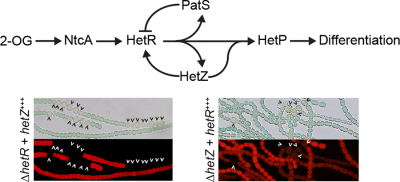
The hetZ gene indirectly regulates heterocyst development at the level of pattern formation in Anabaena sp. strain PCC 7120
Summary
Multicellular development requires the careful orchestration of gene expression to correctly create and position specialized cells. In the filamentous cyanobacterium Anabaena sp. strain PCC 7120, nitrogen-fixing heterocysts are differentiated from vegetative cells in a reproducibly periodic and physiologically relevant pattern. While many genetic factors required for heterocyst development have been identified, the role of HetZ has remained unclear. Here, we present evidence to clarify the requirement of hetZ for heterocyst production and support a model where HetZ functions in the patterning stage of differentiation. We show that a clean, nonpolar deletion of hetZ fails to express the developmental genes hetR, patS, hetP and hetZ correctly and fails to produce heterocysts. Complementation and overexpression of hetZ in a hetP mutant revealed that hetZ was incapable of bypassing hetP, suggesting that it acts upstream of hetP. Complementation and overexpression of hetZ in a hetR mutant, however, demonstrated bypass of hetR, suggesting that it acts downstream of hetR and is capable of bypassing the need for hetR for differentiation irrespective of nitrogen status. Finally, protein–protein interactions were observed between HetZ and HetR, Alr2902 and HetZ itself. Collectively, this work suggests a regulatory role for HetZ in the patterning phase of cellular differentiation in Anabaena.
Graphical Abstract
Anabaena sp. strain PCC 7120 differentiates nitrogen-fixing heterocysts from vegetative cells in a reproducibly periodic and physiologically relevant pattern. HetZ is known to impact heterocyst development. In this study, we clarify the placement of HetZ in the genetic program controlling development and find that it acts downstream of HetR, the master regulator of differentiation and upstream of HetP, a factor involved in the commitment to a differentiated cell fate, at the transition between patterning and commitment.
Introduction
For most multicellular organisms to develop properly, the correct cell types must reproducibly form in the correct locations over time. The required spatiotemporal dynamics of cellular differentiation are largely based upon the de novo formation of biological patterns that dictate the placement of cells capable of differentiation. As originally proposed by Turing (1952), applied by Geirer and Meinhardt (1972) and Meinhardt (2008) and formalized as the activator–inhibitor model of pattern formation, a periodic pattern of cell types can arise from a homogenous field based on lateral inhibition. In this model, an autocatalytic activator is expressed in a source cell and may travel a short distance from the source. This activator is also responsible for the production of its own inhibitor, which travels laterally away from source cells farther than the activator, and functions to stop differentiation in neighboring cells. The interaction of the activator and inhibitor defines the pattern of cells capable of differentiating and has been shown to be a mechanism of de novo pattern formation in many developmental systems (Marcon and Sharpe, 2012; Saga, 2012; Chuong et al., 2013; Robinson and Roeder, 2015). While pattern formation can be studied in complex organisms, it is often more easily accessed in a simple prokaryotic model of multicellular development.
Anabaena sp. strain PCC 7120 (hereafter Anabaena) is a filamentous freshwater cyanobacterium capable of differentiating nitrogen-fixing cells called heterocysts (Wolk, 1996; Kumar et al., 2010; Muro-Pastor and Hess, 2012). When a source of combined nitrogen is available, usually nitrate or ammonia during laboratory growth, filaments are composed entirely of photosynthetic vegetative cells. Upon the removal of combined nitrogen resulting in nitrogen starvation, a periodic pattern of heterocysts is produced. Roughly every 10th cell in the filament will develop into a terminally differentiated, morphologically distinct, nitrogen-fixing heterocyst within about 24 h of nitrogen starvation. Heterocysts shut down photosynthesis, increase respiration and deposit additional extracellular layers to create a microoxic environment for the nitrogenase complex, thus spatially separating oxygen-evolving photosynthesis and oxygen-labile nitrogen reduction. Heterocysts export fixed nitrogen to neighboring vegetative cells in exchange for a source of reductant, due to the mutual dependency of these two cell types in this multicellular context. The production of a single differentiated cell type in a 1-dimensional pattern in a prokaryotic genetic background makes Anabaena a useful model for the study of pattern formation and cellular differentiation.
The process of cellular differentiation in Anabaena can be functionally divided into four phases: the induction of differentiation (0–2 h), formation of a biological pattern (2–9 h), commitment to a differentiated cell fate (9–13 h) and morphogenesis (13–24 h). Differentiation is induced following the removal of combined nitrogen and is perceived by the cell as an increased concentration of 2-oxoglutarate, which initiates the activation of a cascade of regulators that ultimately results in the production of HetR in the patterning phase (Li et al., 2003; Laurent et al., 2005; Zhang et al., 2006). HetR is the master regulator of heterocyst differentiation and is both necessary and sufficient for differentiation in an otherwise wild-type background; deletion of hetR abrogates differentiation and overexpression results in supernumerary heterocysts (Buikema and Haselkorn, 1991, 2001). hetR is an autocatalytic transcriptional regulator that also promotes the expression of its inhibitor, patS, both of which are expressed in a cell-type-specific manner (Black et al., 1993; Yoon and Golden, 2001; Huang et al., 2004). While HetR has not been shown to move away from source cells, PatS moves laterally from its source to cause post-translational HetR degradation (Risser and Callahan, 2009; Corrales-Guerrero et al., 2013; Rivers et al., 2014). The interaction of HetR (activator) and PatS (inhibitor) fulfill the activator–inhibitor model of de novo pattern formation to define the cells along a filament that are capable of differentiation in Anabaena. Patterned cells then irreversibly commit to a differentiated cell fate through an unknown mechanism, the timing and efficacy of which are governed by HetP, its homologs Asl1930, Alr2902 and Alr3234 and their interaction with HetR (Videau et al., 2016). Following commitment, developing heterocysts vastly alter their transcriptome, cease to divide and complete morphogenesis into a mature, functional form. Previous work has indicated a role for the hetZ gene in the process of heterocyst differentiation (Zhang et al., 2007).
The hetZ gene is part of a three-gene cluster (hetZ, patU5 and patU3) involved in heterocyst differentiation (Meeks et al., 2002; Zhang et al., 2007). Several transposon insertions in hetZ have been independently isolated and produce either low percentages of heterocysts or are incapable of differentiation completely (Ning and Xu, 2004; Zhang et al., 2007). Transcriptional fusions of the hetR, patS and hetZ promoters with the green fluorescent protein (gfp) demonstrated that hetZ is required for patterned expression of these genes (Zhang et al., 2007). Assessment of the hetZ promoter region showed that HetR binds directly to an inverted repeat sequence and this sequence is required for hetZ expression (Du et al., 2012, Videau et al., 2014). While these results indicate that hetZ is involved in heterocyst formation, its role in development and placement in the cascade of events leading to differentiation remain unknown. The work described here demonstrates that hetZ is the first gene whose overexpression can fully bypass hetR to produce functional heterocysts. Our results indicate that hetZ is required for the patterning phase of development and works with hetR in a positive feedback loop likely mediated by protein–protein interactions.
Results
The hetZ gene is required for heterocyst differentiation
The hetZ gene (alr0099) in Anabaena encodes a gene product annotated as a hypothetical protein (Kaneko et al., 2001). A previous comparison of hetZ homologs among cyanobacterial genomes suggested that hetZ and a small gene upstream (asr0098) actually encode a single larger protein involved in heterocyst differentiation (Zhang et al., 2007) and this larger hetZ open reading frame was assessed herein (Fig. 1A). A search of cyanobacterial genomes found that HetZ homologs were widely distributed, but few unicellular and no baeocystous genera interrogated harbored homologs (Supporting Information Fig. S1). Alignment of 33 HetZ homologs from various genera showed clustering between those from filamentous, heterocystous and ramified physiologies. Amino acid conservation was fairly high in the middle of the protein while the N- and C-termini tended to have less conservation (Supporting Information Fig. S2). A search for conserved domains did not identify any of significant similarity to those known but did inforce the previous finding that HetZ may be similar to sigma factors (Zhang et al., 2007).
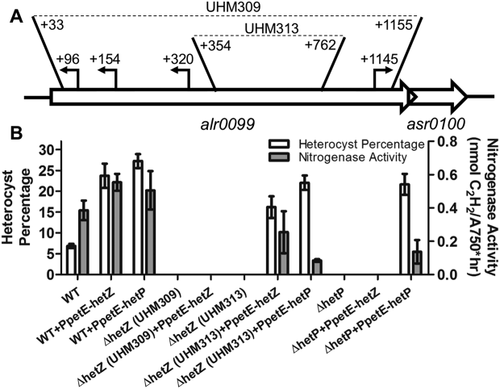
The hetZ gene (alr0099) is required for heterocyst differentiation.
Schematic of the transcriptional start sites (TSS) within hetZ, their proximity to the downstream gene asr0100 (patU5), and the hetZ mutations created in this article (A). TSS are denoted by arrows and the nucleotide positions of the TSS and the boundaries of mutations are presented relative to the + 1 position of the start codon (Anabaena chromosomal nucleotide 103458). Heterocyst percentages (left axis, white bars) and nitrogenase activity (right axis, gray bars), as measured by acetylene reduction assay, for the wild type, ΔhetZ (UHM309), ΔhetZ354–762 (UHM313) or ΔhetP(UHM158) harboring either PpetE-hetZ (pPJAV408) or PpetE-hetP (pSMC224) at 24 h of development (B). All measurements were conducted in triplicate and are presented as the average ± standard deviation.
To date, three studies report on mutations of the hetZ gene that result in varying and pleotropic phenotypes on the timing and efficacy of heterocyst differentiation, which range from slight decreases in total heterocyst percentage produced 48 h after induction to complete abrogation of differentiation (Ning and Xu, 2004; Zhang et al., 2007; Du et al., 2012). These mutations were created either via transposon insertion or homologous recombination to introduce an antibiotic resistance cassette. It is likely that the inconsistent phenotypes reported from ΔhetZ mutants were due to polar effects that altered the expression of the downstream genes, patU5 (asr0100) and patU3 (alr0101), which have also been implicated in heterocyst development (Meeks et al., 2002; Zhang et al., 2007). To more precisely define the role of hetZ in heterocyst differentiation, a clean, in-frame deletion of the coding region was created in which the nucleotides + 33 to + 1155 relative to the published translational start site were deleted (Zhang et al., 2007). This strain (UHM309) produced normal vegetative filaments but was incapable of forming heterocysts after the removal of combined nitrogen (Fig. 1B). Ectopic expression of hetZ from the copper-inducible petE promoter, however, did not complement the deficiency in heterocyst differentiation of UHM309.
To explore this failure to complement UHM309, the hetZ open reading frame was reexamined. A study identifying transcriptional start sites (TSSs) in the Anabaena genome mapped four TSSs within the hetZ coding region (Mitschke et al., 2011): three at the N-terminus that read toward the upstream gene alr0097 and one at the C-terminus that reads downstream toward patU5 (Fig. 1A). Deletion of hetZ nucleotides + 33 to + 1155 in UHM309 removed all of the annotated TSSs and therefore likely created a polar mutation that could not be complemented by the expression of hetZ alone at an ectopic location. A second clean, in-frame mutant was created in which the + 354 to + 762 nucleotides of hetZ were deleted to leave all four TSSs intact (UHM313). As with UHM309, UHM313 displayed normal vegetative filaments and was incapable of developing heterocysts; however, heterocyst differentiation was restored in UHM313 with ectopic expression of PpetE-hetZ (Fig. 1B). The nonpolar nature of the mutation in UHM313 suggests that it represents the hetZ-null phenotype, will be used for the remainder of this study and will be referred to as ΔhetZ354–762. These results support previous studies that describe hetZ as a positive effector of heterocyst differentiation and show its requirement in this process.
hetZ functions before commitment in the process of differentiation
It is possible to determine the relative placement of a factor involved in heterocyst differentiation by defining the point at which the cascade of genetic events governing development fails to progress. Recent work has shown that hetP governs the timing and efficacy of commitment to a differentiated cell fate in Anabaena (Videau et al., 2016). Expression of genes involved in differentiation also displayed wild-type patterning in a ΔhetP mutant (Fig. 2) as previously described (Higa and Callahan, 2010). Despite its role in commitment, a ΔhetP mutant differentiates about 2–3% heterocysts after 48 h of development so an additional positive factor controlling commitment has yet to be elucidated and we hypothesized that hetZ could function in this capacity. To determine the placement of hetZ relative to hetP and commitment in the developmental cascade, PpetE-hetZ and PpetE-hetP were introduced into the wild-type, ΔhetP and ΔhetZ354–762 mutants and complementation was assessed 24 h after the removal of combined nitrogen. Assuming a linear pathway, it would be expected that expression of the later-acting positive effector would induce differentiation in a strain harboring a mutation in an earlier-acting positive effector. Plasmids containing PpetE alone had no effect on differentiation in any of the strains tested (Supporting Information Fig. S3). Introduction of PpetE-hetZ and PpetE-hetP into the wild type resulted in the production of supernumerary heterocysts in nitrogen-replete and -depleted conditions, which indicates that these constructs are functional and both can act as positive effectors of differentiation independent of nitrogen status (Fig. 1B). While PpetE-hetZ rescued the defect in heterocyst development in the ΔhetZ354–762 mutant, no heterocysts were observed in ΔhetP harboring the same construct after 24 h. In contrast, both ΔhetP and ΔhetZ354–762 strains carrying PpetE-hetP produced similar levels of heterocysts. Acetylene reduction assays demonstrated that all heterocysts formed during complementation or bypass in nitrogen-depleted conditions were functional. Because hetZ was unable to bypass the need for hetP, we conclude that hetZ likely functions before hetP in heterocyst differentiation.
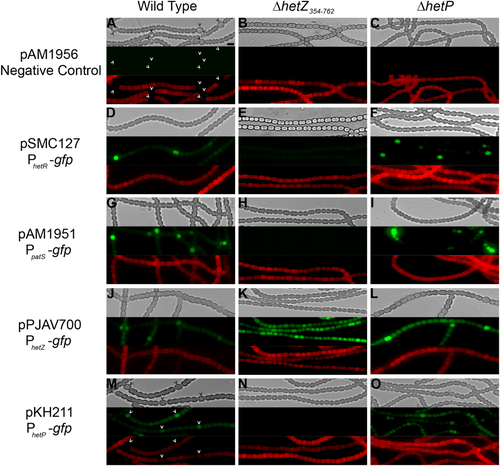
Cell-type specific expression of developmental genes is impaired in a ΔhetZ354–762 mutant (UHM313).
The wild type (A, D, G, J and M), ΔhetZ354–762 (B, E, H, K and N) and ΔhetP (UHM158; C, F, I, L and O) harboring the negative control plasmid pAM1956 with a promoterless gfp (A–C), PhetR-gfp (pSMC127; D–F), PpatS-gfp (pAM1951; G–I), PhetZ-gfp (pPJAV700; J–L) or PhetP-gfp (pKH211; M–O). Strains harboring the negative control plasmid pAM1956 and PhetP-gfp were imaged 24 h after the removal of combined nitrogen while strains harboring the other three constructs were imaged after 12 h of nitrogen starvation. From top to bottom: bright-field, green fluorescence from the promoter regions fused to gfp and red autofluorescence. Carets indicate the location of heterocysts sufficiently developed to be identified. Bar in panel (A), 10 μm.
A functional copy of hetZ is required for proper expression of various developmental genes
Previous work using ΔhetZ mutants harboring different transposon insertions indicated that expression of hetR and patS may be regulated by hetZ, but a consistent phenotype was not presented (Zhang et al., 2007). To assess the role of hetZ in the expression of key developmental genes upstream of commitment and define its position in the stages of differentiation, transcriptional fusions of the patS, hetR, hetZ and hetP promoters to gfp were assessed for cell-type specific expression in wild-type and the ΔhetZ354–762 mutant UHM313. In the wild type, previous work and the results of this study show that expression of patS, hetR and hetZ is patterned in cells that are capable of differentiating by 10–12 h after the removal of combined nitrogen at levels higher than neighboring cells (Fig. 2 and Supporting Information Fig. S4) (Zhang et al., 2007). Patterned hetP expression can be observed about 18–24 h after the induction of differentiation in the wild type (Videau et al., 2016). In the ΔhetZ354–762 mutant, however, none of the introduced transcriptional fusions displayed fluorescence in a periodic pattern. Almost no fluorescence was evident from the patS, hetR and hetP promoter fusions in ΔhetZ354–762 and PhetZ-gfp displayed unpatterned fluorescence in all cells in the same background. Previous studies on patS, hetR and hetZ expression in ΔhetZ insertion mutants have shown that their expression is no longer patterned and that fluorescence from PpatS-gfp decreased while fluorescence from PhetZ-gfp increased in a similar manner to that presented here (Zhang et al., 2007). While previous expression results from PhetR-gfp in ΔhetZ insertion mutants displayed bright but unpatterned fluorescence, fluorescence was greatly reduced from PhetR-gfp in the ΔhetZ354–762 mutant UHM313 used in this study. The difference in hetR expression may be due to differences between the insertional mutants studied previously and the in-frame and nonpolar ΔhetZ354–762 mutant presented here. It has previously been shown that a PhetZ-gfp fusion continues to fluoresce in a ΔhetZ mutant and that HetR is required for proper hetZ expression (Du et al., 2012). Despite the decreased and nonpatterned hetR expression seen in the ΔhetZ354–762 mutant, hetZ expression was observed but was not patterned. It is possible that either the low level of hetR expression observed is sufficient for hetZ expression or that the probable NtcA binding site within the hetZ promoter causes some activation of this gene (located between nucleotides 103158–103389 relative to the + 1 of hetZ at 103458 in the Anabaena genome) (Picossi et al., 2014). Taken together, the defects in expression of genes known to be involved in the patterning phase of differentiation indicate that hetZ is likely required for the creation or maintenance of the biological pattern formed in Anabaena filaments.
Ectopic expression of hetZ bypasses the need for hetR
Based on the requirement of hetZ for patterned hetR and patS expression presented above, it is possible that hetZ functions at or before the level of hetR. If at the same level, then ectopic expression of hetZ could bypass the need for hetR in differentiation. Only one gene, hetP, has been shown to bypass hetR, which resulted in the formation of partially functional heterocysts during ectopic expression in a ΔhetR mutant (Higa and Callahan, 2010). To more precisely determine the stage of differentiation regulated by hetZ, hetZ was ectopically expressed from the petE promoter in a ΔhetR mutant (UHM103) and heterocyst differentiation was assessed. The introduction of a negative control vector carrying PpetE alone failed to produce heterocysts in the ΔhetR mutant regardless of the presence of copper or the nitrogen status of the culture (ammonia, nitrate or diazotrophic; Fig. 3A). In contrast, ectopic expression of hetZ in the presence of copper produced heterocysts in the ΔhetR mutant in the presence or absence of combined nitrogen. Heterocysts were also produced in this strain in the absence of a combined nitrogen source even when the medium was not supplemented with copper, which indicates that only a low level of hetZ expression is necessary to bypass the hetR mutation. The heterocysts that formed lost red autofluorescence, similar to those observed during ectopic expression of hetP in the ΔhetR mutant (Fig. 3D). Unlike the heterocyst-like cells formed during hetP bypass of hetR that only functioned in anaerobic conditions, the heterocysts produced from hetZ overexpression in the ΔhetR mutant fixed nitrogen in aerobic conditions (Fig. 3B). This indicates that the heterocysts produced are functional and that ectopic expression of hetZ can fully bypass the need for hetR in heterocyst differentiation.
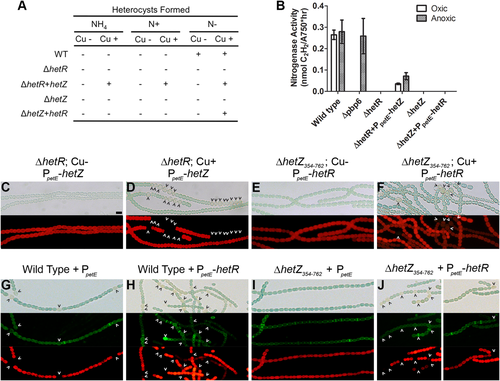
Overexpression of hetZ or hetR in the ΔhetZ354–762 mutant (UHM313) or ΔhetR mutant (UHM103), respectively, results in the production of heterocysts or heterocyst-like cells. The wild type, ΔhetR mutant (UHM103) and ΔhetZ354–762 mutant (UHM313) strains harboring either a negative control PpetE plasmid (pPJAV213), PpetE-hetZ (pPJAV408) or PpetE-hetR (pDR120; A). Cultures were incubated in BG-11 supplemented with ammonia (NH4), nitrate (N+) or no nitrogen source (N–) for 24 h with 2 μM copper (Cu+) or without copper (Cu–). The formation of heterocysts is denoted by a plus and the absence of heterocysts by a minus. Nitrogenase activity of the above strains harboring the specified plasmids, as measured by acetylene reduction assay, was assessed in oxic (white bars) or anoxic (dotted bars) conditions (B). The Δpbp6 mutant is included as a control for nitrogenase activity only in anoxic conditions. All measurements were conducted in triplicate and are presented as the average ± standard deviation. The ΔhetR mutant (C and D) and ΔhetZ354–762 mutant (E and F) harboring either PpetE-hetZ (pPJAV408) or PpetE-hetR (pDR120), respectively, in the absence (C and E) or presence (D and F) of 2 μM copper all in N– conditions. From top to bottom: bright-field and red autofluorescence. The wild type (G and H) and ΔhetZ354–762 mutant (I and J) harboring either a negative control PpetE plasmid (pPJAV213; G and I) or PpetE-hetR (pDR120; H and J) were grown in 2 μM copper in N– conditions. The areas of active peptidoglycan synthesis are highlighted via fluorescently labeled vancomycin (FL-VAN) staining. From top to bottom: bright-field, green fluorescence from the FL-VAN stain and red autofluorescence. Carets indicate heterocysts. Bar in panel (A), 10 μm.
Ectopic expression of hetR partially bypasses the need for hetZ
The ability to functionally bypass a hetR mutation presented above suggests that hetZ functions after hetR in the cascade of events leading to heterocyst differentiation, if a linear pathway of development is assumed. It is also possible that hetZ and hetR may have redundant functions or operate in a positive feedback loop during differentiation. To more precisely define the role of hetZ in differentiation relative to hetR, a construct carrying PpetE-hetR was introduced into the wild-type and the ΔhetZ354–762 mutant to determine whether ectopic hetR expression could complement the ΔhetZ354–762 mutant. Once this construct was introduced, the cultures were maintained on copper-free medium to prevent spurious differentiation. When either nitrate or ammonia was present in the medium, the addition of copper did not induce heterocyst formation (Fig. 3A). Roughly 24 h after the removal of combined nitrogen and the addition of copper, however, cells resembling heterocysts were observed at low levels in the ΔhetZ354–762 mutant harboring PpetE-hetR (0.8 ± 0.4% compared to 20 ± 2.4% in the wild type with the same plasmid; Figs 1B and 3E and F). These cells stained with alcian blue but appeared to have two morphologies; one that was indistinguishable from wild-type heterocysts and a second aberrant morphology that was larger, greener and contained what appeared to be division planes (Fig. 3F and Supporting Information Fig. S6A). These aberrant cells were fairly common and accounted for 29% of heterocysts counted. The properly formed heterocysts lost red autofluorescence, but the aberrant alcian blue-staining heterocyst-like cells maintained low levels of autofluorescence. Nitrogen-starved cultures of ΔhetZ354–762 overexpressing hetR were stained with fluorescently labeled vancomycin (FL-VAN) to determine the areas of active peptidoglycan synthesis and, indirectly, cell division. Previous work with FL-VAN staining of Anabaena has shown that the midcell or poles of vegetative cells stain while only the poles of heterocysts stain (Burnat et al., 2014; Mariscal et al., 2016). Certain cells with heterocyst morphology that had lost autofluorescence displayed bands of FL-VAN staining about the midcell in ΔhetZ354–762 overpressing hetR, which suggests that cell division had or was taking place (Fig. 3G–J). The midcell regions of FL-VAN staining displayed higher fluorescence intensity than nearby membranes or cytoplasmic regions (Supporting Information Fig. S5). In contrast, no heterocyst-like cells were found to stain with FL-VAN at the midcell in nitrogen-starved cultures of ΔhetZ354–762, the wild type and the wild type overexpressing hetR. TLC analysis showed that the heterocyst glycolipids were absent during hetR bypass of hetZ (Supporting Information Fig. S6B), and these cells were incapable of fixing nitrogen aerobically or anaerobically as determined by acetylene reduction assay (Fig. 3B). These results indicate that, though hetR and hetZ likely regulate a similar phase of development, hetZ normally functions in concert with, but slightly later than, hetR. Additionally, we infer that hetZ may have a role in the transition from a dividing to a nondividing cell state.
hetZ engages in protein–protein interactions
The results presented above demonstrate that hetZ is required for hetR expression, heterocysts formed from ectopic expression of hetR in the absence of hetZ are nonfunctional and heterocysts formed from ectopic expression of hetZ in the absence of hetR are functional. To perform its functions, it could be necessary for HetZ to interact with other positive effectors of differentiation. To gain insight into the mechanism used by HetZ to enact differentiation, a network of interaction partners involved in cellular differentiation (HetR, HetZ, HetP, Asl1930, Alr2902 and Alr3234) was assessed using a bacterial two-hybrid assay (BACTH) (Videau et al., 2016). Each protein was translationally fused to a functional domain of adenylate cyclase that, when brought together by protein-protein interaction, result in the production of β-galactosidase (Karimova et al., 1998). Every N- and C-terminal combination of potential interaction partners was assessed for interaction, and β-galactosidase activity was quantified for any pair that resulted in blue colonies during growth on solid media containing X-gal (Table S1). Only three protein combinations resulted in β-galactosidase activity that was significantly higher than the negative control (p < 0.01): HetZ/HetZ, HetZ/HetR and HetZ/Alr2902 (Fig. 4). These results indicate that HetZ is capable of interacting with itself as a dimer or multimer. The interaction of HetZ with HetR and Alr2902, a homolog of HetP and antagonist of HetR involved in defining the timing of commitment, could provide the basis for its function. Previous work has shown that a complex network of protein–protein interactions occurs between HetR, HetP, Asl1930, Alr2902 and Alr3234 and may contribute to the timing and efficacy of heterocyst formation (Videau et al., 2016). We infer that protein–protein interactions may be involved in HetZ function during cellular differentiation in Anabaena.
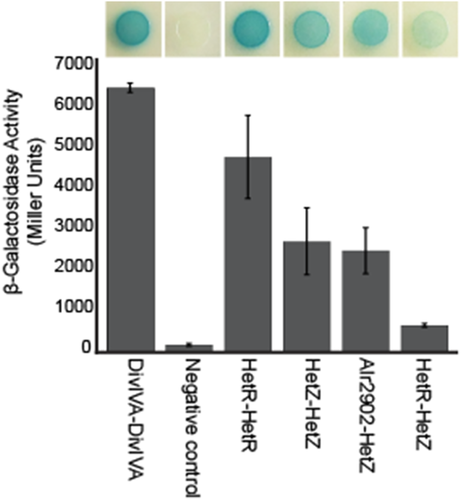
Bacterial two-hybrid assay for protein–protein interactions.
Positively interacting combinations of HetR, HetZ and Alr2902 are represented qualitatively as a blue colony growing on media supplemented with X-gal (top image) and quantitatively as a Miller unit value (bar graph). A negative control of empty vectors and a positive control of DivIVA self-interaction are included for comparison. All measurements were conducted in triplicate and are presented as the average ± standard deviation. Interactions significantly higher than the negative control (t-test; p < 0.01) are shown.
Discussion
For Anabaena to survive nitrogen starvation, heterocysts must be properly spaced along a filament. Without consistent intervals of vegetative cells between heterocysts, the distance needed for fixed nitrogen to reach vegetative cells at the midpoint would be too great, resulting in cell death and a loss of filament integrity. Conversely, heterocysts placed immediately adjacent to one another will fail to receive sufficient reductant from photosynthetic cells of the filament. The optimal placement of heterocysts in Anabaena therefore allows this multicellular cyanobacterium to thrive in nitrogen-limited environmental niches. In this work, we showed that the hetZ gene product is involved in heterocyst differentiation during the patterning phase of development. While previous studies have created ΔhetZ insertion mutants that could be complemented with hetZ on a plasmid, their phenotypes were inconsistent (Ning and Xu, 2004; Zhang et al., 2007). While studying these mutants did provide data to indicate that hetZ is involved in heterocyst differentiation, they pointed to roles for hetZ in either the patterning or commitment phases of development. By creating a clean, nonpolar ΔhetZ mutant (ΔhetZ354–762; UHM313) that can be complemented with hetZ alone, we show that the heterocyst-negative phenotype is likely the true phenotype of Anabaena lacking a functional copy of hetZ (Fig. 1). This result indicates that the published strains harboring insertions in hetZ that retained the capacity to differentiate likely did so by one of the two mechanisms: the creation of a partially functional HetZ protein and by overexpression of the downstream patU5 and patU3 genes, both of which are involved in differentiation, via read-through from elements in the transposons. It is possible that the portion of hetZ remaining in UHM313 after the removal of nucleotides 354–762 could have been expressed and translated. If the undeleted portion of hetZ formed a protein that could contribute to heterocyst differentiation, it is possible that an intermediate phenotype could be seen and some heterocysts produced as has potentially been the case in published ΔhetZ insertion mutants. Because the ΔhetZ354–762 mutant presented here was constructed in a clean and nonpolar manner and no spurious differentiation was observed, we were able to interpret a potential role for hetZ in the cascade of development.
While this article was in review, a related body of work was published that also describes the relationship between hetZ and heterocyst differentiation (Zhang et al., 2018). Our findings corroborate several of their ideas: the phenotype of a hetZ null mutant is Het-, overexpression of hetZ is sufficient to bypass the need for hetR and HetR protein can interact with HetZ protein. Additionally, they show a heterocyst with a division plane resulting from hetR overexpression in a hetZ mutant in Fig. 2. There are, however, several differences between these studies worthy of note. First, we did not observe bypass of a hetP mutant by overexpression of hetZ, while Zhang et al. did. The reason for these opposing results is unknown, but we speculate that differences in how each mutant background was constructed (in-frame versus allelic insertion), as well as the choice of promoter for overexpression (the heterologous PpetE in our work versus the native PhetZ or the HetR-dependent PntcA in theirs), may have contributed. The use of the ntcA promoter makes the results of a developmental pathway difficult to interpret because ntcA is itself in a positive feedback loop with hetR and previous work has demonstrated that PntcA requires hetR for proper expression (Muro-Pastor et al., 2002). As hetR was one of the genetic factors being tested, it is complicated to discern the influence of a gene that contributes to and is dependent on the heterocyst regulatory network being interrogated. To overcome the inherent problems with using a developmentally-regulated promoter to study developmental regulation, we utilized the petE promoter, which is controlled by copper concentration and has never been shown to rely on developmental regulation for expression (Buikema and Haselkorn, 2001). Furthermore, expression was assessed in Zhang et al. by qRT-PCR, a method that produces an mRNA abundance value averaged across a heterogenous population of cells (Zhang et al., 2018). Because differentiation is a cell-type specific process, expression dynamics at the individual cell level, perhaps from differences in promoters used, could have also contributed to differences in phenotypic outcome. Finally, we observed a BACTH interaction between HetZ and itself, while Zhang et al. did not in their Yeast Two-Hybrid assay. The benefit of a bacterial two-hybrid system is that bait and prey interactions can occur in a different cellular location from the activation of reporter gene expression. This allows the detection of interactions that could not physically occur at the DNA (between membrane associated proteins, for example). Yeast Two-Hybrid assays require the bait and prey complex to physically interact with the promoter sequence of the reporter gene. The difference in these two reporter systems could account for the difference in our observations with the BACTH revealing an additional HetZ interaction for this regulatory network.
Based on our results of transcriptional fusions and BACTH analysis, it appears that HetZ interacts with HetR and indirectly influences de novo pattern formation via a positive feedback loop with hetR (Fig. 5). Assessment of each transcriptional fusion of hetR, patS, hetZ and hetP promoters with gfp in the ΔhetZ354–762 mutant showed that cell-type specific gene expression was abrogated (Fig. 2). Based on previous work demonstrating that HetR is required for the expression of patS, hetZ and hetP and binds directly to regions within their promoters, it is reasonable that impaired hetR expression or function would inhibit the expression of genes it regulates (Huang et al., 2004; Higa and Callahan, 2010; Du et al., 2012; Videau et al., 2014). These results implied that HetZ may play a similar role in hetR expression. Overexpression of hetZ in a ΔhetR mutant resulted in the production of functional heterocysts, which indicates that HetZ likely functions after HetR in a linear developmental pathway (Fig. 3 and Supporting Information Fig. S6). Surprisingly, the overexpression of hetR in a ΔhetZ354–762 mutant resulted in the production of heterocyst-like cells that lost autofluorescence, stained with alcian blue and stained with FL-VAN to mark areas of active peptidoglycan synthesis including the midcell but failed to fix nitrogen in aerobic or anaerobic conditions. This result indicated that HetR and HetZ may have some level but not completely overlapping functionality and suggests that these proteins somehow interact with one another in a positive feedback loop of expression. Such a loop could explain the mutual dependence for cell-type specific gene expression as well as the protein–protein interaction observed in the BACTH results. While these results place hetZ within the developmental cascade, they do not entirely point to its function.
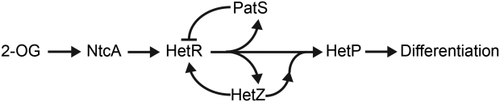
Model of the genetic relationships among factors influencing heterocyst differentiation in Anabaena.
Arrows represent positive interactions, and T bars represent a repressive effect. 2-OG; 2-oxoglutarate.
The BACTH results show that the hetZ gene product is capable of interacting with itself as a dimer or multimer, HetR as discussed earlier, and Alr2902 (Fig. 4). Overexpression of hetR in the clean ΔhetZ354–762 mutant resulted in the formation of enlarged cells that stained with alcian blue, retained some amount of their red autofluorescence from partial degradation of the phycobiliproteins and have a division plane at the midcell (Fig. 3 and Supporting Information Fig. S6). As it seems that hetZ is involved in pattern formation, these results indicate that there is a switch point between patterning and commitment that requires HetZ and/or HetR. Previous work demonstrated that the timing of commitment could be modulated by mutation of hetP and subsets of its three homologs (asl1930, alr2902 and alr3234) and showed protein–protein interactions were common among these gene products (Videau et al., 2016). The interaction of HetZ with Alr2902 further indicates that patterning and commitment are interconnected. It is possible that the transition point where patterned cells commit intersects with the cessation of cell division. The formation of heterocyst-like cells with division planes indicates that the mechanical process of morphogenesis can be separated from the developmental event stopping cell division and that hetR and hetZ are somehow involved directly or indirectly in the cessation of cell division. It is especially interesting that not every cell differentiated in response to hetR overexpression in a ΔhetZ354–762 mutant, which indicates that some other potentially stochastic variable is involved in the progression of the proper developmental program.
During the overexpression of either hetR or hetZ in the ΔhetZ354–762 or ΔhetR mutant, respectively, the influence of nitrogen status in the medium was notable (Fig. 3). Overexpression of hetZ in the ΔhetR mutant resulted in the formation of heterocysts in the presence of either ammonia or nitrate, but the overexpression of hetR in the ΔhetZ354–762 mutant evolved non-functional heterocyst-like cells only in the absence of combined nitrogen. This difference in functionality during varying nitrogen regimes indicates that while HetR is responsive to nitrogen status, HetZ is not. It has been shown that HetR and the nitrogen regulator NtcA function in a positive feedback loop to upregulate their expression during differentiation (Muro-Pastor et al., 2002). It is possible that the interaction of HetR and NtcA, or one of the genes regulated by NtcA, may be required for HetR to advance differentiation in accordance with nitrogen deprivation. Conversely, hetZ overexpression resulted in differentiation in all conditions, irrespective of nitrogen status, implying that HetZ does not require the same conditions, cofactor or binding partner(s) as HetR to function. This may represent a stage of differentiation where the progression of genetic events transitions to proceeding independently of nitrogen status. At such a stage, patterned cells may cease to divide and undertake the processes of morphogenesis. Based on the presence of heterocyst-like cells that have an active division plane at their midcell upon hetR overexpression in the ΔhetZ354–762 mutant, it would appear that the transition from nitrogen-controlled processes to nitrogen-independent differentiation may occur concomitant with the cessation of cell division.
Previous studies in Anabaena have operationally defined commitment to a differentiated cell fate, as the point at which the addition of a fixed nitrogen source to a nitrogen-starved culture fails to halt the process of differentiation. Based on the differing functional responses to nitrogen status observed for HetR and HetZ and the apparent involvement of these regulators in cell division, it is entirely possible that these two gene products aid in the transition from patterning to commitment in the Anabaena developmental system (Fig. 5). In the model presented, there is likely some overlapping functionality between HetR and HetZ, but the roles of each in the process of differentiation are likely not entirely redundant. There are at least two possible ways this could occur: (i) HetZ is a direct transcriptional activator and (ii) HetZ is a positive, non-DNA binding effector of differentiation functioning through protein–protein interactions. It is possible that HetZ is a transcriptional regulator, but, as our and published data indicates, no conserved regulatory domains have been predicted. The observations that HetZ interacts with HetR and a HetP homolog lends some support to the second option in which HetZ is a positive effector functioning through protein-protein interaction, as previously suggested (Videau et al., 2016). The proteins that have been shown to interact point to the possibility that HetZ may interact with low levels of HetP and its homologs in the cell to drive differentiation even in the absence of HetR. Because hetP is dispensable for heterocyst formation in certain genetic backgrounds, we hypothesize that there must be additional, as yet unknown, HetR-dependent positive effectors in addition to the simplified network shown. The functions of these regulators in patterning and commitment are pertinent to the underpinnings of cellular differentiation in this system and are the subject of our continued investigations.
Experimental procedures
Bacterial strains and growth conditions
The growth of Escherichia coli and Anabaena sp. strain PCC 7120 (wild type) and its derivatives, concentrations of antibiotics, the induction of heterocysts in media lacking a source of combined nitrogen, and conditions for photomicroscopy were as previously described (Borthakur et al., 2005; Higa and Callahan, 2010). BG-11 media containing 6 mM ammonia as a nitrogen source was prepared as previously described (Mitschke et al., 2011). Transcription from the copper-inducible petE promoter was induced with the addition of copper to a final concentration of 2 μM (Buikema and Haselkorn, 2001). BG-11 media lacking copper to stop expression from the petE promoter was prepared as previously described (Borthakur et al., 2005). To determine heterocyst percentages, 500 cells were counted and only those that appeared morphologically distinct and stained with alcian blue, a dye that specifically adheres to heterocyst-specific exopolysaccharides (McManus and Mowry, 1960), were recorded as heterocysts (Videau et al., 2015). All results are expressed as the average of three independent replicates. Error bars represent the standard deviation. Plasmids were introduced into Anabaena strains by conjugation from E. coli as previously described (Elhai and Wolk, 1988).
Plasmid construction
The plasmids used in this study are listed in Table 1. The oligonucleotides (herein primers) used in this study are listed in Table 2. The integrity of all PCR-derived constructs was verified by sequencing. The plasmids pST370 and pST442 are suicide vectors based on pRL277 (Black et al., 1993) used to create clean, in-frame deletions of the hetZ coding region from nucleotides + 33 to + 1155 and + 354 to + 762 relative to the published translational start site respectively. Regions up- and downstream of the deleted nucleotides were amplified by PCR from Anabaena chromosomal DNA using the primers hetZ up F, hetZ up R, hetZ down F and hetZ down R and hetZ up F, hetZ + tsp up R, hetZ + tsp II U5U3 down F and hetZ down R, respectively, the fragments were fused by overlap extension PCR (Higuchi et al., 1988), and the products were cloned into the EcoRV site of pBlueScript SK+ (Stratagene). The fused regions were excised as BglII-SacI fragments and cloned into the same sites in pRL277 to create pST370 and pST442.
| Relevant characteristics | Source | |
|---|---|---|
| Strain | ||
| PCC 7120 | Wild type | Pasteur Culture Collection |
| UHM103 | ΔhetR | Borthakur et al. (2005) |
| UHM158 | ΔhetP | Higa and Callahan (2010) |
| UHM309 | ΔhetZ(Δ33–1155) | This study |
| UHM313 | ΔhetZ(Δ354–762) | This study |
| Plasmid | ||
| pBlueScript SK+ | Cloning vector, Apr | Stratagene |
| pAM504 | Shuttle vector for replication in E. coli and Anabaena; Kmr Nmr | Wei et al. (1994) |
| pAM1951 | pAM504 carrying PpatS-gfp | Yoon and Golden (1998) |
| pAM1956 | Shuttle vector pAM504 with promoterless gfp | Yoon and Golden (1998) |
| pDR120 | Shuttle vector carrying PpetE-hetR | Orozco et al. (2006) |
| pRL277 | Suicide vector; Spr Smr | Black et al. (1993) |
| pKH211 | Shuttle vector carrying PhetP-gfp | Higa and Callahan (2010) |
| pSMC127 | Shuttle vector carrying PhetR-gfp | Callahan and Buikema (2001) |
| pSMC224 | Shuttle vector carrying PpetE-hetP | Higa and Callahan (2010) |
| pPJAV153 | pAM504 used for creating copper-inducible C-terminal YFP translational fusions | Rivers et al. (2014) |
| pKT25 | Plasmid carrying the T25 fragment of CyaA for C-terminal protein fusions | Karimova et al. (1998) |
| pKNT25 | Plasmid carrying the T25 fragment of CyaA for N-terminal protein fusions | Karimova et al. (1998) |
| pUT18C | Plasmid carrying the T18 fragment of CyaA for C-terminal protein fusions | Karimova et al. (1998) |
| pUT18 | Plasmid carrying the T18 fragment of CyaA for N-terminal protein fusions | Karimova et al. (1998) |
| pJP41 | pUT18 carrying divIVA from Bacillus subtilis | Patrick and Kearns (2008) |
| pJP42 | pKNT25 carrying divIVA from Bacillus subtilis | Patrick and Kearns (2008) |
| pST558 | pKT25 carrying hetR | Videau et al. (2016) |
| pST565 | pUT18C carrying hetR | Videau et al. (2016) |
| pST572 | pKNT25 carrying hetR | Videau et al. (2016) |
| pST579 | pUT18 carrying hetR | Videau et al. (2016) |
| pRO176 | pT18-C-link carrying C-terminal hetP T18 fusion | Videau et al. (2016) |
| pRO177 | pT18-N-link carrying N-terminal hetP T18 fusion | Videau et al. (2016) |
| pRO178 | pT25-C-link carrying C-terminal hetP T25 fusion | Videau et al. (2016) |
| pRO179 | pT25-N-link carrying N-terminal hetP T25 fusion | Videau et al. (2016) |
| pRO180 | pT18-C-link carrying C-terminal asl1930 T18 fusion | Videau et al. (2016) |
| pRO181 | pT18-N-link carrying N-terminal asl1930 T18 fusion | Videau et al. (2016) |
| pRO182 | pT25-C-link carrying C-terminal asl1930 T25 fusion | Videau et al. (2016) |
| pRO183 | pT25-N-link carrying N-terminal asl1930 T25 fusion | Videau et al. (2016) |
| pRO184 | pT18-C-link carrying C-terminal alr2902 T18 fusion | Videau et al. (2016) |
| pRO185 | pT18-N-link carrying N-terminal alr2902 T18 fusion | Videau et al. (2016) |
| pRO186 | pT25-C-link carrying C-terminal alr2902 T25 fusion | Videau et al. (2016) |
| pRO187 | pT25-N-link carrying N-terminal alr2902 T25 fusion | Videau et al. (2016) |
| pRO188 | pT18-C-link carrying C-terminal alr3234 T18 fusion | Videau et al. (2016) |
| pRO189 | pT18-N-link carrying N-terminal alr3234 T18 fusion | Videau et al. (2016) |
| pRO190 | pT25-C-link carrying C-terminal alr3234 T25 fusion | Videau et al. (2016) |
| pRO191 | pT25-N-link carrying N-terminal alr3234 T25 fusion | Videau et al. (2016) |
| pST370 | pRL277 used to create UHM309 | This study |
| pST442 | pRL277 used to create UHM313 | This study |
| pST560 | pKT25 carrying hetZ | This study |
| pST567 | pUT18C carrying hetZ | This study |
| pST574 | pKNT25 carrying hetZ | This study |
| pST581 | pUT18 carrying hetZ | This study |
| pPJAV408 | pAM504 carrying PpetE-hetZ | This study |
| pPJAV700 | pAM504 carrying PhetZ-gfp | This study |
- Ap, ampicillin; Km, kanamycin; Nm, neomycin; Sp, spectinomycin; Sm, streptomycin.
| Oligonucleotidea | Sequence |
|---|---|
| hetZ-F | TCATGATGTAGCAAATCG |
| hetZ-R | GCGTTTGTTGACAGTGGATG |
| hetZ up F | ATAAGATCTGCACTGATCAAGATAGCTGAATATGC |
| hetZ up R | GAATTACGAACCCGGGGGTTGGAATAGTTGCTGTTG |
| hetZ down F | TATTCCAACCCCCGGGTTCGTAATTCCCTAGTGTCC |
| hetZ down R | ATAGAGCTCCATTACCTTGCAGTGTCAAGATTCC |
| hetZ + tsp up R | GAGTCAAACCCCCGGGGCGCTGAGGGGGATTAATGTA |
| hetZ + tsp II U5U3 down F | TATTCCAACCCCCGGGATGGACTATCTGGAGCAGAAAC |
| hetZ BamHI F A | AGGAGGGATCCGGGTTCCGCTGGCTCCGCTGCTGGTTCTGGCAATTCAGCCGCAACAGCAACTATTC |
| hetZ MunI R A | CTCCTCAATTGCTATTCATGAGTGGATGCACTTG |
| hetZ BamHI F B | AGGAGGGATCCGAATTCAGCCGCAACAGCAAC |
| hetZ MunI R B | CTCCTCAATTGCCGCCAGAACCAGCAGCGGAGCCAGCGGAACCTTCATGAGTGGATGCACTTGATC |
| hetZ2-up-NdeI | AATACATATGAATTCAGCCGCAACAG |
| hetZ-SacI-R | TATATGAGCTCGTTGCAAAGATTCTGAGTCA |
| PhetZ-F | GAGTTTGCCCAAGAAGGAGATTTAGAGCAAGCAC |
| PhetZ-R | CTCAAGCATTGTTGTAGCCGATGCTTGGCTAG |
- a. Oligonucleotides are shown in the 5′–3′ direction.
Plasmid pST560 is a vector based on pKT25 (Karimova et al., 1998) carrying a C-terminal translational fusion of hetZ with the T25 cyaA domain. The coding region of hetZ was amplified by PCR from Anabaena chromosomal DNA with the primers hetZ BamHI F A and hetZ MunI R A. The product was cloned as a BamHI-MunI fragment into the BamHI-EcoRI sites of pKT25 to create pST560.
Plasmid pST567 is a vector based on pUT18C (Karimova et al., 1998) carrying a C-terminal translational fusion of hetZ with the T18 cyaA domain. The coding region of hetZ was amplified by PCR from Anabaena chromosomal DNA with the primers hetZ BamHI F A and hetZ MunI R A. The product was cloned as a BamHI-MunI fragment into the BamHI-EcoRI sites of pUT18C to create pST567.
Plasmid pST574 is a vector based on pKNT25 (Karimova et al., 1998) carrying an N-terminal translational fusion of hetZ with the T25 cyaA domain. The coding region of hetZ was amplified by PCR from Anabaena chromosomal DNA with the primers hetZ BamHI F B and hetZ MunI R B. The product was cloned as a BamHI-MunI fragment into the BamHI-EcoRI sites of pKNT25 to create pST574.
Plasmid pST581 is a vector based on pUT18 (Karimova et al., 1998) carrying an N-terminal translational fusion of hetZ with the T18 cyaA domain. The coding region of hetZ was amplified by PCR from Anabaena chromosomal DNA with the primers hetZ BamHI F B and hetZ MunI R B. The product was cloned as a BamHI-MunI fragment into the BamHI-EcoRI sites of pUT18 to create pST581.
Plasmid pPJAV408 is a mobilizable shuttle vector based on pAM504 (Wei et al., 1994) carrying hetZ transcriptionally fused to the petE promoter. The coding region of hetZ was amplified by PCR from Anabaena chromosomal DNA with the primers hetZ2-up-NdeI and hetZ-SacI-R and the product was cloned into the SmaI site of pBlueScript SK+. The hetZ coding region was excised as an NdeI-SacI fragment and cloned into the same sites of pPJAV153 (Rivers et al., 2014) to create pPJAV408.
Plasmid pPJAV700 is a mobilizable shuttle vector based on pAM504 carrying a transcriptional fusion with the hetZ promoter region to gfp. The hetZ promoter region was amplified by PCR from Anabaena chromosomal DNA with the primers PhetZ-F and PhetZ-R. The product was cloned into the SmaI site of pAM1956 (Yoon and Golden, 1998) and screened for directionality by PCR to create pPJAV700.
Strain construction
The Anabaena strains used in this study are listed in Table 1. Clean, unmarked, in-frame deletions of the hetZ coding region from nucleotides + 33 to + 1155 and + 354 to + 762 relative to the published translational start site were introduced into the wild type using the plasmids pST370 or pST442 respectively (Zhang et al., 2007). Mutant strains were created as previously described (Cai and Wolk, 1990; Callahan and Buikema, 2001; Orozco et al., 2006). To confirm the hetZ mutant constructions, the hetZ-F and hetZ-R primers flanking the mutation and located outside the region of Anabaena DNA used to make the mutations were used to amplify the region of the intended mutation. The resulting sizes of the PCR products, as well as sensitivity to spectinomyin and streptomycin, were used to confirm that the desired deletions had been introduced.
Microscopy and fluorescent vancomycin staining
Anabaena filaments were routinely visualized on a Leica DM3100 brightfield microscope. Fluorescence microscopy was performed using either a Nikon Eclipse Ni epifluorescence microscope with a Nikon DS-Qi2 monochromatic camera and processed with NIS Elements version 4.51.01 imaging software or a Nikon Diaphot 300 as previously described (Borthakur et al., 2005). Images were processed using Adobe Photoshop CS6.
In vivo labeling to show areas displaying active peptidoglycan synthesis was done with fluorescent vancomycin as previously described with slight modifications (FL-VAN; BODIPY FL conjugate; Invitrogen) (Lehner et al., 2013). Anabaena cultures were induced for differentiation in N– BG-11 medium for 24 h and expression from the petE promoter was induced with the addition of copper as described above. Culture aliquots (50 μl) were incubated with FL-VAN at a 1 μg ml−1 final concentration for 1 h in the dark and washed twice with N– BG-11 medium to decrease the fluorescence background. Samples were visualized at the South Dakota State University Functional Genomics Core Facility with an Olympus BX53 microscope equipped with an X-Cite 120LED (Lumen Dynamics) light source using the 40x objective and an Olympus DP73 digital camera. Green fluorescence from FL-VAN was assessed using an Olympus GFP-4050A filter (excitation was 466/40, emission was 525/50). Red autofluorescence fluorescence from vegetative cells or its absence in heterocyst cells was assessed using an Olympus TRITC-B filter (excitation was 543/22, emission was 593/40). Fluorescence exposure times never lasted longer than 1 s to prevent photobleaching and this time was short enough to avoid green autofluorescence. FL-VAN fluorescence was quantified using the ImageJ software.
Thin-layer chromatography
The same cultures that were prepared above for FL-VAN staining were also utilized for heterocyst-specific glycolipids. Two sets of 1 ml culture aliquots were centrifuged at 2000 × g for 3 min, and the medium was decanted. The first aliquot was used to measure the chlorophyll a concentration via methanol extraction (Meeks and Castenholz, 1971). Total glycolipids were extracted from pellets in the second tubes with 2:1 chloroform–methanol. An aliquot containing 0.5 μg of chlorophyll a was spotted about 1 cm above the bottom of a 10 cm tall aluminum-backed silica 60 TLC plate and run with a mobile phase of chloroform, methanol, acetic acid and water in a ratio of 85:15:10:3.7 (Nichols and Wood, 1968). Plates were visualized by charring with 25% sulphuric acid at 125°C for 10 min.
Alcian blue staining, acetylene reduction assays, bacterial two-hybrid assays and beta-galactosidase assays
Heterocyst-specific exopolysaccharide was stained with alcian blue as previously described (McManus and Mowry, 1960; Higa and Callahan, 2010). Aerobic and anaerobic acetylene reduction assays, and the use of the Δpbp6 mutant to control for anaerobic acetylene reduction conditions, were performed as previously described (Ernst et al., 1992; Higa and Callahan, 2010, Videau et al., 2014). Bacterial two-hybrid assays were performed as previously described (Karimova et al., 1998; van den Ent et al., 2006; Videau et al., 2016). Visual assessment of β-galactosidase activity from plate-based assays and quantitative β-galactosidase activity from liquid assays were photodocumented or determined spectrophotometrically as the average of three independent cultures as previously described (Miller, 1972). Error bars represent the standard deviation. Statistics were conducted using the GraphPad Prism software (GraphPad, La Jolla, CA).
Phylogenetic and structural protein analyses
Phylogenetic analysis was performed using the JTT matrix-based model and the Maximum Likelihood method in MEGA7 (Jones et al., 1992; Kumar et al., 2016). The Profile ALIgNEment (PRALINE) multiple sequence alignment application was utilized to align amino acids sequences with the BLOSUM62 exchange weights matrix (Simossis and Heringa, 2005; Simossis et al., 2005). Protein domains were predicted using the ExPASy Prosite and HHPred software using the default settings (Söding et al., 2005; Sigrist et al., 2012).
Acknowledgements
We are grateful to Sean Callahan (University of Hawaii at Manoa) for guidance, resources and feedback; Dan Kearns (Indiana University) for the plasmids pT18-N-link, pT18-C-link, pT25-N-link and pT25-C-link; Dale Droge and Nancy Presuhn (Dakota State University) for thoughtful comments, insight and support and Lizbeth Videau (Duke University) for critical reading of the article. We thank Michael Hildreth and Liping Gu for aid with microscopy and acknowledge use of the SDSU-FGCF supported in part by NSF/EPSCoR Grant No 0091948 and by the State of South Dakota. This work was supported by NSF-PRFB Award 1103610 (to L.M.C.), an Illinois Wesleyan Artistic and Scholarly Development Grant (to L.M.C.), a Dakota State University Arts & Sciences Faculty Research Grant (to P.V.) and a Dakota State University Faculty Research Initiative Grant (to P.V.). The authors declare no conflicts of interest.
Author contributions
P.V., L.M.C., O.S.R. and M.O.G. contributed to the conception or design of the study; all authors were involved in the acquisition, analysis or interpretation of the data; P.V. and L.M.C. wrote the article and all authors edited the article.