The for gene as one of the drivers of foraging variations in a parasitic wasp
Abstract
Foraging behaviours encompass strategies to locate resources and to exploit them. In many taxa, these behaviours are driven by a major gene called for, but the mechanisms of gene regulation vary between species. In the parasitoid wasp Venturia canescens, sexual and asexual populations coexist in sympatry but differ in life-history traits, physiology and behaviours, which could impact their foraging strategies. Here, we explored the molecular bases underpinning divergence in behaviours by testing two mutually nonexclusive hypotheses: first, the divergence in the for gene correlates with differences in foraging strategies, and second, the latter rely on a divergence in whole-genome expression. Using comparative genomics, we showed that the for gene was conserved across insects considering both sequence and gene model complexity. Polymorphism analysis did not support the occurrence of two allelic variants diverging across the two populations, yet the asexual population exhibited less polymorphism than the sexual population. Sexual and asexual transcriptomes split sharply, with 10.9% differentially expressed genes, but these were not enriched in behaviour-related genes. We showed that the for gene was more highly expressed in asexual female heads than in sexual heads and that those differences correlate with divergence in foraging behaviours in our experiment given that asexuals explored the environment more and exploited more host patches. Overall, these results suggested that fine tuning of for gene expression between populations may have led to distinct foraging behaviours. We hypothesized that reproductive polymorphism and coexistence in sympatry of sexual and asexual populations specialized to different ecological niches via divergent optima on phenotypic traits could imply adaptation through different expression patterns of the for gene and at many other loci throughout the genome.
1 INTRODUCTION
Loss of sexuality has been frequently recorded in a diverse array of eukaryotic taxa with three possible origins leading to the emergence of asexual lineages: mutation, hybridization (Normark, 2003) or endosymbiotic infection (Stouthamer et al., 1990). When reproductive modes are exclusive, asexuals no longer exchange gene flow (through meiosis and fecundation) with the sexual population from which they originated and thus begin to diverge by accumulating genetic mutations (Muller, 1964). Competitive interactions should favour one reproductive mode over the other (Lively, 2010). Asexuals have a demographic advantage by producing only females, and they avoid the cost of males (Maynard-Smith, 1978). In contrast, sexual populations maintain greater genetic diversity, which may confer a decisive advantage in changing environments (Otto, 2009). If geographical or ecological heterogeneity allows for ecological specialization, with each lineage performing better in a specific habitat, sexual and asexual populations can coexist in different geographical areas or sympatrically (Bell, 1982; Lynch, 1984). Such coexistence of sexual and asexual lineages is called reproductive polymorphism. In this case, adaptations in behaviour, morphology or life-history traits should distinguish sexual from asexual populations. Reproductive polymorphisms have been widely reported, notably in haplodiploid arthropods (van der Kooi et al., 2017), suggesting that competitive exclusion is not the norm and that ecological conditions allowing their coexistence are frequently met.
In the hymenopteran parasitoid Venturia canescens (Ichneumonoidea: Gravenhorst), asexual populations coexist in sympatry with sexual populations in natural conditions, each better adapted to specific ecological niches. The sexual populations are found in natural or seminatural habitats (e.g., orchards), while asexual populations are mainly found inside buildings (e.g., mills, bakeries; Beukeboom et al., 1999; Schneider et al., 2002). Morphologically, asexuals are indistinguishable from sexuals (Schneider et al., 2002), but they do differ in behaviours, physiology and life-history traits, as reported in numerous population pairs coming from different localities and integrated in a meta-analysis (Amat et al., 2017). In particular, asexual wasps have a higher capacity to find hosts (Liu et al., 2009), a larger egg load and a higher oviposition rate (Pelosse et al., 2007; Thiel et al., 2006). In contrast, sexual wasps fly longer and faster, have a higher energy content and live longer (Lukáš et al., 2010). We predict that these characteristics will affect foraging, which encompasses a broad range of behaviours deployed to access resources—such as food, oviposition sites, shelter and mates (Stephens et al., 2008). In the context of foraging for oviposition sites, we assume that sexual and asexual individuals will differ in their exploration (i.e., behavioural strategy to find host patches at a distance) and exploitation (i.e., behavioural strategy allowing the acquisition of resources once on the host patch). In particular, we expect that sexuals better explore their environment (since in the field host patches are sparsely distributed; Driessen & Bernstein, 1999) due to their higher dispersal abilities and longevity, while asexuals favour exploitation by taking advantage of their potential fecundity (i.e., egg-load) and their greater oviposition rate. In parasitoids, exploration and exploitation are directly linked to oviposition ability and thus determine reproductive success, a relevant proxy of fitness (van Alphen et al., 2003).
The molecular basis of foraging behaviours has been studied the most. In Drosophila melanogaster, the genetic influence on these behaviours has been extensively investigated. In this species, two distinct types of strategies have been characterized (Allen et al., 2017; Anreiter et al., 2017; de Belle & Sokolowski, 1989; Osborne et al., 1997; Sokolowski, 1980). Exploratory individuals that move across multiple patches are called rovers and coexist with individuals that remain on the same single patch to exploit it, called sitters (Sokolowski, 1980). The two strategies are under the influence of a major gene named foraging (for), encoding the protein kinase G (PKG). Despite the many genes involved in generating foraging behaviours (Anreiter et al., 2017), manipulations of for gene expression are sufficient to modify the behavioural responses (Osborne et al., 1997). The influence of foraging by the for gene should be taken in a broad sense as it includes the search for food and for oviposition sites (Edelsparre et al., 2014; McConnell & Fitzpatrick, 2017). The for gene has two allelic variants: rovers have at least one dominant allele (forR) corresponding to a higher for gene expression, while sitters have two recessive alleles (forS) and lower gene expression. The two alternative behaviours are maintained by selection; patchy food and high population densities advantage rovers, while evenly distributed food and low population densities advantage sitters (Sokolowski et al., 1997). The role of the for gene as a single major gene influencing foraging behaviours has been maintained during evolution as it has been characterized in many animal taxa as diverse as nematodes (Hao et al., 2011; Hong et al., 2008), insects (Ben-Shahar et al., 2002; Chardonnet et al., 2014; Ingram et al., 2005; Keating et al., 2013; Lucas et al., 2010; Lucas & Sokolowski, 2009; Tobback et al., 2011; Wenseleers et al., 2008) and mammals (Struk et al., 2019). However, the existence of allelic variants as well as the relationships between for gene expression level and foraging behaviours vary between species. Hymenoptera provide a great illustration of such variations: eusocial species (bees, ants and wasps; Aculeata) display the same age-dependent caste division of labour, where older workers are the foragers while young workers take care of the colony. To date, a single allele of the for gene has been reported in this group, with an expression pattern varying with caste, but in the opposite direction depending on the species. In Apoidea (including Apis mellifera and Bombus terrestris), foragers exhibit higher for gene expression (Ben-Shahar, 2005; Tobback et al., 2011). In contrast, in the ancestral groups of ants (Pogonomyrmex barbatus, Pheidole pallidula; Formicoidea), and wasps (Vespa vulgaris; Vespoidea) expression of for was lower in foragers (Ingram et al., 2005; Lucas & Sokolowski, 2009; Wenseleers et al., 2008). Venturia canescens belongs to Ichneumonoidea, a parasitoid basal superfamily within the Apocrita group that includes all other eusocial hymenopteran species (bees, ants and wasps; Peters et al., 2017). This solitary species, with coexisting populations showing behavioural differences, appears to be a relevant model for studying the genetic bases underlying the variability of foraging behaviours and their evolution within Hymenoptera. We expect to find no divergence in the for allele between the two populations consistently with the description of a single allele in all hymenopteran species so far. Moreover, we expect divergence in for expression patterns across populations: sexual individuals are expected to be better explorers and thus might have lower for expression, consistent with the ancestral pattern described in common wasp and ants. Furthermore, given the wide variations in life-history traits, physiology and behaviours observed between sexuals and asexuals, we assume that a large number of genes may be differentially regulated, including numerous behavioural genes. To test this hypothesis, we extended our comparison of gene expression to the genome scale.
Here, we explored two nonmutually exclusive hypotheses that could explain the divergence in behaviours of V. canescens populations: first, a divergence in the for gene, and, second, a divergence in whole-genome expression. We first proceed to the characterization of the for gene in V. canescens using genomic and transcriptomic sequences: (i) we describe the for orthologues and reconstruct its evolution in insects; (ii) we annotate the full gene model by analysing sexual and asexual transcriptomes; and (iii) we describe allelic variations in sexual and asexual populations. We explored the second hypothesis by studying the differential gene expression between sexual and asexual populations, with a particular focus on the behavioural genes. Finally, by coupling a behavioural experiment with for gene quantification, we tested whether variations in foraging behaviours correlate with variations in for gene expression.
2 MATERIALS AND METHODS
2.1 Field sampling and insect rearing
Venturia canescens is a solitary endoparasitoid of caterpillars of pyralid moths (Salt, 1976). The females used in the experiments come from sexual and asexual populations collected annually near Valence (44°58′21″N, 4°55′39″E). In this unique location, individuals of the sexual population were usually sampled in an orchard, while individuals of the asexual population were mostly collected close to grain silos. Caterpillars of Ephestia kuehniella (Zeller) were left for 1 week exposed to parasitoids and then brought back to the laboratory awaiting the emergence of parasitoids. Virgin emerging V. canescens females were isolated and left with hosts to sex their progeny. In V. canescens, sex determination is haplodiploid: sexual females have a parthenogenetic arrhenotokous reproduction; that is, unfertilized eggs produced haploid males, while diploid females resulted from fertilized eggs. Thus, virgin arrhenotokous females produce only males. In contrast, virgin asexual thelytokous females produce only females following central fusion automictic parthenogenesis, meaning that some genetic recombination occurs during the early stages of oogenesis. Thus, genetic variation still exists between asexual offspring even if an irreversible increase in homozygosity in populations occurs over time (Beukeboom & Pijnacker, 2000; Mateo Leach et al., 2009). Sexual and asexual wasps were maintained separately on the host E. kuehniella fed on semolina, where they produced kairomones attracted to parasitoids from mandibular gland secretions (Castelo et al., 2003). Insects were grown under a constant environment (25 ± 1°C, 55 ± 5% relative humidity, 12:12-h light–dark).
2.2 Annotation of the gene for in the Venturia canescens genome
Orthologues are genes that descended from the same ancestral sequence separated by a speciation event and often have the same function; hence, we first searched the orthologues of the for gene in the genome of V. canescens. A set of 40 orthologous for sequences from 38 insect species, as well as the branchiopoda Daphnia pulex and the mouse (Mus musculus) sequences, were identified using orthologue annotation in the Ensembl Metazoa database and literature (Table S1). To identify the for orthologue in the V. canescens genome, we used the reciprocal best hits with tblastn with a set of 42 for orthologous protein sequences previously described as a query and the V. canescens genome as a database (http://bipaa.genouest.org/sp/venturia_canescens/V.1.0). The for gene that was localized on scaffold 64 contained the longest open reading frame (Vcan27709), comprising 2445 nucleotides encoding 815 amino acids.
2.3 for phylogenetic reconstruction in insects
The putative V. canescens for sequence was added to the set of 42 orthologues and then aligned using muscle (Edgar, 2004). The corresponding protein alignment was highly variable in the N-terminus but conserved in the C-terminus. Alignment was manually curated; most conserved residues were selected using gblock, and the resulting alignment consisted of 533 amino acids. To reconstruct the phylogeny of the for gene and position the V. canescens sequence among other insect sequences, prottest version 3.4.2. (Darriba et al., 2011) was used to determine the best-fit model of protein evolution using Akaike's information criterion (AIC) (Abascal et al., 2005). The JTT model of protein evolution was used, and topology optimization was performed using the best NNI and SPR options. The phylogenetic tree was reconstructed with the maximum-likelihood method using phyml implemented in seaview (version 4.7; Gouy et al., 2010). Default aLRT (SH-like) was used for branch support (Anisimova & Gascuel, 2006).
2.4 RNA extraction and sequencing
Six RNA-sequencing (RNA-seq) libraries were prepared: sexual and asexual populations both consisted of three biological replicates. Each of the three replicates was composed of a pool of 30 individual heads taken from naive females (aged 0 days) flash frozen upon emergence and then used as an input for RNA extraction. Heads were crushed using steel beads and a Qiagen TissueLyser (45 s, 25 Hz). Total RNA was extracted using the Rneasy Mini Kit (Qiagen) following the manufacturer's protocol and including the DNase step. RNA integrity was controlled using gel electrophoresis and quantified with a Nanodrop. After integrity control and quantification, polyadenylated RNAs were enriched from 1 μg of high-quality total RNA with oligo-dT magnetic beads and then fragmented and converted to cDNA (Illumina TruSeq Stranded mRNA Library Prep kit). Fragments of 200 bp were selected, adapters were ligated, and fragments were amplified by PCR (polymerase chain reaction) to generate DNA colonies. Each library was labelled, multiplexed and pooled for sequencing on a HiSeq 2500 Illumina sequencer (Fasteris), with a paired-end protocol (2 × 150 bp).
2.5 For gene model reconstruction
We identified all isoforms of for transcripts and reconstructed the for gene model in V. canescens by screening the six RNA-seq libraries from sexual and asexual populations and focusing on the for reads. kissplice 2.5.4. (Sacomoto et al., 2012) is a method based on De Bruijn graphs that allows for identification of all variants without using a reference genome, including single nucleotide polymorphisms (SNPs), indels and alternative splicing events. In parallel, we built a de novo transcriptome assembly with trinity (Haas et al., 2013).
2.6 Polymorphism analysis at the for locus
To evaluate for gene polymorphisms, we localized all SNPs, insertions and deletions across all isoforms previously identified with kissplice. We then used KisSplice2RefTranscriptome to position each SNP on isoforms. Finally, we used the R package kissde to find SNPs that significantly differed in frequency across sexual and asexual populations (adjusted p < 0.05).
2.7 Differential expression analysis
The genome-wide divergence between sexual and asexual populations was estimated using RNA-seq libraries to identify differentially expressed genes (DEGs) between the two populations. Read quality was first assessed with fastqc, and then reads were trimmed and filtered using trimmomatic with a minimum length set to 75 bp. After filtering, the transcriptomic data set included a total of 91 million reads, of which an average of 92% were successfully aligned to the V. canescens transcriptome using hisat2 (Kim et al., 2019; Table S2). Genes with differential expression between sexual and asexual populations were identified using a negative binomial generalized linear model (GLM) implemented in the program deseq2 (Love et al., 2014). We tested for differential expression of all transcripts with an average level of expression greater than 10 reads per gene (n = 14,106). A gene was considered differentially expressed (DE) when the false discovery rate (FDR)-adjusted p value was <0.05, without applying a supplementary fold change threshold.
2.8 Functional analysis
The de novo transcriptome was annotated using blast and Gene Ontology (GO) tools to assign biological functions to transcripts. Then, we focused our analysis on the transcripts related to the “behaviour” GO term or to any of its child terms, that is all GO terms related to “behaviour” in the GO graph but annotated with a more specific term, thus annotating a functional group of transcripts related to behaviour. This list of transcripts was crossed with the previously established lists of DEGs between the two populations.
2.9 Behavioural experiment
We set up an experimental design to quantify foraging behaviours (i.e., the exploitation and exploration of host patches) of individuals from sexual and asexual populations facing two host patches. A total of 34 females (17 asexual, 17 sexual) aged 1 day were tested in random order. Exploitation was considered the capacity of females to localize and successfully lay eggs on hosts. Host patch exploitation was measured by the means of two parameters: (i) total patch residence time (PRT) was used as a synthetic parameter to summarize the exploitation of the two patches (sum of PRT on patches 1 and 2); and (ii) oviposition success, i.e., the total number of cockings, corresponding to a peculiar movement of the abdomen observed after egg laying when the female loads a new egg at the tip of its ovipositor (Rogers, 1972). Exploration was considered the ability to visit the entire experimental device (i.e., environment) and was quantified using two parameters: (i) the proportion of females that manage to visit the two host patches, considered as the aptitude to locate new resources; and (ii) the number of switches between the two host patches, considered as the ability to navigate between different resources. The experimental device contained two host patches placed 20 cm from each other inside a box (50 × 16 × 8 cm) with two side holes covered with veils to allow ventilation. The two host patches were made of Petri dishes (5.5 cm Ø) containing six 21-day-old larvae of E. kuehniella and semolina to the rim, prepared 7 days before the test and covered with a thin gauze to prevent larvae from escaping. Each host patch was embedded in clean semolina in the middle of a larger Petri dish (13 cm Ø). Every morning, wasps were collected at emergence and placed individually in tubes with one drop of water. The day after, males and females of the sexual strain were gathered in a cage to mate. Females were observed and gradually picked up in a tube as they mated until the behavioural experiment. Emerging asexual females were placed in another cage under the same conditions. At the beginning of the experiment, a single female was inserted into the box and deposited in the middle of the left patch, called “patch 1,” whereas the right patch was called “patch 2.” Foraging behaviours were followed for 20 min by recording four metrics with jwatcher (Blumstein & Daniel, 2007): (i) probing, wasps probed the substrate with ovipositor once presence of hosts detected thanks to kairomones; (ii) cockings; (iii) moving outside of patches (i.e., flying or walking); and (iv) time dedicated to hosts, PRT (i.e., sum of the time spent on patch 1 and patch 2). The female was considered to have left a patch when more than 150 s was spent outside of the patch; hence, PRT included short excursions outside patch boundaries. Immediately after the behavioural experiment, all 34 wasp heads were individually collected to quantify the expression of the for gene, while abdomens were dissected to count the number of eggs in the ovarioles, termed egg load. The heads were stored on ice in 10 μl of RNA-later (Sigma-Aldrich) and then at −20°C until RNA extraction.
2.10 Quantification of for gene expression
We quantified the for gene in each of the 34 wasp heads (17 females aged 1 day from each population) using real-time quantitative PCR (RT-qPCR) to correlate foraging behaviours with for gene expression. All samples were collected immediately after the end of the behavioural test, and the 34 RNA extractions were performed in one batch by series of 12 randomized samples using the protocol described above. First-strand cDNA was synthesized from 70 ng of total RNA using a SuperScript III first-strand synthesis system (Thermo Fisher Scientific) with random hexamer primers, followed by an RNAse-H step. Quantification was conducted on the for gene, together with two reference genes (rpl32 and gapdh) used for normalization between samples to control variations in extraction yield, reverse transcription yield and efficiency of amplification. Reactions were performed on a CFX96 (Bio-Rad) using 1:10 diluted cDNA and SYBR Green master mix (Bio-Rad) according to the manufacturer's instructions. The amplification conditions were a first step of denaturation (95°C, 1 min) followed by 40 cycles of denaturation (95°C, 10 s) and elongation (melting temperature, 30 s). Details on the primers and melting temperatures are listed in Table S3. Fluorescence was quantified at the end of each cycle, and the quantification cycle (Cq) corresponding to the start of exponential phase amplification was measured. Each sample was quantified twice: all duplicated Cq values varied by less than 0.5 cycles, indicating elevated replicability. The expression level of the for gene was determined relative to the expression level of both reference genes rpl32 and gapdh using the ∆∆Cq method (Livak & Schmittgen, 2001). The results were consistent regardless of the reference gene used, and both rpl32 and gapdh provided satisfactory quality control (low Cq values and low variations across samples; Figure S1 and Table S4). Therefore, we finally used the mean Cq between rpl32 and gapdh for normalization to increase the precision of the results. The expression values were expressed using relative values comparing each individual to the median individual, considered as the value 0. Negative values indicated individuals with expression lower than the median, and positive values indicated individuals with expression higher than the median.
2.11 Statistical analysis
The foraging behaviours, decomposed into exploitation and exploration, each measured by a set of parameters previously defined, were analysed using GLMs. The population (sexual or asexual), for expression (fold change), egg load (a proxy for parasitoid fitness; West et al., 1996) and double interactions with the variable population were included as predictor variables. PRT was analysed with a GLM with a gamma distribution for errors and inverse links. The number of switches between host patches was analysed with a GLM with a Poisson distribution and log link. The number of cockings was also analysed with a GLM with a Poisson distribution for error and log link. PRT was added to the full model as cocking probability increases with PRT. for gene expression was analysed with a linear model using population, egg load and their interaction as explanatory variables. The least contributory variables in all models were iteratively removed using backwards selection to select optimal models. All statistical analyses were performed with R (R Core Team, 2017).
3 RESULTS
3.1 Identification of the for gene in the Venturia canescens genome and for gene evolution in insects
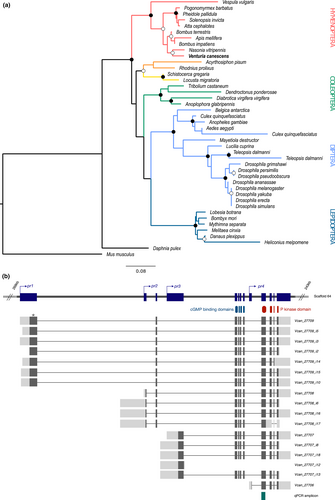
We identified a sequence candidate as the for orthologue in the Venturia canescens genome and then aligned it to a set of orthologues to reconstruct the evolutionary history of the for gene. The resulting maximum-likelihood tree robustly related the major represented insect clades: Hymenoptera, Orthoptera, Coleoptera, Diptera and Lepidoptera (Figure 1a). A majority of one-to-one orthologous relationships were detected, with the exception of rare duplication events. All 10 sequences from hymenopteran species constituted a monophyletic group that was highly supported (bootstrap value > 95%, Figure 1a). Within this group, V. canescens (Ichneumonidae) clustered with Nasonia vitripennis (Chalcidoidea) to constitute the parasitoida group. The phylogenetic reconstruction confirmed that one unique sequence within the V. canescens genome was orthologous to the for gene in Drosophila melanogaster, and was then annotated as the V. canescens for gene (Vcan_for).
3.2 Characterization of the for gene model in Venturia canescens
We produced RNA-seq libraries from sexual and asexual populations with a triple objective: (i) reconstruct the for gene model, that is the region of the gene that is supposed to be transcribed into RNA; (ii) evaluate polymorphism at the for locus within sexual and asexual populations; and (iii) assess genome-wide differences in gene expression between the two populations. To produce an accurate model of the for gene, we screened the RNA-seq libraries from sexual and asexual female heads searching for all reads mapping on this locus and reconstructed all of the transcripts. We identified four separate transcription start sites, supporting a gene model that contains four independent promoters (pr1–pr4) corresponding to four distinct open reading frames (Figure 1b). The longest open reading frame, Vcan27709, started with pr1 and exhibited seven isoforms that mainly differed in their untranslated regions (UTRs). The Vcan27708 transcript started with pr2 and showed four isoforms, while Vcan27707 (pr3) had five isoforms. Finally, the shortest transcript, Vcan27706, starting with pr4, presented one isoform. Overall, a total of 13 exons were identified of which different combinations constituted 17 different isoforms. The nine first exons exhibited alternative splicing; thus, isoforms essentially differed in their 5′ UTR and the corresponding N-terminus coding sequences. In contrast, the last four exons were constitutive of all isoforms (except Vcan27707_i12, Vcan27709_i14 and Vcan27708_i17; which contained an early stop codon) and constituted one unique 3′ extremity (Figure 1b) encoding the C-terminus part of the protein, containing the two cGMP-binding domains as well as the kinase domain.
3.3 Allelic variation at the for locus between sexual and asexual populations
We focused on the population polymorphism at the for locus by screening RNA-seq libraries based on 90 females from sexual and asexual populations, and we identified a total of 15 SNPs (Table 1). Only three SNPs were located within the coding region, including two synonymous SNPs and one single nonsynonymous mutation (in bold in the table). The 12 remaining SNPs were located outside of coding sequences within UTRs. Among the 15 SNPs, 14 variants exhibited significant differences in frequency between sexual and asexual populations (Table 1). These variants were polymorphic in the sexual population, while nine were fixed in the asexual population. Together, these results do not support the existence of two allelic variants that differ between sexual and asexual populations. Rather, we described a variety of polymorphic sites accumulated all along the locus, with an important reduction of polymorphism detected in the asexual population. Moreover, the protein sequence was negligibly affected by polymorphisms, with only one nonsynonymous variant recorded and located outside of the functional sites. Nonetheless, the numerous polymorphic sites reported all along the for gene could affect the transcription or the alternative splicing of the gene rather than the sequence of the encoded protein itself.
| ID | Transcript | Region (position) | Polymorphism | Sexual population | Asexual population | Type of mutation | Amino acid change |
|---|---|---|---|---|---|---|---|
| 1 | All | CDS (1398) | A or G | G(201), A(463) | G(526), A(22) | Synonymous | |
| 2 | Vcan27709 | CDS (3109) | T or C | T(105), C(218) | C(468) | Nonsynonymous | Valine or alanine |
| 3 | Vcan27707 | CDS (1879) | T or C | T(16), C(61) | T(3), C(126) | Synonymous | |
| 4 | Vcan27708 | UTR (2903) | C or G | C(15), G(21) | C(25) | ||
| 5 | Vcan27708 | UTR (2516) | T or C | T(1), C(31) | T(13) | ||
| 6 | Vcan27709 | UTR (3870) | G or A | G(129), A(133) | A(231) | ||
| 7 | Vcan27709 | UTR (3818) | G or A | G(134), A(109) | A(255) | ||
| 8 | Vcan27709 | UTR (3635) | C or T | T(56), C(95) | T(146) | ||
| 9 | Vcan27708 | UTR (990) | A or G | A(352), G(71) | G(474), A(5) | ||
| 10 | Vcan27707 | UTR (2784) | C or T | C(12), T(28) | C(73) | ||
| 11 | Vcan27707 | UTR (2477) | C or T | C(49), T(24) | C(69) | ||
| 12 | Vcan27707 | UTR (2465) | A or G | A(29), G(45) | A(75), G(1) | ||
| 13 | Vcan27707 | UTR (2418) | C or T | C(35), T(26) | C(73) | ||
| 14 | Vcan27707 | UTR (2276) | A or G | A(23), G(15) | A(2), G(52) |
- Characterization of for SNPs that differed in frequency across sexual and asexual populations: The transcript column indicates the isoform cluster containing the variant; the region and position column indicates if the SNP is located within the coding sequence (CDS) or outside (UTR) and its position considering the longest transcript sequence given in paretheses; polymorphism indicates the different bases identified at the SNP position; sexual and asexual population columns contain the number of reads corresponding to each variant in parentheses. In the last two columns, information related to the type of mutation (synonymous or nonsynonymous, when SNPs occurred within CDS), and amino acid change (only for the nonsynonymous mutation) are presented.
3.4 Genome-wide expression divergence across sexual and asexual populations
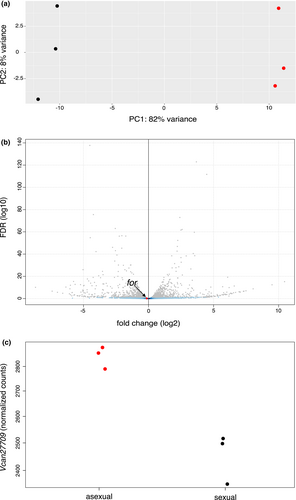
Overall, we found that gene expression diverged strongly according to sexual or asexual population. Principal component analysis based on the expression of all genes showed that the first axis separated the sexual population from the asexual population and explained 82% of the total variance (Figure 2a). Among the 14,106 transcripts that passed the expression filter, a total of 1539 genes were DE (p-adj < 0.05) between the sexual and asexual populations, representing 10.9% of the transcriptome. The for transcript, represented in the transcriptome by its longest isoform (Vcan27709 transcript), was not included within this list of DEGs (rank 2507/14,106, p-adj = 0.168) (Figure 2b). Although the p-adj value was above the significance level, the analysis of normalized counts of the for transcript across the six libraries showed that expression was about 10% higher in the asexual population than in the sexual population (Figure 2c). None of the three other for transcripts (Vcan27706, Vcan27708 and Vcan27709) exhibited significant differential expression between sexual and asexual populations, but all showed the same expression pattern (Figure S2).
3.5 Behavioural gene expression divergence between sexual and asexual populations
The de novo transcriptome assembly was composed of a total of 22,333 transcripts that were annotated using blast and Gene Ontology (GO) tools. Among those, 18,316 obtained a blast hit (82%), and 12,923 obtained at least one GO term annotation (58%). We selected the “behaviour” GO term, as well as all its child-related GO terms. In this way, we annotated 249 transcripts with putative functions associated with behaviour in V. canescens. Among them, we reported 26 transcripts that were DE between the two populations, representing potential candidates in the differences in foraging behaviours observed between sexuals and asexuals (Table 2). The proportion of behavioural genes with differential expression between the two populations was not different compared to the full transcriptome (26/249 vs. 1539/12,567; χ2 = 0.44, p = 0.50). Among those, we noted a majority of transcripts related to sensory behaviour: chemosensory (18 transcripts) or visual (two transcripts). The other functions detected were locomotory behaviour (two transcripts), learning and memory (two transcripts), reproductive behaviour (one transcript) and rhythmic behaviour (one transcript).
| Transcript ID | blast annotation | Gene ontology term | Specific gene ontology term | Population with overexpression (log2FC) | Expression mean (normalized) | p-adj (FDR) |
|---|---|---|---|---|---|---|
| g12192 | Gustatory receptor for sugar taste 64f-like | Chemosensory behaviour | Sensory perception of sweet taste | Asex (−1.19) | 29.7 | 0.01 |
| g12206 | Odorant receptor | Sensory perception of smell | Asex (−1.22) | 22.1 | 0.03 | |
| g12512 | Odorant receptor | Sensory perception of smell | Asex (−0.95) | 54.7 | 0.01 | |
| g13557 | Odorant receptor Or1-like | Sensory perception of smell | Sex (+0.93) | 39.3 | 0.03 | |
| g13718 | Odorant receptor | Sensory perception of smell | Asex (−5.11) | 3.0 | 0.009 | |
| g13720 | Odorant receptor | Sensory perception of smell | Sex (+1.54) | 12.99 | 0.04 | |
| g15418 | Odorant receptor | Sensory perception of smell | Sex (+2.07) | 36.2 | 1.8 e-6 | |
| g15445 | Odorant receptor 13a-like | Sensory perception of smell | Sex (+0.91) | 170 | 0.00001 | |
| g19653 | Odorant receptor | Sensory perception of smell | Asex (−4.11) | 5.8 | 0.009 | |
| g21277 | Odorant receptor | Sensory perception of smell | Sex (+3.32) | 5.8 | 0.02 | |
| g6212 | Odorant receptor 4-like | Sensory perception of smell | Asex (−1.69) | 16.8 | 0.02 | |
| g6216 | Odorant receptor | Sensory perception of smell | Sex (+1.46) | 14.6 | 0.04 | |
| g7031 | Odorant receptor | Sensory perception of smell | Asex (−0.76) | 63.9 | 0.03 | |
| g7034 | Odorant receptor | Sensory perception of smell | Asex (−0.53) | 166.02 | 0.037 | |
| g8746 | Odorant receptor Or2-like | Sensory perception of smell | Asex (−1.13) | 42.5 | 0.005 | |
| g9877 | Odorant receptor | Sensory perception of smell | Asex (−0.59) | 159.8 | 0.016 | |
| g16075 | Odorant receptor | Sensory perception of smell | Sex (+4.79) | 30.5 | 4.8 e-11 | |
| g16076 | Odorant receptor | Sensory perception of smell | Sex (+3.18) | 8.8 | 0.002 | |
| g10000 | Opsin, blue sensitive | Visual behaviour | Visual perception | Asex (−0.47) | 22240.5 | 0.004 |
| g1755 | Trafficking protein particle complex subunit 5 | Visual perception | Asex (−0.49) | 197.1 | 0.027 | |
| g12418 | Protein artichoke-like | Locomotory behaviour | Locomotion | Sex (+0.47) | 695.2 | 0.003 |
| g13961 | Slit homologue 1 protein | Locomotion | Sex (+0.77) | 3109.8 | 2.36 e-08 | |
| g13938 | Replicase polyprotein 1a | Learning and memory | Long-term memory | Asex (−0.89) | 62.9 | 0.008 |
| g15975 | GATA zinc finger domain-containing protein14 | Olfactory learning | Sex (+0.63) | 103.5 | 0.03 | |
| g18212 | Protein yellow-like | Reproductive behaviour | Male mating behaviour | Asex (−0.33) | 845.2 | 0.043 |
| g11163 | Uncharacterized protein LOC122417635 | Rhythmic behaviour | Sleep | Sex (+0.81) | 95.9 | 0.002 |
- Among the 1539 DEGs between sexual and asexual populations, 26 transcripts were annotated as related to the “behaviour” GO term or one of its child GO terms. The population in which the transcript was overexpressed was indicated as well as its fold change (log2), with positive values when overexpressed in the sexual population, and negative values in the asexual population.
3.6 Asexual females exploited more hosts and explored more environments than sexual females
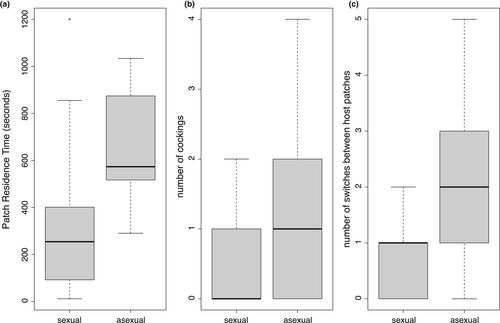
In the behavioural experiment, asexual wasps exploited more hosts than sexual wasps by allocating more time to hosts (Figure 3a; χ2 = 3.81, df = 1, p < 0.01). On average, asexual females spent twice as much time on host patches compared with sexuals (655.5 ± 60.8 s vs. 333.3 ± 80.4 s). Neither egg load, for expression nor their interactions with population were significant. Asexual females laid twice as many eggs as sexual females (Figure 3b; χ2 = 4.94, df = 1, p < 0.05), with on average 1.24 eggs laid by asexuals (±0.32) compared to 0.53 eggs laid by sexuals (±0.17). Time spent on a patch determined the number of eggs laid as PRT has a positive effect on the number of cockings (χ2 = 14.09, df = 1, p < 0.001). However, for expression has a marginal, though not statistically significant, effect on the number of cockings (χ2 = 3.01, df = 1, p = 0.08), with the number of cockings increasing in individuals with higher for expression. The interactions between for expression and population and between for expression and PRT did not explain the number of cockings. Asexual females also explored the environment more than sexual females. We did not detect differences between sexual and asexual populations in the proportion of females finding the second host patch (11/17 vs. 14/17, respectively, Fisher's exact test, p = 0.44). However, asexual females switched more from one patch to another than sexual females (Figure 3c; χ2 = 4.937, df = 1, p < 0.05), with on average three-fold more changes in asexual females (2.12 ± 0.37 in asexuals vs. 0.71 ± 0.14 in sexual females). Switches between host patches were not influenced by other variables or by their interactions with the population. Together, these results showed that asexual females exploited more hosts, with more time spent on host patches and more eggs laid, and explored more of the environment by more often changing host patches.
3.7 Expression of the for gene and correlations with behaviours in sexual and asexual females
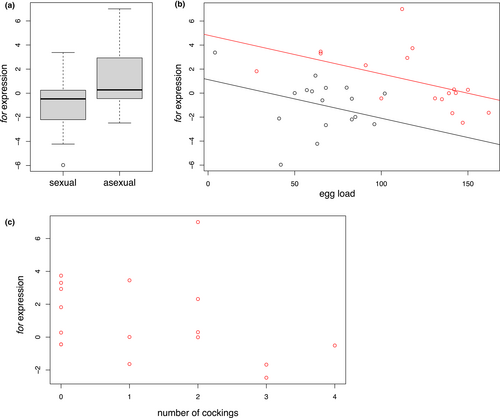
for gene expression was higher in asexual female heads (1.06 ± 0.60) compared to sexual heads (−0.98 ± 0.54; Figure 4a; F = 7.62, df = 1 and 31, p < 0.01). Within each population, for expression decreased with egg load (Figure 4b; F = 6.7, df = 1 and 31, p < 0.05). There was no significant interaction between egg load and population on for gene expression. When analysing both populations separately, the number of cockings increased with PRT in asexual females (χ2 = 6.00, df = 1, p = 0.014) but decreased with for expression (χ2 = 4.96, df = 1, p = 0.026; Figure 4c). In sexual females, the number of cockings was correlated with PRT (χ2 = 8.1061, df = 1, p = 0.004) but not with for expression.
4 DISCUSSION
The for gene exhibited a strong sequence conservation across insects, consistent with function conservation in influencing the foraging behaviours described in numerous insects (Reaume & Sokolowski, 2009). Beyond sequence conservation, we also showed a conservation of the for gene model complexity between Venturia canescens and Drosophila melanogaster with four alternative promoters encoding four proteins differing in their N-termini (Allen et al., 2017) and a total of 17 alternative isoforms sequenced in the female heads. The use of alternative promoters represents a source of diversity and flexibility in the regulation of gene expression and ultimately function, as shown in the for gene, whose promoter variations cause changes in both tissue localization and substrate specificity. Indeed, pr1-for and pr4-for transcripts were expressed within neurons, while pr2-for and pr3-for transcripts were localized in glial cells of the fruit fly central nervous system (Allen et al., 2018; Dason et al., 2020). The isoform pr1-for was presumed to be the only transcript necessary for forage because in mutants, pr1-for expression in neurons was the only transcript required to rescue larval foraging behaviours (Allen et al., 2018). Variations in the N-termini are critical to the specificity of PKG–substrate interactions (Pearce et al., 2010). PKG phosphorylates serine and threonine residues on a dozen proteins known to modulate muscle activity and neuronal signalling pathways (Edelman et al., 1987; Schlossmann & Desch, 2009). Such a variety of substrates may explain the pleiotropic effects of the for gene. Conservation of gene model complexity between the dipteran D. melanogaster and the hymenopteran V. canescens supports the importance of maintaining such complexity to regulate alternative foraging behaviours. The protean nature of the for gene model makes it an excellent support for the plasticity of foraging behaviours. Alternatively, such gene model complexity makes a fine description of the for gene expression challenging. First, the current study was conducted on female heads, each representing a mixture of distinct tissue types. However, expression of the for gene could be located in a specific cell type of the head. Thus, in the ant Pheiole pallidula, for activity is restricted to a very limited cluster of five cells in the anterior brain of workers and is absent in foragers (Lucas & Sokolowski, 2009). Second, the probe used to quantify the for gene is common to 16 of the 17 isoforms described. It is therefore impossible here to have a sufficiently fine resolution to specify either the isoform or the cell type where for expression differs between sexual and asexual populations. Despite these limitations, we detected an increase in for transcription in the asexual population. The use of advanced sequencing technology, such as single-cell sequencing, coupled with immunochemistry will be required to refine the accuracy.
Polymorphism analysis revealed 15 SNPs along the for gene, most of which varied in frequency across populations, and supported a major reduction in genetic diversity that occurred in asexuals rather than the presence of two allelic variants diverging between sexual and asexual populations. Such a reduction of polymorphism in asexuals was expected: in general, thelytokous individuals are more homozygous than arrhenotokous individuals (Beukeboom & Pijnacker, 2000), which was previously shown in V. canescens with a study based on 15 microsatellites that were all homozygous (Mateo Leach et al., 2012). In contrast, some genetic diversity still persisted at the for locus in asexuals. The vast majority of identified SNPs did not affect the protein sequence itself because they occurred outside of the coding region or corresponded to synonymous polymorphisms. A single SNP corresponding to a nonsynonymous mutation was located at the N-terminus part of the predicted PKG, corresponding to the substrate binding region of the protein, outside the kinase and cGMP binding domains. By comparison, rover and sitter alleles differed by more than 300 SNPs segregating in D. melanogaster, also representing regulatory mutations rather than changes in amino acid sequence (Allen et al., 2017). In contrast, the two allelic variants identified in the moth Sesamia nonagrioides differed by only one nonsynonymous SNP located within the kinase domain of the protein, and each variant was associated with different levels of for expression, PKG activity and distinct behaviours (Chardonnet et al., 2014). However, previous studies in hymenopteran species have not shown any evidence for the existence of allelic variants at the for locus, and nor did the present study in V. canescens.
Differences in foraging behaviours recorded between sexuals and asexuals should rely on genome divergence as there is no more gene flow between the 2 populations (Mateo Leach et al., 2012). A previous study revealed such genome divergence as individuals from sexual and asexual populations can be distinguished based on microsatellites. However, how much gene expression diverged at the genome-wide scale between the two populations has not been studied thus far. By comparing head transcriptomes, we reported that the two populations clearly split, with a total of 1539 DEGs. This proportion of 11% of DEGs between two populations from the same species is high and almost as high as that observed in recently diverged species such as Drosophila pseudoobscura pseudoobscura and D. pseudoobscura bogotana (~0.25 million years of divergence and 14.6% DEGs; Gomes & Civetta, 2015). While behavioural divergences are among the most remarkable differences between sexual and asexual wasps, the behaviour-annotated group of genes was not overrepresented within DEGs. Among the behavioural genes for which expression varied between populations, transcripts involved in sensory perception (olfactory, visual) were the most numerous. Chemosensory genes evolved rapidly and played important roles in adaptation (Brand et al., 2015). While the for gene has been implicated in foraging behaviours in various organisms, none of the for isoforms were detected as DE. Analysis of RNA-seq data at this locus showed that all isoforms of the for gene were more highly expressed in the asexual population than in the sexual population, although beyond the significance threshold. Together, these results mean that although the for gene tends to be slightly overexpressed in the asexual population, this gene does not belong to the most divergent part of gene expression. Indeed, whether one considers the intensity of the differences observed, only 10% of the variation between the two populations, or the ranking (2507th out of 14,106 genes tested, well beyond the 1507 significant DEGs), the variations in expression of the for gene would be only one of the many genes with distinct expression profiles between these two populations. Given the extent of transcriptomic divergence, with hundreds of DEGs between sexual and asexual populations, and in the absence of functional analysis, we cannot firmly confirm the functional role played by the slight differences in for expression recorded in the differences of foraging behaviours. Notably, comparison between rovers and sitters in D. melanogaster showed that differences in for expression were small but consistent (Osborne et al., 1997). Drosophila rovers and sitters have differences between their transcriptomes, apart from the single variation in for expression (Kent et al., 2009). Honey bee nurses and foragers differed by ~40% of their brain transcriptome (Whitfield et al., 2003). However, manipulation of for gene expression or the corresponding PKG enzyme activity was sufficient to modify foraging behaviours in the two species (Ben-Shahar et al., 2002; Osborne et al., 1997). Therefore, the differences in for gene expression detected in the current study between sexuals and asexuals, although moderate, might nevertheless have an essential function in the differences in foraging behaviours reported between V. canescens populations.
Globally, the behavioural results were congruent with our predictions; that is, asexuals exploited more host patches because they are faster to choose and travel between hosts, and have a greater egg load (Amat et al., 2017). In contrast, sexual females are better dispersers with both longer and faster flights, and they have greater longevity and higher energy content (Amat et al., 2017). They were thus expected to explore their environment more, yet this prediction was not verified in the current results. The discrepancy could come from the experimental device that might be too small for all exploration-related behaviours to be expressed, in particular dispersal involving long flights with high energy costs (Amat et al., 2012). Field experiments conducted with D. melanogaster showed that rovers exhibit higher dispersion with both greater dispersal tendencies and longer flight distances than sitter flies and that artificial increases in for expression in the brain and nervous system increase dispersal in sitters (Edelsparre et al., 2014). Our study, conducted in the laboratory, does not allow for assessing wasp dispersal ability. Nevertheless, by showing that asexual females switched more frequently between host patches compared with sexual females, the experimental device is relevant to detect differences in some aspects of the exploration between the two populations.
Venturia canescens asexual females present homology with the rover phenotype observed in D. melanogaster both by exploring and exploiting more. The for gene is more highly expressed in asexual wasps than in sexual wasps, consistent with the Drosophila rover model. Previous classification of the for transcript among non-DEGs might be due to the low number of RNA-seq replicates (three), while RT-qPCR was conducted on a greater number of individuals (17), thus increasing statistical power in the detection of DEGs. The higher fecundity is another common characteristic between D. melanogaster rovers (McConnell & Fitzpatrick, 2017) and V. canescens asexuals, here measured by both a higher egg load, corresponding to their potential fitness, and a higher number of eggs laid, corresponding to their effective fitness but measured over a short period. Hence, in these two species, individuals that exploited and explored more were also the more fecund, and were those with higher for expression. A major contribution of the present study is the joint analysis of for gene expression and foraging behaviours measured at the individual scale, which provided information on interindividual variations and allowed for the study of correlations between these traits beyond average measures. Two major results emerged from this approach: first, egg load decreases in females with the highest for expression, and second, there is a decrease in eggs laid by asexual females with the highest for expression. These two correlations were consistent and suggested that an increase in for expression may be costly for females and could result in a decrease in progeny number. In the wasp V. canescens, the cost of reproduction is mostly based on finding hosts to lay eggs, as the egg itself contains little reserve and is not costly to produce (Pelosse et al., 2011). In this case, rather than an energetic cost due to for expression that would directly induce a decrease in fecundity, the cost might be indirect and related to the numerous other functions fulfilled by the highly pleiotropic for gene apart from resource searching behaviours, such as learning, memory or social interactions (Alwash et al., 2021; Reaume & Sokolowski, 2009).
The present study highlights the molecular bases underpinning the variability in foraging behaviours in the parasitoid wasp V. canescens and provides insights into the evolution of foraging behaviours: (i) within populations and (ii) within Hymenoptera. Regarding the first point, asexual populations appear to occur repeatedly from ancestral sexual populations, presumably as a result of mutations leading to the loss of sexual reproduction (Schneider et al., 2002). The numerous adaptations observed in these asexual populations include, among other things, more efficient exploitation behaviour. This difference between efficiency does not require the selection of an allelic variant at the for locus but rather a stronger expression of the gene in asexuals. This increase in for transcription could be facilitated by the complexity of the for gene model and its numerous isoforms, which could be more prone to variations in expression. Increased expression of the for gene in asexual populations may contribute to specialization to anthropogenic environments with high host concentration, where asexuals are most often sampled. Our results also show that the for gene is not the only one to undergo changes in expression between the two populations, as transcriptomic comparison indicated that a proportion of 11% of the genes experience a shift in expression. This dissimilarity observed between the sexual and asexual transcriptomes contrasts with the genetic similarities described thus far between sexuals and asexuals (Mateo Leach et al., 2012; Schneider et al., 2002) and may be a source of adaptations observed between the two populations (Amat et al., 2017). Concerning the evolution of foraging variations within Hymenoptera, to date, studies have focused on social species that acquired eusociality independently: bees, ants and wasps. These studies revealed a caste-specific for expression correlated with foraging intensity but with opposite patterns: honey bee (Apis mellifera) and bumblebee (Bombus terrestris) foragers exhibit a higher for expression than nurses (Ben-Shahar et al., 2002; Tobback et al., 2011), whereas nurses presented a higher for expression than foragers in ants (P. barbatus, P. pallidula) and the common wasp Vespa vulgaris (Ingram et al., 2005; Lucas & Sokolowski, 2009; Wenseleers et al., 2008). The for gene influences social behaviour in various species and may be part of a genetic toolkit involved in the evolution of eusocial insects (Rittschof & Robinson, 2016), whereas the acquisition of eusociality relies on the emergence of a forager caste specialized on foraging tasks that appears to be related to differences in for gene expression. In contrast, selection in bees shows opposite patterns to those described in the ancestral groups of ants and wasps. Parasitoid wasps, which are ancestral to the Apocrita group that includes all social hymenopterans (Peters et al., 2017), are solitary species and therefore do not have foragers. This study suggests that differences in for expression patterns underlying changes in foraging strategies could be ancestral to Apocrita and precede the acquisition of sociality. In this group, variations in for gene expression would not rely on allelic variants. The present work illustrates an original case of divergence in foraging behaviours that is not based on caste differences but is associated with a difference in for expression between populations that also differ in their reproductive mode. However, the adaptations observed in numerous life-history traits in the two populations are not limited to the difference in expression of one gene but could involve differences in the optimum expression pattern of several hundred genes.
AUTHOR CONTRIBUTIONS
A.G., E.D., L.M., C.V.H. and I.A. designed the research; A.G. and D.L. performed the research; A.G., V.L., D.L. and A.E.F. analysed the data; A.G., E.D., L.M., C.V.H. and I.A. wrote the paper.
ACKNOWLEDGEMENTS
We thank Francois Debias for his help during field capture and Elsa Day for her help in insect rearing and experiments. This work was funded by the Agence Nationale de la Recherche JCJC AVOIDINBRED (ANR-17-CE02-0004-01) attributed to A.G. This work was performed using the computing facilities of the CC LBBE/PRABI. We thank the two anonymous reviewers whose constructive comments improved the manuscript.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
BENEFIT-SHARING STATEMENT
Benefits from this research accrue from the sharing of our data and results on public databases as described above.
Open Research
DATA AVAILABILITY STATEMENT
Transcriptomic data sets (six RNA-seq fastq files and transcriptome assembly [fasta file]) were deposited in the GEO repositories from the NCBI database with the accession code GSE194171. The behavioural data set, qPCR data set, count table and associated R scripts were deposited in Dryad (doi:10.5061/dryad.0k6djhb3w).




