Moulded by humans: The domestication of blue-veined cheese fungi
Abstract
It is hard to imagine a world without food-associated microbes. The production of bread, wine, beer, salami, coffee, chocolate, cheese and many other foods and beverages all rely on specific microbes. In cheese, myriad microbial species collaborate to yield the complex organoleptic properties that are appreciated by millions of people worldwide. In the early days of cheese making, these complex communities emerged spontaneously from the natural flora associated with the raw materials, the equipment, the production environment or craftsmen involved in the production process. However, in some cases, the microbes shifted their natural habitat to the new cheese-associated environment. The most obvious cause of this is backslopping, where part of a fermented product is used to inoculate the next batch. In addition, some microbes may simply adhere to the tools used in the production process. These microbial communities gradually adapted to the novel man-made niches, a process referred to as “domestication.” Domestication is associated with specific genomic and phenotypic changes and ultimately leads to lineages that are genetically and phenotypically distinct from their wild ancestors. In this issue of Molecular Ecology, Dumas et al. have investigated a prime example of cheese-associated microbes, the fungus Penicillium roqueforti. The authors identified several hallmarks of domestication in the genome and phenome of this species, allowing them to hypothesize about the origin of blue-veined cheese fungi domestication, and the specific evolutionary processes involved in adaptation to the cheese matrix.
Studies on domestication trace back to Charles Darwin, who was the first to document the striking morphological resemblance between different domesticated animals, despite the lack of close evolutionary relationships between their wild ancestors. During domestication, humans interfere with the natural process of evolution by limiting gene flow to specific subpopulations, often through the creation of artificial, man-made niches, and by selecting traits that do not necessarily increase fitness in natural environments (referred to as “artificial selection”). Only recently, this general principle was extrapolated to microbial species, mainly triggered by the vastly expanding set of genomic data (reviewed by Steensels, Gallone, Voordeckers, & Verstrepen, 2019). Today, microbial domestication is studied intensively, leading to a better understanding of the interaction between humankind and microbes throughout history.
Microbial domestication has been reported in a wide array of organisms. For example, lactic acid bacteria have been shown to have adapted to dairy (most notably Lactococcus lactis), wine (Oenococcus oeni) and meat (Lactobacillus sakei) fermentations (Makarova et al., 2006). Similarly, lineage-specific adaptation to beer, wine, sake and bread have been identified in the yeast Saccharomyces cerevisiae (Gallone et al., 2016, 2019; Peter et al., 2018), while the Koji mould, Aspergillus oryzae, was domesticated for the production of traditional fermented Japanese products from starch-rich grains (e.g., soy bean paste, vinegar and soy sauce) (Gibbons et al., 2012). Despite the huge genetic distance among these organisms, they all share peculiar features that characterize microbial domesticates. First, they are all genetically isolated from wild lineages, indicating limited gene flow between wild and domesticated populations. Second, they are at least partially dependent on, and adapted to, their respective man-made environment. Third, domesticated lineages were often subjected to repeated bottlenecks during backslopping, resulting in a limited genetic diversity. Fourth, they show signs of genome decay (i.e., loss of genes not required in the human-associated niche). Therefore, “feral” strains (i.e., domesticated strains that also thrive in a more natural setting) are generally rare. Lastly, in microorganisms that allow sexual reproduction, such as S. cerevisiae, there is often admixture between distantly related domesticated populations, suggesting human-associated dispersal.
In this issue of Molecular Ecology, Dumas et al. (2020) have identified several of these domestication hallmarks in a set of Penicillium roqueforti strains (n = 148, of which the full genome of 34 was sequenced), isolated from different types of blue-veined cheese and noncheese (silage, lumber, spoiled foods) environments. Using different clustering and phylogenetic methods, the authors estimated the presence of four genetic clusters, two containing exclusively noncheese strains and two including only cheese-associated strains with the exception of two strains isolated from other food-associated environments (brewery and brioche), which were therefore deemed “feral.” Interestingly, of the two cheese-associated P. roqueforti clusters, one represents a more historical population dubbed “Roquefort,” originally shared by multiple farms in the heart of the traditional Roquefort production area; the second one represents a more industrial population dubbed “non-Roquefort” that includes mainly strains used worldwide for the production of other types of blue-veined cheese such as Gorgonzola.
To infer the origin and diversification history of the distinct clusters, 11 demographic models were evaluated. The most likely scenario points towards the presence of two independent domestication events from a common, nondomesticated, ancestral population for the cheese-associated groups, with the “non-Roquefort” population diverging first, followed by the “Roquefort” population (Figure 1). Surprisingly, the noncheese clusters diverged more recently from each other than from the cheese populations, indicating that the closest wild relative of current cheese populations is not present in the sampled collection. In search for wild P. roqueforti strains, old ripening cellars and artisanal cheese companies were sampled by the authors, but no P. roqueforti strains could be identified.
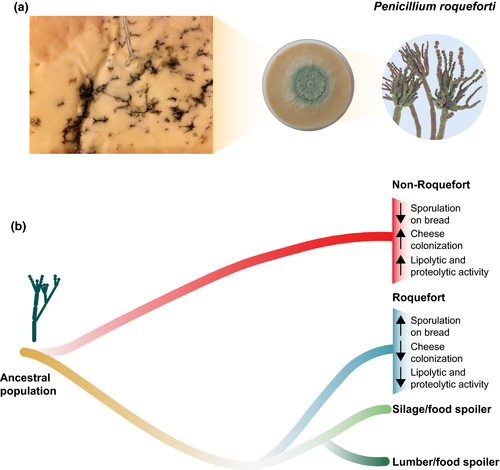
Striking differences in the level of genetic diversity were observed between both cheese-associated and noncheese lineages. In particular, while the genetic diversity of the “Roquefort” lineage is relatively broad (about half of the nucleotide diversity of the noncheese groups), the “non-Roquefort” population shows the lowest genetic diversity, with only ~7,000 segregating sites across the 30-Mb genome. This points to a recent genetic bottleneck, for example the isolation of a single clone. This isolation probably occurred during or after the rise of industrialized cheese making, as the lack of genetic diversification indicates no backslopping, but rather the use of frozen stocks, as is common practice today.
By contrast, the “Roquefort” strains exemplify the role of backslopping in promoting long-term selection of microbial populations. For example, bread was used as q source of spore multiplication before cheese inoculation until the early 19th century, resulting in greater sporulation on bread compared to the “non-Roquefort” strains. In addition, they do not demonstrate fast cheese colonization, their tolerance to salt (a traditional preservative in cheese making) is rather low and their lipolytic and proteolytic activity, crucial for aroma production, does not differ compared to the noncheese strains. The “non-Roquefort” population behaves very differently, with strains exhibiting fast growth in cheese, high salt tolerance and high lipolytic activity (Figure 1). While this reduced fitness in the cheese environment for “Roquefort” strains might seem counterintuitive within a domestication framework, it suggests that these strains may have in fact adapted to life on bread rather than to life in cheese. As an alternative explanation, the authors argue that slow growth on cheese might actually have been selected for. Roquefort cheeses are made of ewe's milk, a product available only between February and July. Therefore, to ensure year-round availability of Roquefort cheese, slow cheese maturation was an important trait for long-term storage in the pre-industrialization era, as there was no refrigeration. For this reason, vigorous fungal growth and lipolytic activity (which would lead to cheese degradation) was undesired. However, fast colonization and high lipolytic activity is beneficial for modern and accelerated production of blue cheese, where refrigeration is available for cold storage, as reflected in the “non-Roquefort” population.
When zooming-in on the genomes of both domesticated lineages, the authors found regions that were population-specific (e.g., acquired by horizontal gene transfer [HGT]). Most notably, the previously identified HGT regions “CheesyTer” and “Wallaby” (Cheeseman et al., 2014; Ropars et al., 2015) were specific only to the to “non-Roquefort” strains. These regions include genes related to carbohydrate metabolism (β-galactosidase and lactose permease genes) and to antagonistic interactions with other microorganisms, which is in line with the faster growth of these strains in cheese. Furthermore, the authors detected genes under potential positive selection, but no clear association with the observed phenotypic differences was established. Compared to plant and animal domesticates, the detection of actual positive selection has proven to be challenging in microbes, partly due to selection acting on larger populations rather than a few individuals, weak species boundaries leading to widespread reticulate evolution and mixed mating systems (Rokas, 2009).
In conclusion, Dumas and colleagues have put forward some interesting hypotheses about the life history of blue-cheese fungi. Remarkably, the results reported reveal that the domestication process in these P. roqueforti lineages is less straightforward and more complex compared to some other domesticated microbes. In particular, the lack of a wild genetic stock hampers the investigation of further genetic and molecular changes that occurred during the transition from wild to domesticated populations in P. roqueforti. Nevertheless, once such stock is discovered, P. roqueforti will be an interesting model to investigate the influence of humans on microbial evolution, and the adaptation to harsh niches with complex microbial communities.




