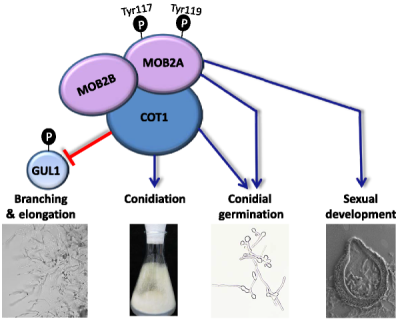
Altering Neurospora crassa MOB2A exposes its functions in development and affects its interaction with the NDR kinase COT1
Summary
The Neurospora crassa Mps One Binder (MOB) proteins MOB2A and MOB2B physically interact with the Nuclear Dbf2 Related (NDR) kinase COT1 and have been shown to have overlapping functions in various aspects of asexual development. Here, we identified two N. crassa MOB2A residues, Tyr117 and Tyr119, which are potentially phosphorylated. Using phosphomimetic mob-2a mutants we have been able to establish that apart from their previously described roles, MOB2A/B are involved in additional developmental processes. Enhanced conidial germination, accompanied by conidial agglutination, in the phosphomimetic mutants indicated that MOB2A is a negative regulator of germination. Thick-section imaging of perithecia revealed slow maturation and a lack of asci alignment in the mutant strains demonstrating a role for MOB2A in sexual development. We demonstrate that even though MOB2A and MOB2B have some overlapping functions, MOB2B cannot compensate for the roles MOB2A has in conidiation and germination. Altering Tyr residues 117 and 119 impaired the physical interactions between MOB2A and COT1, most likely contributing to some of the observed effects. As cot-1 and the phosphomimetic mutants share an extragenic suppressor (gul-1), we concluded that at least some of the effects imposed by altering Tyr117 and Tyr119 are mediated by the NDR kinase.
Graphical Abstract
Mutating residues Tyr117 and Tyr119 of the N. crassa MOB2A protein revealed that in addition to the role MOB2A/B have in hyphal elongation/branching and conidiation, they are involved in regulation of conidial germination. The slow maturation of perithecia and a lack of ascus alignment in the mutants demonstrated a role in sexual development. We propose that the mentioned effects may be a result of the impaired interaction between MOB2A and the key MOB2A/B partner – COT1.
Introduction
Proteins that belong to the Mps One Binder (MOB) family are functionally and structurally conserved from yeast to human. At least two different MOB proteins have been identified in every eukaryote that has been examined. They have been determined to act as activating subunits and control crucial cellular processes such as mitosis, cell death, proliferation and morphology (Hergovich, 2011). In filamentous fungi, some MOB proteins have been shown to be essential for sexual development, hyphal fusion and pathogenicity (Maerz et al., 2009; Bernhards and Pöggeler, 2011; Schmidpeter et al., 2017). Neurospora crassa has four MOB proteins, two of them, MOB2A (NCU03314.7) and MOB2B (NCU07460.7), are functionally similar. Deletion of either mob-2a or mob-2b causes multiple changes in N. crassa's morphology, such as an increase in aerial hyphae formation, a decrease in asexual conidia production along with hyper-branching of hyphae and growth arrest (Maerz et al., 2009). Nonetheless, these mutants are able to create a mature colony and can produce sexual and asexual propagules. However, the Δmob-2a;Δmob-2b double mutant exhibits a severe compact colonial growth phenotype. This morphology is very similar to that of the cot-1 (ts) mutant, which is the founding member of the Nuclear Dbf2-related (NDR) Ser/Thr kinase family, when grown at the restrictive (
 32°C) temperature (Yarden et al., 1992; Maerz et al., 2009). Additionally, COT1 (NCU07296.7) kinase activity was shown to be reduced in both Δmob-2a and Δmob-2b mutants and almost abolished in the Δmob-2a;Δmob-2b double mutant. Both MOB2A and MOB2B have been shown to physically interact with the N-terminal region of COT1 (Maerz et al., 2009; Ziv et al., 2013). Based on their phenotype as well as their physical interactions with COT1, it has been suggested that MOB2A and MOB2B may have many overlapping functions (Maerz et al., 2009). Nonetheless, we have previously established the fact that there are differences in the strength of the physical interactions between COT1 and the two MOB proteins and that MOB2B, but not MOB2A, is involved in negative control of asexual conidial production via the COT1 pathway (Maerz et al., 2009; Ziv et al., 2013). Thus, while overlapping functions exist, under certain conditions, the two proteins lack compensatory capabilities.
32°C) temperature (Yarden et al., 1992; Maerz et al., 2009). Additionally, COT1 (NCU07296.7) kinase activity was shown to be reduced in both Δmob-2a and Δmob-2b mutants and almost abolished in the Δmob-2a;Δmob-2b double mutant. Both MOB2A and MOB2B have been shown to physically interact with the N-terminal region of COT1 (Maerz et al., 2009; Ziv et al., 2013). Based on their phenotype as well as their physical interactions with COT1, it has been suggested that MOB2A and MOB2B may have many overlapping functions (Maerz et al., 2009). Nonetheless, we have previously established the fact that there are differences in the strength of the physical interactions between COT1 and the two MOB proteins and that MOB2B, but not MOB2A, is involved in negative control of asexual conidial production via the COT1 pathway (Maerz et al., 2009; Ziv et al., 2013). Thus, while overlapping functions exist, under certain conditions, the two proteins lack compensatory capabilities.
The N. crassa COT1 protein and its homologues in other fungi have been shown to regulate hyphal elongation and branching (Yarden et al., 1992; Buhr et al., 1996; Dürrenberger and Kronstad, 1999; Mcnemar and Fonzi, 2002; Scheffer et al., 2005; Johns et al., 2006; Ziv et al., 2009; Kodama et al., 2017). While evidence for the role of phosphorylation concerning the function of COT1 and other NDR kinases has accumulated (Millward et al., 1999; Mah et al., 2001; Emoto et al., 2004; Hou et al., 2004; Stegert et al., 2005; Ziv et al., 2009, 2013), no evidence for potential phosphorylative regulation of MOB proteins in N. crassa has been provided to date. Nonetheless, phosphorylative regulation of MOB proteins in other systems has accumulated. In human cells, MOB1 has been found to be phosphorylated, at Thr12/35 within the N-terminal region of the protein, by mammalian sterile20-like (MST) kinase 1 and MST2. This phosphorylation converts the inactive conformation of the protein to an active one, stabilizes MOB-LATS interactions and enhances the ability of MOB1 to activate the NDR kinases it interacts with (Praskova et al., 2008; Bao et al., 2009; Ni et al., 2015). Based on the mouse MOB1 crystal structure, it appears that the N-terminal region of MOB1 can block the LATS1 interacting surface. Phosphorylation of Thr12/35 was shown to eliminate autoinhibition and promote the physical interaction between MOB1 and LATS1 (Kim et al., 2016). In addition to those studied in mouse and human, the mechanism of MOB regulation has also been studied in Drosophila. Mob has been identified as a tumor suppressor protein (Mats) and is a Hpo Ser/Thr protein kinase substrate (Wei et al., 2007). In Saccharomyces cerevisiae, the MOB1 protein has been shown to be phosphorylated by CDC15 (Mah et al., 2001). In Candida albicans, MOB2, which is essential for hyphal development in this organism, was shown to be phosphorylated by CDK1(CDC28) in a cell cycle-dependent manner (Gutiérrez-Escribano et al., 2011). Overall, it appears that phosphorylation is a common regulatory mechanism of MOB proteins in a variety of organisms (Gutiérrez-Escribano et al., 2011).
Structural analysis of the human MOB1 revealed that an acidic surface of MOB1 is involved with its interaction with the NDR kinase (Ponchon et al., 2004). Recently, the structure of the budding yeast MOB2 and the COT1 homologue CBK1 complex was resolved, and the MOB2 H2 and H7 helix, along with the H4–H5 loop, have been determined to interact with the kinase (Gógl et al., 2015). While the COT1 interacting region with the MOB2A/B has been studied in detail (Maerz et al., 2009; Ziv et al., 2013), the region/s on MOB2A/B which are involved in these interactions has not been studied to date.
The proper function of NDR kinases, which in part is dependent on their MOB protein co-activators, is manifested via their downstream effectors. NDR/LATS kinase–MOB targets have been studied in S. cerevisiae. One of the CBK1 (the yeast COT1 homologue) substrates, is Ssd1, which encodes an RNA binding protein that controls translation (Jansen et al., 2009). The N. crassa homolog of SSD-1 is gul-1 (NCU01197.7), which has been shown to be involved in cell wall composition and architecture via the COT1 pathway (Terenzi and Reissig, 1967; Uesono et al., 1997; Seiler et al., 2006). Deletion of gul-1 partially suppresses the abnormal cot-1 (ts) phenotype. The cot-1;gul-1 double mutant exhibits improved growth rate and branching accompanied by a thinner cell wall which resembles that of the wild type (Terenzi and Reissig, 1967; Herold and Yarden, 2017). The observed phenotypic suppression in the gul-1;cot-1 mutant is accompanied by the alteration of gene expression in cot-1 (ts) mutant. Examples include the chitin synthase family members as well as glucan synthase (Herold and Yarden, 2017). The fact that the yeast GUL1 homologue (Ssd1) is a direct substrate of the COT1 homolog (Cbk1) and that at least some of the phenotypic consequences of impairing COT1 can be suppressed by deletion of gul-1 suggests that gul-1 can serve as a proxy for at least part of the NDR kinase's functions.
To further investigate the mechanistic aspects of MOB2 regulation we first used mass spectrometry analysis, which revealed two potentially phosphorylated Tyr residues on the N. crassa MOB2A protein. Using phosphomimetic mob-2a mutants we have been able to demonstrate that in addition to their previously described roles, MOB2A/B are involved in two additional key developmental processes: conidial germination and sexual development. These functions are dependent on the mentioned Tyr residues, which are also important for genetic and physical interactions of MOB2A and COT1. Lastly, we show that at least some of the functions involving MOB2A are mediated by the COT1 substrate GUL1.
Results
Phosphomimetic mutants of MOB2A exhibit defects during early stages of N. crassa development and reveal MOB2A function in conidial germination
We used mass spectrometry analysis of immunoprecipitated His-MYC doubled-tagged COT1 [a construct described by Maerz and colleagues (2009) who found that MOB2A physically interacts with COT1], to show that the protein can potentially undergo phosphorylation on two Tyr residues, Tyr117 and Tyr119. Tyr119, is conserved in a variety of MOB proteins ranging from yeast to human (Fig. 1), suggesting it may have a role in MOB2 regulation and, perhaps, MOB2-NDR kinase interactions. Tyr117 is less conserved. It is present in both the N. crassa MOB2A and MOB2B proteins as well as in those of the related ascomycete fungi Podospora anserina MOB2 (CDP23328.1) and Sordaria macrospora (SMAC_04592), as well as Fusarium oxysporum (FOYG_06803), yet not in some of the other fungi analyzed (e.g., Magnaporthe oryzae, Sclerotinia sclerotiorum and C. albicans).

Sequence alignment of the N. crassa MOB2A (NCU03314.7), MOB2B (NCU07460.7) and MOB proteins from Saccharomyces cerevisiae (CAY79413.1), Schizosaccharomyces pombe (NP_587851.1), Sordaria macrospora (SMAC_04592), Podospora anserina (CDP23328.1), Candida albicans (XP_719006.1), Fusarium oxysporum (FOYG_06803) and Homo sapiens (NP_001231695.1). The potentially phosphorylated MOB2A Tyr117 and Tyr119 are marked.
To analyze the significance of the potentially-phosphorylated MOB2A residues on N. crassa growth and development, Tyr117 and Tyr119 were altered by site directed mutagenesis to produce double mutants harboring two Phe or Glu residues (mimicking the putative unphosphorylated or constantly phosphorylated MOB2A forms respectively). As a basis for constructing these mutated alleles, we used the pLA1 (Aharoni-Kats and Yarden, unpublished) plasmid, which contains mob-2a (NCU03314) tagged with hemagglutinin (HA) at the N-terminus. The resulting products were inserted into pHAN1, which has the appropriate flanking regions that would target the construct to the his-3 locus (Maerz et al., 2009). The obtained constructs (pLA11 and pLA12), as well as an HA-mob-2a control, were transformed into conidia of a Δmob-2a;myc-cot-1;his-3 strain of N. crassa. Thus, isolated transformant homokaryons had only one (either mutated or wild type alleles, along with an HA tag) copy of mob-2a.
strain of N. crassa. Thus, isolated transformant homokaryons had only one (either mutated or wild type alleles, along with an HA tag) copy of mob-2a.
Deletion of the entire mob-2a gene has been shown to result in various morphological effects, including slower hyphal elongation and hyper branching, which results in the formation of a more compact colony than wild type (Maerz et al., 2009). However, these were not significantly altered in the HA-mob-2a(Y117F,Y119F) and HA-mob-2a(Y117E,Y119E) mutants (Fig. 2).
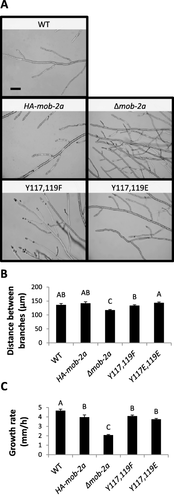
Altering Tyr117 and Tyr119 to Phe or Glu has no significant impact on mature hyphal development.
A. Hyphal morphology of colony edges of wild type, HA-mob-2a, Δmob-2a and the phospho-mimetic mutants, cultured overnight at 34°C. Images of typical morphology were taken using light microscopy. Bar = 100 μm.
B.-C. The effect of mutating Tyr117 and Tyr119 on hyphal branching and growth rate, when cultured at 34°C. The results are an average of three independent experiments. Statistical analysis was performed with Students t-test (P<0.05). Bars indicates standard error.
While no marked changes in mycelial growth were observed in the phosphomimetic mutants, conidiation was clearly affected by the introduced amino acid substitutions. Our results show that similar to that occurring following the disruption of mob-2a, replacing Tyr117 and Tyr119 with Phe or Glu conferred a 40% decrease in the number of conidia formed when compared to the control (Fig. 3A).
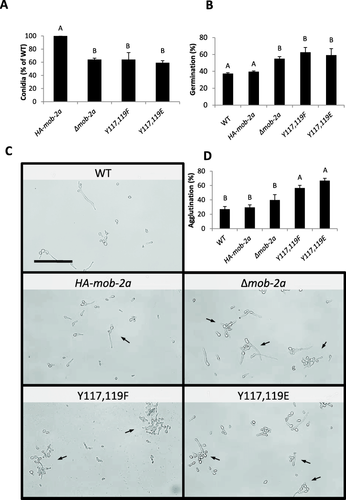
Tyr117 and Tyr119 substitutions mimic the effect of deleting mob-2a on a-sexual conidial formation and germination.
A. Conidiation rates of the mob-2a phospho-mimetic strains. The results are an average of three independent experiments. Statistical analysis was performed with Students t-test (P<0.05). Bars indicates standard error.
B. Percentage of germinating conidia 3 hours after induction. Statistical analysis was performed with Students t-test (P<0.05). Error bars represent standard error of the mean.
C. Germinating conidia (arrows), 3 hours after induction of germination. Bars = 100 µm.
D. Percentage of agglutinated conidia, 3 hours after induction of germination. Statistical analysis was performed with Students t-test (P<0.05). Bars indicate standard error.
Another significant developmental stage in asexual reproduction is conidial germination. To determine whether MOB2A may be involved in germination, and whether altering the Tyr residues may be important for this process, we monitored the germination rates of Δmob-2a and the phosphomimetic mutants along a four-hour time course. The most significant differences between the mutants and the control were observed three hours after induction of germination (Fig. 3B and C). While by this time point 40% of wild type conidia had produced visible germ tubes, deletion of mob-2a resulted in an 18% increase in the number of germinating conidia, suggesting that MOB2A may be a negative regulator of conidial germination. This was also expressed by the fact that the Δmob-2a germ tubes were longer than those of the wild type. While after 2 hrs of growth, germ tube lengths of all the tested mutants ranged 5–8 ± 0.9 µm, significant differences were evident by the third hour. Specifically, while the length of the wild type germ tubes was 6.6 ± 0.6 µm, Δmob-2a germ tubes were significantly longer (21.7 ± 2.8 µm). The length of the phosphomimetic mutant germ tubes was similar to those of the control. Similar in nature to the results obtained while studying conidiation, replacing both Tyr117 and Tyr119 to Phe or Glu clearly mimicked the effect of deleting mob-2a with regard to the effect on conidial germination rate. About 60% of the phosphomimetic mutant strains' conidia had produced a germ tube within three hours after induction. In addition to enhanced germination, germling agglutination was evident in the phosphomimetic mutants, where agglutinated germlings were about 2.5-fold more abundant than in the wild type (Fig. 3D). The changes observed in the two phosphomimetic mutants further demonstrate the importance of Tyr117 and Tyr119 in MOB2A function during asexual reproduction.
Deletion of mob-2b exposes phenotypic attributes conferred by altering Tyr117 and Tyr119 in MOB2A
MOB2A and MOB2B are structurally very similar and have been suggested to have overlapping functions (Maerz et al., 2009), even though some distinct functions of the two proteins have since been identified (Ziv et al., 2013). To further determine what the specific roles of MOB2A may be, and to what effect Tyr117 and Tyr 119 may be significant for these roles, we produced strains of the phosphomimetic mutants in which mob-2b had been deleted. Neither mob-2a(Y117F,Y119F) nor mob-2a(Y117E,Y119E) exhibited abnormal hyphal elongation and branching. However, when mob-2b was no longer active, a significant role for Tyr117 and Tyr119 in hyphal growth was clearly made evident, and a pronounced effect of either of the mutated alleles was observed (Fig. 4). mob-2a(Y117E,Y119E);Δmob-2b [(but not mob-2a(Y117F,Y119F);Δmob-2b)] exhibited a colonial growth phenotype (extremely slow growth and hyper branching) at 34°C (optimal for the wild type), a morphology which strongly resembles that of cot-1 (ts) when grown at the restrictive temperature. To verify whether these dramatic changes in the mob-2a(Y117E,Y119E);Δmob-2b strain are, in fact, due only to the mentioned amino acid substitutions, we complemented the mutant with a copy of the wild type allele of mob-2a. The randomly inserted wild type mob-2a gene was sufficient to confer improved hyphal elongation and branching rates to the mob-2a(Y117E,Y119E);Δmob-2b strain, in a manner which was very similar to that observed in the strain lacking a functional mob-2b (Fig. 4C). This also demostrated the efficacy and functionality of the ccg-1-driven gene at the his-3 locus.
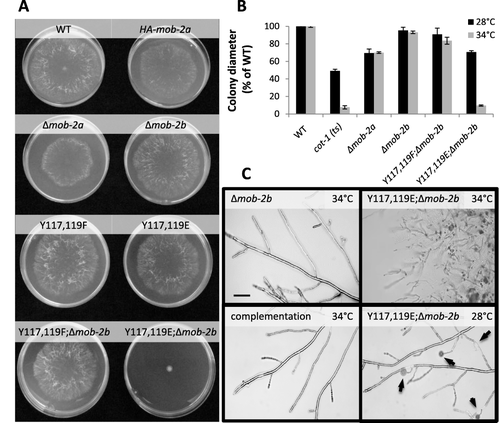
MOB2A Tyr117 and Tyr119 affect mature hyphal growth in a Δmob-2b background.
A. Growth of wild type and phospho-mimetic mutants in a Δmob-2b background. Cultures were grown on Vogel's minimal medium for 22 hours at 34°C.
B. mob-2a(Y117E,Y119E);Δmob-2b is a temperature sensitive mutant. Colony diameter of the phospho-mimetic mutants and cot-1 (ts), compared to the wild type control, grown at the optimal temperature for the wild type (34°C) or (28°C). Bars represent standard error of the mean.
C. Mutant morphology as observed after culturing at restrictive (34°C) or optimal (28°C) temperatures, as indicted. Cytoplasmic leakages are marked by arrows. Transformation of a wild type allele of mob-2a to mob-2a(Y117E,Y119E);Δmob-2b (ts) complemented the mutant's morphology at the restrictive temperature (34°C). Bars = 100 μm.
Δmob-2b markings in the figure designate the mutant in a Δmob-2a;HA-mob-2a background.
We tested whether culturing the mob-2a(Y117E,Y119E);Δmob-2b strain at a lower temperature would alleviate any of the phenotypic abnormalities observed. In fact, when grown at temperatures below 34°C colony morphology was improved, as was made evident by faster hyphal elongation rates and longer distances between branches. 28°C was determined to be the optimum growth temperature for the mob-2a(Y117E,Y119E);Δmob-2b strain, indicating that whatever functional changes had been imposed, these were temperature-sensitive. While improved colony morphology and the formation of aerial hyphae were observed, a large number of the aerial hyphal tips exhibited cytoplasmic leakage, some of which was accompanied by the accumulation of extracellular material (Fig. 4C).
To further analyze the morphological characteristics of the mob-2a(Y117E,Y119E);Δmob-2b temperature sensitive mutant, hyphal growth rate and branching frequency were analyzed in cultures grown at a semirestrictive temperature (32°C). The temperature sensitive mutant displayed a dramatic decrease (∼75%) in hyphal elongation when compared to the control (Fig. 5A) and the average distance between branches was significantly shorter than that measured in the mob-2a(Y117E,Y119E) or Δmob-2b strains (78 μm, 127 μm and 147 μm respectively) (Fig. 5B). However, no significant changes in these parameters were observed between mob-2a(Y117F,Y119F);Δmob-2b, mob-2a(Y117F,Y119F) and the wild type (Fig. 5B), indicating that only the Tyr to Glu substitutions were sufficient to confer a similar change in these growth parameters.
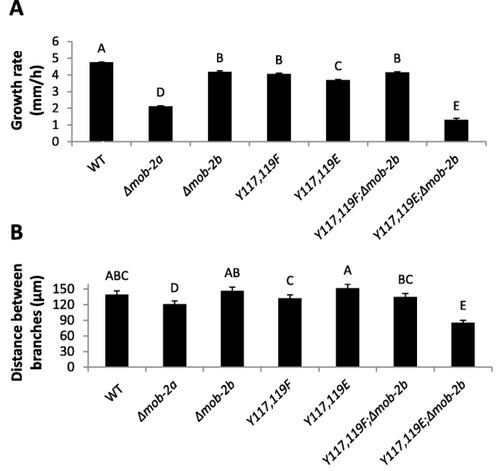
Deletion of mob-2b reveals the effects of altering Tyr117 and Tyr119 in MOB2A on hyphal elongation and branching.
Growth rate (A) and the distance between branches (B) of the mob-2a phosphopho-mimetic mutants in a Δmob-2b background. Statistical analysis was performed with Students t-test (P<0.05). Bars represent standard error of the mean. Δmob-2b markings in the figure designate the mutant in a Δmob-2a;HA-mob-2a background.
Although the MOB2A phosphomimetic mutants did not affect hyphal elongation and branching, it appears that deletion of mob-2b exposed the importance of Tyr117 and Tyr119 during mature hyphal development. Based on these results we concluded that MOB2A and MOB2B may have overlapping functions in these processes, but this is not the case during conidiation and asexual spore germination.
MOB2A and MOB2B are involved in sexual development
Maerz et al. (2009) reported that sexual development of Δmob-2a and Δmob-2b strains was normal and these mutants were female fertile when crossed with wild type or the deletion strains of the opposite mating type. As MOB2A and MOB2B have many overlapping functions, we used the phosphomimetic MOB2A mutants in a Δmob-2b background to examine whether additional roles of MOB2A and MOB2B could be identified during this phase of the fungal life cycle. When the phosphomimetic mutants were crossed with wild type, barrage formation appeared about 5 days after induction, in the proximity of the wild type × wild type control (Fig. 6). Thus, perithecia were typically observed on either side of a clear zone [the barrage (Perkins, 1988)] which were derived from protoperithecia fertilized by nuclei from the strain located on the opposing side of that zone. However, when crosses were performed between wild type and the mob-2a phosphomimetic mutants in a Δmob-2b background, it became clear that MOB2A and MOB2B play a role in normal sexual development. First, a slight decrease in the secretion of melanin to the medium was evident. This may indicate improper melanin biosynthesis, which is part of successful fruiting body development (Lehr et al., 2014). Moreover, the mob-2a(Y117F,Y119F);Δmob-2b double mutant was affected in female fertility. This was also determined in another set of experiments where a marked reduction in protoperithecial development and fertilization was observed following sequential inoculation of the mating partners on the SC medium (Supporting Information Fig. S1). We focused on the analysis of fruiting bodies formed in the co-inoculated crosses and found that only few perithecia appeared in the mob-2a(Y117F,Y119F);Δmob-2b double mutant. Furthermore, these were formed following an approximate delay of 12 h, when compared to the wild type. These perithecia were capable of producing viable, normal shaped, ascospores, albeit some of them where longer than the control (Supporting Information Fig. S2). In fact, 6 days after crossing, a slight decrease in perithecium size can been seen when mob-2a(Y117F,Y119F);Δmob-2b or mob-2a(Y117E,Y119E);Δmob-2b composed the female structures. Normal-sized perithecia were predominant in the proximity of the wild type strain inoculation point 11 days after crossing (Fig. 7B). In addition, 32 days after induction, both phosphomutants and the wild type control ascospores were shot through the perithecial beak. Few ascospores can be seen inside the perithecium structure (Supporting Information Fig. S3).
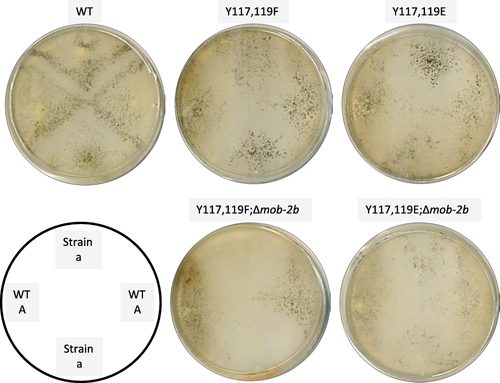
MOB2A and MOB2B are required for proper sexual reproduction. The mob-2a phospho-mimetic mutants and the wild type were plated on synthetic crossing medium. Barrage formation was documented 5 days later.
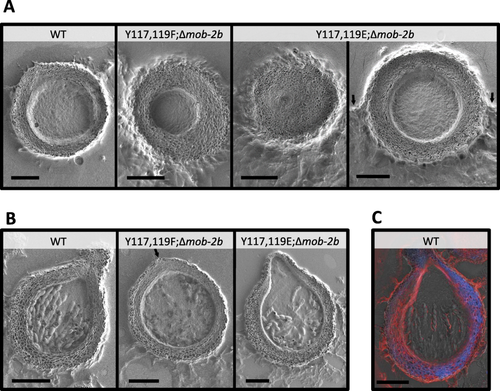
Altering MOB2A Tyr117 and Tyr119 affects perithecial development. Scanning electron micrographs of thick sections of wild type, mob-2a(Y117F,Y119F);Δmob-2b, mob-2a(Y117E,Y119E);Δmob-2b perithecia, 6 and 11 days after induction (panels A and B, respectively). The semi-submerged context of the mob-2a(Y117E,Y119E);Δmob-2b strain perithecium is marked by arrows (A - right panel). The location of the mob-2a(Y117F,Y119F);Δmob-2b ostiole (B) is designated by an arrow. Bars = 100 μm.
C. Scanning electron micrographs of thick-sectioned wild type perithecia 11 days after induction, stained with calcofluor white and congo red. Images were taken using a collaborative microscopy protocol (see experimental procedures for details).
We concluded that mob-2a(Y117F,Y119F);Δmob-2b was able to fertilize the wild type protoperithecia and, subsequently, mature perithecia could be formed. In contrast, in the case of the converse situation, very few mature fruiting bodies were formed, apparently because of impaired protoperithecia production capabilities conferred by the mutations in mob-2a.
To further analyze the perithecia produced by mob-2a(Y117F,Y119F);Δmob-2b and mob-2a(Y117E,Y119E); Δmob-2b, we adapted a correlative microscopy approach for studying the inner structure of the perithecium in higher detail. This was based on subjecting thick (20–30 µm) perithecial sections to scanning electron microscopy (SEM) and, when required, merging the obtained micrographs with images obtained by light and fluorescence microscopy.
Most of the perithecia produced by mob-2a(Y117E,Y119E);Δmob-2b were partially submerged in the agar in a manner that differed from the wild type (Fig. 7A). Furthermore, the formation of the inner fruiting body cavity differed among the strains during this early stage of development. Later, in 11 day-old wild type perithecia, mature asci were aligned along the central perithecial cavity, in a manner in which asci tips faced the ostiole (Fig. 7B). In contrast, when either of the phosphomimetic strains was used as a parent, the characteristic asci alignments were frequently disorganized.
In addition to the ultrastructural analysis, we also used calcofluour white and congo red to determine if marked changes in the perithecial cell wall could be observed. This was based on previous evidence concerning significant changes in cell wall morphology and composition in mutants of the major MOB2A target – COT1 (Gorovits et al., 2000; Herold and Yarden, 2017). Clear differences in the staining patterns of perithecia subjected to the two dyes were observed (Fig. 7C). Most of the perithecial wall was clearly stained with calcofluor white, suggesting it is chitin-rich. The fact that the inner and outer wall layers stained brightly with congo red indicated the likelihood of higher concentrations of amyloids along these tissues. Nonetheless, no marked differences in staining patterns of either dye were observed in the phosphomimetic mutants (Supporting Information Fig. S3).
COT1 is involved in mediating the effects on hyphal elongation and branching in the Tyr117 and Tyr119 MOB2A strains
To verify whether Tyr117 and Tyr119 substitutions may affect the genetic interactions between mob-2a and cot-1, we took an approach which bypasses the hurdle of introducing a cot-1 (ts) allele to these mutant backgrounds. This was due to the lack of a common growth temperature, which would allow for proper morphological analysis of a strain harboring the different temperature-sensitive alleles. To do so, we employed a proposed COT1 substrate-encoding gene, gul-1, as a reporter of genetic interactions with its upstream regulator, cot-1. Many genes are co-affected by cot-1 and gul-1 (Herold and Yarden, 2017; Herold and Yarden, unpublished). After gul-1 had been deleted in the mob-2a(Y117F,Y119F);Δmob-2b MOB2A phosphomimetic mutant, we found that colony morphology and hyphal branching were similar to those observed in the Δgul-1 parent (Figs. 8 and 9). However, deletion of gul-1 in the mob-2a(Y117E,Y119E);Δmob-2b (ts) mutant, partially suppressed the observed hyphal elongation and branching defects. The mob-2a(Y117E,Y119E);Δmob-2b barely grew at 34°C (0.12 mm/h), yet the growth rate of the mob-2a(Y117E,Y119E);Δmob-2b;Δgul-1 strain was more than 10-fold faster. Moreover, the average distance between branches was significantly longer (38 μm and 120 μm respectively). When cultured at various temperatures, ranging from 28°C to 34°C, no significant changes in the colony diameter of the mob-2a(Y117E,Y119E);Δmob-2b;Δgul-1 mutant were observed, indicating that it is not temperature sensitive as its parental mob-2 mutant strain (data not shown).
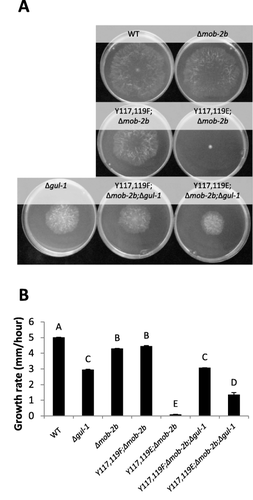
gul-1 is a partial suppressor of mob-2a(Y117E,Y119E);Δmob-2b (ts).
A. Colony morphology of the mob-2a phospho-mimetic mutants in a Δmob-2b background and as affected by the deletion of Δgul-1. Strains were cultured for 20h at 34oC.
B. Growth rate of the mob-2a phospho-mimetic mutants in the presence or absence of gul-1. Statistical analysis was performed with Students t-test (P<0.05). Bars represent standard error of the mean.
Δmob-2b markings in the figure designate the mutant in a Δmob-2a;HA-mob-2a background.
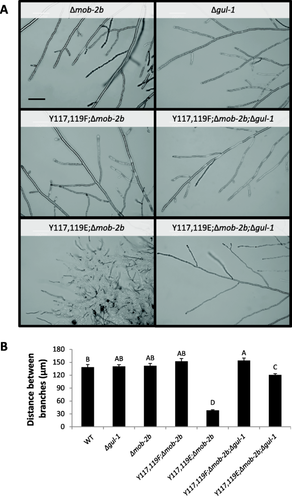
Deletion of gul-1 suppresses mob-2a(Y117E,Y119E);Δmob-2b (ts) hyperbranching.
A. Hyphal morphology at the colony edge of the analyzed strains. Bar = 100 μm.
B. Distance between branches of the mob-2a phospho-mimetic mutants in the presence or absence of gul-1. Statistical analysis was performed with Students t-test (P<0.05). Bars represent standard error of the mean. Δmob-2b markings in the figure designate the mutant in a Δmob-2a;HA-mob-2a background.
Based on these results we concluded that at least part of the dramatic morphological changes seen in the mob-2a(Y117E,Y119E);Δmob-2b (ts) mutant, including hyphal elongation and branching, are due to the potential impairment of the interactions between MOB2 and COT1.
To determine whether the observed genetic interaction between the mob-2a(Y117E,Y119E);Δmob-2b (ts) mutant and gul-1 is also articulated in GUL1 function, we measured the mRNA levels of genes whose expression was found to be increased in cot-1 (ts) and suppressed by gul-1 (Herold and Yarden, 2017; Herold and Yarden, unpublished). Our results show that chs-1 (NCU03611) RNA levels are elevated in both cot-1(ts) as well as in the mob-2a(Y117E,Y119E);Δmob-2b strain, suggesting that the Tyr117 and Tyr119 substitutions may affect COT1 activity. Deletion of gul-1 suppressed the observed changes in chs-1 transcription in mob-2a(Y117E,Y119E);Δmob-2b, in a manner similar to that observed in a cot-1;gul-1 background (Fig. 10A). Analyzing the transcription levels of fks-1 (NCU06871), which encodes the catalytic subunit of glucan synthase, showed similar, albeit less pronounced, results (Supporting Information Fig. S5). In contrast to chs-1 and fks-1, the mRNA levels of dur-3 (NCU09909) are decreased in the cot-1 (ts) strain (Herold and Yarden, unpublished; Fig. 10B). We found a similar decrease in the mob-2a(Y117E,Y119E);Δmob-2b strain, and here too, deletion of gul-1 resulted in an increase of dur-3 expression. These results further support that, at least among these genes, expression patterns in the phospho-MOB2A mutant are dependent on a functional gul-1, and thus involve the COT1 pathway.
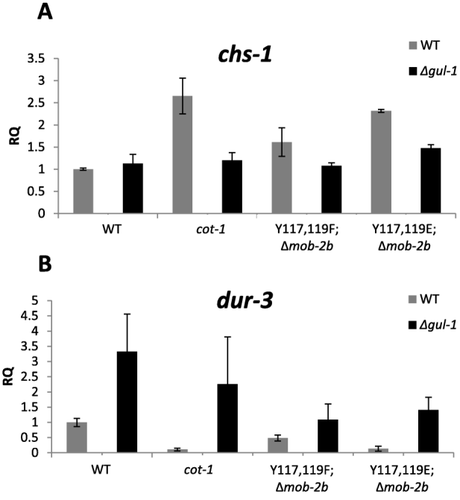
Expression levels of chs-1 (A) and dur-3 (B) in the wild type and mob-2a phospho-mimetic mutants (in a Δmob-2b background) in the presence or absence of a functional gul-1 (grey and black bars respectively).
In addition to fks-1, chs-1 and dur-3, we analyzed the expression of chs-3 (NCU04251), gh18-5 (NCU04554) and chit-1 (NCU02184) that are involved in cell wall remodeling. Even though the high mRNA expression levels of these genes were suppressed by gul-1 in the cot-1 (ts) mutant, deletion of gul-1 did not significantly change their mRNA levels in the mob-2a(Y117E,Y119E);Δmob-2b when compared to the mob-2a(Y117E,Y119E);Δmob-2b;Δgul-1 stain. We, therefore, concluded that the changes introduced to Tyr117 and Tyr119 have a partial functional effect on COT1, as evident via the effects obtained by inactivation of the downstream effector GUL1.
Taken together, it appears that while some of the genes whose expression is altered in a phosphomimetic MOB2A background involve the COT1 pathway, yet not all of the COT1/GUL1 functions are necessarily mediated by MOB2A or involve the amino acid substitutions analyzed here.
Changes in Tyr117 and Tyr119 affect MOB2A-COT1 protein interactions
We previously showed that MOB2A physically interacts with COT1, and that changes in phosphorylation at the N-terminal region of COT1 affect the physical interactions between them (Ziv et al., 2013). To verify whether some of the significant changes in the morphology of the MOB2A phosphomimetic mutants (some of which apparently involve the COT1-GUL1 pathway, as demonstrated above) may be due to interference with the physical interactions between COT1 and MOB2A, we used yeast two hybrid analysis with the corresponding gene products. The growth of the yeast strains, expressing COT1 and MOB2A (Y117F/E,Y119F/E) as well as the wild type allele were analyzed, under medium (SD/-Leu/-Trp) and high selection (SD/-Leu/-Trp/-His/-Ade) conditions. Yeasts harboring Tyr117 and Tyr119 substitutions exhibited decreased growth rates when grown under high selection conditions (data not shown). Moreover, quantitative analysis of the yeast two hybrid interactions showed that replacing MOB2A Tyr117 and Tyr119 with Phe or Glu significantly reduced (∼ 20% and ∼ 50% from control respectively) the secretion of the alpha-galactosidase reporter, when compared to the wild type allele (Fig. 11). Thus, changes in both Tyr residues do not abolish COT1-MOB2A interactions, yet they significantly affect their strength. Based on the importance of MOB2A as a co-activator of COT1, we concluded that the mechanistic aspect of at least some of the morphological consequences observed in this study are likely due to impaired function of COT1 as a result of a defect in the physical interactions between the kinase and its coactivator.
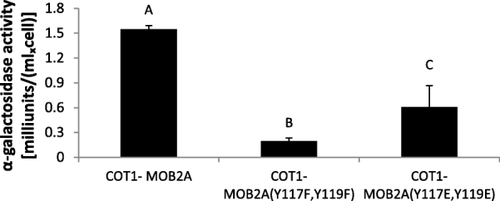
Tyr117 and Tyr119 are required for proper COT1/MOB2A protein interactions as determined by Yeast Two Hybrid analysis. Yeast transformants carrying each of the mob-2a alleles and cot-1, were grown in SD medium lacking Leu and Trp. The supernatant of the yeast culture was assayed for α-galactosidase activity. Statistical analysis was performed with Students t-test (P<0.05).
Discussion
MOB proteins have been determined to function as co-activators of NDR kinases in several biological systems (Komarnitsky et al., 1998; Mah et al., 2001; Weiss et al., 2002; Hou et al., 2003; Bichsel et al., 2004; Bothos et al., 2005; He et al., 2005; Shi et al., 2008; Schmidpeter et al., 2017). Phosphorylation state has been shown to regulate the activity of both the kinase and its co-activator (Millward et al., 1999; Mah et al., 2001; Tamaskovic et al., 2003; He et al., 2005; Seiler et al., 2006; Wei et al., 2007; Praskova et al., 2008; Bao et al., 2009; Ziv et al., 2009; Gutiérrez-Escribano et al., 2011; Ziv et al., 2013). The N-termini of NDR proteins have been shown to be critical for the physical interactions between the kinases and their MOB counterparts (Bichsel et al., 2004; Hergovich et al., 2008; Hergovich et al., 2009; Gógl et al., 2015). In fact, mutating residues at the N-terminus of the N. crassa COT1 kinase resulted in an impaired physical interaction between the kinase and MOB2 proteins, leading to morphological changes during asexual development (Ziv et al., 2013). To date, research has focused on changes in the COT1 partner of the complex. Here, we analyzed the possible effects of altering MOB2A on the mentioned interaction and the possible consequences of interfering with this process.
Altering MOB2A (Tyr117 and Tyr119) affected multiple morphological parameters. During the asexual cycle, N. crassa produces spores, most of which are borne by aerial hyphae. COT1, which has been shown to co-localize with MOB2A at the hyphal tip and partially colocalize with the Spitzenkörper (Maerz et al., 2012), has been shown to affect aerial hyphae formation, as well as different stages of conidiation (Ziv et al., 2009). Moreover, deletion of mob-2b in a phospho-COT1 mutant background is impaired in conidiation (Ziv et al., 2013). Thus, changes in the interactions between MOB2 and COT1 may affect conidiation. In fact, totally abolishing such an interaction by deletion of mob-2a, results in a significant reduction in the number of conidia formed (Maerz et al., 2009). Our current results demonstrate that two Tyr substitutions are sufficient to mimic the impaired conidiation effect observed in the Δmob-2a mutant.
When grown at 28°C, the temperature sensitive mutant mob-2a(Y117E,Y119E);Δmob-2b produced a large number of aerial hyphae. Many of these exhibited cytoplasmic leakage at their tips (Fig. 4C). Such occurrences have also been observed in the case of inactivation of the second N. crassa NDR kinase, dbf-2 (NCU09071.7; Dvash et al., 2010), as well as when the mas-1 gene (NCU03140.7; shown to be involved in cell wall integrity) had been deleted (Koch et al., 2014). One explanation for the leakage phenomenon may be the occurrence of a defect in aerial hyphae quality, such as imposed by a flaw in cell wall integrity. The Δmob-2a mutant is characterized by an increase in aerial hyphae formation, however, a reduction in the amount of asexual conidia. This reduction could well be attributed to the structural impairment of the aerial hyphae (which are required for the biogenesis of the asexual spores). We propose that a reduction in COT1 activity and/or changes in COT1 localization may be involved. MOB2A/B have been shown to control COT1 localization at the hyphal tip and the interactions between these proteins are essential for COT1 kinase activity (Maerz et al., 2009; Maerz et al., 2012). This, in turn, may affect the expression of various cell wall remodeling genes, as has been more recently described (Herold and Yarden, 2017). In fact, preliminary data we have indicates that MOB2A can physically interact with some cell wall remodelling proteins such as the glycoside hydrolase GH-17-3 and chitin synthase 7 (Aharoni-Kats and Yarden, unpublished).
While the involvement of COT1 in conidial germination has been shown, the possible involvement of its coactivators in this process has not been analyzed to date. Changes in the phosphorylation state of the COT1 Ser189, which is located at the N-terminal of this protein, were shown to result in a delay in the establishment of germ tube formation, during the early stages of conidial germination. Furthermore, these changes also affect the physical interactions of the kinase with both MOB2 proteins (Ziv et al., 2013). We now demonstrate that deleting mob-2a also significantly affects the germination process by conferring enhanced conidial germination rates. A similar phenomenon was observed in the Δmob-2b strain, where 2 hrs post induction, germination in the mutant was almost twofold of that observed in the wild type (28% and 15% respectively). To the best of our knowledge, this is the first report in which proteins that belong to the MOB family are shown to be involved in this fundamental stage of fungal development. If defects in COT1 result in delayed germination rates, yet deletion of its coactivators result in the converse, it is likely that MOB2A and MOB2B interact with additional protein/s (other than COT1) as part of their role in the regulation of germination. While MOB2A/B appear to be negative regulators of conidial germination, our observations indicate that the young germ tubes elongate faster in a Δmob-2a background than in the wild type (Fig. 3C). However, mature hyphae of this mutant elongate at a slower rate when compared to the wild type. Nonetheless, the question whether the polarized nature of germination and subsequent events of hyphal extension and branching are controlled by identical or different regulatory networks remains open (Riquelme et al., 2011). Our data support the possibility that even if some of the components (such as cot-1 and mob-2a) are involved in more than one of these morphologically distinct events, they may do so in different manners.
In addition to the faster germination rates, increased conidial agglutination was also observed in the mob-2a(Y117F,Y119F) as well as mob-2a(Y117E,Y119E) mutants. Conidial agglutination/adhesion has been extensively described in human and plant pathogens (Hamer et al., 1988; Braun and Howard, 1994; Mims et al., 1995; Sundstrom, 1999). Nonetheless, this characteristic was also observed in the saprophyte Aspergillus nidulans (Osherov and May, 2000) and was also shown to be a central part in post germination stages (e.g., conidial anastomosis tube formation) in N. crassa (Fu et al., 2011). Based on the results presented here, MOB2A may play a role (direct or indirect) in determining the agglutination properties of N. crassa, in the context of the multiple physiological events accompanying the germination process, including cell wall biosynthesis. Such a link between changes in cell wall properties and agglutination/adhesion have been described in A. fumigatus and C. albicans conidia (Tronchin et al., 1989; Tronchin et al., 1995). In the case of the latter, Tronchin et al. (1995) demonstrated that removal of cell wall polysaccharides suppresses agglutination.
Schmit and Brody (1976) described some of the biochemical and physiological events that occur during conidial germination of N. crassa. Many of the changes described, including those in RNA synthesis, DNA replication, protein synthesis machinery transport and those related the cellular energy were highly correlated with the changes observed in the transcriptional profile of germinating conidia (Kasuga et al., 2005). Thus, future delineation of the biochemical events that lead to the increase in agglutination, along with the rapid germination observed in the phosphomimetic mutants and the alleles analyzed in this study, could well be assisted by transcriptional analysis of the mentioned strains during the initial phases of germination. The mentioned strain and its phenotypic attributes may well assist in the temporal and spatial analysis of events involved in the attraction, agglutination, CAT formation and cell wall remodeling and complement what has been elegantly established so far (Fu et al., 2011, Herzog et al., 2015; Serrano et al., 2017).
In a previous report, deletion of mob-2a or mob-2b was shown to not affect female fertility or ascospore abundance and shape (Maerz et al., 2009). Although the functional link between MOB2A/B and sexual development has not been established, Lehr et al., (2014) found changes in mob-2a and mob-2b expression levels during perithecia formation. In this study, while using double mutants, in which mob-2a was altered in a Δmob-2b background, we were able to expose a role for MOB2A/B during sexual development of the fungus. These results further support the fact that MOB2A and MOB2B have overlapping functions during this process. Thus, we now establish that in addition to MOB1 and MOB3 (Maerz et al., 2009) the two remaining members of the N. crassa MOB family of proteins are also involved in regulation of the sexual cycle. Another example is MOB3, which has been shown to be essential for completion of the sexual phase in the phylogenetically-related, yet homothallic, S. macrospora (Bernhards and Pöggeler, 2011). As COT1 has not been shown to regulate sexual development, we suggest that MOB2A/B interact with additional proteins, which are not in the COT1 pathway, as part of their role in the regulation of sexual development. To date, our ultrastructural analyses of NDR kinase and interacting proteins mutants was based on standard SEM techniques (Shomin-Levi and Yarden, 2018; Yatzkan and Yarden, 1999; Dvash et al., 2010; Ziv et al., 2009). Here, some of the observations of the effects imposed by the phosphomimetic mutants were based on the use of correlative microscopy, which has been previously used for the analysis of fungal structures (Lord and Read, 2011; Szczepanowska et al., 2015). In the future, we envision expanding these techniques to include both spatial as well as temporal views of developmental events of structures that have cell walls (Kaminskyj and Dahms, 2008; Sarkar et al., 2009; Kumar and Elbaum, 2018). This could well include further analysis of perithecial development, using model systems like Neurospora and Podospora (Harris et al., 1975; Raju and Leslie, 1992; Lehr et al., 2014; Teichert et al., 2014) as well as other fungi.
While the mob-2a(Y117E,Y119E) mutant exhibits normal hyphal morphology, deletion of mob-2b in this mutant resulted in a dramatic increase in branching and extremely slow radial growth of the colony, as was evident on solid medium. This observation leads us to conclude that MOB2B can compensate for the lack of MOB2A function during hyphal elongation and branching. This result is in agreement with that described concerning overlapping functions of the two MOB2 proteins (Maerz et al., 2009). Since then, the overlap has been shown not to be complete (Ziv et al., 2013), and in this study we have expanded on the array of non-overlapping functions of these proteins. These include developmental processes in the asexual phase of the fungus' growth. In particular, and in contrast to mature hyphal growth, substitutions of Tyr117 and Tyr119 affected conidiation and germination (Fig. 3), indicating that MOB2B cannot compensate for the lack of MOB2A during early stages of N. crassa colony formation. Interestingly, both Tyr residues appear to be involved in the developmental processes analyzed here, as transforming the mob-2a(Y117E,Y119E);Δmob-2b strain with either a mob-2a(Y117E) or a mob-2a(Y119E) allele, was not sufficient to complement the temperature-sensitive phenotype described above (Aharoni-Kats and Yarden, unpublished). In addition, preliminary results showed that mob-2a and mob-2b deletion mutants exhibits differential resistance to the serine-palmitoyl transferase inhibitor Myriocin, the first, enzyme of the sphingolipid synthesis pathway (Aharoni-Kats and Yarden unpublished). Taken together, it appears that in addition to some overlapping functions, MOB2A and MOB2B also have distinct ones during fungal growth and development.
Deletion of gul-1 in a cot-1 (ts) background partially suppressed the pleiotropic morphology of cot-1 (ts) at the restrictive temperature. The phenotypic changes were accompanied by a reduction in the intensity of changes in the expression of genes, some of which are associated with cell wall integrity and were elevated or reduced in the cot-1 strain (Terenzi and Reissig, 1967; Herold and Yarden, 2017). Based on the interactions between the S. cerevisiae GUL1 and COT1 homologues [SSD1 and Cbkp respectively; (Jansen et al., 2009)] it is likely that GUL1 is a COT1 substrate and has a similar function as a translational regulator involved in cell wall remodeling (Herold and Yarden, 2017). Using GUL1 we studied the functional link between mob-2a(Y117E,Y119E);Δmob-2b and COT1. Deleting gul-1 in a mob-2a(Y117E,Y119E);Δmob-2b (ts) background significantly improved hyphal elongation and branching while culturing this strain at the restrictive temperature. The observed phenotypic suppression was also accompanied by changes in gene expression as observed in cot-1(ts) and cot-1;Δgul-1 mutants. Thus, mob-2a(Y117E,Y119E);Δmob-2b exhibited changes in the expression of chs-1, fks-1 and dur-3 genes which resembled that observed in cot-1 (ts) [Herold and Yarden, 2017 and Herold and Yarden, unpublished respectively] and these changes were suppressed by gul-1. Nevertheless, gul-1 only partially suppressed the mob-2a(Y117E,Y119E);Δmob-2b phenotype. It is very likely that similar to that found in yeast, GUL1 is not the only COT1 substrate (Mazanka et al., 2008; Gógl et al., 2015) and overcoming the defects observed involves additional genes/gene products. Our results also suggest that MOB2A interacts with additional proteins and is involved in regulation of additional pathways that are not dependent on COT1 (e.g., sexual development). Taken together, we conclude that some of the effects of altering Tyr117 and Tyr119, such as hyphal growth and morphology, are mediated by COT1.
In addition to the described genetic interactions between mob-2a(Y117,Y119) and cot-1 we also showed that changes in these predicted phosphorylation sites in both mob-2a(Y117F,Y119F) and mob-2a(Y117E,Y119E) mutants resulted in a weaker physical interaction between MOB2A-COT1, as determined in vitro. Based on the fact that both alterations resulted in a similar effect, we suggest that either the interactions require the presence of intact Tyr residues (regardless of potential phosphorylation state) or that one of the introduced substitutions does not fully mimic the potential phosphorylation state. The latter possibility was also suggested by Praskova and colleagues (2008), who showed that the conversion of two Thr to Ala in the human MOBKL1A nearly eliminated binding to its NDR kinase counterpart LATS1. At the same time, as substitution to Glu led to a similar effect, they suggested that this is not a result of mimicking a phosphorylated form of the protein.
Despite the fact that mimicking the phosphorylated and unphosphorylated state of the Tyr residues led to similar effects on MOB2A/COT1 interaction, the mutants' morphological attributes (in a Δmob-2b background) was very distinct. One explanation for these results is the involvement of other protein-protein interactions with MOB2A.
Interestingly, significant changes in the MOB2A-COT1 protein interactions were observed in the phosphomimetic mutants yet, the substituted residues are predicted to be in a region which is not within the expected physical interface of the two proteins, as determined by Gógl and colleagues (2015) to occur between MOB2 and CBK1, the respective S. cerevisiae homologues. The human MOB1-LATS1 crystal structure revealed that MOB1 phosphorylation sites Thr12/Thr35 are not positioned at the physical interface between MOB1 and LATS1 even though altering them affects the interaction between these proteins (Praskova et al., 2008; Ni et al., 2015). We suggest that substituting Tyr117 and Tyr119 may result in conformational changes of the protein in a manner that also affects residues which actually interact with the kinase. Such changes may well affect interactions of the MOB2A protein with additional proteins.
Most of the mechanistic analysis of MOB regulation by phosphorylation has been studied in human cells. It has been shown that MOB1 is phosphorylated on at least three Thr residues. These residues, Thr12/35/74, are phosphorylated by MST1 and MST2 (Hirabayashi et al., 2008; Praskova et al., 2008; Bao et al., 2009) and are not conserved in the N. crassa MOB2A. In this study, we identified two Tyr residues which are potentially phosphorylated. While the location of previously described phosphorylated residues in human and C. albicans were mainly in the N-terminal region of the MOB proteins (Praskova et al., 2008; Bao et al., 2009; Gutiérrez-Escribano et al., 2011), the N. crassa MOB2A Tyr residues are distal to the mentioned N-terminal region of the protein.
An additional difference between phosphorylation of human and fungal MOB/NDR proteins involves the function of MSTs. While in human, the MST kinase can phosphorylate both MOB (Praskova et al., 2008) and the hydrophobic motif of the NDR (or LATS) kinase (Chan et al., 2005; Stegert et al., 2005; Vichalkovski et al., 2008; Hergovich et al., 2009), in N. crassa, POD6, which is also a member of the STE20 kinase family, phosphorylates COT1 (Seiler et al., 2006), yet does not phosphorylate MOB2A (Maerz et al., 2012). Moreover, POD6, most likely, has no physical interaction with the NDR kinase coactivator, as shown by yeast two hybrid analysis (Maerz et al., 2012). Overall, MOB signaling pathways are more complex in human cells than in fly, yeast and N. crassa. This may be due to the larger number (6) of MOB proteins present in human, resulting in more potential interactions between the NDR kinases and their co-activators (Hergovich, 2011), as well as with additional proteins. For example, the human MOB2 protein may have a different role than the other MOB proteins, and under specific conditions can even act as a negative regulator of the NDR kinase (Kohler et al., 2010; Couzens et al., 2017). Taken together, we assume the human MOB1 model is different from that of MOB2 in N. crassa. In contrast, due to the conserved structure and function of MOB in other fungi, we assume that the mechanism of MOB2 regulation in N. crassa may be very similar in additional filamentous fungi.
In this study, we have shown that changes in the phosphorylation state of MOB2A Tyr117 and Tyr119 have a different effect on various stages of N. crassa development. We assume that under natural conditions MOB2A phosphorylation is governed by the appropriate kinases/phosphatases that function in a developmental stage-dependent manner. This was also shown in the case of C. albicans, where MOB2 phosphorylation is growth mode-dependent. Moreover, it has been suggested that the CBK1/MOB2 complex is regulated by differential phosphorylation of MOB2 (Gutiérrez-Escribano et al., 2011). In addition, we speculate that in N. crassa only small amount of MOB2A is phosphorylated at every given moment, perhaps in a manner similar to that described in the case of the human MOB1B (Bao et al., 2009). Thus, the constitutive phosphomimetic state of the MOB2A examined here might exacerbate some of MOB2A functions that have led to the effects we have observed. This may also be true for other phosphomimetic mutants in other proteins/systems.
Experimental procedures
Strains, media and growth conditions
General procedures and media used in the handling of N. crassa have been previously described (Davis, 2000) or are available through the Fungal Genetics Stock Center, (http://www.fgsc.net/Neurospora/NeurosporaProtocolGuide.htm). N. crassa strains used in this study are listed in Table 1. Strains were grown in either liquid or solid (supplemented with 1.5% agar) Vogel's minimal medium with 1.5% (w/v) sucrose. When required, the medium was supplemented with 100 µg ml−1 Histidine (Sigma Aldrich) 10 µg ml−1 Nourseothricin (Werner BioAgents, Jena, Germany) or 100 µg ml−1 Hygromycin B (Calbiochem, Riverside, CA). All analyses of sexual development were carried out on cultures grown on synthetic crossing medium (SC). Strains were either co-inoculated or inoculated sequentially (with a 5-day interval between the mating partners), as indicated.
| Strain | Genotype | Source |
|---|---|---|
| Wild type | 74-OR23-1 Mat A | FGSC#987 |
| Wild type | ORS-SL6 Mat a | FGSC#4200 |
| cot-1(ts) | cot-1(C102t) | FGSC#4066 |
| myc-cot-1 | myc-cot-1(183-4) | Ziv and colleagues (2009) |
| Δmob-2a; myc-cot-1 | hph:: NCU03314.2Δ myc-cot-1 | Ziv and colleagues (2013) |
| Δmob-2b; myc-cot-1 | hph:: NCU07460.2Δ myc-cot-1 | Ziv and colleagues (2013) |
| Δgul-1 | hph::gul-1Δ | FGSC#11288 |
| Δmob-2a; myc-cot-1;his3- | hph:: NCU03314.2Δ myc-cot-1 his3- | This study |
| HA-mob-2a;Δmob-2a; myc-cot-1 | his-3::Pccg-1-HA-mob-2a hph:: NCU03314.2Δ myc-cot-1 | Ziv and colleagues (2013) |
| HA-mob-2a(Y117F,Y119F);Δmob-2a; myc-cot-1 | his-3::Pccg-1-HA-mob-2a(Y117F,Y119F) hph:: NCU03314.2Δ myc-cot-1 | This study |
| HA-mob-2a(Y117E,Y119E);Δmob-2a; myc-cot-1 | his-3::Pccg-1-HA-mob-2a(Y117E,Y119E) hph:: NCU03314.2Δ myc-cot-1 | This study |
| HA-mob-2a;Δmob-2a;Δmob-2b;myc-cot-1 | his-3::Pccg-1-HA-mob-2a NCU03314.2Δ NCU07460.2Δ myc-cot-1 | This study |
| HA-mob-2a(Y117F,Y119F);Δmob-2a;Δmob-2b;myc-cot-1 | his-3::Pccg-1-HA-mob-2a (Y117F,Y119F) NCU03314.2Δ NCU07460.2Δ myc-cot-1 | This study |
| HA-mob-2a(Y117E,Y119E);Δmob-2a;Δmob-2b;myc-cot-1 | his-3::Pccg-1-HA-mob-2a (Y117E,Y119E) NCU03314.2Δ NCU07460.2Δ myc-cot-1 | This study |
| HA-mob-2a(Y117F,Y119F);Δmob-2a;Δmob-2b; cot-1(ts) | his-3::Pccg-1-HA-mob-2a (Y117F,Y119F) NCU03314.2Δ NCU07460.2Δ cot-1(C102t) | This study |
| HA-mob-2a(Y117E,Y119E);Δmob-2a;Δmob-2b; cot-1(ts) | his-3::Pccg-1-HA-mob-2a (Y117E,Y119E) NCU03314.2Δ NCU07460.2Δ cot-1(C102t) | This study |
| HA-mob-2a(Y117F,Y119F);Δmob-2a;Δmob-2b; Δgul-1 | his-3::Pccg-1-HA-mob-2a (Y117F,Y119F) NCU03314.2Δ NCU07460.2Δ gul-1Δ | This study |
| HA-mob-2a(Y117E,Y119E);Δmob-2a;Δmob-2b; Δgul-1 | his-3::Pccg-1-HA-mob-2a(Y117E,Y119E) NCU03314.2Δ NCU07460.2Δ gul-1Δ | This study |
| HA-mob-2a(Y117F,Y119F);HA-mob-2a;Δmob-2a;Δmob-2b;myc-cot-1 | his-3::Pccg-1-HA-mob-2a(Y117F,Y119F) NCU03314.2Δ NCU07460.2 myc-cot-1(183-4) HA-mob-2a NatR | This study |
| HA-mob-2a(Y117E,Y119E);HA-mob-2a;Δmob-2a;Δmob-2b;myc-cot-1 | his-3::Pccg-1-HA-mob-2a(Y117F,Y119F) NCU03314.2Δ NCU07460.2 myc-cot-1(183-4) HA-mob-2a NatR | This study |
| HA-mob-2a(Y117E,Y119E);HA-mob-2a(Y117E);Δmob-2a;Δmob-2b;myc-cot-1 | his-3::Pccg-1-HA-mob-2a(Y117E,Y119E) NCU03314.2Δ NCU07460.2 myc-cot-1(183-4) HA-mob-2a(Y117E) NatR | This study |
| HA-mob-2a(Y117E,Y119E);HA-mob-2a(Y119E);Δmob-2a;Δmob-2b;myc-cot-1 | his-3::Pccg-1-HA-mob-2a(Y117E,Y119E) NCU03314.2Δ NCU07460.2 myc-cot-1(183-4) HA-mob-2a(Y119E) NatR | This study |
Producing MOB2A phosphomimetic mutants
mob-2a (NCU03314) strains with point mutations were produced using the pLA1 plasmid (containing a mob-2a fragment tagged with HA at the N-terminus and targeted to the mob-2a locus) as a template. Mutations were inserted to replace Tyr 117 and/or Tyr119 to Phe or Glu [commonly used to mimic the unphosphorylated and phosphorylated state of the residue respectively (Piedra et al., 2001; Oh et al., 2010; Shimozato et al., 2015; Sidibé et al., 2014)]. Point mutagenesis was performed by PCR using Phusion® High-Fidelity DNA Polymerase (Thermo Scientific) and the oligonucleotides listed in Supporting Information Table S1. The resulting amplification products were purified using The Wizard® SV Gel and PCR Clean-Up System according to the manufacturer's protocol and were then digested with DpnI (Thermo Scientific). The resulting plasmids, together with the pHAN1-mob-2a [allowing the expression of HA-mob-2a from the his-3 locus (Maerz et al., 2009)], were digested with SpeI and Eco47III (Thermo Scientific) and the fragments carrying the mutation site/s were ligated with the pHAN1-mob-2a fragment to form a mutated HA-mob-2a fusion which can be targeted to the his-3 locus. The resulting plasmids and the pHAN1-mob-2a control were linearized with SspI (Thermo Scientific) and transformed to the Δmob-2a; his-3-;myc-cot-1 strain. Transformants were first selected by their ability to complement his-3 to auxotrophy. Two microconidia-derived homokaryon strains found to harbor each of the HA-tagged mob-2a alleles were isolated. The presence of the HA-tagged alleles was verified by PCR and the presence of the relevant point mutations were confirmed by sequencing.
Determining growth rate, length between branches, conidiation and conidial germination rate
For growth-rate measurements, race tubes containing 20 ml Vogel's sucrose minimal medium were inoculated with 6 μl of a conidial suspension (2 × 106 conidia/ml). The race tubes were incubated for several days at 32°C or 34°C. Hyphal growth was measured three times daily.
To determine colony diameter, 6 μl of a conidial suspension (2 × 106 conidia/ml) were placed at the center of plates containing 15 ml VgS medium, in triplicate. Plates were incubated at 28°C or 34°C for about 20 h. Colony diameter was measured (3 measurements for each colony) using a ruler.
To determine conidiation rate, 107 conidia of each strain were inoculated into 100 ml Erlenmeyer flasks containing 20 ml solid Vogel's sucrose minimal medium. The flasks were incubated for 7 days at 34°C and an additional 3 days at room temperature. Conidia were harvested by adding 50 ml of distilled water and vigorously shaking the flasks. The conidial suspensions were filtered through cheesecloth and subsequently centrifuged for 5 min at 4000 g and the supernatant aspirated. The conidia were then resuspended in 5 ml of distilled water and the number of total asexual propagules was determined by use of a hemocytometer.
To determine the length between branches, conidia of the different N. crassa strains were inoculated on glass slides covered with 1 ml Vogel's sucrose minimal medium and incubated overnight at 34°C or 32°C under high humidity conditions. The edges of the growing colonies were observed microscopically and the entire colony edge was documented in approximately 20 photographs. Each of the photographs was analyzed using ImageJ 1.37V (Rasband, W.S., U.S. National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997–2006). The length between each two successive branches of leading hyphae was determined and at least 300 measurements were performed for each strain. The entire data set of each strain was used to calculate the average length between branches. The presented data (and calculated standard error) is based on the averages of at least three independent experiments performed with each strain.
To determine the extent of conidial germination of the different strains, 5 ml Vogel sucrose minimal medium were inoculated with a conidial suspension (final concentration of 5 × 106 conidia/ml) and incubated (34°C) at 160 r.p.m. Cultures were sampled 3 h after induction of germination and examined by light microscopy. Conidia were considered “germinated” once a clear germ tube was visible at a 40× magnification. At least 250 conidia were analyzed for each strain and the experiment repeated twice. In addition to germination rate, the extent of germling agglutination was also measured. At least 350 conidia of each strain were accounted for. When at least 3 conidia were observed as a single entity, they were considered agglutinated. For measuring germling length, cultures were sampled 2 and 3 h after induction. At least 80 conidia were analyzed for each strain, using ImageJ 1.37V.
Real time PCR
For gene expression analysis experiments, conidia were cultured (105 conidia/ml) in liquid Vogel's minimal medium with 1.5% (w/v) sucrose. Cultures were grown at 25°C for 16 h and then shifted to 34°C for additional 4 h (160 r.p.m.). Mycelia were collected and RNA was extracted with the RNeasy Plant Mini Kit (Qiagen, Germany). cDNA was produced using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA) according to the manufacturer's instructions. Fast SYBR green Master mix (Applied Biosystems, CA, USA) was used together with relevant primers (10 µM) (Supporting Information Table S2), 2 µl of target cDNA solution and nuclease free water to prepare a final volume of 10 μl per reaction, in MicroAmp Fast Optical 96-well reaction plates (Applied Biosystems, China). All reactions were performed in triplicate on an ABI StepOnePlus Real-Time PCR Sequence Detection System and software (Applied Biosystems). Real Time PCR relative quantification was analyzed using 2−ΔΔct (Livak and Schmittgen, 2001). β-tubulin (NCU04054) was used as the reference gene to calculate total cDNA abundance.
Mass spectrometry analysis
For the identification of MOB2A phosphorylation sites, protein gel bands were de-stained and digested in-gel with sequencing grade trypsin (10 ng/μl trypsin, 50 mM ammonium bicarbonate, pH 8.0) at 37°C, overnight. The resulting tryptic peptides were extracted with 5% formic acid/50% acetonitrile and 0.1% formic acid/75% acetonitrile sequentially and then concentrated to ∼ 20 μl. The peptides were separated on a homemade analytical capillary column (50 μm × 10 cm) packed with 5 μm spherical C18 reverse phase material (YMC, Kyoyo, Japan). The peptides were eluted by an Agilent 1100 binary pump with the following HPLC gradient: 0%–5% B in 5 min, 5%–40% B in 25 min, 40%–100% B in 15 min (A = 0.1 M acetic acid in water, B = 0.1 M acetic acid/70% methanol). The eluted peptides were sprayed directly into a QSTAR XL mass spectrometer (MDS SCIEX, Toronto, Canada) equipped with a nano-ESI ion source. The mass spectrometer was operated in data dependent mode with one MS scan followed by three MS/MS scans for each cycle. Database searches were performed on an in-house Mascot server (Matrix Science, London, UK). Methionine oxidation, phosphorylation of Ser, Thr and Tyr were set as variable modifications.
Yeast two hybrid assays
For yeast two hybrid analyses, the Matchmaker Two-Hybrid system 3 (Clontech, USA) was used, following the manufacturer's instructions. The construction of the pGADT7 vector (containing the GAL4 activation domain) and pGBKT7 (containing the DNA-binding domain) expressing either COT1 [the cDNA of the long transcript(Gorovits et al., 1999)] or MOB2A was described in Maerz et al. (2009). The pGADT7-MOB2A cassette was mutagenized to introduce the Tyr117 and Tyr119 point mutations using the primers described in Supporting Information Table S1. The resulting fusion proteins were expressed together with pGBKT7-COT1 in S. cerevisiae AH109 cells and potential physical interactions were determined using α-Gal quantitative assay as described by Ziv and colleagues (2013). At least three independent transformants were analyzed for each interaction that was tested.
Microscopy
Light microscopy was performed with a Zeiss Axioscope microscope, equipped with a Nikon DXM1200F digital camera or EVOS FL Auto Cell Imaging System (Life Technologies).
Fluorescence microscopy was performed with an EVOS FL Auto Cell Imaging System (Life Technologies). Images were taken using a Dapi and TxRed filters for calcofluor white and congo red staining respectively.
For the cryostat procedure, 6 µl conidial suspension (2 × 106 conidia/ml) of the two mating types were cultured on SC, supplemented with 0.01 µg ml−1 calcofluor white [binds to the cell wall component chitin (Ram and Klis, 2006)] , for 6, 11 and 32 days. Samples were then fixed, for 1 h, with 4% Paraformaldehyde solution in PBS (Santa Cruz biotechnology). The samples were washed three times with 25% sucrose every 10 min and then maintained overnight at 4°C. Several drops of Tissue-Tek O.C.T (Sakura, Japan) were then added to each sample. The samples were placed on dry ice for 10–15min until the resin solidified and then maintained at −20°C, overnight, prior to sectioning. Fixed samples (5–10 fruiting bodies of each strain) were sectioned with a Leica CM1860 cryostat (20–30 µm each section) and were stained with congo red (1 gr/l) which interacts with cell wall (Ram and Klis, 2006). Images were taken using a fluorescence microscopy (details are mentioned above). Samples were then coated with iridium (Ir) as mentioned above and SEM images were taken using either a High resolution JSM-7800F Field Emission Electron Microscope or a JSM-IT100 scanning electron microscope (JEOL SEM technologies), in which images were taken with an accelerating voltage of 20 kV. Photoshop (Adobe Systems, San Jose) was used to layer images obtained by light, electron and fluorescence microscopy (as required) for correlative microscopy.
Statistical analysis
Statistical analysis was performed by Student's t test, using the JMP statistical package (version 13). A two-tailed p value less than 0.05 was considered significant.
Bioinformatics
Multiple Alignment was performed using Clustal Omega (Sievers et al., 2011).
Acknowledgements
We thank Carmit Ziv for her comments on the manuscript. This work has been supported by the Israel Science Foundation.