Demographic history shapes genomic variation in an intracellular parasite with a wide geographical distribution
Handling Editor: Elin Videvall
Abstract
Analysing variation in a species’ genomic diversity can provide insights into its historical demography, biogeography and population structure, and thus its ecology and evolution. Although such studies are rarely undertaken for parasites, they can be highly revealing because of the parasite's co-evolutionary relationships with hosts. Modes of reproduction and transmission are thought to be strong determinants of genomic diversity for parasites and vary widely among microsporidia (fungal-related intracellular parasites), which are known to have high intraspecific genetic diversity and interspecific variation in genome architecture. Here we explore genomic variation in the microsporidium Hamiltosporidium, a parasite of the freshwater crustacean Daphnia magna, looking especially at which factors contribute to nucleotide variation. Genomic samples from 18 Eurasian populations and a new, long-read-based reference genome were used to determine the roles that reproduction mode, transmission mode and geography play in determining population structure and demographic history. We demonstrate two main Hamiltosporidium tvaerminnensis lineages and a pattern of isolation-by-distance, but note an absence of congruence between these two parasite lineages and the two Eurasian host lineages. We suggest a comparatively recent parasite spread through Northern Eurasian host populations after a change from vertical to mixed-mode transmission and the loss of sexual reproduction. While gaining knowledge about the ecology and evolution of this focal parasite, we also identify common features that shape variation in genomic diversity for many parasites, such as distinct modes of reproduction and the intertwining of host–parasite demographies.
1 INTRODUCTION
Uncovering the ecological and evolutionary forces that drive variation in genetic diversity within and among populations across a species’ range is important for understanding biological processes of basic and applied interest. Factors such as historical conditions (e.g., phylogeography and population structure), environmental conditions (e.g., adaptation to local climate) and stochastic processes (e.g., genetic drift and founder events) may contribute to patterns of genetic variation in a species (Bellis et al., 2020; Levicoy et al., 2021; Sendell-Price et al., 2021). For parasites, such studies take on an additional level of complexity, as parasite evolution is influenced by its dependence on the host and by issues such as host demography, parasite epidemiology, and co-evolutionary interactions between hosts and parasites (Ebert & Fields, 2020). While a great deal of research has examined the effect of parasitism on host evolution, we know little about the factors that drive changes in the parasite's genomic diversity, such as parasite life history, host shifts, mode of transmission and mode of reproduction (Janssen et al., 2004; Nieberding et al., 2008; Sandrock et al., 2011). The phylogeography of human parasites, which has been successfully studied to illuminate genetic epidemiology and variation in several systems, has proved to be a powerful tool in understanding, for example, Mycobacterium tuberculosis and viruses such as the recently emerged coronavirus SARS-CoV-2 (Dellicour et al., 2021; Gagneux & Small, 2007). For the Gram-negative bacterium Helicobacter pylori, a known risk factor for stomach cancer in humans, the link between host and parasite biogeography and the parasite's mode of transmission was shown to be an important factor in the evolution of the system (Montano et al., 2015). Similarly, He. pylori, like its human host, shows a continuous loss of genetic diversity with increasing geographical distance from East Africa. Its vertical mode of transmission probably contributed to the geographical distribution of genetic diversity shared between the parasite and its host. The interplay between co-phylogeography and mode of transmission here highlights the importance of studying interactions between factors that drive genetic diversity when trying to understand host and parasite evolution. In this article, we present a population genomic study of a parasitic microsporidium, focusing on the distribution of genomic diversity and relating it to key factors of parasite biology.
Genetic variation in parasites and their hosts may be influenced by the same or different factors; moreover, they might influence each other's evolution in both specific and general ways (Langerhans, 2008). In He. pylori, for example, the parasite's genomic signature of co-evolution with the human host was so strong that it could be used to infer the history of the host across the Pacific islands (Moodley et al., 2009). However, investigating interrelationships between multiple factors such as mode of transmission and biogeography in He. pylori is challenging, as both host and parasite need to be considered; therefore, such studies are still rare in parasite evolutionary biology.
One taxon that has recently gained attention as a potential model for parasite evolution is the microsporidia, due to its high variation in many aspects relevant to evolutionary and ecological theory (Murareanu et al., 2021; Wadi & Reinke, 2020). Microsporidia are obligate intracellular parasites with a phylogenetic position close to the fungi (James et al., 2013), although they show many highly derived features: for example, they have no mitochondria, have fewer genes and a very small genome size compared to fungi, including the smallest known eukaryote genomes (Corradi, 2015). However, the latter two features are highly variable among microsporidia (Wadi & Reinke, 2020; B. A. Williams et al., 2008). Not only do microsporidia genomes vary tremendously in length and in gene numbers, but de novo genome comparisons of microsporidia also found high levels of intraspecific genomic diversity (Pelin et al., 2015; Pombert et al., 2013; T. A. Williams et al., 2016). Moreover, microsporidia show high variation in their modes of reproduction and transmission, with ~18% of species being thought to transmit vertically (Murareanu et al., 2021). Because disentangling and understanding the factors that drive genomic diversity may be easier in such a fast-evolving clade, microsporidia present a powerful model clade for evolutionary biology research.
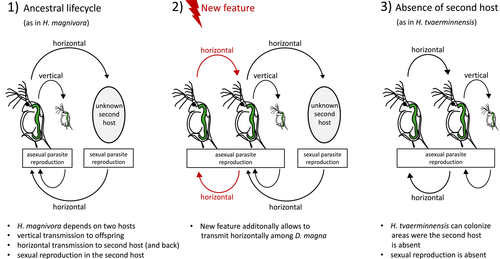
The freshwater planktonic crustacean Daphnia magna is a well-established model system in ecology and evolution, including studies of host–parasite interactions (Altermatt & Ebert, 2008; Ebert, 2008; Orlansky & Ben-Ami, 2019). D. magna is parasitized by different species of microsporidia (Ebert, 2005), including the microsporidian genus Hamiltosporidium, of which the two species, H. magnivora and H. tvaerminnensis, infect D. magna exclusively (Haag et al., 2011). Host and parasites are widespread across Eurasia. Both Hamiltosporidium species have, as compared to other microsporidia, relatively large, gene-sparse genomes, a feature that has been linked to a high number of repetitive, transposable elements (Parisot et al., 2014) that may have spread in the Hamiltosporidium genome due to the parasite's ability to transmit vertically, which leads to bottlenecks and thus a decreased effective population size, Ne, and reduced effectiveness of selection (de Albuquerque et al., 2020; Haag et al., 2020). Previous studies have found that H. tvaerminnensis reproduces asexually and has a mixed-mode transmission (i.e., horizontal and vertical), whereas its sister species, H. magnivora, reproduces sexually and transmits among D. magna hosts only vertically (Haag et al., 2011, 2013b). H. magnivora is believed to have a second, as yet unknown host, to which it is transmitted horizontally (Mangin et al., 1995). While diversity in aspects such as modes of reproduction and transmission makes the genus Hamiltosporidium a good model for studying parasite evolution in general, it might represent a more unique system in terms of understanding the consequences of gaining or losing a second host and of changing the amount of recombination following a switch in reproductive mode.
Several studies on the distribution of genetic variation in microsporidia in diverse hosts have shown that host demography contributes to parasite phylogeny, population structure and demographic history (e.g., Gómez-Moracho et al., 2015; Shafer et al., 2009; Wang et al., 2019). Existing knowledge regarding the demographic history of D. magna (Fields et al., 2018; Orsini et al., 2012, 2013; Stollewerk, 2010) allows us to go one step further and investigate the parasite's genetic demography in relation to that of its host, looking at host–parasite co-phylogeny, co-biogeography and co-evolution. Previous studies on the D. magna–microsporidia host–parasite system have focused on mode of reproduction, demographic history and transmission mode (Haag et al., 2020; Haag, Traunecker, et al., 2013) in an effort to understand the system's colonization history and its transition from sexual to clonal reproduction. Here, we go a step further, re-evaluating these questions using entire genomes sampled from a much larger swath of the species’ range, including Europe and Asia, and combining various approaches to investigate the evolutionary history and underlying mechanisms shaping genomic variation in this system. First, we ask how much intraspecific genomic variation exists in the microsporidian species H. tvaerminnensis using a new, highly improved long-read-based reference genome. Based on a genomic study that estimated genomic variation in the microsporidium Nosema ceranae (Pelin et al., 2015), a close relative of H. tvaerminnensis with the same mode of transmission (Haag et al., 2020), a reasonable expectation was to find similar magnitudes of genetic diversity in H. tvaerminnensis. Second, using a multicontinental data set, we ask how historical processes such as demography and phylogeography can explain the current distribution of genetic variation in H. tvaerminnensis across continental biogeographical scales? Knowing the phylogeography and demographic history of the host allowed us to test the co-phylogeography hypothesis. A finding that the parasite shares the same population structure and pattern of isolation-by-distance (IBD) as has been reported in the host (Andras et al., 2018; Fields et al., 2015) would support a model of phylogeographical co-cladogenesis. Since effects of demography and selection on genetic diversity are often hard to distinguish (Pavlidis et al., 2008), and nonadaptive processes may be an especially powerful force in the focal microsporidian genus (Haag et al., 2020), we also quantified the strength of positive selection in H. tvaerminnensis. Confirming weak selection strength would give us more confidence in our demography-focused hypothesis and support previous findings (Haag et al., 2020).
2 MATERIAL AND METHODS
2.1 Daphnia magna diversity panel
The parasites examined in this study were derived from material collected within the framework of a large-scale biogeographical study of the host species, D. magna (Fields et al., 2015, 2018, 2022; Seefeldt & Ebert, 2019). Animals collected from across the species range were brought to the laboratory, and one iso-female line was created from each population (these iso-female lines are termed “clones” for the course of this study). Animals from each clone were checked for microsporidia infections with phase-contrast microscopy, using squash-preparations or samples of the gut. The D. magna clones were sequenced with Illumina paired-end (PE) sequencing using a HiSeq 2500 device, with the respective parasite genome simultaneously sequenced in several cases. Whole-genome sequences of three Hamiltosporidium magnivora and 15 Hamiltosporidium tvaerminnensis, each from a different host clone collected from a different population, were obtained and used in the present study (Table 1). Sequences from clone IL-G-3 were reused from Haag et al. (2020) (NCBI database; SRA accession: SRP211974, Bioproject ID: PRJNA419750). H. tvaerminnensis and H. magnivora are closely related, but are distinct species based on morphological traits (Haag et al., 2011; Larsson et al., 1998). A genome-wide assessment of their relatedness has not been conducted thus far. Here, one common reference genome for the two Hamiltosporidium species was used.
| Sample ID | Country | Latitude (N) | Longitude (E) | Parasite species | Coverage |
|---|---|---|---|---|---|
| BE-OM−2 | Belgium | 50.863280 | 4.721370 | H. magnivora | 594× |
| BE-T1-2 | Belgium | 50.823050 | 4.593940 | H. magnivora | 145× |
| BE-WH2-11 | Belgium | 51.334780 | 3.348130 | H. magnivora | 149× |
| CY-PA3-2 | Cyprus | 35.034060 | 33.954930 | H. tvaerminnensis | 598× |
| ES-DO1-1 | Spain | 36.978360 | −6.477640 | H. tvaerminnensis | 282× |
| ES-OM−5 | Spain | 37.254353 | −6.969114 | H. tvaerminnensis | 348× |
| FI-BR1-39–6 | Finland | 59.845253 | 23.274526 | H. tvaerminnensis | 219× |
| FI-FHS2-11–3 | Finland | 60.273780 | 27.218750 | H. tvaerminnensis | 94× |
| FI-OER−3–3 | Finland | 59.788590 | 23.174140 | H. tvaerminnensis | 1097× |
| IL-G−3 | Israel | 32.228840 | 34.831140 | H. tvaerminnensis | 703× |
| IL-PS−6 | Israel | 32.255710 | 34.853270 | H. tvaerminnensis | 689× |
| IL-SH−4 | Israel | 31.701030 | 34.713760 | H. tvaerminnensis | 600× |
| RU-BAYA1-1 | Russia | 53.017833 | 106.886833 | H. tvaerminnensis | 756× |
| RU-TY1-3 | Russia | 50.255500 | 89.546830 | H. tvaerminnensis | 389× |
| RU-TY2-2 | Russia | 50.212170 | 89.717830 | H. tvaerminnensis | 523× |
| RU-ZB1-1 | Russia | 50.413170 | 114.724703 | H. tvaerminnensis | 374× |
| SE-G4-20 | Sweden | 60.253040 | 18.306090 | H. tvaerminnensis | 562× |
| SE-H1-4 | Sweden | 58.342330 | 11.218030 | H. tvaerminnensis | 336× |
2.2 Reference genome assembly
To obtain D. magna free of microbiota, except microsporidia, we followed a procedure described by Fields et al. (2015), using Qiagen Genomic Tips to obtain high-molecular-weight DNA from the FI-OER-3–3 clone, which gave consistently high amounts of H. tvaerminnensis sequencing reads in the past. We used a Blue Pippin to isolate DNA fragments >15 kb. A standard PacBio library was prepared, and one SMRTCELL was sequenced in CLR mode on a Sequel I at the D-BSSE (Basel, Switzerland).
After receiving the raw data in a subread BAM file, we initially mapped all reads to the D. magna reference genome (BioProject ID: PRJNA624896; Fields et al., in prep) using minimap2 version 2.17 (H. Li, 2018). Unless otherwise stated, we used default parameters for bioinformatics programs. To achieve the most complete assembly of H. tvaerminnensis, we used canu version 2.1.1 (Koren et al., 2017) with high-sensitivity parameters to effectively work out the H. tvaerminnensis genome as part of the larger metagenome of D. magna and H. tvaerminnensis in the read set. We used blobtoolkit version 2 (Challis et al., 2020) to further isolate contigs specific to H. tvaerminnensis. By mapping Illumina data from an uninfected host genotype, we were able to further disentangle suspected H. tvaerminnensis contigs from contigs that probably belonged to the host. Afterwards, we used purge_haplotigs version 1.1.1 (Roach et al., 2018) to haplodize the assembly of contigs that presumably derived only from the parasite genome. Finally, we polished the resulting H. tvaerminnensis assembly using genomic consensus version 2.3.3 (Chin et al., 2013) and pilon version 1.23 (Walker et al., 2014).
To annotate the new H. tvaerminnensis genome, we mapped annotations from the previously published assembly (NCBI database; Assembly name: FIOER33 v1; GenBank assembly accession: GCA_004325045.1, Bioproject ID: PRJNA419750; Haag et al., 2020) to the new one using ragoo version 1.1 (Alonge et al., 2019) and evaluated the biological completeness of this new genome draft using busco version 4.0.1 (Seppey et al., 2019) and its microsporidia_odb10 (Creation date: August 5, 2020).
2.3 Mapping and variant calling
Raw reads were assessed for quality with fastqc version 0.11.7 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) and subsequently trimmed with trimmomatic version 0.38 (Bolger et al., 2014) to remove low-quality sequences and adapter contamination. Trimming success was assessed by doing a second run of fastqc. Quality trimmed reads were mapped to the reference genome with bwa mem version 0.7.17 (H. Li, 2013). samtools version 1.9 (H. Li et al., 2009) was used to convert the SAM files to BAM files, coordinate sort individual BAM files and remove unmapped reads. Read groups were added and duplicates marked for individual BAM files using picard toolkit version 2.18.16 (Broad Institute, 2019). The average read depths were computed using samtools function depth. gatk version 3.8 HaplotypeCaller was used to generate variant calls (McKenna et al., 2010; Van der Auwera et al., 2013), specifically GVCFs, which were first generated for individual BAM files. We used the gatk function GenotypeGVCF to combine GVCFs into all-site VCFs. Variant calls were generated for H. tvaerminnensis–H. magnivora in the form of a VCF file, which was filtered to exclude INDEL variants with vcftools version 0.1.16 (Danecek et al., 2011).
2.4 Coding sequences—single-copy orthologues
Protein coding regions are of particular interest for phylogenetic analyses and for identifying signals of selection (including its efficacy) across the genome. To characterize variation in these particular subsections of the genome, we needed to extract subsets of the genome-wide VCF. Protein sequences of H. magnivora (BEOM2 v1; GenBank accession: PITI00000000.1) were downloaded from NCBI and protein data sets of H. tvaerminnensis and H. magnivora were used as input for orthomcl version 2.0.9 to find one-to-one orthologues between the two (L. Li et al., 2003, following specifically the automation pipeline described at the Github repository: https://github.com/apetkau/orthomcl-pipeline). The orthologous sequences of H. tvaerminnensis and H. magnivora were aligned with prank version 170427 (Löytynoja, 2014) using a custom script adapted from Fields et al. (2022). After an initial survey of pairwise alignment quality, we implemented a masking step, in which excessively divergent or poorly aligned sequences (divergence > 0.5%) were excluded from downstream analysis. We then used the R version 3.5.1 (R Core Team, 2018) package seqinR version 3.4–5 (Charif & Lobry, 2007) to import FASTA alignments and PopGenome version 2.7.1 (Pfeifer et al., 2014) to manipulate the VCF files. To produce multiple sequence alignments, pseudohaplotypes (i.e., a random assignment of single nucleotide polymorphisms [SNPs] to one or the other haplotype) were generated by recoding the structure of the VCF file. One variant-imputed version of the reference per pseudohaplotype was produced for each H. tvaerminnensis sample with the function vcf2fasta of vcflib version 1.0.0-rc2 (Garrison, 2019), and then coding sequences of interest were cut out of the variant-imputed versions of the reference with the script gff2fasta.pl (https://github.com/ISUgenomics/common_scripts). The resultant coding sequences were aligned to the initial H. tvaerminnensis and H. magnivora alignments using mafft version 7.407 (Katoh et al., 2002; Katoh & Standley, 2013) and its --add option. Next, all positions masked in the two species reference alignments were masked in the multisequence alignments with generate_masked_ranges.py (https://gist.github.com/danielecook) and bedtools version 2.27.1 (Quinlan, 2014) function maskfasta.
2.5 Sequence variation and population genetic analyses
Per-site nucleotide differences (π) were calculated along the genome for H. tvaerminnensis in 1-kbp windows using pixy version 0.95 (Korunes & Samuk, 2021), and the difference between lineages was statistically tested using a Wilcoxon signed rank test in R. Alleles of less than half and more than double the average sample coverage were filtered out for the calculation of π. Similarly, π was calculated for the coding sequences and for concatenated four-fold degenerate sites of single copy orthologues of H. tvaerminnensis, identified with mega version 7.16.0617 (Kumar et al., 2016), using the script selectionStats.py (https://github.com/tatumdmortimer/popgen-stats). Both methods consider invariant sites in their calculations, making calculations more consistent with theoretical expectations and more comparable among species (Korunes & Samuk, 2021). π was separately calculated for H. magnivora. Nucleotide identity between H. tvaerminnensis and H. magnivora, and between each pair of H. tvaerminnensis was calculated from the coding sequence alignments as the Hamming distance relative to the total length using custom code.
2.6 Population structure and phylogenetic analyses
We conducted both a principal component analysis (PCA) and a cluster analysis with the Hamiltosporidium whole-genome polymorphism data using the R packages SNPRelate version 1.14.0 and gdsfmt version 1.16.0 (Zheng et al., 2012). Additionally, the pattern of IBD was tested for H. tvaerminnensis by comparing the pairwise genetic differentiation of the samples with their pairwise geographical distance. The R base stats function dist was used to calculate the pairwise Euclidean distance, while geodist version 0.0.3 (Padgham & Sumner, 2019) was used to calculate the geographical distance between the samples. VCFR and adegenet version 2.1.2 (Jombart, 2008) were used in R for file import and format conversions. The correlation between differentiation and geographical distance was tested with a dbMEM (distance-based Moran's eigenvector maps) analysis by redundancy analysis (RDA). Specifically, we transformed the explanatory variable, geographical distance, into dbMEMs using the R package adespatial version 0.3–14 (Dray et al., 2021) and decomposed the response variable, genetic differentiation, into principal components using the R base stats function prcomp. The RDA was done in R using the package vegan version 2.5–7 (Oksanen et al., 2020) with significance assessed with 1,000 permutations. In addition to looking at whole-genome diversity, we also examined the phylogenetic signal in protein-coding regions of the genome directly. As the aforementioned pseudohaplotypes are inappropriate for a number of phylogenetic methods that rely on haplotype information, we used ambiguity codes, an alternative method for unknown phase. We used the gatk functions SelectVariants and FastaAlternateReferenceMaker to generate variant-imputed versions of the H. tvaerminnensis genome with ambiguity codes. Single copy orthologue genes were extracted from the alternative references using gff2fasta.pl and concatenated into a single sequence. The larger alignment of these individual samples was filtered for four-fold degenerate sites with mega. A Bayesian phylogenetic analysis was conducted using beast2 version 2.5.1 (Bouckaert et al., 2019; Drummond et al., 2005) with the ambiguity code option enabled, Yule model as tree prior, JC69 as site model, a strict clock, and a Markov chain Monte Carlo (MCMC) chain of 10,000,000 iterations. Thereby, inputs were prepared using beauti and the posterior sample of trees was summarized to a maximum clade credibility tree using treeannotator with 10% of the MCMC chain discarded as burn-in. The obtained tree was combined with each sample's geographical coordinates using the R package phytools version 0.7–20 (Revell, 2012). Similarly, for the host–parasite co-phylogeny, we applied the same methodology using the host genome (version 3.0; Daphnia Genome Consortium).
2.7 Demographic history
We attempted to reconstruct the demographic history of the H. tvaerminnensis and H. magnivora samples by characterizing the unfolded site frequency spectrum (uSFS) of single-copy orthologues, distinguishing the uSFS for synonymous and nonsynonymous sites. The script siteFrequencySpectrum.R (https://github.com/tatumdmortimer/popgen-stats) was used to interface with PopGenome and modified to compute the uSFS for both site classes. In the uSFS of H. tvaerminnensis, we used H. magnivora as an outgroup to determine the state of the variants (ancestral or derived), and vice versa. We used the function gap.barplot from the R package plotrix version 3.7-7 (Lemon, 2006) to plot the uSFS.
To estimate changes in the effective population size history of Hamiltosporidium samples, we used psmc version 0.6.5 (H. Li & Durbin, 2011). Therefore, all BAM files were downsampled to an average whole-genome coverage of 50× with the samtools function view. Afterwards, one consensus sequence per sample was produced using samtools option mpileup, bcftools version 1.9 option call (H. Li, 2011) and vcfutils.pl vcf2fq, which is included in bcftools. SNPs with a coverage of less than a third or more than double of the average whole-genome coverage were filtered as suggested by the developer (https://github.com/lh3/psmc). The script plotPsmc.r (Liu & Hansen, 2017) was used for plotting the inferred historical dynamics of Ne. Similarly, “pseudodiploid” samples were analysed. Therefore, downsampled BAM files of two samples were jointly input to samtools to produce two-sample VCF files using bcftools. whatshap version 0.18 (Martin et al., 2016) was then used for read-based phasing. Unphased sites were masked and haplotypes were combined in all four possible ways before converting them to four FASTQ files using vcf2fq.
2.8 Rate of adaptive nucleotide substitutions
The magnitude of positive selection, or the rate of adaptive substitution (α), was inferred for H. tvaerminnensis by using both the classical McDonald–Kreitman test (MKT; McDonald & Kreitman, 1991) and more recently derived extensions. Thereby, intraspecific diversity and divergence counts as compared to the outgroup H. magnivora (i.e., counts of polymorphisms segregating within the species and counts of fixed differences between the ingroup and the outgroup) were created from a multisequence alignment of single-copy orthologues. These alignments were first concatenated and then used as input for imkt version 0.2 (Murga-Moreno et al., 2019), a web-based application (accessed May 21, 2021) to perform standard MKTs (McDonald & Kreitman, 1991). We also used imkt to estimate α based on the asymptotic MKT approach of Haller and Messer (2017). This approach differs from a traditional MKT in that it makes an explicit attempt to control for the confounding effects of low-frequency, deleterious allele classes. Finally, we estimated the distribution of fitness effects to acquire a model-based estimate of α and ωA (an estimate of adaptation that may sometimes be more useful than α) using dfe-alpha version 2.16 (Eyre-Walker & Keightley, 2009; Keightley & Eyre-Walker, 2007), after masking internal stop codons with macse version 2.03 (Ranwez et al., 2011). We ran dfe-alpha using a wrapper script adapted from Fields et al. (2022).
3 RESULTS
3.1 A reference genome of H. tvaerminnensis based on long reads
As the tissue of the host and the intracellular parasite are difficult to separate, we sequenced host and Hamiltosporidium tvaerminnensis together using long-read PacBio technology. Our initial assembly of the host and parasite genomes resulted in an assembly length of 123 Mbp. While the host genome had an average coverage <50×, the parasite genome had a coverage mostly >100× (Figure S1A). The H. tvaerminnensis genome had an assembly length of 28.5 Mbp and showed nearly 0× coverage when we analysed reads from a sequenced Daphnia magna host known to be uninfected by H. tvaerminnensis (Figure S1B). After applying purge_haplotigs to this assembly, the final, haploid assembly size of H. tvaerminnensis was 21.6 Mbp.
This new reference genome significantly improved the contiguity of the previous H. tvaerminnensis genome, which was based solely on short-read Illumina sequencing (Haag et al., 2020) and which had a total length of 18.3 Mbp, a maximum contig length of 0.06 Mbp, an N50 of 0.01 Mbp and was composed of 2,915 contigs. In contrast, our new long-read-based assembly had a total length of 21.6 Mbp, a maximum contig length of 2.7 Mbp, an N50 of 1.03 Mbp and 27 contigs. Along with the improved contiguity, the biological completeness of the new reference genome improved, with an ~15% increase in both genome length and busco completeness score (Table S1). However, without karyotype cytological data we cannot be certain that this improved assembly represents chromosomal contigs.
3.2 Samples, mapping and sequence variation
Infected clonal host lines were sequenced together with the parasites, resulting in 15 genomic samples, each from a different Eurasian population with sufficient representation of H. tvaerminnensis reads to describe its genomic variation (Table 1). Genomic samples from three populations of our outgroup Hamiltosporidium magnivora were also included to extend the number of analytical approaches for characterizing genome-wide variation in the H. tvaerminnensis sample. The percentage of sequencing reads mapped to the parasite's new reference genome ranged from 6.28% to 63.70%; most of the remaining reads were from the D. magna host. The average whole-genome coverage was greater than 94× in all cases of Hamiltosporidium infections. The overall SNP density in H. tvaerminnensis was 25 SNPs per kbp, with the total number of SNPs being 554,935. The average whole-genome π (Nei & Li, 1979) value was 0.009, or ~1%, and that of four-fold degenerate sites of single-copy orthologues was 0.008. The estimates for H. magnivora were similar (SNP density = 30 SNPs per kbp, π = 0.013 and πfour-fold = 0.012), but from a much smaller (geographical) sample. The nucleotide identity between H. tvaerminnensis and H. magnivora was 86% and above 98% for within-species comparisons (Table S2).
3.3 Population structure and phylogenetic analyses
We assessed the amount of genomic variation that differed or was shared between samples by applying a PCA to the whole-genome SNP data. The first two eigenvectors of the PCA (Figure S2) divided the Hamiltosporidium samples into three clusters: PC1 (explaining 36.09% of the variance) separated H. tvaerminnensis samples from those of H. magnivora; and PC2 (22.35%) split H. tvaerminnensis samples into two clusters, one Northern (N = 9) encompassing Scandinavian and East Asian samples, and one Southern (N = 6) encompassing all Mediterranean samples. A cluster analysis applied to the whole-genome SNP data resulted in the same groupings (Figure S2), as did a Bayesian phylogenetic tree estimation based on four-fold degenerate sites of the 2,229 single-copy orthologues (223,212 bp, Figure 1; Figure S3). After finding these groupings consistently in all of our population structure analyses, we refer to them as Northern and Southern H. tvaerminnensis lineages.
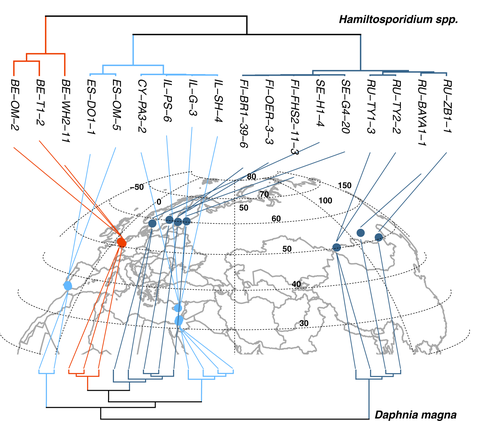
We inferred the demographic history for the two H. tvaerminnensis lineages separately. The SNP density and the π value showed that the Northern lineage (11 SNPs per kbp) was considerably less SNP-dense and less diverse (π = 0.005) than the Southern lineage (19 SNPs per kbp; π = 0.008; Wilcoxon signed rank test: p <.001). We found a positive correlation between pairwise differentiation and geographical distance for the overall H. tvaerminnensis data and the species’ lineages using dbMEM analysis by RDA (overall: R2 =.15, p =.197; Northern: R2 = .43, p = .001; Southern: R2 =.82, p =.003), which has been suggested as preferable over the formerly used Mantel tests (Legendre et al., 2015). The much higher correlation coefficients within the lineages, as compared to the total data set, strongly underline the importance of considering the phylogeography within the lineages.
3.4 Demographic history
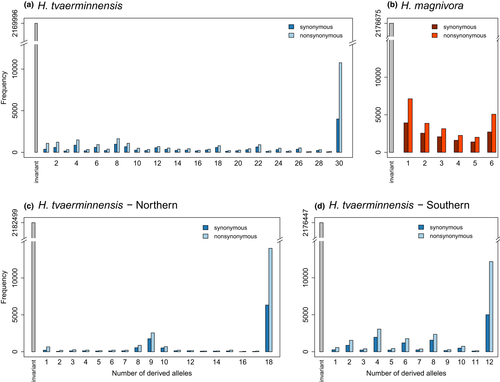
We examined the demographic history of H. tvaerminnensis looking at both the full data set and each of the Northern and Southern lineages individually. The uSFS of H. tvaerminnensis suggests strong deviation from what one would expect in a Wright–Fisher population (Crawford & Lazzaro, 2012), that is a panmictic population in mutation–drift balance (Figure 2a,c,d). Specifically, we do not observe any left-skewed distribution in H. tvaerminnensis, although it was seen in H. magnivora (Figure 2b). The Northern H. tvaerminnensis lineage had a clear peak around intermediate frequencies consistent with the earlier observation of fixed heterozygosity at many northern European sample sites (Haag, Traunecker, et al., 2013). Such a clear peak was not observed in the Southern H. tvaerminnensis lineage, however, which showed a more even spread than expected from a Wright–Fisher population.
To further understand the evolution of these lineages, we analysed the changes of Ne across time using software based on a Pairwise Sequentially Markovian Coalescent (PSMC) model to infer population history. Additionally, we did a very rough time calibration using publicly available estimates of mutation rate from the well-studied “red yeast” fungus Rhodotorula toruloides (1.90 × 10−10 per site per cell division; Long et al., 2016) and generation time of microsporidia (63 hr per generation; Kramer, 1965). PSMC revealed a relatively consistent pattern across samples from the same species and lineage (Figure 3a). Ancient Ne (i.e., around 100 thousand to 1 million years ago) was similar among all species and lineages but diverged in more recent times, with Ne for H. magnivora being less dynamic across time than Ne for all H. tvaerminnensis samples. The biggest difference between the species was the recent, strong reduction in Ne a few thousand years ago, seen only in the H. tvaerminnensis samples. There were also differences among the H. tvaerminnensis samples. Specifically, a more ancient decrease in Ne, between around 15,000 and 50,000 years ago was seen in all H. tvaerminnensis samples except those from Southeast Europe/Middle East, suggesting distinct population histories for the eastern and western samples of the Southern lineage. Apart from that, the consistency within the Northern lineage and the two groups of the Southern lineage gives us confidence about the quality of this population history measure.
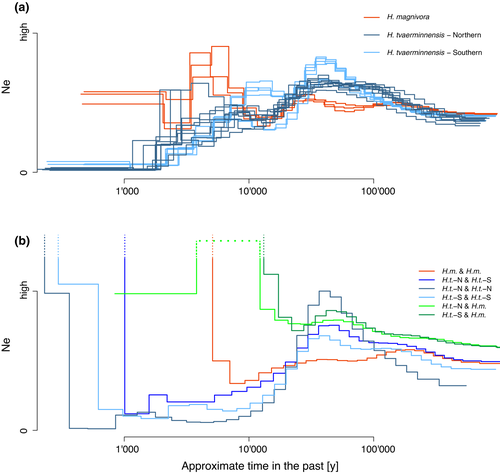
According to the PSMC results, neither Hamiltosporidium species showed extreme upward vertical trajectories in Ne, which one would expect in a diploid species that reproduces asexually over a long period of time and has nonrecombining chromosomes that separately accumulate mutations (i.e., the Meselson effect; Ceplitis, 2003; Weir et al., 2016). This result could suggest that the supposedly asexual H. tvaerminnensis experiences some recombination, as is believed to be true for the sexual H. magnivora. To further understand how mode of reproduction evolved, we ran the PSMC method with in silico, “pseudodiploid” samples, that is single phased haplotypes of our diploid samples that we combined in a pairwise manner. While this method is frequently used to estimate the relative divergence times of species (Cahill et al., 2016; Mattle-Greminger et al., 2018), we used it to investigate the degree of separate chromosomal evolution within and between Hamiltosporidium species. The PMSC results using “pseudodiploids” differed strongly from the results using the original samples (compare Figure 3a,b), especially in recent times. Ne in ancient times was similar in both analyses, with none of the intraspecies “pseudodiploid” samples’ PSMC results showing extreme upward vertical trajectories; however, in recent times, Ne showed extreme upward vertical trajectories in all the intraspecies “pseudodiploid” samples (Figure 3b). This confutes long-time asexuality and represents spatially based population differentiation. Of the intraspecies combinations, H. magnivora showed the deepest upward vertical trajectories in time (orange in Figure 3b), suggesting the highest differentiation (see also tree in Figure 1); however, upward vertical trajectories in interlineage and interspecies “pseudodiploid” samples are further in the past (green lines in Figure 3b), which is expected for more diverged haplotype combinations. Interestingly, there may have been lineage-specific events, including hybridization with H. magnivora (light green in Figure 3b): in contrast to the other “pseudodiploid” haplotype combinations, the PSMC results of Northern H. tvaerminnensis–H. magnivora “pseudodiploid” haplotype combinations show upward vertical trajectories in Ne followed by a return to lower values, which we interpret as putative gene flow between the species.
3.5 Rate of adaptive nucleotide substitutions
As genomic signals of demography and selection are often hard to disentangle, we assessed the importance of selection in H. tvaerminnensis by estimating the rate of adaptive substitutions (α) using a standard MKT. The α values of H. tvaerminnensis ranged from 0.121 to 0.227 (Table 2). Asymptotic MKT-derived α values, which are expected to be less affected by demographic factors, ranged from 0.222 to 0.279. However, the asymptotic methodology needed more data in some frequency categories than were available in our study (i.e., more neutral and selected diversity) to perform the asymptotic fit over the data (J. Murga-Moreno, pers. comm.). Therefore, the estimation of α using asymptotic MKT resulted in large confidence intervals and was unfeasible for the Northern lineage. The α values of H. tvaerminnensis estimated using dfe-alpha ranged from −0.026 to 0.203. Estimates of α have previously been found to be above 0.5 in many species and to reach up to 0.9 (Galtier, 2016). The consistently low estimates of α for H. tvaerminnensis support previous findings that genomic variation in Hamiltosporidium spp. might be predominantly driven by nonadaptive processes rather than by adaptive evolution (Haag et al., 2020).
| Lineage | Standard MKT | |
|---|---|---|
| α | p | |
| H. tvaerminnensis—all | 0.227 | <.001 |
| H. tvaerminnensis—north | 0.121 | <.001 |
| H. tvaerminnensis—south | 0.202 | <.001 |
| Lineage | dfe-alpha | ωA | ωA low | ωA high | ||
|---|---|---|---|---|---|---|
| α | α low | α high | ||||
| H. tvaerminnensis—all | 0.203 | 0.142 | 0.272 | 0.066 | 0.041 | 0.093 |
| H. tvaerminnensis—north | −0.026 | −0.104 | 0.061 | −0.008 | −0.030 | 0.021 |
| H. tvaerminnensis—south | 0.139 | 0.004 | 0.156 | 0.047 | 0.001 | 0.054 |
4 DISCUSSION
A core aim of population genetics is to understand genetic variation over space and time and link it to variation in the ecology of the species. To understand genetic variation in parasites, it is further helpful to know the host's biology and features that determine interactions, such as parasite transmission and host–parasite co-evolution. Our population genomic study provides insights into the variation of genomic diversity, population structure and demographic history in a highly specific parasite that exclusively infects the planktonic freshwater species Daphnia magna, a model system in host–parasite evolutionary ecology. This parasite, the microsporidium Hamiltosporidium, is widely spread and locally very common (Decaestecker et al., 2005; Ebert et al., 2001; Goren & Ben-Ami, 2013). Our analyses use samples spanning the Eurasian region of the host's Holarctic range where D. magna forms two clades, a Western Eurasian and an East Asian clade (Fields et al., 2018). We also find two clades for Hamiltosporidium tvaerminnensis, but the host and parasite clades are not congruent with each other, and thus are not the result of co-cladogenesis. Furthermore, we find relatively low levels of adaptive evolution, which is consistent with the hypothesis that a genome expansion may have resulted from high levels of genetic drift, corroborating a mechanism for the evolution of highly divergent genome architectures in microsporidia in general (Haag et al., 2020; Lynch, 2007). Within the genus, we hypothesize that H. tvaerminnensis might be derived from Hamiltosporidium magnivora (de Albuquerque et al., 2020), as H. tvaerminnensis, which presumably no longer depends on a second host, has been able to colonize geographical regions inaccessible to H. magnivora. This apparently became possible with the evolution of direct horizontal transmission among D. magna individuals (only vertical in H. magnivora), thus eliminating the need for a second host (H. magnivora is still believed to depend on a second, as yet unknown, host for horizontal transmission). Living without a second host also resulted in a loss of sexual reproduction (Figure 4), which has been suggested to take place in the second host (Mangin et al., 1995), with profound consequences for the evolution of genetic diversity of H. tvaerminnensis.
4.1 No pattern of host–parasite (co-)phylogeography
Because microsporidian parasites and their hosts interact closely, it might be assumed that parasite and host phylogeography should be congruent—the within-species equivalent of the Fahrenholz rule (Fahrenholz, 1913), which states that host and parasite phylogenies are expected to be congruent with each other (i.e., show a pattern of co-cladogenesis). It is now known, however, that this is rarely the case (Page, 2003). A strict co-phylogeography may be expected if the parasite's mode of transmission is uniquely vertical (Werren et al., 2008) and when host and parasite co-disperse (Page, 2003). A previous attempt by Pelin et al. (2015) to reconstruct a phylogeography based on whole-genome data for the microsporidium Nosema ceranae remained inconclusive due to high, human-driven migration and lack of IBD. In a different parasite of D. magna, the horizontally transmitted bacterium Pasteuria ramosa, a pattern of IBD was observed (Andras et al., 2018; Fields et al., 2015), but the issue of co-phylogeography was not addressed in the Pasteuria–Daphnia system. In the H. tvaerminnensis–D. magna system studied here, vertical transmission is common, and natural dispersal as well as co-dispersal seem likely (Haag, Traunecker, et al., 2013), especially because the unstable pond habitat of D. magna requires a high propensity for migration. We therefore hypothesized that we would find a pattern of host–parasite co-phylogeography and co-cladogenesis.
Although D. magna shows two main clades in Eurasia, a Western Eurasian and an East Asian clade (Fields et al., 2018), our phylogeographical analysis suggests that the split between the two main lineages of H. tvaerminnensis is clearly distinct from that of the host. Instead of a Western Eurasia/Eastern Asia split, we found a north–south split, a signal consistent across all the phylogeographical analyses we conducted (Figure 1; Figure S2). Thus, the phylogeography of D. magna and H. tvaerminnensis is nonoverlapping and cannot be explained by vertical transmission and co-dispersal alone. Nevertheless, a pattern of IBD—as has been shown for D. magna by Fields et al. (2015) and Andras et al. (2018)—was observed for H. tvaerminnensis, supporting the assumption of low or moderate gene flow between populations and reinforcing the intimate host–parasite association.
4.2 The divergence of Northern and Southern parasite lineages
Our phylogenetic analysis of Hamiltosporidium revealed a clear separation between a Northern and a Southern clade of H. tvaerminnensis. Not surprisingly, therefore, the correlation between pairwise genetic differentiation and geographical distance was clearer within these two parasite lineages than in the combined data set. We speculate that the Northern H. tvaerminnensis lineage resulted from the parasite spreading after the host expanded northwards out of its glacial refugium about 10,000 years ago when the ice retreated from the north. This spread of the Northern lineage of H. tvaerminnensis might have accompanied a population bottleneck, which resulted in reduced genetic diversity. Furthermore, the uSFS of Northern H. tvaerminnensis indicates fixed heterozygosity at many positions (Figure 2c), a finding consistent with Haag, Traunecker, et al. (2013) suggestions that the spread to the north coincided with the emergence of asexuality and a subsequent population expansion. The Southern lineage's samples, in comparison, are more divergent from each other, even though their currently known geographical spread is smaller than that of the Northern lineage. This could have resulted from an older transition to asexual reproduction or an additional glacial refugium other than the one in Southeast Europe/Middle East for D. magna (Fields et al., 2018). However, this needs further investigation. Additionally, estimating divergence times between the parasite lineages and comparing them to the host clades’ divergence time could indicate whether co-dispersal (i.e., range expansion of host and parasite at a similar time and in the same direction) played an essential role for the evolution of the host and H. tvaerminnensis.
4.3 Relative importance of selection
Models to determine the demographic history of a population are typically based on the assumption of neutrality, expecting that deviations from neutrality will lead to biased results (Johri et al., 2021). Discussing demographic history with regard to selection is therefore important. In Hamiltosporidium spp., it has been proposed that nonadaptive processes reduce the efficiency of adaptive evolution (Haag et al., 2020). Briefly, horizontal transmission is thought to have been the transmission mode of the ancestors of Hamiltosporidium until, at some point, an ancestral lineage developed the ability to transmit vertically among D. magna as well, which is now the case for all Hamiltosporidium species (Haag et al., 2020). A shift from horizontal transmission to mixed-mode transmission, especially if vertical transmission predominated and played an important role in co-dispersal, could have reduced effective population size, thus making it easier for slightly deleterious mutations to increase in frequency by genomic drift and making purifying and positive selection less efficient (Haag et al., 2020). Consequently, the number of nonsynonymous substitutions fixed by genomic drift increases while the proportion of substitutions fixed by positive selection (α) decreases.
To test this theory, we used our whole-genome data to determine the relative strength of selection in Hamiltosporidium. Of the different methods used to estimate α in our study, dfe-alpha relies on a specific demographic model of sexual species, any deviation from which might confound this method. The SFS as well as historical changes in Ne were inferred (see section below; Figures 2 and 3) to determine how the demography of the Hamiltosporidium samples could violate dfe-alpha’s underlying demographic model. Although we think that H. tvaerminnensis is predominantly reproducing asexually, given the PSMC results and that the sister species is sexual, this is probably not the case for a long time. However, Northern H. tvaerminnensis samples exhibited a clear recent bottleneck in their SFS and demographic history. The estimated α value of this lineage was negative (Table 2), suggesting that (i) positive selection is either weak or constrained, or (ii) the underlying likelihood model of dfe-alpha might not have been appropriate for these data. Test and reference regions were similarly affected by demography, as the synonymous and nonsynonymous SFS have the same shape (Figure 2). To ensure a demographically robust analysis for our estimation of α, we conducted both a standard MKT and a more recent approach called asymptotic MKT (Haller & Messer, 2017). It has been suggested that this latter approach can accommodate a wider range of demographic histories by accounting for the effects of low-frequency, deleterious variants. α values estimated using MKTs were similar to those using dfe-alpha. Also, the overall data set always had the highest estimate, while the Northern lineage always had the lowest. Furthermore, among all the species for which α and the rate of adaptive divergence relative to neutral divergence (ωA) has been estimated, H. tvaerminnensis is on the lower end of the selection efficacy distribution (e.g., Galtier, 2016; Rousselle et al., 2020). Taken together, our findings support the hypothesis of weak selection efficacy, increased genetic drift and a resulting genome expansion in Hamiltosporidium spp. proposed by Haag et al. (2020). Within the genus Hamiltosporidium, selection might be more efficient in H. magnivora relative to H. tvaerminnensis due to its constant sexual reproduction. However, with our sample, we could not estimate alpha in H. magnivora.
4.4 Historical changes in Ne
Historical processes are a major contributor to present-day distribution of genetic variation, influencing both similarities and differences in the co-phylogeography that a parasite might share with its host. The effective population size history of H. tvaerminnensis constructed with the PSMC method showed a rather consistent pattern across samples from the same lineage but revealed differences among lineages (Figure 3a). Since the mutation rates and generation times of H. tvaerminnensis and H. magnivora are unknown, we used available estimates for related species to estimate time intervals of interest. The more ancient decrease in Ne, which is observable in many species (Hewitt, 1996; Holder et al., 1999; Stewart et al., 2010), is thought to stem from the species’ range contraction, which happened when the glaciers expanded at the beginning of the last glaciation about 110,000 years ago. It is followed by an increase in Ne, presumably after the glaciers retreated about 10,000 years ago, as also described for the host D. magna (Fields et al., 2018). Samples that originated from hosts in the Middle East, a region where glaciers had no direct impact and where the presumed glacial refugia of the Western Eurasian D. magna clade were located, consistently seem to be more stable during the whole period of glacial expansion and recession. Numerous other species have shown patterns consistent with southern refugia during this period (Stewart et al., 2010). Importantly, our Spanish samples of the Southern lineage show an Ne pattern of change that is more similar to non-Middle Eastern samples (Figure 3a), coinciding with the absence of a glacial refugium for hosts in southwestern Eurasia (Fields et al., 2018). Thus, Daphnia hosts and their parasites presumably recolonized Western Europe from the same refugium, including the Hispanic peninsula after the last glaciation.
A second decrease where Ne dips to very low estimates is observed in more recent times for all H. tvaerminnensis samples but not for H. magnivora. A number of potential factors can decrease the effective population size: specialization of the parasite to a single host species; a clonal mode of reproduction (i.e., a switch to asexuality); an epidemic dynamic of boom and bust; restricted and local dispersal; frequent population extinctions and recolonizations; a short-lived, annual or ephemeral host; and small, fragmented host populations (Barrett et al., 2008). Although several of these factors probably contributed to the Ne dynamics of H. tvaerminnensis, it is unclear which of them predominated. For example, D. magna varies strongly in susceptibility to H. tvaerminnensis, with the most susceptible hosts occurring in unstable habitats that have a high propensity to dry up in summer (e.g., ephemeral rock or desert pools) (Cabalzar et al., 2019; Lange et al., 2015). These unstable habitats are also associated with frequent population extinctions and recolonizations as well as a loss of diversity because of frequent population bottlenecks.
However, as previous analyses have suggested that H. tvaerminnensis transitioned from a sexual to an asexual reproduction mode at some point in its recent history (Haag et al., 2013a), we hypothesize that the more recent decrease in its Ne might be related mainly to the evolution of horizontal transmission directly from D. magna to D. magna (Figure 4). The loss of sexual reproduction in the absence of a second host, which is believed to be necessary for sexual reproduction in H. magnivora (Mangin et al., 1995), reduced Ne. However, the transition from sexual to exclusively asexual reproduction would leave a very distinct historical pattern of change in Ne as inferred via PSMC. Specifically, because individual homologous chromosomes can no longer recombine, one would expect to see an upward, nearly vertical trajectory in Ne as mutations accumulate on separate chromosomal copies, which our reconstructed history of H. tvaerminnensis’ population size does not show, or at least not deep enough in time when such a transition would be easier to pin down with the PSMC method. This implies that exclusive asexuality is probably not ancient, coinciding with the SFS of the Northern lineage, which shows a distinct peak at 0.5 caused by fixed heterozygosity. Being exclusive asexual for a long period of time would provide sufficient time for individual haplotypes to accumulate their own distinct mutations, which is not what the SFS shows (Figure 2). Alternatively, although H. tvaerminnensis may be a mostly asexual species, it could still show occasional recombination, for example when it encounters its now presumably facultative second host. A loss of sexual reproduction with occasional recombination would explain the low estimates of Ne without extreme upward vertical trajectories of nonrecombining chromosomes, using the PSMC method. However, any discussion about the second decrease in Ne should be undertaken with the mindfulness that estimates of PSMC from the recent past are generally more uncertain (H. Li & Durbin, 2011), and that PSMC has not often been used for understanding the demographic history of parasitic species (but see, e.g., Hecht et al., 2018).
When comparing H. magnivora and H. tvaerminnensis, we found that their histories of Ne do not align precisely. Along with differences in their demographies, differences in mutation rate and generation time, both of which used here may not be optimal for each species, can also cause aberrations with the PSMC method. The PSMC results for the in silico, “pseudodiploid” samples support the idea that hybridization events occurred between H. magnivora and the Northern H. tvaerminnensis lineage. The combinations of H. tvaerminnensis and H. magnivora haplotypes show the expected, nearly vertical trajectories of no longer recombining lineages in deeper time (Figure 3b). However, combinations of the Northern H. tvaerminnensis lineage and H. magnivora reveal a demographic signal consistent with gene flow between the two species. Overall, our analyses support the suggestion that an ancestral H. tvaerminnensis became able to transmit horizontally directly from D. magna to D. magna (H. magnivora is unable to do this) and thus was able to persist in habitat without its second host. This allowed H. tvaerminnensis to colonize Northern habitats, but also made it reliant almost exclusively on asexual reproduction, with the latent ability to recombine if the second host were present.
4.5 Comparatively high genomic variation in Hamiltosporidium
Large-sized microsporidia genomes are generally difficult to assemble due to a high number of repetitive elements (Parisot et al., 2014). H. tvaerminnensis has been the target of previous assembly trials using shotgun and Illumina sequencing (Corradi et al., 2009; Haag et al., 2020). Here, we were able to produce a very contiguous genome assembly of this microsporidium with a large genome size using long-read PacBio sequencing. H. tvaerminnensis has a whole-genome SNP density of ~25 SNPs per kbp, which is more diverse than its close relative N. ceranae (12.7) and on the high end compared to other microsporidia and fungi (Pelin et al., 2015). A probable reason why the multicontinent N. ceranae sample has a lower density estimate is because the species was recently introduced into the sampled region and may have experienced a bottleneck during range expansion (Pelin et al., 2015). In contrast, D. magna, the host of H. tvaerminnensis, is native to Eurasia.
5 CONCLUSION
The evidence presented here of a nonoverlapping phylogeography between two partners in an intimate host–parasite system with a wide geographical distribution has several implications. It suggests that the parasite, Hamiltosporidium tvaerminnensis, might be a rather young parasite of its host, Daphnia magna. Otherwise, it could not be simultaneously widespread and have a phylogeography distinct from its host. Also, it implies that co-dispersal of host and parasite, while common, is not the only form of dispersal. Thus, our research underlines the importance and potential of using samples from the whole-species range in population genomic studies. Regarding microsporidia with long genomes, we could strengthen the assumption of reduced selection efficacy, probably as a result of high levels of genetic drift that ultimately caused genome expansion (Haag et al., 2020). By quantifying genomic variation and selection efficacy in other microsporidia, more evidence for this hypothesis and for the evolution of different genome architectures in this taxon could be provided. However, while our study reveals some general principles on the evolution of genomic variation in a parasite, it also shows how specific factors shape genomic variation, some of these (or their combination) being possibly unique to this system (e.g., loss of the second host and the shift to exclusive asexuality in the absence of the second host). It therefore provides a case study for the genomic evolution of a microsporidium at a fine scale, which awaits comparison with other systems that will help paint a bigger picture of the evolution of specific obligate parasites.
ACKNOWLEDGEMENTS
We thank Jürgen Hottinger, Urs Stiefel, Michelle Krebs and Andrea Cabalzar for help in the field and laboratory. We are grateful to members of the Ebert group for providing feedback on the study and the manuscript. We thank Elin Videvall and three anonymous reviewers for helpful comments on the manuscript. The scheme of D. magna was drawn by Dita Vizoso. This work was supported by the Swiss National Science Foundation (SNSF) (grant nos. 310030B_166677 and 310030_188887 to D.E.).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
All authors designed the study. P.A. analysed the data and wrote the manuscript. All authors reviewed the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All analysis scripts as well as raw and processed data are available at https://github.com/pascalangst/Angst_etal_2022_MolEcol, NCBI SRA database (BioProject ID PRJNA780787) and NCBI GenBank (BioProject ID PRJNA778105).




