. A TMHMM search (http://www.cbs.dtu.dk/services/TMHMM/) revealed that XcsB/Tlr0903 and EmrA share an N-terminal transmembrane helix for anchoring in the plasma membrane (XcsB residues ∼28–50). Homology modeling also revealed that a trimer of TolC/Tlr1605 forms 12-stranded β-barrel and α-helical cylinder domain, which are almost identical to the trimer of E. coli TolC (Supporting Information Fig. S11). These results strongly suggest that XcsA/Tll0007 in the plasma membrane is connected to the TolC/Tlr1605 cylinder in the outer membrane via the periplasmic adaptor XcsB/Tlr0903, like the typical tripartite efflux systems such as AcrAB-TolC pump and MacAB-TolC pump (Du et al., 2014; Fitzpatrick et al., 2017). To date, the tripartite efflux systems, which are widely distributed among bacteria, have been categorized into three according to the inner membrane transporters as ABC transporters, MFS and resistance-nodulation-division (RND) (Hinchliffe et al., 2013). Now, the cyanobacterial XcsA system may belong to a novel category of the tripartite secretion systems, although more evidence is needed for further discussion about its tripartite model. Because HlyD-like proteins have been reported in some operons of cellulose synthase and glucan synthase (Römling and Galperin, 2015), the fourth type of the secretion system may not be limited to cyanobacteria. Our tripartite secretion complex model (XcsAB-TolC) contrasts with the established cellulose synthase BcsA-BcsB (Morgan et al., 2013; Krasteva et al., 2017), which is functionally connected to the outer membrane porin BcsC [Supporting Information Fig. S11, (McNamara et al., 2015; Nojima et al., 2017)]. This ‘bacterial’ cellulose synthase system (BcsAB-BcsC) is mostly found in highly diverged proteobacteria (Römling and Galperin, 2015). In this model, BcsC, the outer membrane porin with periplasmic tetratricopeptide repeats (TPRs), is necessary for secretion of the cellulose chain across the outer membrane of Gram-negative bacteria (Wong et al., 1990). Critical roles of the porin and TPRs may also be shared in alginate synthase system in Pseudomonas aeruginosa, and poly β-1,6-N-acetylglucosamine synthase system in E. coli (Whitney et al., 2011).
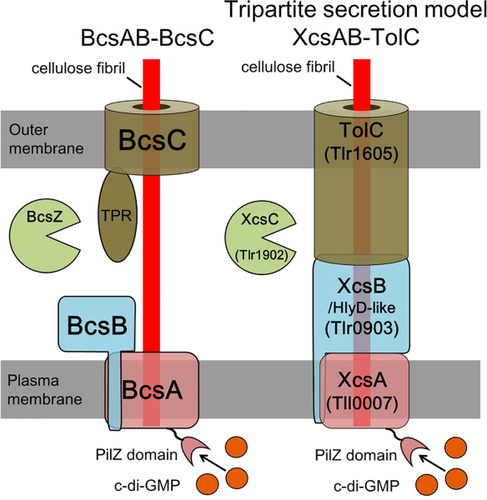
Model for the cyanobacterial extracellular cellulose (Xcs) complex compared with the BcsAB-BcsC model established for some proteobacteria.
Details are described in the text. Model of the bacterial cellulose synthase Bcs complex is redrawn based on Ramsey and Wozniak (2005).
Another significant finding of this work is the remarkable upregulation of the xcsB/tlr0903 transcript at low temperature, although the other two xcs genes were also upregulated, but less so. The importance of the XcsB/Tlr0903 induction at low temperature was supported by the overexpression data: xcsB/tlr0903 in combination with tlr1210 (which encodes the c-di-GMP-producing enzyme, Tlr1210) was sufficient for the accumulation of cellulose and cell aggregation at the normal temperature in white light (Figs 3 and 7).
Dual regulatory model for cell aggregation in T. vulcanus
We propose a dual regulatory model for the low-temperature/blue–green light-induced aggregation of T. vulcanus cells (Fig. 9). The light signal is recognized by three cyanobacteriochrome-type photoreceptors, namely SesA, SesB and SesC, and transduced into c-di-GMP signalling (Enomoto et al., 2015). SesA is the main photoreceptor that recognizes blue light and produces c-di-GMP. SesB is the antagonistic photoreceptor that recognizes teal light and subsequently degrades c-di-GMP. SesC is a unique dual sensor that, upon stimulation with blue or green light, induces diguanylate cyclase or phosphodiesterase activity respectively. Under blue light, the combination of these three photosensory systems elevates the c-di-GMP level, which in turn activates the cellulose synthase XcsA/Tll0007 via its C-terminal PilZ domain. Nevertheless, even if cells are illuminated with blue light, cell aggregation and cellulose accumulation remain low at the normal temperature. A reduction in temperature induces transcriptional upregulation of xcsB/tlr0903 via yet unknown signalling. It is likely that XcsB/Tlr0903 binds to XcsA/Tll0007 to activate its synthase activity. Thus, cellulose production and cell aggregation are induced only when two independent signals are transmitted simultaneously. Species of Thermosynechococcus and the related Synechococcus are often found as the most abundant phototrophs in microbial mats in hot springs, where the phototrophic niche is very limited (Ward et al., 1998). We assume that the signals-dependent cellulose-mediated cell aggregation is the principal mechanism for the formation of phototrophic microbial mats. A study as to how these mats are formed and maintained under natural conditions is of particular interest from the viewpoint of the regulation of cellulose biosynthesis.
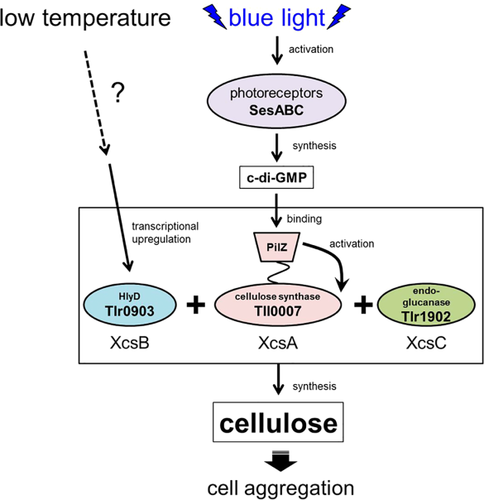
Induction model for extracellular cellulose synthesis and cell aggregation in T. vulcanus. [Color figure can be viewed at wileyonlinelibrary.com]
The other novel key factor is the putative endoglucanase XcsC/Tlr1902, classified as Glucoside Hydrolase family 10 (GH_10) and formerly described as the cellulase family F [Carbohydrate-Active enZYmes Database, CAZy, http://www.cazy.org/(Henrissat, 1991; Lombard et al., 2014)]. It is well known that optimal biosynthesis of cellulose requires cellulase activity (endo-1,4-β-d-glucanase). For example, BcsZ (GH_8, formerly cellulase family D) is needed for cellulose biosynthesis in the typical BcsAB-type cellulose synthase. The GH_9 endoglucanase KORRIGAN plays important roles in the trafficking of the cellulose synthase complex in plants (Lei et al., 2014; Vain et al., 2014). Such endoglucanases may contribute to the reorganization and crystallization of nascent cellulose microfibrils (Nakai et al., 2013). Accordingly, we speculate that the putative GH_10 endoglucanase XcsC/Tlr1902 may play a similar and crucial role in the cellulose synthase XcsA/Tll0007.
Phylogenetic analysis and classification of prokaryotic cellulose synthases
We carried out a phylogenetic analysis of the prokaryotic cellulose synthases and related β-glucan synthases using the plant cellulose synthase CesA as the outgroup (Fig. 10). They were separated into at least four major clusters: cluster 1 corresponds to the ‘bacterial’ cellulose synthase consisting of BcsA-BcsB in many proteobacteria and some cyanobacteria (Römling and Galperin, 2015); cluster 2 includes XcsA/Tll0007 (Kawano et al., 2011) and related proteins, which are mainly found in cyanobacteria; cluster 3 corresponds to curdlan ((1→3)-β-d-glucan) synthase and other β-glucan synthases of several proteobacteria (Stasinopoulos et al., 1999; Perez-Mendoza et al., 2015); and cluster 4 includes the cell wall-type cellulose synthase of certain cyanobacteria (Zhao et al., 2015) and related synthases of certain proteobacteria. The PilZ domain for binding of c-di-GMP is found in clusters 1 and 2 but is absent from clusters 3 and 4. Genes of clusters 2 and 3 are accompanied by an hlyD-like gene. HlyD-like bgsB (for cluster 3) was essential for production of the mixed linkage (1→3)(1→4)-β-d-glucan in Sinorhizobium meliloti (Perez-Mendoza et al., 2015). It may suggest that the mixed linkage glucan is secreted through a TolC pore on the outer membrane. According to the phylogeny, the cell wall-type cellulose synthase (cluster 4) first diverged and then the rest further diverged into clusters 2/3 and cluster 1, depending on the pathway to cross the outer membrane (TolC in clusters 2 and 3 vs. BcsC porin in cluster 1). In other words, the phylogenetic divergence of the cellulose synthases of cluster 1 from cluster 2 may reflect the different structural partners of the synthase on its periplasmic side. We must keep in mind that the linkage specificity of these glucan synthases might independently have changed in evolution as seen in genes of cluster 3. Finally, because most cyanobacteria contain genes of clusters 2 and/or 4, the phylogenetically common cellulose synthase genes may have originated in a subset of cyanobacteria and were subsequently transferred to other bacteria.
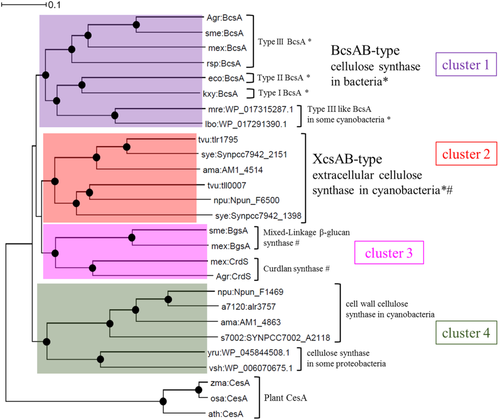
Phylogenic tree for prokaryotic cellulose synthase proteins.
This tree is based on full-length amino acid sequences and was constructed by the neighbour-joining method. The plant cellulose synthase, CesA family, was defined as the outgroup. Black circles: reliability of the junctions based on bootstrap method (>95%); *Proteins with PilZ domain; #protein for which the corresponding gene is linked with hlyD-like gene on the genome; coloured boxes: clusters 1–4. Note that in cluster 3, two BgsA proteins are mixed-linkage (1→3)(1→4)-β-d-glucan synthases. Types I–III of BcsAB-type cellulose synthase were based on the classification of Römling and Galperin (2015). a7120, Anabaena sp. PCC 7120; npu, Nostoc punctiforme ATCC 29133; mre, Mastigocladopsis repens; ama, Acaryochloris marina MBIC11017; lbo, Leptolyngbya boryana; s7002, Synechococcus sp. PCC 7002; tvu, Thermosynechococcus vulcanus; sye, Synechococcus elongatus PCC 7942; sme, Sinorhizobium meliloti; mex, Methylobacterium extorquens; agr, Agrobacterium sp. ATCC 31749; rsp, Rhodobacter sphaeroides; kxy, Komagataeibacter xylinus; eco, Escherichia coli K-12; yru, Yersinia ruckeri; vsh, Vibrio shiloi; ath, Arabidopsis thaliana; zma, Zea mays; osa, Oryza sativa Japonica Group.
Experimental procedures
Plasmid construction
Primers are listed in Supporting Information Table S2. For plasmid construction, see (Maeda et al., 2014; Enomoto et al., 2015). In brief, for overexpression of the diguanylate cyclase Tlr1210 in E. coli: tlr1210 was amplified by PCR from the T. elongatus genomic DNA with primers tlr1210-1F/2R, cloned into an intermediate vector and finally, using NdeI and BamHI, subcloned into pET28a (Novagen, Madison, WI), which encoded N-terminal His-tagged Tlr1210. For overexpression of tlr1210 in T. vulcanus, the tlr1210 fragment was subcloned into a plasmid that harbours a homologous recombination platform near tlr1977 derived from T. vulcanus, a chloramphenicol resistance cassette (CmR) and a strong promoter of photosystem I component psaA (PpsaA) in vector pT7Blue (Novagen). We used the corresponding listed primers and generated pT77CAtlr1210. The neutral site in this platform was an HpaI site located in the intergenic region between tlr1977 and tll1978. For overexpression of tll0007 plus tlr1210, tll0007 from T. vulcanus was combined with a kanamycin-resistance cassette, the strong promoter trc for tll0007 and a ribosome-binding site for tlr1210. This construct replaced the psaA promoter in pT77CAtlr1210, resulting in pT77KTtll0007-tlr1210. Inactivation of tlr1210 was carried out as in Supporting Information Fig. S3. Deletion of tll0007 was as described (Enomoto et al., 2015). The overexpression and deletion of tlr0903 and tlr1902 were carried out as shown in Supporting Information Figs S5 and S6, and Table S1. Briefly, the native promoter regions of tlr0903 or tlr1902 were replaced with the synthetic spectinomycin-resistance cassette (SpR, Supporting Information Figs S5 and S12) and the trc promoter for overexpression and replaced with a cassette that carried a stop codon (TAG) instead of the initiation codon (ATG) of the target gene for inactivation. All plasmid constructs were confirmed by DNA sequencing.
Protein preparation and Western blotting
For expression and purification of His-tagged Tlr1210, we used E. coli strain C41(DE3) (Enomoto et al., 2014; Maeda et al., 2014). The target protein was induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside and shaking at 20°C overnight. After disruption with a French press, the protein was purified by Ni-affinity column chromatography. Proteins subjected to SDS-PAGE were stained with Coomassie Brilliant Blue R-250 or analysed by western blotting using His-probe (Pierce, Rockford, IL).
Measurement of diguanylate cyclase activity
The diguanylate cyclase activity of Tlr1210 was determined at 25°C by measuring the byproduct, pyrophosphate, using a pyrophosphate assay kit (Molecular Probes, Life Technologies, Carlsbad, CA) as described (Maeda et al., 2014). Each reaction contained 20 mM Tris-HCl (pH7.5), 10 mM MgCl2 and 100 μM GTP. Protein concentrations were measured by using the Bradford method (Bradford, 1976) with bovine serum albumin as the standard.
Strains and cultures
The thermophilic cyanobacterium T. vulcanus strain RKN (equivalent to NIES-2134) that shows positive phototaxis was cultured at 45°C (normal temperature) or 31°C (low temperature) in BG11 medium (Stanier et al., 1971) with bubbling air containing 1.0% (v/v) CO2. Cultures were continuously illuminated with white light (white fluorescent lamps, 30 μmol photons m−2 s−1), red light (red LED, λmax 634 nm, 30 μmol photons m−2 s−1) or blue light (blue LED, λmax 448 nm, 5 μmol photons m−2 s−1).
Transformation of T. vulcanus
T. vulcanus cells were transformed with plasmid DNAs (Iwai et al., 2004). Antibiotics used for screening and maintaining mutants were 5 μg ml−1 chloramphenicol, 80 μg ml−1 kanamycin, 5 μg ml−1 streptomycin and 10 μg ml−1 spectinomycin. Complete segregation of the transformed DNA in the multicopy genome was confirmed by PCR using the primers listed in Supporting Information Table S2.
Cell aggregation and cellulose accumulation
The cellulose-dependent cell aggregation of T. vulcanus was evaluated as the aggregation index (Kawano et al., 2011). In short, the index representing the ratio of aggregated cells was obtained by the formula: Aggregation index (%) = (ODtotal – ODNA)/ODtotal × 100, with ODNA (NonAggregated) = cell density (OD730) after spontaneous precipitation of aggregated cells for 30 min and ODtotal = OD730 after complete dispersion of the aggregates by treatment with cellulase (Worthington). The cellulose surrounding the cells was stained with the β-glucan-specific fluorescent dye, calcofluor white (Fluorescent Brightener 28, SIGMA); upon excitation with UV light, fluorescence at 440 nm was detected.
Cellulose quantification
The cellulose quantification was as described (Kawano et al., 2011), except that the extraction protocol was improved by selective solubilization with the ionic liquid, N-ethyl-N'-methyl-imidazolium dimethyl phosphonate (Sigma-Aldrich) (Fukaya et al., 2008). Briefly, cells were harvested by centrifugation, washed with Milli-Q water, desiccated and then extracted with the ionic liquid at 45°C for 1.5 h. The cell debris was removed by centrifugation at 20,000 × g for 1 h, and the polysaccharides in the supernatant fraction were precipitated by addition of ethanol [final 83% (v/v)], collected by centrifugation at 20,000 × g for 15 min, and washed with 70% ethanol. The remaining polysaccharide fraction was digested three times overnight with final 4 U ml−1 glucoamylase at 37°C to remove glycogen and then with cellulase (final concentration, 200 U ml−1) at 37°C for 96 h. The released glucose was quantified enzymatically using a glucose assay kit (Glucose CII-test, Wako).
Sequence alignment and phylogram
Protein sequences were obtained from NCBI (http://www.ncbi.nlm.nih.gov/), and CyanoBase [(Fujisawa et al., 2017); http://genome.microbedb.jp/cyanobase]. The domain architectures were searched using the Simple Modular Architecture Research Tool, SMART (Letunic et al., 2015). The classifications of cellulose synthase and glycoside hydrolases were based on CAZy database (http://www.cazy.org/) (Henrissat, 1991; Lombard et al., 2014). Amino acid sequence similarity was evaluated by NCBI BLAST search. Sequence alignments were performed using ClustalX2 (Larkin et al., 2007), and from these, we constructed phylogenetic trees by the bootstrap neighbour-joining method in ClustalX2.
Real-time qPCR
Cells were harvested by centrifugation at 5,000 × g for 10 min at 4°C. We used the RNeasy Mini kit for bacteria (Qiagen) for cell disruption and RNA extraction and mechanical homogenization with zirconia beads (0.1 mm diameter) in a micro-homogenization system (Micro Smash MS-100, Tomy) at 5,000 r.p.m. for 40 s, five times. For cDNA preparation, RNA was reverse transcribed using random primers (PrimeScript RT reagent kit with gDNA eraser, Takara). Real-time qPCR was performed using THUNDERBIRD SYBR qPCR Mix (Toyobo) and the Thermal Cycler Dice Real Time System II (Takara). The expression level of each gene was normalized to the internal control (rnpB) and induction at 31°C was given as a relative value to that at 45°C. The primers are listed in Supporting Information Table S2.
Acknowledgements
This work was supported by Grant-in-Aid for the CREST program from JST (to M.I.), and Grant-in-Aid for JSPS Fellows 15J07605 (to K.M.). The authors have no conflict of interests to declare.