The global regulator of pathogenesis PnCon7 positively regulates Tox3 effector gene expression through direct interaction in the wheat pathogen Parastagonospora nodorum
Summary
To investigate effector gene regulation in the wheat pathogenic fungus Parastagonospora nodorum, the promoter and expression of Tox3 was characterised through a series of complementary approaches. Promoter deletion and DNase I footprinting experiments identified a 25 bp region in the Tox3 promoter as being required for transcription. Subsequent yeast one-hybrid analysis using the DNA sequence as bait identified that interacting partner as the C2H2 zinc finger transcription factor PnCon7, a putative master regulator of pathogenesis. Silencing of PnCon7 resulted in the down-regulation of Tox3 demonstrating that the transcription factor has a positive regulatory role on gene expression. Analysis of Tox3 expression in the PnCon7 silenced strains revealed a strong correlation with PnCon7 transcript levels, supportive of a direct regulatory role. Subsequent pathogenicity assays using PnCon7-silenced isolates revealed that the transcription factor was required for Tox3-mediated disease. The expression of two other necrotrophic effectors (ToxA and Tox1) was also affected but in a non-dose dependent manner suggesting that the regulatory role of PnCon7 on these genes was indirect. Collectively, these data have advanced our fundamental understanding of the Con7 master regulator of pathogenesis by demonstrating its positive regulatory role on the Tox3 effector in P. nodorum through direct interaction.
Graphical Abstract
The expression of the Tox3 effector gene from the wheat pathogen Parastagonospora nodorum is positively regulated through the direct of the PnCon7 transcription factor with an upstream element within the promoter. A variety of techniques, including GFP transcriptional fusion analysis, were used to identify the Tox3-URE element 230bp upstream of the Tox3 transcriptional site. Gene silencing of PnCon7 resulted in the loss of Tox3-mediated disease on wheat.
Introduction
Plant pathogens are key contributors to significant yield losses globally and pose an ongoing threat to food security. The ability of pathogens to successfully colonise plants hinges on their ability to circumvent host defence measures to facilitate growth in planta and cause disease. Plants though are well equipped to prevent pathogen growth through a combination of innate defence systems (Jones and Dangl, 2006; Dodds and Rathjen, 2010). First, the pathogen must overcome pattern-associated molecular pattern-triggered immunity (PTI) whereby the plant senses the presence of specific pathogen signature molecules (e.g., chitin, Flg22) and initiates a passive defence response. Successful pathogens are able to circumvent PTI through the secretion of effector proteins which function to inhibit PTI or mask the pathogen's recognisable molecular patterns. Plants, in turn, have evolved processes to recognise the pathogen effectors and elicit a more robust defence response stopping pathogen growth (effector-triggered immunity, ETI). ETI is considered the outcome of the gene-for-gene model whereby pathogen recognition by a host leads to resistance. Pathogens though can avoid recognition through effector evolution resulting in non-recognition by the host leading to successful colonisation. Consequently, pathogen effector function and recognition are crucial to the outcome of a plant-pathogen interaction.
Seminal work over the last decade has demonstrated how effector proteins secreted by Parastagonospora nodorum govern a strict gene-for-gene relationship with wheat (Oliver et al., 2012). The three genes encoding effector genes described to date are ToxA, Tox1 and Tox3 (Friesen et al., 2006; Liu et al., 2009, 2012). The encoded products of each of these genes interact specifically (directly or indirectly) with the wheat dominant susceptibility loci Tsn1, Snn1 and Snn3 respectively resulting in disease. The absence of either the effector or the host susceptibility gene gives rise to an incompatible or resistant response of the host plant, and as such, this interaction is regarded as an inverse gene-for-gene system (Oliver and Solomon, 2010).
Recent studies in P. nodorum and related pathogens have significantly advanced our functional understanding of these effectors during infection. These reports have described that whilst each induces cell death in the presence of its corresponding dominant susceptibility locus, they also have roles in host defence suppression. Tox1 is a chitin binding protein which is localised to the surface of mycelia and to protect the fungus from wheat chitinases during defence response (Liu et al., 2016). Tox3 and ToxA have recently been shown to interact with wheat PR-1 proteins and that this interaction influences pathogen growth during infection (Lu et al., 2014; Breen et al., 2016).
Whilst studies on how the necrotrophic effectors facilitate disease are advancing, the genetic regulation of effector gene expression is comparatively poorly understood (Tan and Oliver, 2017). Effector characterisation studies have demonstrated that the expression of each of the encoding genes (ToxA, Tox1 and Tox3) is much higher during infection than axenic growth (Liu et al., 2009, 2012; Faris et al., 2011). Several reports have now provided evidence that the expression of ToxA and Tox3 is regulated, either directly or indirectly, by transcription factors. A Zn2Cys6 zinc finger transcription factor (PnPf2) has been found to positively regulate the expression of ToxA and Tox3 and isolates of P. nodorum lacking PnPf2 results in loss of pathogenicity on the ToxA and Tox3 sensitive wheat lines (Rybak et al., 2017). PnPf2 is an ortholog of the Alternaria brassicicola AbPf2 which is also a Gal4-like Zn2Cys6 zinc finger transcription factor; similarly, deletion of this gene causes loss of pathogenicity (Cho et al., 2013). An earlier study also reported that the expression of Tox3 in P. nodorum is regulated by the APSES transcription factor SnStuA (IpCho et al., 2010). Despite this, how these transcription factors regulate Tox3 is unknown.
In this study, we have sought to understand the basis of Tox3 gene regulation in P. nodorum. Through a series of complementary approaches, we have identified that the C2H2 zinc finger transcription factor PnCon7 directly interacts with the Tox3 promoter to positively regulate the expression of Tox3 and control Tox3-mediated disease.
Results
A cis-regulatory element required for Tox3 expression is encoded at position −213 to −237
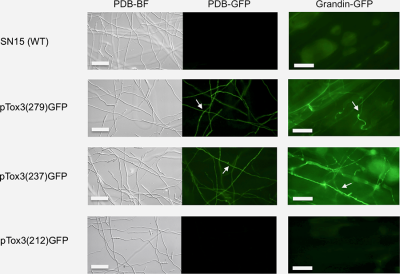
Tox3 promoter fragments of varying lengths were amplified and fused to GFP to generate a series of transcriptional fusion constructs (Fig. 1A). Each of the constructs was transformed into P. nodorum SN15 with a minimum of 20 transformants generated per construct. PCR screening confirmed the presence of the expression cassette in at least eight transformants per construct and these were chosen for further analysis. Subsequent analysis during growth in axenic culture and in planta of the transformants revealed strong GFP fluorescence in the transformants harbouring the 279 bp and 237 bp upstream regions of the Tox3 promoter. However, no fluorescence was observed when the promoter length was reduced to 212 bp or less, suggesting that a required cis-regulatory element for Tox3 expression resided within −213 to −237 of the promoter region (Fig. 1B).
Promoter deletion assay identified a cis-regulatory element on the Tox3 promoter.
A. Diagram of the Tox3 promoter:GFP constructs. The 5′ end points of the different constructs are indicated relative to the translation start site (ATG).
B. GFP expression in Tox3 promoter:GFP transformants. Tox3 promoter:GFP constructs were transformed into P. nodorum SN15. Numbers in bracket represented the Tox3 promoter length. Conidia of the transformants were incubated either in PDB for 24 h or inoculated on Grandin for 24 h. The expression of GFP was observed by epifluorescence microscopy. Arrows denote hypha. Scale Bar = 50 μm.
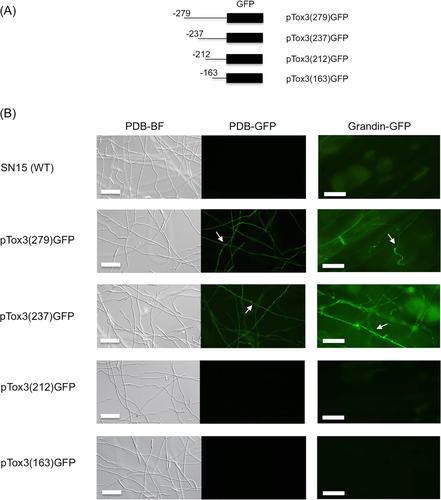
To confirm the presence of a putative regulatory element within −213 to −237 bp of Tox3, DNase I footprinting was used as a second independent approach. DNase I footprinting is a DNA-protein interaction technique working on the basis that an interacting protein will protect the bound DNA from DNase I cleavage which can then be monitored by a variety of techniques. Based on the identification of the putative interaction site above, a 6-FAM labelled flanking region around the putative cis-regulatory element (–164 to −279 bp) of the Tox3 promoter was incubated with a crude protein extract derived from 3-day-old P. nodorum in Fries medium then subjected to DNase I digestion. Fries medium was chosen as the preferred growth environment as it induced maximal Tox3 expression compared to other media, and therefore it was assumed that any interacting positive transcription factor would also be present (Supporting Information Fig. S1). Subsequent analysis revealed a protection pattern (much lower peaks in the presence of P. nodorum proteins compared with absence of proteins) from position −236 and three hypersensitive sites (much higher peaks in the presence of proteins compared with absence of proteins) at the flanking region (–237, −238, −239) (Fig. 2A). Furthermore, the dideoxynucleotide sequencing reactions indicated the hypersensitive sequence as TAC and protein bound to C from −235 (Fig. 2B). These data provide further evidence of a cis-regulatory element (5′-CTCCACCTATCCTAATCTAGTTAAA-3′) between −213 and −237 bp from the Tox3 translation start site (ATG). Consequently, this region was defined as Tox3-URE (Supporting Information Fig. S2).
Analysis of DNase I-digested Tox3 promoter fragment. The y-axes are in relative fluorescence units (3730 DNA Analyzer) which represent fluorescence from 6-FAM. Sequence positions are marked with reference to the start codon of the Tox3 gene.
A. Overlay of electropherograms of DNase I-digested DNA in the presence of P. nodorum SN15 protein (blue) and without protein (red). The horizontal bracket indicates the protected region, and hypersensitive sites are indicated with red asterisks.
B. Overlay of DNase I-digested DNA with the dideoxynucleotide sequencing reaction using the same 6-FAM-labelled primer. In the upper panel, the protein protected trace (blue) and without protein trace (red) were superimposed to show the protected region. In the lower panel, the traces for the dideoxynucleotide sequencing reactions were superimposed. Precise alignment of size standards (Liz-500, orange peaks) allows assignment of DNA fragments to specific bases. [Color figure can be viewed at wileyonlinelibrary.com]
A putative transcription factor binds specifically to the Tox3-URE cis-regulatory element on the Tox3 promoter
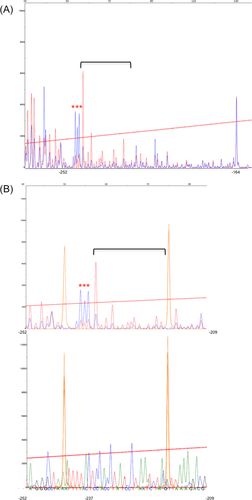
A yeast one-hybrid approach was used to screen for DNA binding regulatory proteins that interact with the Tox3-URE region identified above. First, two repeats of Tox3-URE were cloned into a bait vector, pINT1-HIS3NB prior to transformation into the yeast strain Y187, generating the bait strain yTox3-URE. Another yeast strain (yp53BS) harbouring the human p53 binding site was generated and used as negative control to exclude non-specific binding. The prey cDNA library in Y2HGold for mating with the bait strain was constructed with pGADT7-rec using RNA from P. nodorum grown in Fries medium (Fig. 3A). Subsequent assays revealed that only one cDNA clone resulted in specific interaction with the Tox3-URE (growth on medium lacking leucine and histidine) while no interaction occurred in the negative controls with either yp53BS or empty vector (Fig. 3B). Sequence analysis of the open reading frame against the annotated P. nodorum genome revealed that the interacting plasmid harboured the gene SNOG_08362. This result confirmed the specific interaction of the SNOG_08362 protein and Tox3-URE.
SNOG_08362 specifically interacted with the Tox3-URE.
A. Design of bait and prey constructs for Y1H screening.
B. yTox3-URE expressing SNOG_08362 grew on the -Leu -His medium. Yeast cells were grown into stationary phase and adjusted to 1, 10−1 and 10−2 times of dilution before spotting onto appropriate plates. The indicated selective media were incubated at 30°C for 3 days.
SNOG_08362 is an ortholog of the Con7 transcription factor with four alternative splicing variants
BlastP analysis of SNOG_08362 revealed a sequence similarity to Con7p from Magnaporthe oryzae and Con7-1 from Fusarium oxysporum, of 46% and 54.8% respectively (Supporting Information Fig. S3A). As such, SNOG_08362 was designated as PnCon7. The predicted PnCon7 protein was most similar to uncharacterised putative orthologs in Setosphaeria turcica and Bipolaris sorokiniana with over 85% amino acid identity (Supporting Information Fig. S3B). Indeed, Con7 was present in most ascomycetes and was highly conserved within Pleosporales group. All Con7 orthologs harboured a highly conserved C2H2 zinc finger, a nuclear localization signal and a coiled-coil region (Supporting Information Fig. S4).
Previous studies on Con7 have reported the gene to have different splicing variants (Odenbach et al., 2007; Ruiz-Roldán et al., 2015). By analysing previously published RNA-seq data from P. nodorum (Syme et al., 2016), four alternative splicing variants of PnCon7 were identified. The 1553bp PnCon7 pre-mRNA was processed to 4 variants, which differ only at the boxed 5′ regions (alternative splicing region) (Fig. 4A, Supporting Information Fig S5). For variant 1 (PnCon7-1), three exons were identified in this region, resulting in a protein of 330 amino acids. Two exons with different lengths were identified in the boxed regions of both variant 3 (PnCon7-3) and variant 4 (PnCon7-4) resulting in each encoding proteins of different sizes (PnCon7-3, 355 amino acids and PnCon7-4, 346 amino acids). Variant 2 (PnCon7-2) was the largest alternative splicing variant of PnCon7. Only one large exon was identified in the boxed region of variant 2 (PnCon7-2) and the whole transcript was translated into 377 amino acids.
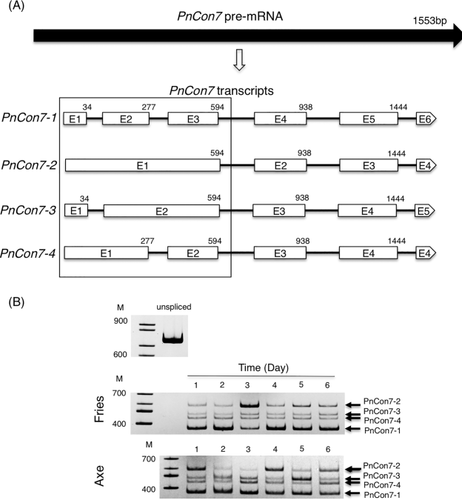
PnCon7 was subjected to alternative splicing and produced 4 hypothetical protein variants.
A. Structure of PnCon7-1, PnCon7-2, PnCon7-3 and PnCon7-4 transcripts. PnCon7-1 was produced by splicing of five introns from the PnCon7 pre-mRNA. PnCon7-2 was produced by splicing of the last three introns and retention of the first and second introns. PnCon7-3 was produced by splicing of the last three introns and retention of the second intron. PnCon7-4 was produced by splicing of the last three introns and retention of the first intron. E1, exon 1; E2, exon 2; E3, exon 3; E4, exon 4. E5, exon 5. E6, exon 6. Numbers indicated intron position from the start codon. Boxed: alternative splicing region.
B. Daily change in PnCon7 transcripts (RT-PCR products) after incubating in Fries (in vitro) or Axe (in planta). unspliced: PnCon7 unspliced PCR product. Marker DNAs (M) are in 100 base pair increments. Identical amount of total RNA was used in every RT-PCR reaction. Results are representative of at least three biological replicates per treatment.
The abundance of the four alternative splicing variants of PnCon7 was assessed during growth in axenic culture and infection by RT-PCR. PCR and sequencing analysis confirmed the presence of each transcript variant under all conditions assayed (Fig. 4B).
PnCon7-2 is not able to interact with Tox3-URE
PnCon7-4 was the splicing variant of PnCon7 identified in the initial Y1H screening. To verify whether the other three variants were able to interact with the Tox3-URE, cDNA sequences from PnCon7-1, PnCon7-2 and PnCon7-3 were cloned into the yeast expression vector pGADT7. The resulting plasmids were transformed into either the yTox3-URE or the yp53BS bait strains. Yeast cells carrying the plasmid containing cDNA of either PnCon7-1 or PnCon7-3 grew on medium lacking His and Leu, but those carrying the plasmid containing the cDNA of PnCon7-2 did not (Supporting Information Fig. S6A). There was no interaction of any of the PnCon7 protein variants with the negative control bait p53BS substantiating their specific interaction with Tox3-URE. yTox3-URE cells with PnCon7-2 showed reduced growth on -Leu medium and large amount of protein was observed on the Western blot (Supporting Information Fig. S6B). In contrast, yp53BS with PnCon7-2 showed normal growth on -Leu medium suggesting that PnCon7-2 was not toxic in yeast. Western blot analysis confirmed that all variants were expressed in the bait strains. Furthermore, the distinct size of each protein variant revealed that the cDNA was not processed to form other variants in yeast. These results indicate that PnCon7-1, PnCon7-3 and PnCon7-4 but not PnCon7-2, specifically interacted with Tox3-URE.
Tox3 expression is dose-dependent on PnCon7 expression
Having confirmed that three splicing variants of PnCon7 were able to interact with the Tox3-URE cis-regulatory element within the Tox3 promoter, we determined if this interaction positively or negatively regulated Tox3 expression. To do this, we attempted to remove the PnCon7 gene from P. nodorum genome through homologous recombination with a selectable marker. However, despite being an established approach in P. nodorum, we were unable to generate transformants lacking PnCon7. Nearly, 300 transformation colonies were screened for the mutation at the PnCon7 locus but all were found to harbour only ectopic integrations.
Consequently, RNA silencing was undertaken to down-regulate the expression of PnCon7 in P. nodorum. Various species of RNA, such as sense, antisense, double strands and hairpin, have been demonstrated to trigger RNA silencing in fungi (Kadotani et al., 2003). Here, RNA silencing was undertaken by introducing antisense PnCon7 into the P. nodorum SN15 (Fig. 5A). Over 30 transformants were selected on the medium containing the selectable marker bialaphos. The intact silencing cassette was confirmed in each transformant by PCR (data not shown). These transformants were then designated con7i. Subsequent analyses revealed the con7i transformants were phenotypically comparable to SN15 (Supporting Information Fig. S7).
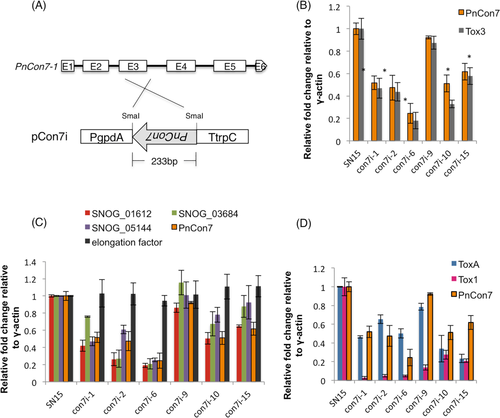
Gene silencing of PnCon7 resulted in down-regulation of Tox3, ToxA and Tox1. Genes with Tox3-URE on the promoter regions were also down-regulated.
A. Schematic representation of the silencing vector pCon7i. pCon7i was used for PnCon7 silencing in SN15. PgpdA, Aspergillus nidulans gpdA promoter; 233 bp inverted PnCon7-1 exon3; TtrpC, A. nidulans trpC terminator.
B. Expression of PnCon7 and Tox3 in the con7i transformants as determined by qRT-PCR.
C. Expression of genes with Tox3-URE on the promoters in comparison to PnCon7. Translation elongation factor (SNOG_11663) was used as control.
D. Expression of ToxA and Tox1 in comparison to PnCon7. Values shown in y-axis represent the relative fold change of gene expression. For all genes scored, the relative fold change was calculated by dividing the mutant value by the wild type SN15 value. All gene expression value was first normalized to the endogenous control γ-actin. Biological triplicates were assayed using technical replicates. Standard error bars are shown. * denotes significantly reduced (p < 0.05).
The expression level of PnCon7 was examined in six con7i transformants by qRT-PCR (Fig. 5B). The results showed that in most transformants, the expression of PnCon7 was reduced to about 50% of the expression level in wild type SN15. con7i-6 was most reduced with only approximately 20% of the wild type expression level observed. In contrast, the expression of PnCon7 in con7i-9 was 90% of the wild type. Subsequent qRT-PCR analyses revealed that the expression of Tox3 in each of the con7i transformants was significantly lower than that observed in wild-type P. nodorum. It was striking to observe that the expression of Tox3 closely correlated with PnCon7 expression in the con7i transformants (R2 = 0.97) supporting the claim that PnCon7 directly regulates the expression of Tox3 in a dose-dependent manner (Supporting Information Fig. S8).
PnCon7 regulates the expression of multiple genes
The 25 bp Tox3-URE sequence was used in a Blastn analysis against 500 bp upstream of each predicted gene in the P. nodorum genome. As expected, an identical match was found within the Tox3 promoter. Weaker matches were identified in a number of other genes (data not shown). Of these blast matches, a 10 bp region (CCACCTATCC) of Tox3-URE appeared conserved within some of these promoter regions. A subsequent motif search using this conserved region revealed its presence (with up to a 1 bp mismatch) in the promoter regions of 340 genes. Nine of these promoters harboured the exact sequence whilst 330 contained the sequence with a single mismatch. The nine genes with the exact sequence in their respective promoters are listed in Supporting Information Fig. S9. There appeared to be no commonality of these genes in terms of function or role although it is interesting to note that of those nine, three were predicted (including Tox3) to encode effector proteins as determined by EffectorP (Supporting Information Table S1; Sperschneider et al., 2016).
Three of the genes harbouring the CCACCTATCC sequence (SNOG_01612, SNOG_03684 and SNOG_05144) were randomly selected to determine if like Tox3, their expression was regulated by Con7. As observed for Tox3, there was a significant decrease in the expression of each gene in the different con7i transformants (Fig. 5C). Subsequent analysis confirmed that this decrease directly correlated with that PnCon7 in the con7i mutants (R2 = 0.89, 0.81 and 0.77 respectively) providing evidence that these conserved 10 bp within Tox3-URE play a key role in the direct regulation of gene expression (Supporting Information Fig. S8). In contrast, the expression of the gene encoding elongation factor (Ef1α), which does not harbour a Tox3-URE site in its promoter region, was unaffected by the reduced PnCon7 transcription (R2 = 0.14).
Despite not harbouring a Tox3-URE (or the conserved 10 bp motif) site in their promoter regions, the expression of the ToxA and Tox1 effector genes was also examined in the con7i transformants (Fig. 5D). Subsequent analysis revealed that the expression of ToxA was reduced to varying levels in all the transformants. More striking though was the strong reduction of Tox1 expression in all con7i transformants. However unlike the examples above harbouring a Tox3-URE, there was only a weak correlation between the levels of expression of ToxA and Tox1 with PnCon7 in the con7i transformants (R2 = 0.14, 0.11 respectively) implying that the influence PnCon7 was not directly causal to the reduced expression of ToxA and Tox1 (Supporting Information Fig. S8).
con7i transformants are less pathogenic on wheat
It was hypothesised that the down-regulation of Tox3 in the con7i transformants would negatively impact on disease development. To test this, the wheat cultivars Corack and Axe were infected with the con7i transformants. Corack is dominant for Snn3 and therefore susceptible to P. nodorum isolates expressing Tox3. However, Corack is recessive for Tsn1 and Snn1 meaning that ToxA and Tox1 play no discernible role in disease development on this cultivar. In contrast, Axe is susceptible to both Tox3 and ToxA and would be expected to be more susceptible to disease than Corack when infected with the con7i strains. Subsequent disease scoring on plants seven days after inoculation revealed that pathogenicity on Corack was largely reduced in con7i-1 and con7i-6 while only slightly decreased in con7i-9 (Fig. 6). These variations in disease development correlate with levels of PnCon7 (and Tox3) gene expression and demonstrate the requirement of PnCon7 for Tox3-mediated disease development. However, the same con7i isolates were significantly more pathogenic when inoculated onto Axe compared to Corack. This confirms that the reduced disease on Corack was due to the down-regulation of Tox3 and demonstrates the requirement of PnCon7 for Tox3-mediated disease development.
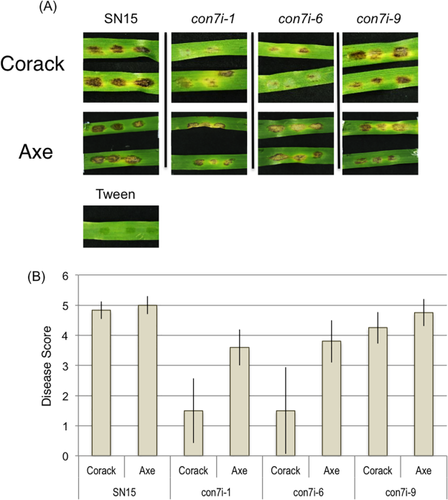
con7i transformants were less pathogenic on wheat.
A. Detached leaf assays on Corack and Axe at 5 dpi. The images of diseased leaves represent infections of the P. nodorum wild type SN15 and con7i.
B. The histogram represents the average disease ratings from 0 to 5 which 0 means resistant and 5 means susceptible. Standard error bars are shown (n = 10).
Discussion
Despite the significant advances recently achieved in dissecting the role of P. nodorum effectors during infection, the mechanisms underlying their regulation are poorly understood. In this study, we have exploited independent approaches to demonstrate that the C2H2 transcription factor, PnCon7, direct interacts with the cis-regulatory element Tox3-URE on the Tox3 promoter to positively regulate its expression.
Con7 was first identified through a forward genetics approach in Magnaporthe oryzae. Con7 mutants were found to generate conidia of abnormal cell shape with reduced sporulation (Shi and Leung, 1995). Appressorial formation was also significantly affected leading to a complete loss of pathogenicity on rice. Con7 was independently verified as a pathogenicity factor in M. oryzae through a later forward genetics approach (Odenbach et al., 2007).Subsequent sequence and homologous based searches identified the product of Con7 to harbour a C2H2-Zn2+ finger family DNA binding motif and to be localised to the nucleus, consistent with its role as a predicted transcription factor. As previously demonstrated, Con7 was found to be required for normal growth and also disease development on rice. A subsequent study confirmed that Con7 had comparable roles in disease and growth in the tomato pathogen Fusarium oxysporum (Ruiz-Roldán et al., 2015).
In this study, PnCon7 was identified through its direct interaction with a cis-regulatory element (described as Tox3-URE) in the promoter of the Tox3 effector. Multiple attempts to inactivate the gene through homologous recombination failed. This approach has been the basis behind many successful reverse genetics studies in P. nodorum (Oliver et al., 2012), and as such, our conclusion is that PnCon7 is likely to be required for viability in P. nodorum. To circumvent this, we used RNA silencing to down-regulate the expression of PnCon7. This was successful and multiple mutants were generated demonstrating varying levels of PnCon7 expression. Analysis of these mutants showed that unlike in M. oryzae and F. oxysporum, there were no discernible differences in the growth or morphology of the con7i silenced mutants compared to the wild-type. However, it is very possible that the residual amount of PnCon7 gene expression observed in silenced mutants could be sufficient to maintain growth phenotypes (e.g. sporulation) in vitro.
These data though suggest that the Con7 transcription factor has distinct roles in P. nodorum compared to these other pathogens. Analysis of the Con7 protein sequences has shown that those from M. oryzae and F. oxysporum appear relatively distant to that from P. nodorum (and others with the Pleosporales) supporting potentially diverse roles for these proteins amongst different plant pathogens. This is not without precedent with other genes having been described (e.g. Tps1, trehalose 6-phosphate synthase) as being functionally unique in P. nodorum and M. oryzae (Wilson et al., 2007; Lowe et al., 2009).
PnCon7 is not the first transcription factor shown to have a demonstrable effect on Tox3 expression. IpCho et al. (2010) reported that the inactivation of SnStuA transcription factor resulted in the down-regulation of Tox3. It should though be noted that the P. nodorum mutants lacking SnStuA displayed a multitude of phenotypes including poor growth in axenic culture suggesting that the effect of the transcriptional factor was causal to other pleiotropic effects. More recently, Rybak et al. (2017) showed that the GAL4-like PnPf2 transcription factor was required for both ToxA and Tox3 expression. Unlike the SnStuA mutants, the PnPf2 mutants in P. nodorum appeared phenotypically comparable to the wild-type strain implying that the regulatory role of the transcription factor is more specific. However the mechanisms underlying PnPf2 regulation have yet to be determined and yeast 2-hybrid analysis was unable to demonstrate an interaction of PnPf2 with the Tox3 promoter region (data not shown).
No attempt was made in this study to use a transcriptomics approach to identify the array of genes regulated by PnCon7. This was primarily due to the fact that it is very difficult to determine using such approaches the genes are directly regulated from those in which gene expression changes are causal to the inactivation of the transcription factor. Rather, we searched for genes directly regulated by PnCon7 by looking for the Tox3-URE sequence within the promoters of P. nodorum genes. Through this approach, nine genes were identified as harbouring a conserved 10 bp sub-region of Tox3-URE. It is acknowledged that this 10 bp is unlikely to represent the exact interaction sequence and that further experimentation would be required to resolve the precise motif. It did though provide a starting point in which to identify genes putatively directly regulated by PnCon7.
Expression analysis revealed that four randomly selected genes from the nine identified were down-regulated in the PnCon7-silenced mutants. Furthermore, their expression appeared dose-dependent on the level of PnCon7 transcript present in the different con7i mutants tested supporting the scenario that these genes are directly regulated through interaction with the transcription factor. Whilst initially disappointed that we were unable to make a PnCon7 gene disruption mutant, the generation of different RNAi silenced isolates (with varying levels of PnCon7 expression) proved very useful in directly linking the expression of PnCon7 with that of the gene of interest.
It was surprising to find that the expression of ToxA and Tox1 was affected in the con7i mutants. However, in contrast to Tox3, we suggest that this change in expression was an indirect consequence of reduced PnCon7 expression and that neither ToxA nor Tox1 are directly regulated by the transcription factor. Careful analysis of the promoter regions of both effectors found no evidence of the Tox3-URE sequence. In addition, the expression of either effector gene was not dose-dependent on PnCon7 transcript levels as was evident for the other genes harbouring Tox3-URE in their promoters suggesting that there is an alternative basis for the effect on ToxA and Tox1 gene expression in the con7i transformants. One possibility is that PnCon7 may act upstream of ToxA and Tox1 and influence other regulatory proteins. Indeed, previous studies have identified multiple transcription factors have a role regulating ToxA and Tox3 expression (IpCho et al., 2010; Rybak et al., 2017). Moreover, there is no evidence to suggest a transcription factor regulates Tox1 expression. It has though been reported that Tox3 expression is epistatic to Tox1 implying that Tox1 has a transcriptional suppression role over Tox3 expression (Rybak et al., 2017). There have been no reports to date on the influence of Tox3 on Tox1 expression.
Previous studies have shown that Con7 does regulate effector genes in other fungal pathogens. For example, Con7 in M. oryzae was shown to control the expression of several chitin binding proteins whilst, the expression of the SIX8 and SIX9 effector genes in F. oxysporum were regulated by Con7 (Odenbach et al., 2007; Ruiz-Roldán et al., 2015). It is interesting to consider that three of the nine genes harbouring the 10 bp motif within Tox3-URE in P. nodorum are predicted through a validated machine learning approach to encode effectors suggesting a common role for the transcription factor in regulating pathogenesis (Sperschneider et al., 2016).
An analysis of previously published RNAseq data revealed four alternative splicing variants of PnCon7 (Syme et al., 2016). These analyses were subsequently verified through experimental approaches as described above. Alternative splicing has also been identified in other fungal orthologs. In M. oryzae, two different Con7 transcripts were observed. Similarly, alternative splicing at the 5′ end of F. oxysporum Con7-1 generated four different transcripts, which were revealed to produce four proteins containing specific phosphorylation sites (Odenbach et al., 2007; Ruiz-Roldán et al., 2015). The alternative splicing pattern of PnCon7 identified in this study is identical to that reported for F. oxysporum Con7-1. Comprehensive research into alternative splicing events in humans has identified several different mechanisms of alternative splicing (Keren et al., 2010). The type of alternative splicing observed for PnCon7 is consistent with that defined as intron retention whereby an intron sequence is kept in the mature RNA or spliced out. Previously, intron retention was considered as a spurious event of little biological significance (Kim et al., 2008). However recently, intron retention has been shown to have regulatory roles, such as stabilize RNA structure, affect translational efficiency, modify biological function, facilitate RNA polymerase II pausing (Ner-Gaon et al., 2004; Galante et al., 2004; Braunschweig et al., 2014). Moreover, transcripts harbouring intron retention were shown to be highly expressed and be potentially involved in tumor formation in humans (Sakabe and de Souza, 2007; Dvinge and Bradley, 2015). These studies support the theory that intron retention of PnCon7 is biologically significant as all of the alternative splicing variants exist in vitro and in planta, regardless of harbouring the DNA binding C2H2 zinc finger and that further studies are required to elucidate the roles of each of each transcripts.
Previous studies in M. oryzae and F. oxysporum have suggested that the Con7 transcription factor acts as a master regulator of pathogenesis, however it was unclear how. In this study, we have conclusively demonstrated that the C2H2 transcription factor PnCon7 regulates the expression of Tox3 by direct interaction with the Tox3-URE motif within the Tox3 promoter. Other genes harbouring the Tox3-URE sequence (or the embedded conserved 10 bp motif) in their promoters were also positively regulated by PnCon7. These data have significantly advanced our understanding of effector gene expression and, for Tox3 at least, identified a key regulatory target that is required for disease development. More importantly, this study sheds light on the underlying nature of the Con7 transcription factor and provides a platform to dissect effector regulation in other pathogens.
Experimental procedures
Fungal material and growth condition
Parastagonospora nodorum SN15 (Solomon et al., 2003) was derived from previous studies. Fungal strains were routinely maintained on V8-PDA plates (Campbell's V8 juice, 150 ml l−1; potato dextrose agar, 10 g l−1; CaCO3, 3 g l−1; agar, 15 g l−1) and incubated at 22°C under 12-h cycles of light.
Plant materials
Wheat lines (Triticum aestivum), Grandin (Friesen et al., 2006), Axe and Corack (Australian Grain Technologies, Australia) were used in this study for infection assays. All plants were grown at 20°C under a 14-h day/night cycle in a controlled growth chamber.
Plasmids construction and fungal transformation
The plasmids, pGpD-eGFP (Sexton and Howlett, 2001) and pAN7-1 (Punt et al., 1987) were used to generate Tox3 promoter:GFP constructs. pAN7-1 carries a hygromycin-resistance gene under the control of the Aspergillus nidulans gpdA promoter and trpC terminator. The Tox3 279 bp promoter was first amplified from P. nodorum SN15 using the primer pair PAN-TOX3PRO-F279 and TOX3PRO-GFP-R1. The subsequent promoter deletion fragments were amplified with each upstream primer PAN-TOX3PRO-F237, PAN-TOX3PRO-F212 and PAN-TOX3PRO-F163 and a common downstream primer TOX3PRO-GFP-R1. eGFP was amplified from pGpD-eGFP with the primer pair TOX3-GFP-F1 and GFP-TOX3TER-R1. The Tox3 408bp terminator region was amplified with the primer pair GFP-TOX3TER-F1 and TOX3TER-PAN-R1. To develop a Tox3 promoter:GFP expressing plasmid, the PCR products of Tox3 promoter, eGFP, and Tox3 terminator were then cloned into the plasmid pAN7-1 using Gibson assembly system (New England Biolabs). The constructs were named pTox3(279)GFP, pTox3(237)GFP, pTox3(212)GFP and pTox3(163)GFP, according to the length of each promoter fragment.
For building the PnCon7 RNA silencing construct, the vector pBARGPE1-LIC (Chooi et al., 2013) containing the gpdA promoter and the trpC terminator, was cut using SmaI. An antisense fragment of PnCon7 was amplified from cDNA with primers, pBAR-Con7-F1 and pBAR-Con7-R1. The 233bp fragment was then introduced in pBARGPE1 SmaI site using Gibson assembly (New England Biolabs). The resulting plasmid was designated pCon7i. The plasmid was transformed to P. nodorum and the resulting transformants were selected on medium containing 5 μl ml−1 glufosinate ammonium aqueous phase extracted from commercial herbicide Basta (Bayer Crop Science Australia) with chloroform.
The fungal protoplasting and PEG-mediated transformation methods were manipulated as described (Solomon et al., 2003). Gene insertion was confirmed by PCR.
Fluorescence detection of GFP
GFP fluorescence was observed using the fluorescent microscope Leica DM5500 equipped with a filter set consisting of a blue excitation filter (BP 480/40 nm), a dichromatic mirror (DM 505 nm), and a suppression filter (BP 527/30 nm). Spores were first collected from two-week-old P. nodorum culture and suspended in 0.02% Tween 20 until reaching the concentration of 106/ml. A 10 μl of spore solution was then inoculated on a detached Grandin leaf or in 10 μl potato dextrose broth (PDB, Difco) and kept in 22°C wet chamber before microscope observation. At least eight independent transformants from each transformation were examined for GFP expression.
Measurement of relative GFP expression was conducted using a fluorometer (Tecan Infinite M1000 Pro) with an excitation wavelength of 485 nm and an emission wavelength of 515 nm. Absorbance was read at 415 nm. The spores from two-week-old cultures were collected and washed twice with distilled water. Spores were added to the media to a final concentration of 105 ml−1. The media used were PDB and two previously reported media [liquid minimal medium (Solomon et al., 2003) and liquid Fries 3 medium (Todd et al., 2014)], except the trace element used in this study for Fries 3 medium was the same as the minimal medium. A 200 μl of the tested media with spore suspension was added to 96-well plates with black walls (Costar, Corning, NY). Plates were incubated at 22°C under 12 h cycles of light for three days. Uninoculated media was included as a control to subtract baseline fluorescence from the sample wells. Each experiment was performed with three technical repeats and three biological repeats. Statistical significance was assessed by ANOVA followed by t-tests.
Preparation of protein extracts
Parastagonospora nodorum SN15 was grown at 22°C in liquid Fries 3 medium and harvested after three days growth. Mycelium was collected and was ground in liquid nitrogen, and the following steps were subsequently performed on ice. A 100 mg of sample was resuspended in 1 ml of extraction buffer (20 mM HEPEs, 100 mM KCl, 0.2 mM EDTA, 20% glycerol, 2mM DTT, 1 mM PMSF, protease inhibitor cocktail, 0.1% NP-40, pH 7.9) following by the addition of 1/10 volume saturated ammonium sulfate solution. After 30 min incubation, cell debris was separated by centrifugation (maximum speed, 30 min) and proteins in the supernatant were precipitated with 70% saturated ammonium sulfate. The pellet was resuspended in 40% glycerol and de-salted by Sephadex G-25 (Sigma-Aldrich). Yeast total protein extraction was performed as described (Kushnirov, 2000).
DNase I footprinting
DNase I footprinting analysis was performed according to the method of Zianni et al. (2006) with modifications. −279 to −164 of the Tox3 promoter was amplified by polymerase chain reaction (PCR) with the primers M13-Tox3–279-F1 and Tox3–163-R1. A universal primer, FAM-M13-F, was used in the PCR reaction to add 6-FAM and M13 sequence on the 5′ side of the PCR product. The PCR was performed for 30 cycles under the following conditions: 98°C for 5 min, 98°C for 30 s, 60°C for 45 s, and 72°C for 20 s. And further 8 cycles under the following condition: 98°C for 30 s, 53°C for 45 s, and 72°C for 20 s. Varying amounts of P. nodorum crude proteins ranging from 0 to 1 μg were incubated with 10 ng of fluorescently labelled PCR product and 1.5 μg of Poly(dI-dC) for 20 min at 4°C in binding buffer (1 mM EDTA, 10 mM DTT, 2.5% glycerol, 10 mM Tris, 50 mM NaCl, 2.5 mM MgCl2, 0.5 mM CaCl2 pH 7.5). 0.032 units of DNase I (NEB) was added to the reaction and incubated for 2 min at 37°C. The reaction was stopped by incubating at 75°C for 10 min. Control digestions were performed in the absence of protein. The DNA fragments were precipitated by ethanol precipitation and eluted in 3 μl H2O. The samples were analyzed with a 3730 DNA Analyzer, running an altered genotyping module that increased the injection time to 30 s and the injection voltage to 3 kV. Fragment data were analyzed using Peak Scanner Software (Applied Biosystems, Life Technologies Corporation). Dideoxynucleotide sequencing reaction was performed with the Thermo Sequenase Dye Primer Manual Cycle Sequencing Kit (USB, Cleveland, Ohio) according to the manufacturer's instructions. The resulting product was mixed with HiDi and GeneScan-500 LIZ size standards and analyzed with a 3730 DNA Analyzer.
Yeast-one-hybrid assay
The construction of yeast reporter strain and yeast-one-hybrid screening was performed as previously described (Meijer et al., 1998; Ouwerkerk and Meijer, 2001; Ouwerkerk and Meijer, 2011). Yeast-one-hybrid (Y1H) bait constructs were prepared by cloning three repeats of the putative binding site of p53 (5′-AGACATGCCT-3′) (Ouwerkerk and Meijer, 2011) or 2 repeats of the Tox3-URE (5′-CTCCACCTATCCTAATCTAGTTAAA-3′) into the pINT1-HIS3NB vector (kindly provided by Dr. P.B.F Ouwerkerk), each of which was synthesized with NotI-SpeI overhangs (IDT, Singapore). The oligonucleotides with complementary sequences were annealed by heating to 95°C then slowly cooling down to room temperature. The resulting overhangs were used for cloning into the NotI-SpeI sites of the pINT1-HIS3NB vector. Constructs with inserts were selected and sequenced to confirm integration of the oligonucleotides. The construct was then digested with NcoI and XcmI to yield a linear fragment containing the putative binding sites. Integration of the fragment into the yeast (Y187, Clontech) genomic DNA was performed as described (Ouwerkerk and Meijer, 2001). Bait strains, designated yTox3-URE and yp53BS, were spread on plates with selective media (-His) containing 0–100 mM 3-amino-1,2,4-triazole (3-AT, Sigma-Aldrich) to test possible leaky expression of HIS3 gene.
Total RNA from 3-day-old P. nodorum SN15 grown in Fries 3 medium was prepared by using the Qiagen miRNA kit (Qiagen). cDNA was synthesized according to the protocol of Make Your Own ‘Mate &PlateTM’ Library System (Clontech). The cDNA library was introduced into Y2HGold (Clontech) and stored at −80°C until use.
Mating of the yeast bait strains with the prey cDNA library was conducted by mixing them together and spread on the YPAD medium (10 g l−1 yeast extract, 20 g l−1 Bacto Peptone, 20 g l−1 glucose, 40 mg l−1 Adenine sulfate, 20 g l−1 Bacto agar). After 4 h of incubation at 30°C, the yeast cells were harvested, washed and resuspended in 300 μl of water. The suspension was spread on selective synthetic drop-out medium [6.8 g l−1 of yeast nitrogen base, 20 g l−1 of glucose, 20 g l−1 agar, and 1.46 g l−1 yeast synthetic drop-out medium supplement without leucine and histidine (Sigma)].
Mated yeast cells were screened on the synthetic drop-out medium lacking leucine and histidine. After 4 days, putative positives were isolated and re-streaked on media with x-α-galactose to test α-galactosidase activity. The remaining positives without α-galactosidase activity were then examined for integrated cDNA length and digestion pattern with the restriction enzyme HaeIII. The remaining positives were further analyzed by sequencing. To identify the dependence of HIS3 gene activation on plasmid proteins, plasmids were isolated and re-transformed into yTox3-URE and yp53BS following the Frozen-EZ Yeast Transformation II Kit™ (ZymoResearch) as the manufacturer's instructions.
Western blot
Yeast total proteins were resolved by 11% SDS–PAGE and proteins were transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA) using the Mini Trans-Blot® system (Bio-Rad) as previously described by the manufacturer. HA-protein fusions were detected by anti-HA High Affinity from rat (Roche, Castle Hill, NSW, Australia) followed by an anti-rat IgG HRP from goat (Sigma-Aldrich®). Protein bands on blot were detected using ECL substrate (Bio-Rad) and visualized by ImageQuant4000 (GE Healthcare, Silverwater, TX, USA).
Alternative splicing transcript analysis
Analyses of PnCon7 alternative splicing transcripts were performed by RT-PCR. Total RNA was obtained by using ZR Fungal/Bacterial RNA MiniPrep™ (Zymo Research). cDNA was synthesized from 0.5 μg of RNA, using a cDNA synthesis kit (Promega) with gene-specific primers 08362-F3 and 08362-R2. All splicing products were confirmed by cloning and sequencing. PnCon7 cDNA with the alternative splicing region was produced from different RNA samples with primers 08362-F5 and 08362-R4. An identical amount of cDNA was used in each treatment. PCR products were run on a 5% TBE acrylamide gel. After electrophoresis, the proportion of each splicing variants in individual RNA sample was estimated quantitatively on the basis of the band intensities using the BIORAD Gel Documentation System.
Sequence analysis and phylogenetic trees
Sequence comparisons and phylogenetic trees were made using the Geneious software with sequences obtained from the NCBI database. 500 bp upstream regions of P. nodorum genes were extracted from the NCBI database. The sequences were then imported into Geneious and used as the database for a Blastn analysis using the Tox3-URE sequence (CTCCACCTATCCTAATCTAGTTAAA). Based on the outcomes of the Blastn analysis, the 10 bp motif CCACCTATCC was used for a Motif search with Geneious against the P. nodorum genome.
The alignment of Con7 sequences was conducted by MAFFT. The phylogenetic tree was built by using RAxML (Stamatakis et al., 2005). The nuclear localization signal was predicted using NucPred (http://www.sbc.su.se/~maccallr/nucpred/). C2H2 Zinc finger and Coiled-coil regions were identified with the database InterPro (http://www.ebi.ac.uk/interpro/).
Real-time quantitative PCR
Parastagonospora nodorum was grown in liquid Fries 3 medium for three 3 days. The mycelium was then harvested by centrifugation and total RNA was isolated using TRIzol (Life Technologies, CA, USA). Possible DNA contamination was removed by using a DNA-free reagent (Life Technologies) according to the manufacturer's instructions. cDNA was synthesized from 0.2 μg of RNA, using a cDNA synthesis kit (Promega). Quantitative PCR (qPCR) was performed using the ViiA™ 7 Real-Time PCR System (Applied Biosystems) and the Fast SYBR Green Master mix (Applied Biosystems) in reaction volumes of 10 μl. qPCR primers used in this study were listed in Supporting Information Table S1. Each sample was amplified in quadruplicate. The thermal cycling condition involved a pre-run at 95°C for 20 s, 40 cycles with a 1 s denaturation step at 95°C followed by a 60°C annealing and an extension step for 20 s according to the Fast SYBR Green manual. A non-template control (negative control) was also included in each assay. Relative fold change of gene expression was calculated by normalizing against the expression of γ-actin first, then against the expression of each tested gene in SN15.
Pathogenicity assay
Fungal inoculation was conducted at the two-leaf stage of wheat. Spores were collected after growing for two weeks on V8-PDA and washed twice in distilled water. Spores were then resuspended in distilled water with 0.02% Tween 20 to a final concentration of 106 ml−1. Detached leaf assays (DLAs) were performed as described (Solomon et al., 2004). Briefly, the spore suspension was sprayed on the second leaves of 14-day-old wheat (Axe and Corack) with a paint sprayer. The wheat leaves were sectioned to approximately 3 cm in length and placed on water agar containing 150 mg l−1 benzimidazole with the ends embedded in the agar. The plates were incubated at 20°C in a 12 h light/12 h dark cycle. Disease ratings followed the 0–5 disease scale with 0 being highly resistant and 5 as highly susceptible (Liu et al., 2004).
Acknowledgements
YHC is an Australian Research Council (ARC) Future Fellow. We thank Dr. P.B.F Ouwerkerk (Leiden University), who kindly provided the vector pINT1-HIS3NB for use in the yeast one-hybrid assay.
Author contributions
SYL, YHC and PSS designed the research and analysed the data. SYL and YHC performed the experiments. SYL drafted the preliminary draft of the manuscript. PSS and YHC wrote the submitted version of the manuscript. PSS directed the research.