Roles of three FurA paralogs in the regulation of genes pertaining to peroxide defense in Mycobacterium smegmatis mc2155
Summary
Mycobacterium smegmatis mc2155 has three genes (MSMEG_6383, furA1; MSMEG_3460, furA2; MSMEG_6253, furA3) encoding FurA (ferric-uptake regulator A) paralogs. Three FurA paralogs in M. smegmatis are functionally redundant and negatively regulate expression of a subset of genes involved in peroxide detoxification such as ahpC, katG1 and katG2, as well as their own genes. The FurA paralogs sense H2O2 via metal-catalyzed His oxidation (MCHO) in the same way as PerR. The propensity of FurA2 and FurA3 for MCHO is greater than that of FurA1. The three furA genes are transcribed into leaderless mRNAs lacking the Shine-Dalgarno (SD) sequence. FurA1 and FurA3 have the quaternary structure of homodimers like most Fur homologs, whereas FurA2 occurs as a monomer. The monomeric structure of FurA2 is determined by the C-terminal region of its dimerization domain. FurA2 monomers appear to cooperatively bind to the FurA-binding site with an inverted repeat configuration and have a broader binding specificity for the target DNA than dimeric FurA1 and FurA3. Comparative transcriptomic analysis revealed that the FurA paralogs do not regulate genes related to iron homeostasis in M. smegmatis, and that expression of SigF-regulated genes is significantly decreased in a furA triple mutant relative to the wild-type strain of M. smegmatis.
Graphical Abstract
Introduction
In most aerobic bacteria, the regulation of oxidative stress responses is critical for their survival and growth fitness. Reactive oxygen species (ROS) including superoxide anion (O2·-), hydrogen peroxide (H2O2) and hydroxyl radical (OH·) are produced either endogenously during aerobic respiration or exogenously by environmental competitors or eukaryotic host cells (Beckman and Koppenol, 1996; Imlay, 2008, 2013). ROS cause oxidative damages to biological macromolecules such as proteins, nucleic acids and lipids (Imlay, 2003, 2013). To cope with these deleterious effects, bacteria have evolved sophisticated ROS-defense systems such as mechanisms for the protection and repair of macromolecules, as well as enzymes involved in detoxification of ROS. These adaptive responses to ROS are typically controlled by ROS-sensing regulatory systems such as OxyR, PerR and SoxRS in bacteria (Mongkolsuk and Helmann, 2002; Imlay, 2015).
OxyR is a global peroxide-sensing regulator present in many Gram-negative bacteria (Dubbs and Mongkolsuk, 2012). It senses H2O2 by redox-sensitive Cys oxidation and induces expression of a subset of genes including those related to H2O2 detoxification (Christman et al., 1985; Zheng et al., 1998; Aslund et al., 1999; Zheng et al., 2001; Seib et al., 2007). In contrast, a majority of Gram-positive bacteria contain peroxide regulon repressors (PerR) as functional equivalents of OxyR (Horsburgh et al., 2001; Lee and Helmann, 2007; Gryllos et al., 2008; Hillmann et al., 2009; Dubbs and Mongkolsuk, 2012; Ji et al., 2015). The functional homologs of PerR are also found in some Gram-negative bacteria (Van Vliet et al., 1999; Wu et al., 2006; Palyada et al., 2009). The regulon of Bacillus subtilis PerR (PerRBs) includes oxidative stress-responsive genes such as katA (catalase), ahpC-ahpF (AhpC: alkyl hydroperoxide reductase and AhpF: peroxiredoxin reductase) and perR (Chen et al., 1995; Bsat et al., 1996; Fuangthong et al., 2002; Helmann et al., 2003). Expression of this regulon is known to be induced even by relatively low concentrations of H2O2 or under metal limitation conditions (Chen et al., 1995; Herbig and Helmann, 2002; Helmann et al., 2003).
PerR is a prototype of metal-dependent peroxide sensors, which belongs to the Fur (ferric-uptake regulator) family (Lee and Helmann, 2007; Fillat, 2014). The Fur family includes Fur, PerR, zinc-uptake regulator (Zur), nickel-uptake regulator (Nur), manganese-uptake regulator (Mur) and iron response regulator (Irr).
In most cases, Fur family proteins contain two metal-binding sites, one for a regulatory metal ion and the other for a structural Zn2+. The regulatory metal-binding site is generally located at the interdomain region between the N-terminal DNA-binding and C-terminal dimerization domains and coordinates its corresponding regulatory metal ion such as Fe2+, Zn2+, Ni2+ or Mn2+ (Fillat, 2014). The Fur proteins coordinate Fe2+ as a regulatory metal ion and regulate the expression of genes involved in iron homeostasis (Hantke, 2001; Troxell and Hassan, 2013). The Fe2+ iron at the regulatory metal-binding site of PerRBs is penta-coordinated by three His and two Asp residues (His37, Asp85, His91, His93 and Asp104) (Jacquamet et al., 2009). Although both Fe2+ and Mn2+ can bind to the regulatory metal-binding site, only Fe2+-bound PerR senses H2O2 by means of the metal-catalyzed His oxidation (MCHO) mechanism (Herbig and Helmann, 2002; Lee and Helmann, 2006b). Local Fenton-like reaction of Fe2+ bound to the regulatory metal-binding site with H2O2 leads to the rapid oxidation of either His37 or, to a lesser degree, His91 into the 2-oxo form, resulting in the loss of repressor activity (Lee and Helmann, 2006b; Traore et al., 2009). The Zn2+-binding site at the dimerization domain is usually composed of two Cys-X-X-Cys motifs (CXXC), and Zn2+ binding at this site is essential for holding PerR and Fur proteins in a dimeric structure (Lee and Helmann, 2006a; Traore et al., 2006; Vitale et al., 2009). It was previously reported that the Cys residues of the CXXC motifs in PerRBs are not oxidized in vivo even after treatment with high concentrations of H2O2 (10 mM) (Lee and Helmann, 2006a). In contrast, expression of PerR regulons was shown to be induced in response to low concentrations of H2O2 (∼100 μM) (Fuangthong et al., 2002; Helmann et al., 2003). Based on these facts, it has been suggested that CXXC motifs function as structural Zn2+-binding sites rather than H2O2 sensing sites.
Alkyl hydroperoxide reductase C (AhpC) is a member of the peroxiredoxin family that catalyzes reduction of organic peroxides and H2O2 into corresponding alcohols and water respectively (Wood et al., 2003). The ahpC gene and its downstream gene ahpD encoding peroxiredoxin reductase form an operon in Mycobacterium smegmatis (Daugherty et al., 2011). Since AhpC is capable of detoxifying peroxide species and peroxynitrite (ONOO−) through its enzymatic activity catalyzing the reduction of the peroxide linkage (OO), it has been suggested that this enzyme plays an important role in protection of mycobacterial cells from harmful effects of ONOO− (Master et al., 2002).
The oxyR genes are present in many mycobacteria and divergently located to the ahpC genes (Pagan-Ramos et al., 1998). The genetic organization of a divergently transcribed regulator and its target gene is typical of transcriptional regulators of the LysR family to which OxyR belongs (Schell, 1993). However, M. smegmatis and members of the Mycobacterium tuberculosis complex, including M. tuberculosis (Mtb), Mycobacterium bovis BCG, Mycobacterium africanum and Mycobacterium microti, lack a functional oxyR gene (Deretic et al., 1995, 1997). Despite the lack of OxyR, synthesis of proteins related to ROS detoxification such as AhpC and KatG has been shown to be induced in response to peroxides in M. smegmatis (Dhandayuthapani et al., 1996; Milano et al., 2001; Lee et al., 2014), which is indicative of the presence of (a) peroxide-sensing regulatory system(s) apart from OxyR in this organism. Moreover, ahpC expression has been shown to be positively regulated by Crp (cAMP receptor protein, MSMEG_6189) in M. smegmatis (Lee et al., 2014). However, the precise mechanism by which expression of ahpC is induced by peroxides in M. smegmatis remains unclear.
The mycobacterial furA genes encoding Fur homologs are located immediately upstream of katG encoding catalase/peroxidase (Pagan-Ramos et al., 1998; Milano et al., 2001; Zahrt et al., 2004). The furA and downstream katG genes form operons in Mtb H37Rv and M. smegmatis (MSMEG_6383 and MSMEG_6384) (Pym et al., 2001; Zahrt et al., 2004; Sala et al., 2003). Expression of the furA-katG operon is negatively regulated by FurA and induced by H2O2 in Mtb H37Rv (Pym et al., 2001; Sala et al., 2003).
In this study, we characterized three FurA paralogs in M. smegmatis and demonstrated that they are the functional PerR homologs that are specialized as H2O2-sensory transcriptional factors.
Results
Identification of three FurA paralogs in M. smegmatis
The four genes encoding Fur homologs (MSMEG_3460, MSMEG_4487, MSMEG_6253 and MSMEG_6383) are present in the M. smegmatis mc2155 genome sequence (Santos et al., 2008). Through phylogenetic analysis using their amino acid sequences, we found that MSMEG_3460, MSMEG_6253 and MSMEG_6383 form the same clade with FurA (Rv1909c, FurAMtb) of Mtb H37Rv (Fig. 1A). MSMEG_3460 among them is most similar to FurAMtb (85% sequence similarity). MSMEG_6253 and MSMEG_6383 also show 71% and 81% sequence similarity to FurAMtb respectively. In contrast, MSMEG_4487 forms the same clade with FurB (Rv2359) of Mtb H37Rv (76% sequence similarity) that is known to function as Zur (Maciag et al., 2007).
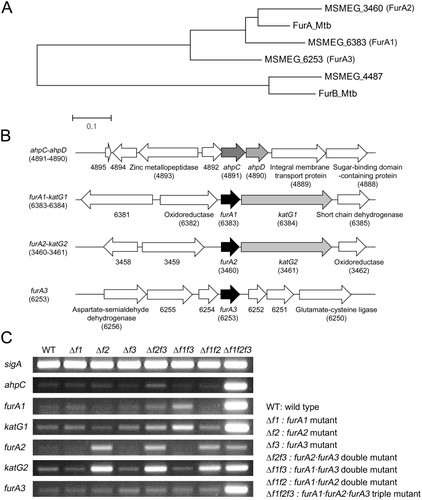
The presence of three FurA paralogs in M. smegmatis and determination of transcription levels of the ahpC, furA and katG genes in the WT and furA combinatorial mutant strains.
A. The neighbor-joining phylogenetic tree analyzing the Fur homologs of M. smegmatis and Mtb H37Rv. Phylogenetic analysis was conducted using the MEGA software (v4.0). The given distance scale indicates 0.1 amino acid substitutions per site.
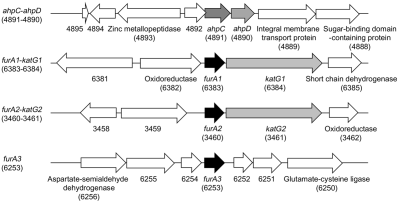
B. The diagram depicting the genetic organization of the ahpC and furA genetic loci. The open reading frames are indicated by arrows. The locus tag numbers of the genes are given in parentheses below the gene names. The genes encoding hypothetical proteins are indicated by their locus tag numbers alone.
C. The WT and furA combinatorial mutant strains were aerobically grown to an OD600 of 0.45–0.5 and transcription levels of the genes (ahpC, furA1, katG1, furA2, katG2 and furA3) in the strains were determined by RT-PCR. The RT-PCR result for sigA was included as a loading control.
Since the MSMEG_6383 gene had been annotated as furA (Zahrt et al., 2004), we annotated the three genes encoding MSMEG_6383, MSMEG_3460 and MSMEG_6253 as furA1, furA2 and furA3 respectively. In many mycobacterial species, the furA homologous genes are juxtaposed with the downstream katG genes that encode catalase/peroxidase (Pagan-Ramos et al., 1998; Zahrt et al., 2004). The MSMEG_6384 and MSMEG_3461 genes, whose products show 80% and 78% sequence similarity to KatG of Mtb H37Rv, respectively, are located downstream of furA1 and furA2 respectively (Fig. 1B). Therefore, we annotated the MSMEG_6384 and MSMEG_3461 genes as katG1 and katG2 respectively. Neither the katG homologous gene nor other catalase genes were found in the region encompassing 10 kb upstream and downstream of furA3.
Negative regulation of genes involved in peroxide detoxification by the FurA paralogs in M. smegmatis
We recently reported that expression of the ahpC-ahpD (MSMEG_4891 and MSMEG_4890) operon is positively regulated by Crp and likely to be regulated by an oxidative stress-responsive transcriptional regulator in M. smegmatis (Lee et al., 2014). Two inverted repeat sequences were identified in the upstream region of ahpC and characterized. One serves as the Crp-binding site, and the other functions as a cis-acting element for the negative regulation of ahpC. The latter inverted repeat sequence is quite similar to the FurA-binding sequence identified in Mtb (Sala et al., 2003; Lee et al., 2014). These results imply that FurA is a transcriptional repressor that controls the regulation of ahpC expression in response to oxidative stress in M. smegmatis. To ascertain the roles of the three FurA paralogs in regulation of the ahpC gene, a set of combinatorial furA deletion mutants was constructed, and the transcriptional level of ahpC was determined in the wild-type (WT) and combinatorial furA mutant strains by reverse transcription-PCR (RT-PCR). To examine whether the three furA genes (furA1, furA2 and furA3) are auto-regulated by their products, expression levels of three furA genes and two katG genes (katG1 and katG2) were determined in the WT and mutant strains (Fig. 1C). RT-PCR analysis revealed that expression of ahpC, furA1, katG1 and furA3 was significantly derepressed only when three furA genes were inactivated simultaneously (Δf1f2f3), indicating that the three FurA paralogs are functionally redundant to negatively regulate expression of ahpC, furA1-katG1 and furA3 in vivo. It is worth noting that derepression of furA2 occurred only in the strains with furA2 mutation (Δf2, Δf2f3, Δf1f2 and Δf1f2f3) among the furA combinatorial mutants. The expression pattern of furA2 was identical to that of katG2, implying that furA2 forms an operon with katG2 and that expression of the furA2-katG2 operon is negatively controlled exclusively by FurA2.
To examine the functionality of the FurA paralogs and their responsiveness to H2O2 in vivo, expression levels of ahpC, katG1, katG2 and furA3 were determined in the WT, Δf1f2f3 and three furA double mutant strains that were exposed to 15 mM H2O2 for 15 min. As controls, the strains not treated with H2O2 were included in the experiment. As shown in Fig. 2A, expression of ahpC was induced by 15 mM H2O2 in the WT and all furA double mutant strains. Interestingly, there were noticeable variations in the induction level of ahpC expression among furA double mutants in the presence of 15 mM H2O2. The highest induction level of ahpC expression was observed in the Δf1f3 mutant expressing FurA2, while the lowest and intermediate induction levels of ahpC expression were observed in the Δf2f3 and Δf1f2 mutants respectively. Using quantitative real-time PCR (qRT-PCR), expression of ahpC was quantitatively determined in the Δf2f3, Δf2f3 and Δf2f3 mutant strains that were subjected to treatment of 1 and 10 mM H2O2 (Fig. 2B). The induction patterns of ahpC observed in qRT-PCR analysis was consistent with the RT-PCR result. These expression and regulation patterns for ahpC were also observed for katG1 and furA3. In contrast, expression of katG2 was repressed only in the strains containing the intact furA2 gene (WT and Δf1f3), confirming that expression of katG2 is repressed only by FurA2 (Fig. 1C). Interestingly, katG2 expression remained repressed in the Δf1f3 mutant exposed to H2O2, while expression of ahpC, katG1 and furA3 was derepressed in the same mutant by H2O2 treatment. These results indicate that oxidized FurA2 can still repress expression of furA2-katG2, but not that of ahpC, furA1-katG1 and furA3. The functionality and responsiveness of each FurA paralog to H2O2 were confirmed using the Δf1f2f3 mutant strains complemented with each of three furA genes (Δf1f2f3 + furA1, Δf1f2f3 + furA2 and Δf1f2f3 + furA3) (Fig. 2C). The expression patterns of ahpC and katG2 in the Δf1f2f3 + furA1, Δf1f2f3 + furA2 and Δf1f2f3 + furA3 strains were same as those in the Δf2f3, Δf1f3 and Δf1f2 mutant strains respectively.
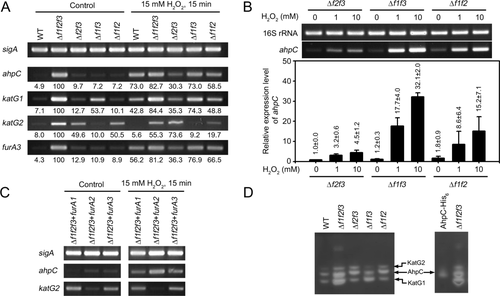
Regulation of ahpC, katG1, katG2 and furA3 by three FurA paralogs.
A. RT-PCR analysis showing transcription levels of ahpC, katG1, katG2 and furA3 in the WT, Δf1f2f3 and three furA double mutant strains (Δf2f3, Δf1f3 and Δf1f2). The M. smegmatis strains were aerobically grown to an OD600 of 0.45–0.5 (control). To investigate the responsiveness of each FurA paralog to H2O2 in vivo, aerobic cultures of the M. smegmatis strains grown to an OD600 of 0.45–0.5 were treated with 15 mM H2O2 for 15 min. Transcription levels were quantitated by densitometry using the ImageJ software (v1.37). After background correction, the values were normalized by the transcription level of sigA.
B. The expression level of ahpC in the Δf2f3, Δf1f3 and Δf1f2 mutant strains was quantitatively determined by qRT-PCR. The strains were aerobically grown to an OD600 of 0.45–0.5 and treated with 1 or 10 mM H2O2 for 15 min. The expression level of ahpC in the H2O2-untreated Δf2f3 strain is set at 1, and the relative values are expressed for the other strains. The result of RT-PCR is present above the qRT-PCR graph. The RT-PCR result for 16S rRNA was included as a loading control. All values provided are the averages of the results from three independent experiments. The error bars indicate the standard deviations.
C. RT-PCR analysis showing the expression levels of ahpC and katG2 in the Δf1f2f3 strains complemented with each of the intact furA paralog genes (Δf1f2f3 + furA1, Δf1f2f3 + furA2 and Δf1f2f3 + furA3), which were grown under H2O2-untreated (control) or treated (15 mM H2O2) conditions.
D. Catalase activity in 20 μg of crude extracts of the M. smegmatis strains was detected by activity staining. To identify the band corresponding to AhpC, 20 μg of crude extracts of E. coli BL21 (DE3) overexpressing His6-tagged AhpC of M. smegmatis were subjected to activity staining.
To assess whether expression patterns of ahpC, katG1 and katG2 correlate with the amounts of their products at the protein level, activity staining was performed to detect catalase activity in cell lysates of the WT, Δf1f2f3 and three furA double mutant strains (Fig. 2D). Three bands containing catalase activity were detected in all strains, and the intensity of all three bands was markedly increased in the Δf1f2f3 mutant relative to the WT strain. The cell lysate of the Δf1f2f3 mutant showed one additional band of unknown nature. The band corresponding to AhpC was identified from the activity staining of the cell lysate of Escherichia coli BL21 (DE3) overexpressing His6-tagged AhpC of M. smegmatis. The bands corresponding to KatG1 and KatG2 were predicted from the expression patterns of katG1 and katG2 in the furA double mutant strains presented in Figs. 1C and 2A. Two major bands detected in the Δf1f2f3 mutant were KatG1 and AhpC, indicating that under FurA inactivation conditions they are major catalase/peroxidase enzymes in M. smegmatis. Taken together, the RT-PCR and activity staining results indicate that KatG1 and AhpC are likely to play major roles in peroxide detoxification under FurA inactivation conditions, and that katG2 is constitutively expressed at low levels without induction under peroxide stress conditions.
Phenotypic characteristics of the Δf1f2f3 mutant
To investigate the effects of inactivation of the three furA genes on in vitro growth of M. smegmatis, the growth curves of the WT and Δf1f2f3 mutant strains in 7H9 medium were measured, and their doubling time was calculated (Fig. 3A). The Δf1f2f3 mutant strain showed a slower growth rate than the WT strain (the doubling times of the WT and mutant strains were 4.2 ± 0.4 and 4.9 ± 0.4 h respectively).
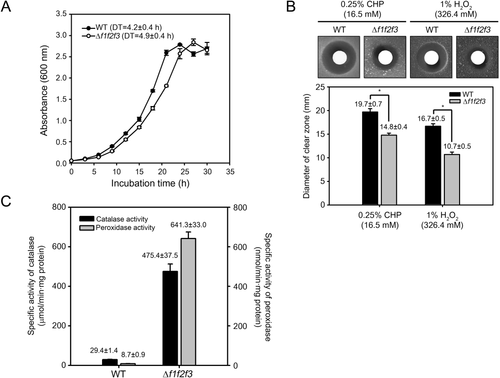
Comparison of growth and susceptibility to peroxides between the WT and Δf1f2f3 mutant strains of M. smegmatis.
A. The growth curves of the WT and Δf1f2f3 mutant strains under aerobic growth conditions. The doubling time (DT) of the strains was calculated from the growth curves. All values provided are the averages of the results from three independent experiments. The error bars indicate the standard deviations.
B. Zone inhibition assay with the WT and Δf1f2f3 mutant strains to compare their susceptibility to CHP and H2O2. The size of clear zones around the discs is related to susceptibility of the strains to the peroxides. All diameter values provided are the averages of the results from five (CHP) and three (H2O2) independent experiments. The error bars indicate the standard deviations. Single asterisk indicates significant differences between two experimental groups at p < 0.001.
C. Catalase and peroxidase activities in the WT and Δf1f2f3 mutant strains. The strains were aerobically grown to an OD600 of 0.45–0.5. The enzyme activities were measured as described in section “Experimental procedures”. All values were obtained from three independent experiments. The error bars indicate the standard deviations. Abbreviation: CHP, cumene hydroperoxide.
To examine the effects of disruption of the three furA genes on susceptibility of M. smegmatis to H2O2 and cumene hydroperoxide (CHP, an organic peroxide), zone inhibition assays were performed using the WT and Δf1f2f3 mutant strains (Fig. 3B). The Δf1f2f3 mutant gave rise to smaller inhibition zones for both H2O2 and CHP than the WT strain.
We also determined catalase and peroxidase activities in the WT and Δf1f2f3 mutant strains grown aerobically without treatment of H2O2 (Fig. 3C). Cell crude extracts of the Δf1f2f3 mutant strain showed 16.2- and 73.7-fold higher catalase and peroxidase activities than the WT strain, respectively, which accounts for low peroxide susceptibility of the Δf1f2f3 mutant relative to the WT strain.
Phylogenetic analysis of Fur homologous proteins from various bacterial species
Since the FurA paralogs of M. smegmatis regulate expression of their target genes in response to H2O2, we assumed that the FurA paralogs share common features with H2O2-sensing PerR orthologs. To verify this assumption, multiple alignment of Fur family proteins coordinating Fe2+ at the regulatory metal-binding sites (Fur, PerR and FurA) was conducted (Supporting Information Fig. S1A). Ten Fur orthologs, eight PerR orthologs and seven actinobacterial FurA proteins from various bacterial species were used in this analysis. As shown in Supporting Information Fig. S1A, the amino acid residues involved in coordination of the regulatory and structural metal ions are well conserved in most Fur family proteins. Of five amino acids comprising the regulatory metal-binding site, which corresponds to His37, Asp85, His91, His93 and Asp104 of PerRBs, three His residues are strictly conserved in the Fur family proteins. The Asp residue corresponding to Asp104 of PerRBs, which is essential for H2O2 sensing through MCHO (Parent et al., 2013), is conserved only in the PerR orthologs and actinobacterial FurA proteins. It is substituted by Glu in the Fur orthologs. The amino acid corresponding to Asp85 of PerRBs is well conserved in the PerR orthologs except for PerR3 of Bacillus licheniformis. This acidic amino acid is conservatively substituted by Glu in actinobacterial FurA proteins except for CatR of Streptomyces coelicolor (CatRSc). With the exception of Pseudomonas aeruginosa Fur that contains no structural Zn2+ ion (Lewin et al., 2002), the structural metal-binding site, which provides ligands for Zn2+ and is composed of two CXXC motifs, is well conserved in the Fur, PerR and FurA orthologs. Two additional Cys residues corresponding to Cys105 and Cys112 of FurA1 are conserved in actinobacterial FurA proteins except for CatRSc. Moreover, FurA3 contains one additional Cys (Cys65) in its DNA-binding recognition helix.
Next, phylogenetic analysis of the Fur family proteins used in the multiple alignment analysis was performed (Supporting Information Fig. S1B). Clade 1 consists of the well-studied Fur proteins of E. coli and B. subtilis, as well as many Fur orthologs from other bacteria (Fillat, 2014). Clade 2 includes well studied PerRBs and various PerR orthologs of other bacteria which serve as H2O2-responsive transcription factors. The FurA proteins, which are found in actinobacteria and involved in oxidative stress response, form clade 3. The results of the multiple alignment and phylogenetic analyses demonstrate that the primary structures of the Fur homologs provide sufficient information to categorize Fur family proteins into the functional subgroups, Fur, PerR and actinobacterial FurA groups.
Inactivation of the FurA paralogs by H2O2-dependent His oxidation
To investigate H2O2-dependent inactivation of M. smegmatis FurA proteins in vivo, we monitored protein oxidation by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) using E. coli strains overexpressing each of the three FurA paralogs as described in section “Experimental procedures” (Fig. 4). When the E. coli strains were treated with 0.1 mM H2O2 for 1 min, FurA1 exhibited oxidation at two tryptic peptides, T5 (from Ser28 to Arg42, m/z = 1681.76) and T12* (from Val85 to Arg95, m/z = 1329.61), as judged by an increase of 16 Da in their mass. H2O2-dependent oxidation also occurred at two tryptic peptides of FurA2, T5 (from Ile23 to Arg45, m/z = 2524.25) and T11* (from Val85 to Arg95, m/z = 1329.62), although oxidation of the T11* peptide was marginal. FurA3 also showed H2O2-dependent oxidation at the tryptic peptides T5 (from Val25 to Arg47, m/z = 2421.26) and T11* (from Thr87 to Arg97, m/z = 1345.63) with the marginal oxidation of T11*. We mapped the sites (amino acids) of oxidation responsible for the 16 Da mass increase using liquid chromatographic-electrospray ionization tandem mass spectrometry (LC-ESI MS/MS). The sites of oxidation for T5 + 16 and T12*+16 from FurA1 were mapped to His34 and His89 respectively (Supporting Information Figs. S2 and S3). Those for T5 + 16 and T11* + 16 from FurA2 were mapped to His34 and His89, respectively (Supporting Information Figs. S4 and S5), and those for T5 + 16 and T11* + 16 from FurA3 were mapped to His36 and His91 respectively (Supporting Information Figs. S6 and S7). An increase of 16 Da in the mass of the tryptic peptides containing the His residues corresponding to His37 and His91 of PerRBs indicates that one oxygen atom was incorporated to the His residues involved in Fe2+ coordination like PerRBs (Lee and Helmann, 2006b). The tryptic peptides of the FurA paralogs with the His residues corresponding to His91 of PerRBs (T12* of FurA1 and T11* of FurA2 and FurA3) showed a relatively lower propensity for H2O2-dependent His oxidation than those with the His residues corresponding to His37 of PerRBs (T5 of the three FurA proteins), indicating that the His residues in T5 fragments are the major oxidation sites. Interestingly, relatively high fractions of FurA2 and FurA3 were oxidized by H2O2 relative to FurA1, which was also observed even without exogenous H2O2 treatment. These results imply that FurA2 and FurA3 are more easily oxidized by MCHO than FurA1.
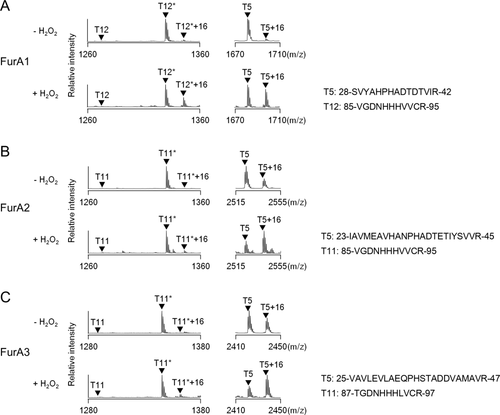
In vivo H2O2-induced His oxidation of three FurA paralogs in E. coli. Oxidation of FurA1 (A), FurA2 (B) and FurA3 (C) by H2O2. E. coli cells expressing C-terminally His6-tagged FurA1, FurA2 or FurA3 were grown in LB medium under aerobic conditions and treated with 100 μM H2O2 for 1 min (+ H2O2). The same strains without H2O2 treatment were included in the experiment (− H2O2). Oxidation of proteins was analyzed by MALDI-TOF MS after SDS-PAGE and subsequent in-gel tryptic digestion. This result is representative of three independent experiments. Asterisks represent the peptides containing carboxyamidomethylated Cys residue(s).
Quaternary structure of three FurA proteins
The members of the Fur family such as Fur, PerR and Zur are present mostly as dimers in solution (Pohl et al., 2003; Traore et al., 2006; Lucarelli et al., 2007; Sheikh and Taylor, 2009; Dian et al., 2011; Makthal et al., 2013). To determine the quaternary structure of the FurA paralogs, His6-tagged FurA proteins were purified and subjected to analytical size exclusion chromatography (Fig. 5A). The size exclusion chromatogram of FurA1 showed that the major species was eluted as the dimeric form, whereas that of FurA2 showed the major peak corresponding to the monomeric form. The chromatogram of FurA3 displayed the peak for the major species eluted at the volume corresponding to the dimer. The shape of the FurA3 peak was broader than that of the FurA1 and FurA2 peaks, indicating that purified FurA3 might be a mixture of dimers and other species with different structural states.
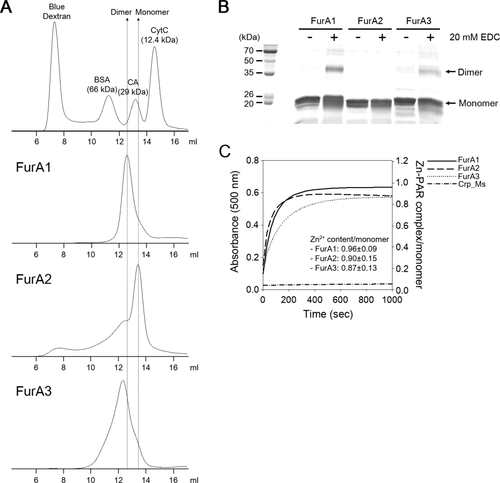
The quaternary structure of three FurA paralogs.
A. The elution profiles of His6-tagged FurA proteins. Gel filtration chromatography of the purified FurA proteins (15 nmol) was conducted with a Superose 12 10/300 GL column equilibrated with 20 mM Tris-HCl (pH 8.0) containing 200 mM NaCl at 8°C. To extrapolate the molecular mass of eluents from the elution profiles, the following standard proteins were subjected to gel filtration chromatography. The result is representative of three independent experiments. Abbreviation: bovine serum albumin (BSA), carbonic anhydrase (CA) and cytochrome c (CytC).
B. Crosslinking analysis of the FurA paralogs. Purified FurA proteins were incubated in 20 mM MOPS (pH 8.0) buffer containing 150 mM KCl and 20 mM EDC for 2 h at room temperature in the dark. The crosslinked products were separated by SDS-PAGE with a 12.5% (wt/vol) acrylamide gel. The result is representative of two independent experiments. Abbreviation: EDC, ethylene carbodiimide.
C. Release of Zn2+ from purified FurA proteins by H2O2. Zn2+ release from the purified FurA paralogs was monitored by PAR assay. Purified His6-tagged FurA proteins (10 μM) were treated with 100 mM H2O2 in the presence of 100 μM PAR. The dissociated Zn2+, which was chelated by PAR, was monitored spectrophotometrically at 500 nm at intervals of 30 s for 1000 s. Purified His6-tagged Crp (cAMP-receptor protein) of M. smegmatis was used as a negative control. The plot for PAR assay was drawn using the averaged data from five independent experiments (purifications and PAR assays). The Zn2+ contents of the FurA proteins presented inside the plot are expressed as mean ± standard deviation. Abbreviation: PAR, 4-(2-pyridylazo) resorcinol.
To confirm the results of size exclusion chromatography, a crosslinking experiment was performed using purified FurA proteins and ethylene carbodiimide (EDC) that forms a covalent linkage between a carboxyl group and an amine group in a salt bridge connection (Fig. 5B). Treatment of purified FurA1 with 20 mM EDC gave rise to the band corresponding to the crosslinked dimeric product with a molecular mass of approximately 40 kDa on the SDS-PAGE gel. The 40 kDa band was also observed in the EDC-treated FurA3 sample, although the band intensity was weaker than that of FurA1. In contrast, no noticeable band corresponding to the dimer appeared when purified FurA2 was treated with EDC. The obtained results are in good agreement with those of size exclusion chromatography showing that FurA1 and FurA3 are primarily present as dimers, while FurA2 is present as a monomer.
Two CXXC motifs coordinating Zn2+ are known to be well conserved in members of the Fur family and required for the maintenance of the dimeric state (Lee and Helmann, 2006a; Traore et al., 2006; Vitale et al., 2009). To determine the Zn2+ contents of the purified FurA proteins, the amounts of Zn2+ released from the purified His6-tagged FurA proteins by 100 mM H2O2 were spectrophotometrically monitored using 4-(2-pyridylazo) resorcinol (PAR, a Zn2+ chelator) (Fig. 5C). The Zn2+ contents of the FurA proteins were determined using both the extinction coefficient of 66,000 M−1 cm−1 at 500 nm for Zn2+–PAR complex (Hunt et al., 1985) and the maximum absorbance in the presence of 100 mM H2O2. The C-terminally His6-tagged Crp protein of M. smegmatis, which is known not to contain Zn2+, was included in the experiment as a negative control. The Zn2+ contents of the purified FurA paralogs per monomer were determined to be 0.96 ± 0.09 for FurA1, 0.90 ± 0.15 for FurA2 and 0.87 ± 0.13 for FurA3.
Identification of the protein region responsible for differences in the quaternary structure of the three FurA paralogs
Despite its high sequence similarity to FurA1 and FurA3 (FurA1 and FurA2, 77%; FurA2 and FurA3, 72%) that have the quaternary structure of dimer, FurA2 exists as a monomer. The monomeric FurA2 and the dimeric FurA1 and FurA3 showed local sequence differences in the C-terminal part of their dimerization domains (the boxed region shown in Fig. 6A). To investigate whether the monomeric structure of FurA2 is attributable to this sequence difference in the dimerization domain, the boxed C-terminal region of FurA2 (33 amino acids) was swapped with the corresponding region of FurA1 (40 amino acids), generating a chimeric protein, FurA2_A1 (Fig. 6B). Size exclusion chromatography using purified FurA2_A1 showed that the major peak corresponding to the dimeric species appeared in the chromatogram of FurA2_A1 (Fig. 6C). The effect of the subdomain swapping of FurA2 and FurA1 clearly indicates that the C-terminal region of FurA2 (from Thr114 to Pro146 in the dimerization domain) primarily determines the monomeric structure of FurA2, and that the corresponding region of FurA1 appears to be important for the maintenance of the dimeric structure.
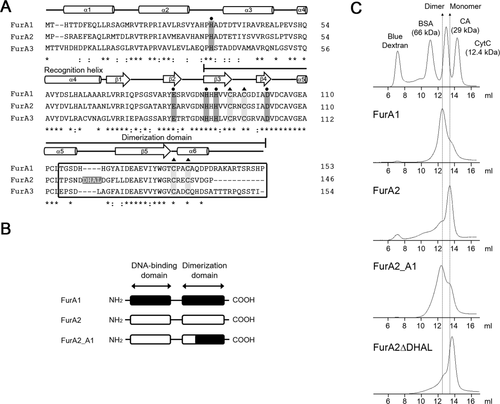
Identification of the region responsible for determination of the oligomeric state of FurA paralogs.
A. Multiple alignment of three FurA paralogs was generated using ClustalW. The position of secondary structure elements was predicted from the three-dimensional structure of PerRBs (Traore et al., 2006). The secondary structure elements are indicated by cylinders and arrows for α-helices and β-strands respectively. The dimerization domain is indicated. The four amino acids of FurA2 (DHAL) between α5 and β5 are shown as white letters in the gray background. The identical amino acids are indicated with asterisks. The colons and dots denote conserved and semiconserved substitutions respectively.
B. The diagram depicting the construction of the chimeric FurA, FurA2_A1.
C. The elution profiles of the chimeric FurA2_A1 protein and the mutant form of FurA2 with deletion of the four amino acids (FurA2ΔDHAL). Purified FurA1 and FurA2 proteins were used as markers for dimeric and monomeric species respectively. The purified proteins (15 nmol) were loaded onto a Superose 12 10/300 GL column equilibrated with 20 mM Tris-HCl (pH 8.0) containing 200 mM NaCl.
The spacer region between the two CXXC motifs coordinating Zn2+ is four amino acids longer in FurA2 than those in FurA1 and FurA3 (Fig. 6A). To examine whether the difference in the quaternary structure between FurA2 and FurA1 (FurA3) is due to this four amino acid extension, the four amino acids were removed from the FurA2 protein (FurA2ΔDHAL), and purified FurA2ΔDHAL was subjected to size exclusion chromatography. As shown in Fig. 6C, purified FurA2ΔDHAL was present as a monomer like FurA2, suggesting that the monomeric structure of FurA2 is not attributable to the longer spacer region between the two CXXC motifs.
Analysis of the upstream sequences of the ahpC and three furA genes
The transcription start points (TSPs) of ahpC and furA1 were previously determined by conventional methods such as S1 nuclease protection and primer extension analyses (Dhandayuthapani et al., 1996; Milano et al., 2001). Based on the finding that there is strong concordance between the reported TSPs for ahpC and furA1 and the first nucleotide positions with a rapid increase in read counts of the ahpC and furA1 mRNAs in the Δf1f2f3 mutant, the TSPs of furA2 and furA3 were predicted from the RNA sequencing data (Fig. 7A and Supporting Information Fig. S8). The predicted TSPs of furA2 (G) and furA3 (T) were shown to precede their start codons by only three and one nucleotides, respectively, in a similar way as furA1. These results indicate that three furA genes are transcribed as leaderless mRNAs that do not contain the canonical ribosomal binding sites (Shine-Dalgarno [SD] sequences) (Grill et al., 2000; Moll et al., 2002).
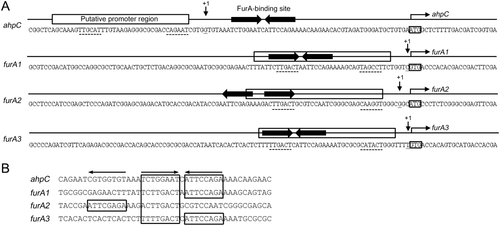
Analysis of the upstream sequences of ahpC, furA1, furA2 and furA3 encompassing their putative promoters and FurA-binding sites.
A. The upstream regions of ahpC, furA1, furA2 and furA3. The TSPs (+1) of ahpC and the furA1-katG1 operon were reported previously (Dhandayuthapani et al., 1996; Milano et al., 2001) and are indicated by the downward arrows. The TSPs of furA2 and furA3 were predicted from RNA deep sequencing data of the whole transcriptomes of the Δf1f2f3 mutant strain (Supporting Information Fig. S8). The putative promoter regions of the four genes were deduced from the position of their TSPs and indicated by the rectangular boxes above the sequences. The putative −35 and −10 regions are underlined with the dotted lines. The predicted FurA-binding sites are marked by the thick horizontal arrows above their sequences. The start codons of the genes are boxed, and the arrows above them indicate the transcriptional direction.
B. Multiple alignment of the nucleotide sequences of the FurA-binding sites upstream of the ahpC and three furA genes. The octamer motifs corresponding to the half site of the FurA-binding sites are indicated by the arrows above the alignment are boxed.
The putative promoter regions for ahpC, furA1, furA2 and furA3, which are similar to those of the mycobacterial −35 and −10 regions, were predicted from the identified TSPs (Fig. 7A). As previously reported, the FurA-binding site upstream of ahpC is an inverted repeat composed of two octamer motifs in a forward-to-reverse (FR) configuration separated by one nucleotide (TCTGGAAT-C-ATTCCAGA) (Fig. 7B). This site is located 31-bp upstream of the ahpC start codon and 7-bp downstream of the TSP. The furA1 and furA3 genes also possess the putative FurA-binding sites with FR configurations in their regulatory regions. Interestingly, two octamer motifs located 31-bp upstream of furA2 are spaced by three nucleotides and arranged in a reverse-to-forward (RF) configuration (ATTCGAGA-AAG-ACTTGACT) (Fig. 7B). The putative FurA-binding sites upstream of furA1, furA2 and furA3 completely overlap the −35 regions of their putative promoters, which accounts for negative autoregulation of the three furA genes.
Binding of FurA1, FurA2 and FurA3 to the FurA-binding sites
To determine if purified FurA1, FurA2 and FurA3 can bind to the FurA-binding sites with a FR or RF configuration, Electrophoretic mobility shift assays (EMSA) were conducted using purified FurA proteins and 112-bp DNA fragments (160 fmol) encompassing either the ahpC or furA2 regulatory region, as well as 60-bp DNA fragment (80 fmol) without the FurA-binding sequence (non-specific DNA) (Fig. 8A). When the DNA fragments containing the ahpC regulatory region were employed, the amounts of FurA–DNA complexes were increased in proportion to the concentration of the three FurA proteins, while the non-specific DNA was not shifted by the FurA proteins. Conspicuously, FurA3 bound to the DNA fragments to a much lesser extent than FurA1 and FurA2. The result implies that purified FurA3 contains a large fraction of inactivated forms that lost its DNA-binding ability, which might result from the high tendency of FurA3 for His oxidation (Fig. 4).
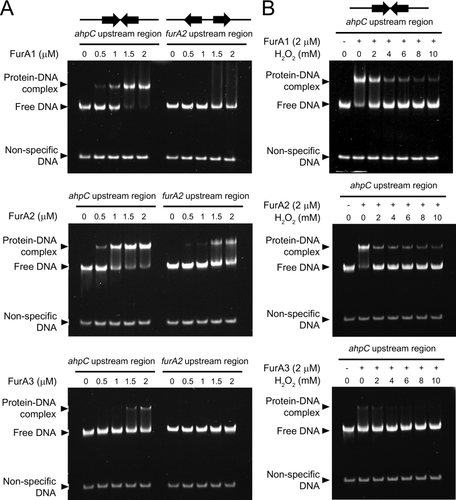
EMSA showing the binding of three FurA paralogs to the upstream regions of ahpC and furA2.
A. The 112-bp DNA fragments (160 fmol) containing either the upstream region of ahpC (ahpC upstream region) or furA2 (furA2 upstream region) and 60-bp non-specific DNA fragments without the FurA-binding sequence (80 fmol) were incubated with various concentrations of purified FurA proteins for 20 min at 25°C.
B. To investigate the effects of H2O2 treatment on FurA binding to the ahpC upstream region, DNA–FurA reaction mixtures were incubated in the presence of H2O2 (0–10 mM) for 20 min at 25°C. The concentrations of FurA used in EMSA are given above the lanes. The DNA–FurA reaction mixtures were subjected to native PAGE (8% [wt/vol] acrylamide gel). Gels were stained with SYBR green staining solution after electrophoresis.
When the furA2 regulatory region was used in EMSA, the distinct retarded bands were only observed in the reaction with FurA2. FurA2 bound to the furA2 regulatory region with a lower affinity than to the ahpC regulatory region (Fig. 8A). Although the amounts of free DNA were slightly decreased with increasing concentrations of FurA1, no distinct band corresponding to the FurA1–DNA complex appeared, indicating weak binding of FurA1 to the furA2 regulatory region. Purified FurA3 did not bind to the furA2 regulatory region under our experimental conditions. The results of EMSA indicate that FurA2 monomers, as well as FurA1 and FurA3 dimers can bind to the FurA-binding site consisting of two octamer motifs in an inverted repeat (FR) configuration, while only FurA2 monomers can bind to the FurA-binding site with an everted repeat (RF) configuration strongly enough to repress the expression of furA2. To examine the effects of H2O2 treatment on the DNA-binding ability of the FurA proteins, various concentrations of H2O2 (2–10 mM) were added to the protein–DNA reaction mixtures (Fig. 8B). Treatment of FurA1, FurA2 and FurA3 with H2O2 resulted in a decrease in retarded FurA–DNA complexes, which is indicative of inactivation of the FurA proteins by H2O2. FurA2 was more sensitively inactivated by H2O2 than FurA1 (we could not evaluate the EMSA result of FurA3 due to a weak DNA-binding ability of purified FurA3).
We next examined the effects of modification of the FurA-binding site on ahpC expression (Fig. 9). The DNA fragments containing the ahpC regulatory region with the WT or mutated FurA-binding sites were fused with the promoterless lacZ gene, yielding ahpC-lacZ transcriptional fusion plasmids (Fig. 9B). pNCahpC is a pNC-based ahpC-lacZ transcriptional fusion plasmid, and pNCM3 has the same construction as pNCahpC except for a 12-bp deletion within the FurA-binding site (Lee et al., 2014). The spacer ‘C’ of the inverted repeat within the FurA-binding site was replaced with six nucleotides (TAGACT), yielding pNCM5. The spatial arrangement of two octamer motifs separated by the extended spacer was expected to make the FurA-binding site unsuitable for serving as the binding site of dimeric FurA proteins. pNCM8 contains the ahpC regulatory region with only the right half of the FurA-binding site. The WT strains of M. smegmatis harboring pNCahpC, pNCM3, pNCM5 or pNCM8 were grown aerobically without treatment of H2O2, and the promoter activity of ahpC was determined by β-galactosidase assay (Fig. 9C). When the FurA-binding site was deleted from the ahpC control region (pNCM3), expression of ahpC was derepressed by 15.9-fold relative to that from pNCahpC. The WT strain harboring pNCM5 showed 55% of β-galactosidase activity detected in the WT strain with pNCM3, indicating that expression of ahpC is still significantly repressed in the WT strain with pNCM5. The WT strain harboring pNCM8 showed a marginal reduction in β-galactosidase activity relative to the WT strains containing pNCM3. Interestingly, the differences in ahpC expression observed in the WT strains harboring pNCM3, pNCM5 and pNCM8 were abolished in the Δf2 mutant of M. smegmatis that did not express FurA2 (Fig. 9C). The results imply that the partial repression of ahpC in the WT strain with pNCM5 is probably due to the binding of FurA2 monomers to two octamer motifs spaced by six nucleotides, and that the binding of one FurA2 monomer to a single octamer motif in pNCM8 elicits only marginal repression of ahpC.
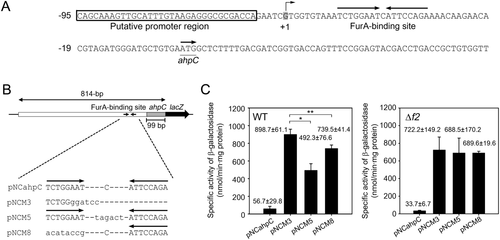
Effect of mutations in the FurA-binding site on ahpC expression.
A. The upstream sequence of ahpC. The putative FurA-binding site is marked by two head-facing arrows above the sequence. The start codon of ahpC is underlined, and the arrow above it denotes the transcriptional direction. The numbers on the left of the sequence indicate the position of the leftmost nucleotides relative to the start codon of ahpC. +1 indicates the TSP of ahpC.
B. ahpC–lacZ transcriptional fusion plasmids carrying the ahpC regulatory region with the WT (pNCahpC) or mutated FurA-binding sites (pNCM3, pNCM5 and pNCM8). The mutations introduced into the FurA-binding site are depicted. The substituted or inserted nucleotides are lettered in lower case.
C. Effect of the mutations within the FurA-binding site on the promoter activity of ahpC in the WT and Δf2 mutant strains. The M. smegmatis strains harboring pNCahpC, pNCM3, pNCM5 or pNCM8 were grown aerobically to an OD600 of 0.45–0.5. Cell crude extracts were used to determine β-galactosidase activity. All values are the means of three independent experiments. The error bars indicate the standard deviations. Single and double asterisks indicate statistically significant differences at p < 0.01 and p < 0.05 respectively.
Identification of the FurA regulon in M. smegmatis
To identify the FurA regulon in M. smegmatis, comparative analysis of transcriptome profiles of the aerobically grown WT and Δf1f2f3 mutant strains was performed by RNA deep sequencing. Changes in gene expression in the Δf1f2f3 mutant relative to the WT strain were expressed as fold changes of expression. The genes with a p value of ≤ 0.01 and a log2 fold change (log2FC) of ≥ 3 and ≤ −3 were considered as differentially expressed genes in this report (Fig. 10).
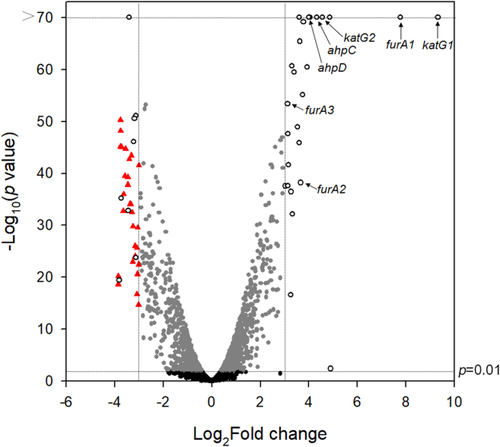
Volcano plot showing the differentially expressed genes in the Δf1f2f3 mutant strain relative to the WT strain. RNA sequencing was performed using RNA prepared from three independent replicate cultures of the WT and Δf1f2f3 strains grown aerobically in 7H9 medium to an OD600 of 0.45–0.5. The volcano plot displays the log2FC of gene expression in the Δf1f2f3 mutant strain relative to the WT strain. The −log10 (p value) indicates statistical significance in differential gene expression between the WT and Δf1f2f3 mutant strains. The vertical dotted lines on the graph indicate the border lines showing log2FC values of −3 and 3. The genes with p value < 0.01 and |log2(mutant/WT)| > 3 are indicated by open circles. The genes discussed in this report are marked. The genes, which are significantly repressed (log2FC < −3) in the Δf1f2f3 mutant strain and at the same time belong to the SigF regulon, are indicated by red triangles.
We identified 26 significantly induced genes (Supporting Information Table S1) in the Δf1f2f3 mutant compared with the WT strain. One fourth of 26 induced genes are related to peroxide detoxification (the genes encoding alkyl hydroperoxide reductase, catalase/peroxidase and three FurA paralogs).
We also identified 37 significantly repressed genes in the Δf1f2f3 mutant relative to the WT strain (Supporting Information Table S2). Interestingly, a large portion (29 genes, 78.4%) of 37 repressed genes overlaps the known SigF regulon of M. smegmatis (Fig. 10 and Supporting Information Table S2) (Singh et al., 2015). The significantly repressed genes other than the SigF regulon and the genes of unknown function include aceA (MSMEG_0911) encoding isocitrate lyase (McKinney et al., 2000; Munoz-Elias and McKinney, 2005), as well as glpK (MSMEG_6759) and glpD (MSMEG_6761) encoding glycerol kinase and glycerol-3-phosphate dehydrogenase, respectively (Seno and Chater, 1983; Titgemeyer et al., 2007), implying downregulation of the glyoxylate shunt and glycerol metabolism in the Δf1f2f3 mutant.
Comparison of expression levels of furA1, furA2 and furA3
Based on the reads per kilo base pair per million mapped reads (RPKM) values obtained from RNA sequencing analysis, expression levels of furA1, furA2 and furA3 in the aerobically grown WT and Δf1f2f3 mutant strains of M. smegmatis were compared (Supporting Information Fig. S9). Because the Δf1f2f3 mutant has deletions (198, 181, 164 bp) within furA1, furA2 and furA3, respectively, the RPKM values of furA1, furA2 and furA3 in the mutant were calculated using the length of the mutated furA genes with deletion in place of that of the intact genes.
In the WT strain, furA2 and furA3 showed 3.1- and 4.6-fold higher expression levels than furA1. In the Δf1f2f3 mutant strain that mimics FurA-inactivation conditions such as H2O2-treated conditions, expression of furA1, furA2 and furA3 was shown to be significantly induced, and the furA1 expression level was highest. These results indicate that FurA2 and FurA3 are the major FurA proteins in the WT strain under H2O2-untreated conditions, while FurA1 is the most abundant FurA protein in the WT strain under H2O2-treated conditions. Given that expression of furA2 is not induced by H2O2 (see katG2 expression in Fig. 2A), the cellular level of FurA2 in the WT strain under H2O2-treated conditions is likely to be significantly low as compared with that of FurA1 and FurA3.
Discussion
M. smegmatis contains three genes (MSMEG_3460, MSMEG_6253 and MSMEG_6383) encoding the FurA paralogs. Using various furA mutant strains of M. smegmatis, we demonstrated that all three FurA paralogs are functional in vivo to negatively regulate the expression of target genes such as ahpC, furA1 and furA3 that have a 8–1–8 palindrome sequence (TCTGGAAT-N-ATTCCAGA) or its derivative sequences in their upstream regulatory regions. Comparative analysis of transcriptomes of the Δf1f2f3 mutant and WT strains revealed that expression of a subset of genes involved in peroxide detoxification such as furA1-katG1, furA2-katG2, furA3 and ahpC-ahpD was strongly derepressed (by more than eightfold) in the Δf1f2f3 mutant, whereas expression of genes related to iron metabolism and homeostasis (mbt and fxb, mycobactin and exochelin synthesis; exiT and fxu, exochelin transport; bfrA, bacterioferritin) was not noticeably changed in the Δf1f2f3 mutant when compared with the WT strain (Supporting Information Tables S1 and S3). Similarly, it has been reported that FurA of Mycobacterium avium subspecies paratuberculosis is involved in oxidative stress responses, but not in iron homeostasis (Eckelt et al., 2015). In the high-GC Gram-positive bacteria including mycobacteria, the iron-dependent transcriptional regulator (IdeR) protein belonging to the diphtheria toxin regulator (DtxR) family is known to be implicated in the regulation of genes related to iron metabolism and homeostasis (Fiss et al., 1994; Ratledge, 2004; Cornelis et al., 2011). In addition to AhpC, KatG1 and KatG2, M. smegmatis possesses several defense systems that counteract imposing oxidative stress. The bacterium contains six additional catalase genes (MSMEG_3486, MSMEG_3708, MSMEG_3729, MSMEG_4204, MSMEG_6213 and MSMEG_6232) and three superoxide dismutase genes [sodC (MSMEG_0835), sodA1 (MSMEG_6427) and sodA2 (MSMEG_6636)], as well as the ohr (MSMEG_0447) and osmC (MSMEG_2421) genes whose products are known to contribute to detoxification of alkyl or organic peroxides (Lesniak et al., 2002, 2003; Saikolappan et al., 2011). With regard to defense against thiol-oxidative stress in mycobacteria, both mycothiol as a functional equivalent of glutathione and the Trx-TrxB (thioredoxin-thioredoxin reductase) system play an important role (Raman et al., 2001; Manganelli et al., 2002). With the exception of ahpC-ahpD, furA1-katG1 and furA2-katG2, no significant increase in gene expression was observed for genes related to oxidative stress defense such as catalase genes (MSMEG_3486, MSMEG_3708, MSMEG_3729, MSMEG_4204, MSMEG_6213 and MSMEG_6232), superoxide dismutase genes (sodC, sodA1 and sodA2), ohr, trx, osmC, dps, egtD and genes involved in mycothiol biosynthesis (mshA, mshB, mshC and mshD) in the Δf1f2f3 mutant of M. smegmatis when compared with the WT strain (Supporting Information Table S3). Expression of regulatory genes related to oxidative stress responses, for example, ohrR encoding organic hydroperoxide stress resistance regulator (Saikolappan et al., 2015), rbpA encoding a regulatory RNA polymerase-binding protein (Hu et al., 2016), oxyS (Li and He, 2012) and alternative sigma factor genes (sigE, sigF and sigH) (Fernandes et al., 1999; Raman et al., 2001; Manganelli et al., 2002; Humpel et al., 2010; Singh et al., 2015; Hu et al., 2016), was not significantly altered in the Δf1f2f3 mutant when compared with the WT strain. Taken together, our results suggest that the FurA proteins in M. smegmatis are not the Fur orthologs pertaining to iron metabolism and homeostasis, but the PerR orthologs that selectively regulate the expression of ahpC, katG1 and katG2 among oxidative stress defense genes.
Multiple alignment analysis revealed that three His and two acidic residues constituting the regulatory metal-binding sites of PerR and Fur proteins are well conserved in three FurA paralogs of M. smegmatis (His34, Glu82, His89, His91 and Asp102 in FurA1) (Supporting Information Fig. S1). The Asp residues (Asp102, Asp102 and Asp104 in FurA1, FurA2 and FurA3, respectively) corresponding to Asp104 of PerRBs, which is conserved specifically in PerR orthologs and has been suggested to play a crucial role in MCHO (Parent et al., 2013), are conserved in the three FurA paralogs of M. smegmatis, reinforcing that the three FurA paralogs belong to the PerR subfamily. Mass spectrometry analyses showed that the short treatment (1 min) of the E. coli strains expressing M. smegmatis FurA proteins with low concentrations (100 μM) of H2O2 led to the oxidation of considerable fractions of the three FurA paralogs by the incorporation of an oxo group into one of two His residues coordinating the regulatory metal (Fig. 4 and Supporting Information Figs. S2–S7). The level of His oxidation observed for the three FurA paralogs was comparable to that of PerR proteins treated by H2O2 under the same experimental conditions (Won et al., 2010; Kim et al., 2016). The high sensitivity of the three FurA paralogs to His oxidation by H2O2 suggests that they are rapidly inactivated by H2O2 through MCHO like PerR proteins. To the best of our knowledge, this is the first demonstration of MCHO in mycobacterial FurA proteins. It is noteworthy that oxidation of the His residues in the FurA paralogs occurs with the favored incorporation of the oxygen atom to the His residues corresponding to His37 of PerBBs relative to the His residues corresponding to His91, which is similar to the oxidation pattern of PerRBs (Traore et al., 2009). FurA2 and especially FurA3 were shown to be oxidized by endogenously produced H2O2 to a larger degree than FurA1 in H2O2-untreated E. coli cells (Fig. 4). Furthermore, purified FurA3 was shown to include larger fractions of inactivated forms than purified FurA2 and FurA3 (Fig. 8). These findings imply that FurA2 and especially FurA3 are more easily oxidized than FurA1 when exposed to low concentrations of H2O2. It has been reported that the channel for H2O2 leading to the regulatory metal-binding site of PerRBs is surrounded by the carbonyl groups of Ala36, Phe86, Ser89 and Val103, the hydroxyl group of Thr88 and the amino group of Lys101. This hydrophilic environment was suggested to be related to the preference of PerRBs for H2O2 over hydrophobic organic peroxides (Jacquamet et al., 2009). The Thr and Lys residues corresponding to Thr88 and Lys101 of PerRBs are relatively well conserved in PerR orthologs (Supporting Information Fig. S1). Interestingly, only FurA3 among three FurA paralogs of M. smegmatis contains Thr87 and Arg101 corresponding to Thr88 and Lys101 of PerRBs, respectively, which might provide a plausible explanation for the highest propensity of FurA3 for MCHO by H2O2 (Fig. 4).
The structural Zn2+ ion is known to be coordinated by four Cys residues that are arranged as two CXXC motifs in all PerR orthologs. These CXXC motifs are also conserved in the three FurA paralogs of M. smegmatis. Purified FurA1, FurA2 and FurA3 proteins were shown to contain 0.87–0.96 Zn2+ per monomer as judged by the result of the PAR assay (Fig. 5C). These findings indicate that FurA monomers coordinate Zn2+ ions by two CXXC motifs with a 1:1 stoichiometry like PerR proteins. The rate of Zn2+ release from PerRBs by H2O2 was reportedly too slow to explain in vivo peroxide response of B. subtilis (Lee and Helmann, 2006a). Moreover, it has been demonstrated that the Cys residues coordinating Zn2+ of PerRBs were not oxidized in vivo by treatment with even high concentrations (10 mM) of H2O2 in contrast to MCHO (Lee and Helmann, 2006a), indicating that the in vivo inactivation of PerRBs by H2O2 is not the result of Cys oxidation. These facts regarding PerRBs inactivation, together with our finding that the Fe2+-coordinating His residues in the FurA proteins are rapidly oxidized by treatment of micromolar concentrations of H2O2 (Fig. 4), allowed us to suggest that the three FurA paralogs in M. smegmatis exposed to H2O2 rapidly lose their DNA-binding ability by MCHO, rather than by the oxidation of the Cys residues coordinating Zn2+.
In the presence of millimolar concentrations of H2O2, the M. smegmatis strain expressing only FurA2 showed the highest expression level of ahpC, katG1 and furA3 among M. smegmatis strains expressing only one of three FurA paralogs, while the expression level of the genes was lowest in the M. smegmatis strain expressing only FurA1 (Fig. 2). The different responsiveness of the M. smegmatis strains expressing one of three FurA paralogs to H2O2 is likely caused by the different sizes of active FurA pools, which are determined by the inactivation and synthesis rates of each FurA paralog. Under H2O2 treatment conditions the cellular level of active FurA2 in M. smegmatis is assumed to be smaller than that of active FurA1 and FurA3. This is because expression of furA2 is not induced by H2O2 in contrast to furA1 and furA3 (see katG2 expression in Fig. 2A), and FurA2 has a higher propensity for MCHO than FurA1 (Fig. 4). In contrast, the lowest responsiveness of FurA1 to H2O2 could be explained by strong induction of furA1 expression under H2O2 treatment conditions (see katG1 expression in Fig. 2A and Supporting Information Fig. S9) and high reluctance of FurA1 to MCHO relative to FurA2 and FurA3 (Fig. 4).
The dimeric FurA1 and FurA3 were shown to negatively regulate expression of ahpC-ahpD, furA1-katG1 and furA3, but not that of furA2-katG2. However, the monomeric FurA2 could repress furA2-katG2 in addition to ahpC-ahpD, furA1-katG1 and furA3 (Fig. 2). The FurA-binding sites upstream of ahpC, furA1 and furA3 consist of the inverted repeat sequences with two octamer motifs arranged in an 8–1–8 configuration (TCTGGAAT-N-ATTCCAGA), while the binding site upstream of furA2 is composed of two octamer motifs arranged in an everted repeat (Fig. 7). From these findings, together with the results of Figs. 8 and 9, we assumed the followings: (i) the helix-turn-helix DNA-binding domains of three FurA paralogs recognize and bind to the same octamer motif. (ii) The dimeric FurA1 and FurA3 can use exclusively the 8–1–8 inverted repeat sequences as operator sequences, and their binding to two octamer motifs arranged differently from the 8–1–8 configuration or a single octamer motif appears not to be strong enough to elicit repression. (iii) The FurA2 monomer binds to the single octamer motif more strongly than the dimeric FurA1 and FurA3 due to its small size and molecular mass. (iv) The presence of two or more octamer motifs in the vicinity enables FurA2 to repress gene expression more strongly. These assumptions might explain a broader binding-specificity of FurA2 for the target DNA than the dimeric FurA1 and FurA3 that use exclusively the 8–1–8 inverted repeat as a binding-sequence. Our EMSA showed that FurA2 monomers bind to the inverted repeat sequence upstream of ahpC with a higher affinity than to the everted repeat sequence in the promoter region of furA2-katG2 (Fig. 8), which might indicate the cooperative binding of FurA2 monomers to the 8–1–8 inverted repeat sequence.
Dissociation of the regulatory metal from the regulatory site as a result of MCHO has been demonstrated to lead to conformational changes of dimeric PerRBs from a caliper-like structure to an open structure that is unsuitable for the binding of PerRBs to the target DNA sequence arranged in the inverted repeat configuration (Traore et al., 2006). This paradigm can be also applied to the dimeric FurA1 and FurA3, as judged by the results of mass spectrometry analyses and EMSA (Figs. 4 and 8). Similarly, it is likely that FurA2 monomers inactivated by MCHO cannot simultaneously bind to FurA-binding sites with the 8–1–8 inverted repeat configuration due to the mutual steric hindrance, which accounts for derepression of ahpC, furA1-katG1 and furA3 in the H2O2-treated Δf1f3 strain expressing only FurA2 (Fig. 2). Constitutive repression of furA2-katG2 even in the H2O2-treated M. smegmatis strains expressing FurA2 (Fig. 2) suggests that two oxidized FurA2 monomers can still bind to the everted repeat sequence to repress expression of furA2-katG2.
The inverted repeat sequences within the FurA-binding sites upstream of ahpC, furA1 and furA3 are flanked by A/T-rich sequences (Fig. 7B), which is also the case for the FurA-binding sites upstream of the furA orthologous genes of Mtb, M. bovis, Mycobacterium fortuitum, Mycobacterium leprae and Streptomyces reticuli (Ortiz de Orue Lucana and Schrempf, 2000; Sala et al., 2003). The presence of the consecutive A/T sequence is known to cause DNA curvature and the formation of narrower minor grooves (Asayama et al., 1999; Deng et al., 2015). It has been suggested that Lys15 of Magnetospirillum gryphiswaldense Fur contributes to both recognition of and binding to the target DNA through the insertion of its side chain into the narrower minor grooves that flank the inverted repeat sequence of the Fur-binding site. Interestingly, this Lys15 residue is conserved as Lys or Arg in all Fur, PerR and mycobacterial FurA proteins as shown in Supporting Information Fig. S1 (Arg17, Arg17, Arg19 in FurA1, FurA2 and FurA3, respectively). When 10 nucleotides were inserted between the FurA-binding inverted repeat and the AAA sequence in the ahpC control region, expression of the gene was significantly derepressed in M. smegmatis (data not shown), indicating the importance of the A/T-rich sequence in FurA binding. The A/T-rich sequences flanking the FurA-binding sites could also contribute to stabilization of FurA–DNA interactions by inducing the formation of DNA curvature.
Three furA genes of M. smegmatis appear to be transcribed into leaderless mRNAs without the SD sequence in their upstream regions (Fig. 7A). Leaderless mRNAs were shown to be translated with a lower efficiency than mRNAs containing the SD sequence (Grill et al., 2000). In this respect, the three FurA paralogs of M. smegmatis appear to restrict their cellular abundances through translation from leaderless mRNAs in addition to the negative autoregulation of their genes. Since the furA1 and furA2 genes are the first genes of the furA1-katG1 and furA2-katG2 operons, respectively, and the downstream katG1 and katG2 genes possess their own SD sequences, transcription of the operons into leaderless mRNAs appears to result in a high expression ratio of KatG1 (KatG2) to FurA1 (FurA2) at the protein level.
Differences in the sensitivity of three FurA paralogs to MCHO and their cellular abundance give some insights into physiological implications of the presence of three FurA paralogs in M. smegmatis. FurA2 and FurA3, which are more sensitive to MCHO than FurA1, appear to be the predominant FurA proteins in M. smegmatis grown under H2O2-untreated conditions, as judged by relative expression levels of furA1, furA2 and furA3 (Supporting Information Fig. S9). Although we do not have any direct evidence for heterodimerization of FurA1 and FurA3, high sequence similarity (70.3%) between the dimerization domains of FurA1 and FurA3 suggests a possibility of their heterodimerization. If this were true, the major species of FurA proteins under H2O2-untreated conditions would be FurA3 homodimers, FurA1-FurA3 heterodimers and FurA2 monomers that are likely to be more easily inactivated by MCHO at low concentrations of H2O2 than FurA1 homodimers, whereas FurA1 homodimers might be almost depleted due to sequestration of FurA1 through its heterodimerization with abundant FurA3. When M. smegmatis cells are exposed to H2O2, FurA1 homodimers, which are most resistant to MCHO among the FurA paralogs, likely become the predominant species as a result of the highest expression of furA1. This speculation allows us to hypothesize the followings: as M. smegmatis cells transition from H2O2-nonstress conditions to H2O2-stress conditions, they can elicit H2O2-defense responses in the early phase of transition (or at low concentrations of H2O2) using the H2O2-sensitive FurA3 and FurA2. In M. smegmatis cells exposed to H2O2, an increase in FurA1 homodimer fractions might contribute to preventing the excess expression of the FurA regulon. Given that the Δf1f2f3 mutant showed a slower growth rate than the WT strain (Fig. 3A), full derepression of the FurA regulon appears to be disadvantageous to growth and fitness of M. smegmatis.
Interestingly, our comparative analysis of transcriptome profiles between the WT and Δf1f2f3 mutant strains revealed that expression of the genes belonging to the SigF regulon was significantly reduced in the Δf1f2f3 mutant relative to the WT strain (Supporting Information Table S2). SigF is known to be involved in cell surface stress (heat, SDS, antibiotics, etc.) and oxidative stress responses, as well as the adaptation to stationary growth phase (Provvedi et al., 2008; Humpel et al., 2010; Singh et al., 2015). This finding implies that the genes of the SigF regulon are positively regulated by FurA in an indirect way. Currently we do not know how FurA influences expression of the SigF regulon in M. smegmatis. The study on crosstalks between SigF and FurA is now under way.
Experimental procedures
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Supporting Information Table S4. Procedures for the construction of various furA mutants are described in Supporting Information. E. coli strains were cultivated in Luria-Bertani (LB) medium on a gyratory shaker (200 rpm) at 37°C. M. smegmatis strains were grown aerobically in Middlebrook 7H9 medium (Difco, Sparks, MD) supplemented with 0.2% (wt/vol) glucose as a carbon source and 0.02% (vol/vol) Tween 80 as an anticlumping agent on a gyratory shaker (200 rpm) at 37°C. Ampicillin (100 μg ml−1 for E. coli), kanamycin (50 μg ml−1 for E. coli and 15 μg ml−1 for M. smegmatis), chloramphenicol (34 μg ml−1 for E. coli) and hygromycin (200 μg ml−1 for E. coli and 50 μg ml−1 for M. smegmatis) were added to the medium when required. To expose M. smegmatis cultures to oxidative stress, the cultures were grown until the optical density at 600 nm (OD600) reached 0.45–0.5, and the cultures were further incubated for 15 min following the addition of H2O2 to the cultures to a final concentration of 1, 10 or 15 mM.
DNA manipulation and electroporation
Standard protocols and manufacturers' instructions were followed for recombinant DNA manipulations (Sambrook and Green, 2012). The transformation of M. smegmatis with plasmids was carried out by electroporation as described elsewhere (Snapper et al., 1990).
Reverse transcription-PCR and quantitative real-time PCR
RNA isolation from M. smegmatis strains, preparation of cDNA and RT-PCR were conducted as described elsewhere (Kim et al., 2010). The sigA or 16S rRNA gene was used as an amplification control. qRT-PCR was performed in a 20-μl mixture containing 5 μl of the template cDNA, 15 pmol of each primer, 10 μl of TB GreenTM Premix Ex TaqTM (Tli RNase Plus) (TaKaRa, Japan), 0.4 μl of the ROX passive fluorescent dye and 2.6 μl of distilled water. Thermal cycling was initiated with 1 cycle at 95°C with 30-s hold for denaturation followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. The primers used in RT-PCR and qRT-PCR were listed in Supporting Information Table S5.
Zone inhibition assay
M. smegmatis strains were grown aerobically in 7H9 medium to an OD600 of 0.45. 7H9-glucose plates were spread with 5 ml of cultures and the excess liquid was drained off. The plates were then tapped on a paper towel to remove the rest of liquid, after which they were dried for 4 h at room temperature. Paper discs were soaked with 15 μl of the described concentrations of H2O2 or CHP and then placed onto the plates. The plates were incubated for 3 days at 37°C until clear zones of growth inhibition arose around the discs.
Catalase and peroxidase assay
The WT and Δf1f2f3 mutant strains were aerobically grown in 7H9 medium to an OD600 of 0.45–0.5. Catalase activity was assayed spectrophotometrically at 240 nm by determining the reduction rate of H2O2 (ɛ240 = 0.0435 mM−1 cm−1). The reaction mixture (1 ml) is composed of appropriate volume (50–100 μl) of cell-free crude extracts and 50 mM potassium phosphate buffer (pH 7.0) containing 10 mM H2O2. Peroxidase activity was measured spectrophotometrically at 460 nm by monitoring o-dianisidine oxidation (ɛ460 = 11.3 mM−1 cm−1). The reaction mixture (1 ml) is composed of appropriate volume (50–100 μl) of cell-free crude extracts and 50 mM potassium phosphate buffer (pH 7.0) containing 0.1 mM o-dianisidine (Sigma, St. Louis, MO) and 23 mM tert-butyl hydroperoxide (Sigma). The enzyme assay was performed at 25°C.
Activity staining for catalase
Activity staining for catalase was conducted using the method described by Wayne and Diaz (1986). Briefly, 20 μg of crude extracts of M. smegmatis were subjected to native gel electrophoresis using 7.5% (wt/vol) polyacrylamide gels. After electrophoresis, gels were washed three times for 15 min with distilled water. The gels were soaked in 100 ml of distilled water containing 10 μl of 30% (wt/vol) H2O2 and shaken gently for 10 min. The H2O2 solution was drained off, and the gels were rinsed with distilled water. Next, 30 ml of freshly prepared solution containing 2% (wt/vol) ferric chloride and 2% (wt/vol) potassium ferricyanide was poured over the gels. The gel container was then shaken constantly until the gels began to turn into deep green. The solution was removed from the gels rapidly, after which the gels were washed with distilled water. The protein bands with catalase activity were detected as achromatic bands in the deep green background.
Protein purification and determination of the protein concentration
C-terminally His6-tagged FurA proteins were expressed in E. coli BL21 (DE3) strains harboring the pET29b derivative plasmids (pET29bfurA1, pET29bfurA2, pET29bfurA3, pET29bfurA2_A1 and pET29bfurA2ΔDHAL). The strains harboring the pET29b derivatives were cultivated aerobically at 37°C in LB medium containing 50 μg ml−1 kanamycin to an OD600 of 0.4–0.6. Overexpression of the furA genes was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) to the cultures to a final concentration of 0.5 mM, after which they were incubated at 30°C for an additional 3 h. Cells were harvested from 800 ml cultures and resuspended in 20 ml buffer A (20 mM Tris-HCl [pH 8.0] and 500 mM NaCl) containing 100 Kunitz units of DNase I and 10 mM MgCl2. The resuspended cells were disrupted twice using a French pressure cell, and cell-free crude extracts were obtained by centrifugation twice at 23,708 × g for 15 min. The crude extracts were loaded into a column packed with 1 ml of the 50% (vol/vol) slurry of Ni-Sepharose high-performance resin (Amersham Biosciences, Piscataway, NJ). The resin was washed with 75 bed volumes of buffer A containing 5 mM imidazole and washed further with 75 bed volumes of buffer A containing 10 mM imidazole. His6-tagged FurA proteins were eluted from the resin with six bed volumes of buffer A containing 250 mM imidazole. The eluted proteins were desalted using a PD-10 desalting column (GE Healthcare, Sweden) equilibrated with appropriate buffer. C-terminally His6-tagged Crp was purified as previously described (Lee et al., 2014). The protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard protein.
MALDI-TOF MS and LC-ESI MS/MS analysis
The oxidation status of FurA proteins was analyzed by MALDI-TOF MS and LC-ESI MS/MS. As described previously (Kim et al., 2016), aliquots of E. coli BL21 (DE3) cells (1.8 ml of cultures of the strains harboring pET29bfurA1, pET29bfurA2 or pET29bfurA3) were treated with either 100 μM H2O2 (final concentration) or the same volume of distilled water for 1 min. Cells were harvested by centrifugation after the addition of 200 μl of 100% (wt/vol) trichloroacetic acid (TCA) solution. The harvested cells were resuspended with 500 μl of 10% (wt/vol) TCA solution and then sonicated. Following centrifugation, the pellets were resuspended with 20 μl of IA buffer (50 mM iodoacetamide, 0.5 M Tris pH 8.0, 5% [vol/vol] glycerol, 100 mM NaCl, 1 mM EDTA, 2% [wt/vol] SDS) and incubated for 1 h in the dark to alkylate free thiols. The samples were separated on 13.3% (wt/vol) Tris-tricine SDS-PAGE, after which the protein bands corresponding to monomeric FurA proteins were cut and subjected to in-gel tryptic digestion. MALDI-TOF MS analyses were subsequently performed using an AXIMA Confidence MALDI-TOF MS analyzer (Shimadzu, Japan). LC-ESI MS/MS analyses were conducted using a nanoflow HPLC system connected to a LTQ Orbitrap-XL mass spectrometer (Thermo Scientific, Waltham, MA), and the oxidation sites were identified using the SeeMS program.
PAR assay and determination of Zn2+ contents in the FurA proteins
PAR (Sigma) absorbs light at 500 nm with ɛ500 of 66,000 M−1 cm−1 when it forms a complex with Zn2+ ion. In this study, 10 μM aliquots of purified FurA proteins were prepared in 500 μl of buffer composed of 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS) (pH 8.0), 150 mM KCl and 100 μM PAR. The mixtures of PAR and proteins were incubated for 30 min at room temperature to remove Zn2+ that had been non-specifically bound to the purified proteins. After the addition of H2O2 to the protein-PAR mixtures to a final concentration of 100 mM, changes in the absorbance at 500 nm were monitored at intervals of 30 s for 1000 s at room temperature.
Size exclusion chromatography using FPLC
The quaternary structure of purified FurA proteins was determined by size exclusion chromatography using an ÄKTA FPLC system (GE Healthcare) at a flow rate of 0.2 mL min−1 and 8°C. After the column (Superose 12 10/300 GL, GE Healthcare) was equilibrated with 20 mM Tris-HCl (pH 8.0) containing 200 mM NaCl, 15 nmol of the purified proteins were subjected to chromatography. The molecular mass of the FurA proteins was extrapolated from the standard curve generated using the following standard proteins (Sigma): bovine serum albumin, carbonic anhydrase and cytochrome c.
EDC crosslinking
The purified protein samples (0.6 nmol) in 20 mM MOPS (pH 8.0) buffer containing 150 mM KCl were treated with 20 mM EDC (Sigma) for 2 h at room temperature in the dark. The total volume of the reaction mixture was 50 μl. The EDC-treated samples were subjected to SDS-PAGE.
RNA sequencing and gene expression profiling
Three independent replicate cultures of the WT and Δf1f2f3 strains were grown aerobically to an OD600 of 0.45–0.5. Total RNA of each culture was isolated as described previously (Kim et al., 2010). After extraction of total RNA, bacterial rRNA was removed from each total RNA sample using a MICROBExpress bacterial mRNA enrichment kit (Ambion, Austin, TX). RNA sequencing libraries were created using an Illumina TruSeq™ RNA sample prep kit (Illumina, San Diego, CA) with the standard low-throughput protocol. Sequencing was conducted on an Illumina HiSeq2000 instrument at the National Instrumentation Center for Environmental Management (Seoul, Korea). The data described in this study have been deposited in NCBI's Gene Expression Omnibus and are accessible through the GEO Series accession number GSE97620. Paired-end reads were then mapped to the reference genome sequence of M. smegmatis mc2155 (NC_008596.1) downloaded from the NCBI genome database (http://www.ncbi.nlm.nih.gov/genome/) using the BWA-MEM algorithm (Li and Durbin, 2010). Mapped reads per annotated gene were counted by Bam2readcount. Summarized statistics of RNA sequencing alignment are listed in Supporting Information Table S6. Differentially expressed genes were subsequently identified pair-wise by the edgeR package in R language (Robinson et al., 2010). In this analysis, the genes with a p value ≤ 0.01 and |log2(mutant/WT)| ≥ 3 were regarded as differentially expressed genes.
Electrophoretic mobility shift assay (EMSA)
EMSA was performed using an EMSA kit (Invitrogen, Carlsbad, NJ). Three types of DNA fragments were used in the assay. An 112-bp DNA fragment containing the upstream region of ahpC was amplified by PCR using pNCahpC as a template and the primer set, ahpC_100_F (5′-ATATGGTACCAGTTGCATTTGTAAGAGG-3′) and ahpC_100_R (5′-ATATAAGCTTAAAAGAGCCATTCACAGC-3′). In the same way, an 112-bp DNA fragment with the upstream region of furA2 was amplified by PCR using pNBV1furA2 as a template and the primer set, furA2_100_F (5′-ATATGGTACCCTCCCATCCGAGCTCCCAG-3′) and furA2_100_R (5′-ATATAAGCTTATGCCGCCCACCTTGCTC-3′). A 60-bp non-specific DNA fragment without the FurA-binding site was amplified by PCR using pUC19 as a template and the primer set, pUC19_EMSA_F (5′-CCTCTAGAGTCGACCTGC-3′) and pUC19_EMSA_R (5′-AGGAAACAGCTATGAACCATG-3′). Purified FurA proteins were incubated with 160 fmol of the DNA fragments containing either the ahpC or furA2 upstream region and 80 fmol of the non-specific DNA fragments in buffer (20 mM MOPS and 150 mM KCl [pH 8.0]) in a reaction volume of 10 μl for 20 min at 25°C. To examine the effect of H2O2 on the binding of FurA to the DNA fragments, H2O2 was added to DNA–protein mixtures. After the addition of 2 μl of 6× loading buffer (included in the kit), 9 μl of the DNA–protein mixtures were analyzed on non-denaturing PAGE [8% (wt/vol) acrylamide] using 0.5× TBE buffer (41.5 mM Tris-borate and 0.5 mM EDTA [pH 8.3]) at 100 V for 2 h 40 min at 4°C. The gels were stained with SYBR green staining solution for 1 h.
β-galactosidase assay
The β-galactosidase activity was measured spectrophotometrically as described previously (Oh and Kaplan, 1999).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF2012R1A1A2004243 and NRF2017R1A2B4008404).
Author contributions
Conception or design of the study: JIO, HNL
Acquisition of the data: HNL, CJJ, HHL, JP, YSS
Analysis or interpretation of the data: HNL, CJJ, JWL, JIO
Writing of the manuscript: HNL, CJJ, HHL, JIO.