Climate warming alters the structure of farmland tritrophic ecological networks and reduces crop yield
Abstract
It is unclear how sustained increases in temperature and changes in precipitation, as a result of climate change, will affect crops and their interactions with agricultural weeds, insect pests and predators, due to the difficulties in quantifying changes in such complex relationships. We simulated the combined effects of increasing temperature (by an average of 1.4°C over a growing season) and applying additional rainwater (10% of the monthly mean added weekly, 40% total) using a replicated, randomized block experiment within a wheat crop. We examined how this affected the structure of 24 quantitative replicate plant–aphid–parasitoid networks constructed using DNA-based methods. Simulated climate warming affected species richness, significantly altered consumer–resource asymmetries and reduced network complexity. Increased temperature induced an aphid outbreak, but the parasitism rates of aphids by parasitoid wasps remained unchanged. It also drove changes in the crop, altering in particular the phenology of the wheat as well as its quality (i.e., fewer, lighter seeds). We discuss the importance of considering the wider impacts of climate change on interacting species across trophic levels in agroecosystems.
1 INTRODUCTION
Climate change is expected to have profound impacts on food production systems over the coming decades (Lobell et al., 2008). Crops will be adversely affected by a combination of both abiotic (e.g., heat, drought, salinity and submergence in water) and biotic (e.g., pests and pathogens) stresses (Baulcombe et al., 2009; Bebber, Holmes, & Gurr, 2014; Lesk, Rowhani, & Ramankutty, 2016; Maxmen, 2013), posing significant threats to food security (Godfray et al., 2010). Despite the growing research demonstrating the impacts of climate change on species abundances and distributions, community composition and organismal physiology (Garcia, Cabeza, Rahbek, & Araujo, 2014; Parmesan, 2006; Sala et al., 2000), the effects on the network of interactions among species are poorly understood (Tylianakis, Didham, Bascompte, & Wardle, 2008), particularly in agroecosystems. This is largely due to the difficulties in quantifying changes in interactions compared with changes in biodiversity (McCann, 2007). Yet, complex networks of biotic interactions, such as insect pollination and parasitism, play an important role in the maintenance of biodiversity (Bascompte, Jordano, & Olesen, 2006), provide valuable ecosystem services (Pocock, Evans, & Memmott, 2012) and can mediate ecosystem responses to environmental change (Brooker, 2006; Sydes & Miller, 1988). Species interactions may, however, be more susceptible to climate change, as they are sensitive to the phenology, behaviour, physiology and relative abundances of multiple species (Memmott, Craze, Waser, & Price, 2007; Suttle, Thomsen, & Power, 2007; Tylianakis, Tscharntke, & Lewis, 2007).
Combining advances in both network theory and molecular ecology offers unprecedented opportunities to describe interactions between species, the structure of communities and the function and stability of ecosystems (Evans, Kitson, Lunt, Straw, & Pocock, 2016). Ecological networks provide a quantitative framework to unify the study of biodiversity and ecosystem function (Thompson et al., 2012) and have been successfully used to quantify the ecosystem-level consequences of global environmental change (Tylianakis, Laliberte, Nielsen, & Bascompte, 2010). There is growing interest in developing these approaches to provide a more holistic, system-based understanding of agroecosystems that could be used to maximize the ecosystem services provided by farmland biodiversity, as well as for anticipating and mitigating future scenarios (Bohan et al., 2013). For example, Macfadyen et al. (2009) constructed quantitative plant–herbivore–parasitoid networks on paired organic and conventional farms and showed that the organic farms had more species across the three trophic levels and significantly different network structure. However, such networks take considerable effort to construct and can be subject to bias because of the limitations of taxonomically selective rearing success as well as the reliance on accurate morphological identification (Evans et al., 2016). Advances in DNA sequencing technologies provide enormous potential to determine hitherto difficult-to-observe species interactions and thus to produce highly resolved ecological networks (Derocles et al., 2018; Evans et al., 2016; Wirta et al., 2014). An accurate and cost-effective PCR diagnostic approach has recently been developed to allow the rapid construction of quantitative ecological networks of farmland aphid–parasitoid interactions (Derocles, Plantegenest, Simon, Taberlet, & Le Ralec, 2012; Derocles et al., 2014) providing new opportunities to examine the impacts of environmental change on network structure and complexity.
In northern Europe, climate models predict significant warming and an increase in both precipitation (mainly in winter) and the frequency of extreme weather events (IPCC 2014), which are likely to cause significant damage to agroecosystems (Olesen et al., 2011). With increasing evidence that present climate change is altering geographical ranges, population dynamics and phenologies of some insects (Altermatt, 2010; Morris, Sinclair, & Burwell, 2015), there is growing concern that global food security is threatened by the emergence and spread of crop pests and pathogens (Maxmen, 2013). Given the ecological and economic importance of phytophagous insects and their natural enemies, a greater understanding of their direct and indirect interactions and how these respond to experimental manipulation is needed (van Veen, Morris, & Godfray, 2006), particularly in the context of climate warming.
Experimental manipulations of temperature and precipitation have provided important insights into the responses of terrestrial ecosystems, with climate warming generally stimulating total net primary productivity, increasing ecosystem photosynthesis and respiration (see Wu, Dijkstra, Koch, Peñuselas, & Hungate, 2011 for a review). Real-world experimental climate manipulations can help to fill the knowledge gap between highly controlled, closed-system laboratory studies (e.g., Le Lann, Lodi, & Ellers, 2014) that tend to focus on a small number of species, and large-scale open-field experiments that rely on variations in temperature along environmental gradients (see Romo & Tylianakis, 2013; de Sassi & Tylianakis, 2012). To date, most field-based simulated warming experiments have used infrared heating devices (see de Sassi, Staniczenko, and Tylianakis (2012) who used underground heating cables) but have mainly focused on plant responses to elevated temperatures. To our knowledge, none have examined the impacts on networks of interacting species across multiple trophic levels. Within grasslands, de Sassi and Tylianakis (2012) demonstrated that in a tritrophic system of plants, herbivores and parasitoids, each trophic level responded differently to warming, and overall, the community was increasingly dominated by herbivores. Within arable crops, a small number of individual simulated climate warming studies have demonstrated a reduction in wheat yield (Fang, Su, Liu, Tan, & Ren, 2013) and increases in aphid pests (Dong, Hou, Ouyang, & Zhang, 2013) and insect predators (Berthe, Derocles, Lunt, Kimball, & Evans, 2015). Thus, it is unlikely that climate warming will affect species richness within arable crops; rather, it will alter network structure and complexity, in particular consumer–resource asymmetries (e.g., network “generality”—the mean effective number of lower trophic level species per higher trophic level species) and interaction evenness, driven by changes in the abundances and frequency of interactions between plants, aphids and parasitoids. However, predicting the specific impacts on the complex pattern of interactions among species in a community remains a pressing challenge (Staniczenko et al., 2017).
Here, we experimentally increase temperature and rainwater within farmland plots consisting of spring-sown wheat and common uncultivated plant (weed) species. The study is framed in the context of understanding climate change implications as it relates to policy targets (e.g., limiting warming to 2°C) within North European agriculture (Olesen et al., 2011). We examine the responses of quantitative plant–aphid–parasitoid networks, constructed using DNA-based methods, as well as the impacts on crop yield. Although predicting the direct and indirect responses of plants, phytophagous insects and their natural enemies to perturbation is a major challenge, quantitative ecological networks are particularly well suited for assessing direct and indirect interactions in the first instance (van Veen et al., 2006). Our objectives are threefold. (a) To construct replicated, quantitative tripartite food webs describing the interactions between crop and non-crop plants, aphids and parasitoids. We apply a DNA barcoding approach to accurately and cost-effectively quantify the interactions of Aphidiinae endoparasitoids with their aphid hosts. (b) To examine the combined effects of a 1.4°C temperature elevation and increase in rainwater on measures of network structure and complexity. We use suspended infrared heaters, which have been effectively applied in other habitats for climate change simulation experiments (Harte, Saleska, & Levy, 2015; Price & Waser, 2000; Wan, Luo, & Wallace, 2002) to warm farmland plots in situ, and apply extra rainwater following established protocols (Rollinson & Kaye, 2012). We predict no impacts on total species richness, but significant increases in aphid abundances in warmed plots due to a positive direct effect on population growth rate (Barton & Ives, 2014) and a corresponding increase in the frequency of parasitoid interactions, potentially leading to changes in network consumer–resource asymmetries and interaction evenness. As aphids and parasitoids are highly specialized in agroecosystems (Derocles et al., 2014; Le Ralec, Ribule, Barragan, & Outreman, 2011), we do not expect an increase in network connectance (a measure involving the number of interactions) in the short term, as this would indicate an expansion of generalism of the species involved. We test this for both bipartite and tripartite networks. (c) To investigate the overall effects of warming on crop yield and whether any changes can be mediated by an increase in rainwater (either as precipitation or as added irrigation).
2 MATERIALS AND METHODS
2.1 Experimental layout
The study was conducted in 2013 at Stockbridge Technology Centre (STC), North Yorkshire, UK (53°49′N–1°9′W), a conventional farm consisting of meadows and cereal crops used for field experiments. The climate is temperate oceanic, with a mean minimum and maximum annual temperature and precipitation of 5.5–14°C (8.6–19.1°C during the experiment) and 537.7 mm (156.6 mm during the experiment), respectively. We established a replicated, randomized block open-field experiment consisting of six replicates of four simulated climate change treatments in a field of spring wheat (Triticum aestivum cultivar Tybalt) (see Berthe et al., 2015; Supporting Information Figure S1). The four treatments consisted of: (W) 1.4°C increase in temperature; (P) increase in precipitation/rainwater by adding 10% of the monthly mean, per week, based on historic records (40% total); (WP) warming and precipitation treatments combined; and (C) control (ambient conditions). We refer to “climate warming” when reporting the effect of warmed treatments and “precipitation” when reporting the effect of additional rainwater treatments. Treatments were randomly allocated to 2 × 2 m experimental plots that were each separated by 2 m of wheat to provide a buffer and allow the free movement of insects. The W and WP treatments involved suspending 240 V infrared heaters 1.5 m above each plot (following Rollinson & Kaye, 2012), consistently heating throughout the day and night: this primarily drives plant phenology rather than heating the column of air (Kimball, 2005; White, Kimball, Wall, Ottman, & Hunt, 2011). A “dummy” heater of the same size and shape was suspended in the non-heated plots to account for any possible effects of shading/shelter.
A real-time proportional-integrative-derivative feedback system ensured constant temperature plot warming through infrared radiometer (IRR) monitoring of surface temperatures in warmed plots. Soil-surface temperatures were monitored by six infrared remote temperature sensors (IR120; Campbell Scientific; Loughborough, UK), positioned 1.10 m above the plots and directed to the middle of the plot and connected to a data logger (Campbell Scientific; Loughborough, UK) to record the temperatures every 10 s and to control the constant output of the infrared heaters. Their positions were selected randomly, three within a heated plot (W, WP) and three within an unheated plot (C, P). Our original aim was for the system to increase the temperature in the warmed plots by 2°C. Over the course of the experiment, temperatures were raised, on average, by 2.2°C (standard deviation 0.6) in block 1; 1.1°C in block 2 (standard deviation 0.6) and 1.1°C in block 3 (standard deviation 0.8) that most likely reflected subtle microclimate differences within the field. This provided a mean temperature increase of 1.4°C (standard deviation 0.9) across all the plots. Increased rainwater was simulated in the P and WP plots by manually adding 10% of the mean monthly rainfall, in collected rainwater, each week based on STC mean monthly rainfall data collected between 2002 and 2012. Thus, we actually increased precipitation/rainwater in absolute terms by 40%. This can either be interpreted as representing weather conditions in a warm and wet summer, or a farmer increasing irrigation to mitigate the effects of a warm and drier summer. We added the following water each week: 13 L in April, 19 L in May, 24 L in June, 26 L in July and 30 L in August, amounting to 407 L in total for each plot. During the course of the experiment, just 156.6 mm of rainfall was measured at Stockbridge Technology Centre, well below the seasonal average. The experimental area in which the plots were located received herbicide applications on 2 April and 13 May (pendimethalin, metsulfuron-methyl and thifensulfuron-methyl); our aim was to allow some weed growth without out-competing the wheat. Experimental treatments commenced immediately after the sowing of spring wheat on 13 April and stopped with the harvest of the crop on 16 August.
2.2 Plant surveys and crop yield
Plants were identified to the species level, with a small proportion to the genus or family, and the percentage cover of each was recorded weekly (18 surveys) in each plot. The date of emergence of the first leaf and the date of emergence of the first ear for T. aestivum were recorded in each plot and converted into Julian date for statistical analysis. At harvest, a 0.5 × 0.5 m quadrat was placed in the area directly below the heaters/dummy heaters (we selected this area because the heating pattern is likely to be more consistent; Kimball, 2005) and the number of T. aestivum ears counted. The density of wheat (number of wheat ears/m2) was then calculated for each plot. We also harvested five ears randomly from each plot, which were dried in an oven at 80°C for 48 hr in the laboratory. The seeds were counted, and the total seed weight was measured for each ear. For each plot, crop yield (g/m2) was calculated as: (total seed weight/ear) × density of wheat.
2.3 Insect surveys
Plant–aphid interactions were recorded by systematically searching each plot and counting the total number of aphids and visibly parasitized aphids (“mummies”) on each plant species every week (18 surveys) throughout the sampling period. We collected up to 30 aphid individuals per colony and placed them in a 1.5-ml tube filled with 95% ethanol and then stored at −20°C in the laboratory for later identification (see below). All aphid mummies were collected and stored in 1.5-ml tubes, but without 95% ethanol. Instead, these were stored under laboratory conditions and observed for 10 days for the emergence of adult parasitoids. Adult parasitoids and aphid mummies where parasitoids did not emerge were then stored individually in a 1.5-ml tube filled with 95% ethanol at −20°C.
2.4 Insect identification
Aphids were first identified morphologically following Blackman and Eastop (1994, 2000, 2006). We extracted the DNA of all the aphids collected using a hotshot DNA extraction (Montero-Pau, Gomez, & Munoz, 2008). Aphid identification was confirmed with DNA barcoding: a fragment 658 bp from cytochrome c oxidase subunit I [COI] was amplified and sequenced with the PCR conditions described by Derocles, Le Ralec, et al. (2012) and the following primer pairs: LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′; Folmer, Black, Hoeh, Lutz, & Vrijenhoek, 1994) and the degenerate reverse primer HCO2198-puc (5′-TAAACTTCWGGRTGWCCAAARAATC-3′; Cruaud et al., 2010). Adult parasitoids and non-emerged parasitoids from the mummies (n = 181) were identified using the DNA barcoding tool described by Derocles, Le Ralec, et al. (2012): a fragment 658 bp from COI was amplified and sequenced to identify these parasitoids. Aphid–parasitoid interactions and parasitism rates were determined using two different molecular methods based on the extracted DNA of aphids. First, we used a multiplex PCR approach developed by Traugott et al. (2008) on the aphid species collected on T. aestivum (Sitobion avenae and Metopolophium dirhodum) to detect both primary and secondary parasitoids. We used nine primary parasitoid and two hyperparasitoid species-specific primer pairs to detect and identify immature primary and secondary parasitoids within cereal aphid hosts. Second, for all the other aphid species, we used the approach developed by Derocles, Plantegenest, et al. (2012) that uses the sequences of a 210-bp fragment from the 16S gene to identify to species level (in most cases) the immature Aphidiinae parasitoids within an aphid host. To improve the reliability, we added an “in tube control” to determine whether an absence of parasitoid detection is due to either a true absence of parasitism or a technical problem during DNA extraction or PCR amplification. For this, we followed the PCR protocol for parasitoid detection described by Derocles, Plantegenest, et al. (2012) and we added in the PCR-mix the aphid COI barcode assay described above. A detection of a parasitoid within an aphid host is characterized by two bands on a 1.5% agarose electrophoresis gel: a band of 658 bp (COI, aphid and parasitoid) and a band of 210 bp (16S parasitoid); an unparasitized aphid is characterized only by the band of 658 bp, from the aphid DNA. An absence of band indicates a failure from either the DNA extraction or the PCR amplification. In this case, the PCR amplification is performed a second time. If after a second PCR amplification a failure is observed, the individual is removed from the analysis. Sixteen aphids were removed from the analysis following two PCR failures. We used two hyperparasitoid species-specific primer pairs (Traugott et al., 2008) to detect the secondary parasitoids in non-crop aphid species. We compared parasitism rate determined using this method vs. the conventional approach (i.e., (the number of aphid mummies collected)/(number of aphid counted)).
2.5 Insect abundance, species richness and parasitism rates
For each plot, we pooled data across the sampling period to calculate:
- percentage of aphids sampled: number of aphids sampled/ number of aphids counted;
- aphid abundance: total number of aphids counted on each plant species throughout the sampling period;
- total species richness;
- species richness per trophic level (i.e., plants, aphids, parasitoids);
- parasitism rate using the DNA-based method: (the number of aphid mummies collected + number of parasitized aphids detected)/(number of aphid mummies collected + number of aphids collected);
- parasitism rate using the conventional method (no DNA-based method): (the number of aphid mummies collected)/(number of aphid counted);
- multiparasitism rate using the DNA-based method: (number of aphids parasitized by at least two detected primary parasitoid species/number of aphids collected + number of mummies collected);
- hyperparasitism rate using the DNA-based method: (number of aphids parasitized by a secondary parasitoid + number of secondary parasitoids identified in mummies/number of aphids collected + number of mummies collected).
2.6 Ecological network construction, visualization and description
Plant–aphid–parasitoid quantitative networks were constructed for each plot by pooling data collected during the course of the experiment. We visualized the tripartite interactions for each of the four treatments (by pooling replicate data from replicate plots) using the “hiver” package (Krzywinski, Birol, Jones, & Marra, 2011) in r 3.3.1 (R Core Team, 2016). We were particularly interested in how the experimental treatments affect consumer–resource asymmetries, classically described in network ecology as vulnerability and generality (i.e., the mean number of consumers per prey and the mean number of prey per consumer, respectively), as well as standard measures of complexity (Bersier, Banasek-Richter, & Cattin, 2002). They are well suited for describing antagonistic interactions and the extent to which consumers are specialized to the resource and how the resource is attacked by the higher trophic level (Wirta et al., 2014). For each of the 24 tripartite networks, we calculated the following qualitative, unweighted quantitative and weighted quantitative network descriptors described by Bersier et al. (2002) using “cheddar” (Hudson et al., 2013) and “bipartite” packages in r 3.3.1 (Dormann, Fruend, Bluethgen, & Gruber, 2009): linkage density (average number of links per species: LD, LD'q, LDq); connectance (proportion of possible links between species that are realized: C, C'q, Cq); vulnerability (mean effective number of higher trophic level species per lower level species: V, V'q, Vq) and generality (mean effective number of lower trophic level species per higher trophic level species: G, G'q, Gq). We based our analysis on weighted quantitative network descriptors (LDq, Cq, Vq and Gq) to specifically examine changes in network complexity and consumer–resource asymmetries as they are commonly used in ecological network studies and less prone to sampling biases (Macfadyen et al., 2009; Tylianakis et al., 2007; Wirta et al., 2014). As interaction evenness may be ecologically important and that these network descriptors are relatively insensitive to differences in the evenness of the distribution of link magnitude, we calculated the quantitative tripartite interaction evenness (IEq) following Albrecht, Duelli, Schmid, and Müller (2007). To examine whether plant–herbivore and herbivore–parasitoid interactions react differently to climate change, we also calculated network descriptors for the plant–aphid and aphid–parasitoid bipartite networks separately.
2.7 Statistical analysis
Statistical analysis was performed in r 3.3.1 (R Core Team, 2016). The effects of treatment on the plants (including yield), insects, parasitism rates and network descriptor response variables were examined using generalized linear models (GLMs) with a Gaussian family (except for aphid abundance data, where a Poisson family was used). To account for the intercorrelation between the network descriptors and to reduce the probability of a type I error, we used a Bonferroni-corrected α of 0.01 to assess the level of significance for the five network descriptors (i.e., LDq, Cq, Vq, Gq and IEq, following Tylianakis et al., 2007). This correction was used when assessing the effect of treatments on tripartite network descriptors and on bipartite (plant–aphid and aphid–parasitoid) network descriptors.
The effects of treatment on the crop phenology (Julian dates of emergences of first leaf and first ear) were examined with Mann–Whitney tests. The effects of treatment on the crop (i.e., number of seeds/ear, the seed weight/ear, the density of wheat and yield) were examined using GLM with a Gaussian family. In addition to the climatic treatments, biological interactions may also affect the crop yield (see Gagic et al., 2016). Non-crop plants are competitors for space and resources with the crop (Fahad et al., 2015). Sitobion avenae and Metopolophium dirhodum are aphid species feeding on the wheat, which may alter the yield (van Emden & Harrington, 2007). In order to examine the potential impact of uncultivated plants on the density of the wheat, a second GLM was performed with the percentage cover of non-crop plants included as a covariate. Similarly, when analysing the yield data, we included the abundance of wheat aphids S. avenae and M. dirhodum as a covariate in a separate model and compared the model fit with and without the covariates using the Akaike information criteria (AIC). In summary, we compared the AIC of the following models:
- the effect of precipitation and increased temperatures on the density of the wheat vs. the effect of precipitation, increased temperatures and percentage cover of non-crop plants on the density of the wheat;
- the effect of precipitation and increased temperatures on the crop yield vs. the effect of precipitation, increased temperatures and the abundance of wheat aphids on the crop yield.
In addition, ANOVA was performed on these two model comparisons to test whether the inclusion of covariates provided a significantly better fit to the model.
3 RESULTS
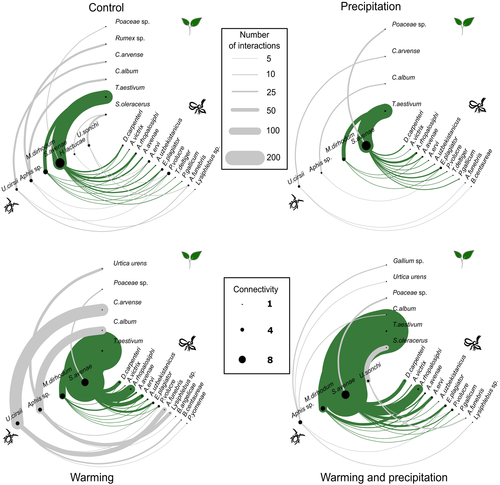
We quantified 2,836 interactions between eight plant species (six plants identified to species level, one to the genus level and one to the family level), 1,946 aphids (1,765 living aphids and 181 aphid mummies) belonging to six species, 761 primary parasitoids from 13 species and 129 secondary parasitoids from two species. Of the 129 secondary parasitoids identified, only 41 primary parasitoid–secondary parasitoid interactions were recovered. Consequently, primary and secondary parasitoids were considered as belonging to the same trophic level and separate primary parasitoid–secondary parasitoid interactions were not examined (Figure 1). Overall, the 1,946 aphids included in the ecological network analysis represented 56.3% of the total aphids counted in the experimental plots.
3.1 Plant cover and richness
We found no effect of treatment on plant species richness (GLM, warming: F = 1.577, df = 1, p = 0.223; precipitation: F = 3.09, df = 1, p = 0.093; Table 1), but climate warming significantly reduced crop percentage cover (GLM, warming: F = 11.746, df = 1, p = 0.003; precipitation: F = 1.043, df = 1, p = 0.319). The overall non-crop species cover was significantly increased in the warmed plots (GLM, warming: F = 4.78, df = 1, p = 0.04; precipitation: F = 1.519, df = 1, p = 0.231).
| Plant richness | Weed Cover | Wheat Cover | Aphid richness | Aphid abundance | Wheat aphid abundance | Parasitism rate | Multiparasitism rate | Hyperparasitism rate | Parasitoid richness | Total species richness | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2 ± 0.632 | 20.5 ± 4.806 | 77.167 ± 4.215 | 2.667 ± 1.033 | 46.167 ± 19.682 | 31.167 ± 7.494 | 0.339 ± 0.077 | 0.037 ± 0.038 | 0.082 ± 0.072 | 8.333 ± 2.16 | 12.833 ± 3.488 |
| Precipitation | 1.667 ± 0.816 | 19 ± 4.195 | 78.833 ± 1.602 | 2.167 ± 0.753 | 59.667 ± 55.479 | 36 ± 13.387 | 0.347 ± 0.111 | 0.042 ± 0.028 | 0.063 ± 0.053 | 7.833 ± 1.472 | 11.667 ± 2.066 |
| Warming | 2.667 ± 1.033 | 33.333 ± 16.525 | 62.5 ± 14.053 | 2.833 ± 0.753 | 183.333 ± 109.485 | 86.333 ± 49.443 | 0.39 ± 0.093 | 0.041 ± 0.014 | 0.067 ± 0.033 | 7.833 ± 1.941 | 13.333 ± 3.077 |
| W+P | 1.833 ± 0.753 | 24.5 ± 10.635 | 68.333 ± 10.801 | 2.667 ± 0.816 | 286.167 ± 267.655 | 97.167 ± 55.315 | 0.362 ± 0.067 | 0.031 ± 0.023 | 0.084 ± 0.044 | 5.5 ± 0.548 | 10 ± 1.265 |
| ↑P F-value | 3.09 | 1.519 | 1.043 | 0.966 | 0.079 | 0.082 | 0.044 | 4.247 | 4.413 | ||
| ↑P p-value | 0.093 | 0.231 | 0.319 | 0.337 | <0.001 | 0.609 | 0.782 | 0.777 | 0.948 | 0.052 | 0.048 |
| ↑T° F-value | 1.577 | 4.78 | 11.746 | 0.966 | 0.851 | 0.091 | 0.02 | 4.247 | 0.297 | ||
| ↑T° p-value | 0.223 | 0.04 | 0.003 | 0.337 | <0.001 | 0.009 | 0.367 | 0.766 | 0.889 | 0.052 | 0.592 |
Notes
- Significant effects at α of 0.05 are in bold. W+P: warming and precipitation; ↑P: increase in precipitation; ↑T°: increase in temperature.
3.2 Aphid abundance and parasitism rates
We found no effect of treatment on aphid species richness, but climate warming resulted in significant aphid outbreaks (GLM, df = 1, p < 0.001; Table 1), with four times as many aphids in the warmed plots compared to control plots. The abundance of the wheat aphids S. avenae and M. dirhodum doubled as a result of warming (GLM, df = 1, p = 0.009; Figure 1, Table 1). Molecular analyses revealed high rates of parasitism (based on parasitoid detection within aphids and mummies sampled; mean 36 ± 1.7%) compared to the conventional “mummy” collection/rearing method (based solely only on mummies sampled; mean 9.9 ± 1.8%). Climate warming did not significantly change parasitoid species richness, although we did detect a trend (GLM, df = 1, F = 4.247, p = 0.052). There were neither significant effects of treatment on parasitism rates nor multiparasitism (two primary parasitoids within a single aphid) and hyperparasitism rates (aphids parasitized by secondary parasitoids), which were relatively low across the treatments (3.77 ± 0.01% and 7.38 ± 0.01%, respectively; Table 1).
3.3 Tripartite ecological network structure
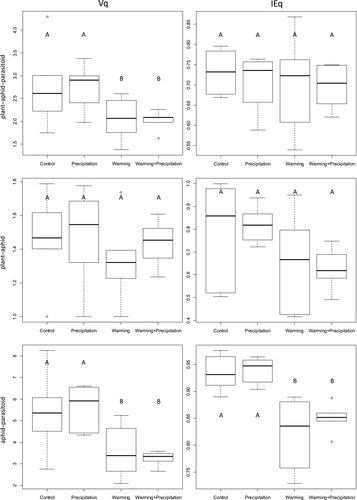
We found a significant effect of climate warming on total species richness across trophic levels (Table 1, Figure 1). Precipitation did not affect quantitative tripartite network descriptors Vq (GLM, F = 0.003, df = 1, p = 0.959), IEq (GLM, F = 0.274, df = 1, p = 0.606; Figure 2), LDq, Cq and Gq (Table 2). Simulated climate warming did not affect qualitative network descriptors (Supporting Information Table S1); however, it did significantly decrease quantitative tripartite Vq (GLM, F = 10.063, df = 1, p = 0.005; Figure 2) and LDq, but did not affect Cq, Gq (Table 2) and IEq (GLM, F = 0.362, df = 1, p = 0.554; Figure 2). Within the 24 networks, both aphids and parasitoids never consumed more than three different species from the lower trophic level (Figure 1).
| Plant–aphid–parasitoid networks | Plant–aphid networks | Aphid–parasitoid networks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LDq | Cq | Gq | LDq | Cq | Gq | LDq | Cq | Gq | |
| Control | 1.945 ± 0.452 | 0.157 ± 0.045 | 1.142 ± 0.1 | 1.284 ± 0.175 | 0.297 ± 0.085 | 1.111 ± 0.183 | 3.305 ± 0.828 | 0.317 ± 0.095 | 1.231 ± 0.163 |
| Precipitation | 1.978 ± 0.22 | 0.175 ± 0.039 | 1.197 ± 0.201 | 1.322 ± 0.197 | 0.380 ± 0.104 | 1.164 ± 0.234 | 3.445 ± 0.504 | 0.354 ± 0.072 | 1.266 ± 0.174 |
| Warming | 1.593 ± 0.208 | 0.125 ± 0.035 | 1.131 ± 0.093 | 1.223 ± 0.116 | 0.245 ± 0.096 | 1.113 ± 0.123 | 2.369 ± 0.579 | 0.228 ± 0.056 | 1.170 ± 0.124 |
| Warming + precipitation | 1.589 ± 0.136 | 0.162 ± 0.028 | 1.159 ± 0.128 | 1.251 ± 0.11 | 0.306 ± 0.094 | 1.066 ± 0.148 | 2.307 ± 0.221 | 0.285 ± 0.042 | 1.361 ± 0.185 |
| ↑P F-value | 0.015 | 2.789 | 0.474 | 0.239 | 3.018 | 0.002 | 0.024 | 2.409 | 2.373 |
| ↑P p-value | 0.905 | 0.11 | 0.499 | 0.63 | 0.097 | 0.966 | 0.878 | 0.136 | 0.138 |
| ↑T° F-value | 9.168 | 1.859 | 0.161 | 0.944 | 2.284 | 0.37 | 16.985 | 6.724 | 0.053 |
| ↑T° p-value | 0.006 | 0.187 | 0.693 | 0.342 | 0.146 | 0.549 | <0.001 | 0.017 | 0.821 |
Notes
- Significant effects at a Bonferroni-corrected α of 0.001 are in bold. SD: standard deviation; W+P: warming and precipitation; ↑P: increase in precipitation; ↑T°: increase in temperature.
3.4 Plant–aphid bipartite network structure
Precipitation did not affect plant–aphid quantitative network descriptors Vq (GLM, F = 0.425, df = 1, p = 0.522), IEq (GLM, F = 0.0001, df = 1, p = 0.991; Figure 2), LDq, Cq and Gq (Table 2). Likewise, climate warming did not affect plant–aphid quantitative network descriptors Vq (GLM, F = 0.753, df = 1, p = 0.395), IEq (GLM, F = 5.574, df = 1, p < 0.029; Figure 2), LDq, Cq and Gq (Table 2).
3.5 Aphid–parasitoid ecological network structure
Precipitation did not affect aphid–parasitoid quantitative network descriptors Vq (GLM, F = 0.005, df = 1, p = 0.944), IEq (GLM, F = 1.091, df = 1, p = 0.308; Figure 2), LDq, Cq and Gq (Table 2). However, climate warming significantly decreased aphid–parasitoid quantitative network descriptors Vq (GLM, F = 18.456, df = 1, p < 0.001) and LDq, but did not affect Cq and Gq. Climate warming negatively affected aphid–parasitoid IEq (GLM, F = 37.599, df = 1, p < 0.0001). This suggests that higher trophic interactions are more sensitive in our system and was likely caused by an increase in the frequency of interactions between wheat aphids and two primary parasitoid species: Aphidius rhopalosiphi and Aphidius ervi.
3.6 Wheat phenology
First leaves emerged three days earlier in the warmed plots (Mann–Whitney, W = 118, p = 0.002). First ears emerged eleven days earlier on average in the warmed plots (Mann–Whitney, W = 144, p < 0.001). Precipitation affected neither the emergence of the first leaves (Mann–Whitney, W = 63.5, p = 0.586) nor the first ears (Mann–Whitney, W = 72, p = 1).
3.7 Crop yield
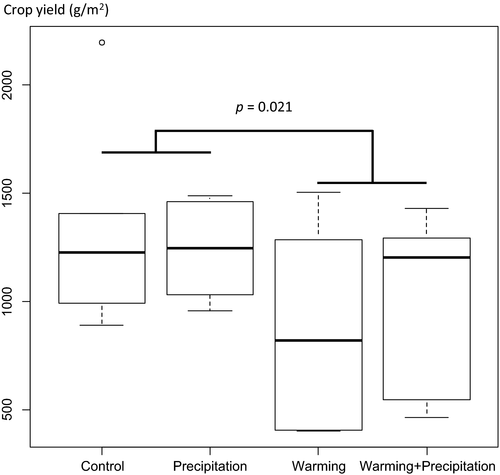
Climate warming significantly reduced the seed number (GLM, F = 4.272, df = 1, p = 0.041, Table 3) and the seed weight (GLM, F = 3.049, df = 1, p = 0.012; Table 3) but not the density of the wheat (GLM, F = 2.109 df = 1; p = 0.161; Table 3), resulting in no overall reduction in crop yield (GLM, F = 3.835, df = 1, p = 0.064; Table 3). However, when including the detrimental effect of non-crop plants or wheat aphid abundance in the models, we found a significant decrease in wheat density (GLM, F = 11.606, df = 1, p = 0.003; Table 4) and crop yield (GLM, F = 6.33, p = 0.021, df = 1; Figure 3; Table 4). Yield loss was not compensated by increased rainfall (GLM, F = 0.066, df = 1; p = 0.8; Table 4). These models, which including wheat aphid abundances and non-crop cover, provided a significantly better fit to the data than the models considering experimental treatment alone (ANOVA; wheat density: F = 95.582, p < 0.001; crop yield: F = 14.663, df = 1, p = 0.001) (Table 5).
| Number of seeds | Seed weight/ear (g) | Density of wheat | Yield (g/m2) | |
|---|---|---|---|---|
| Control mean ± SE | 46.67 ± 1.72 | 2.46 ± 0.11 | 534.7 ± 57.5 | 1323.7 ± 192.4 |
| Precipitation mean ± SE | 44.5 ± 2.18 | 2.37 ± 0.12 | 519.3 ± 27.5 | 1239.3 ± 97 |
| Warming mean ± SE | 40.77 ± 1.7 | 2.1 ± 0.11 | 401.3 ± 82.4 | 872.5 ± 209.7 |
| W+P mean ± SE | 42.7 ± 1.81 | 2.14 ± 0.12 | 478.7 ± 61.5 | 1024.8 ± 167.7 |
| ↑Precipitation F-value | 0.0039 | 0.0166 | 0.2677 | 0.0399 |
| ↑Precipitation p-value | 0.9502 | 0.8509 | 0.6103 | 0.8435 |
| ↑Warming F-value | 4.2722 | 3.0485 | 2.1088 | 3.8351 |
| ↑Warming p-value | 0.0410 | 0.0119 | 0.1612 | 0.0636 |
| df | Density of wheat | Crop yield (g/m2) | |||
|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | ||
| Warming | 1 | 11.6064 | 0.002797 | 6.3304 | 0.020518 |
| Precipitation | 1 | 1.4736 | 0.238918 | 0.0659 | 0.799986 |
| Non-crop cover | 1 | 95.5818 | <0.0001 | Not included in this GLM | |
| Wheat aphid abundance | 1 | Not included in this GLM | 14.6634 | 0.001048 | |
| AIC GLM1: Treatments only | AIC GLM2: Treatments with covariate | |
|---|---|---|
| Density of wheat | 312.3637 | 272.2618 |
| Crop yield (g/m2) | 362.4188 | 351.2199 |
4 DISCUSSION
We provide the first experimental evidence, to our knowledge, of the impacts of climate warming on the structure of tripartite ecological networks, constructed using a DNA barcoding approach. Experimental warming altered total species richness across trophic levels (but not plant and aphid species richness, respectively), and it significantly reduced crop percentage cover and substantially increased aphid abundance (the abundance of the economically important aphids S. avenae and M. dirhodum doubled as a result of warming). This affected quantitative network structure and complexity, including aphid–parasitoid interaction evenness. Molecular analyses revealed much higher rates of parasitism compared to traditional rearing/identification methods, with generally fewer natural predator species in the warmed plots. However, there were neither significant effects of treatment on parasitism rates nor multiparasitism and hyperparasitism rates. Thus, in the short term at least, natural pest control (assessed here using a molecular approach to determine parasitism rate) provided by parasitoids appears unaffected, although studies of aphid and parasitoid population dynamics over the long term are needed. Overall, we show that wheat grown 1.4°C above ambient temperature produced significantly fewer and lighter seeds resulting in a reduction in crop yield, with the best fitting model including aphid abundance and non-crop cover as covariates. We found no statistically significant effect of increased rainwater on any of our response variables, despite it being a very low rainfall season.
4.1 Study limitations
Despite the advances made by our study, there are important limitations to our experiment. First, the 4-m2 plots sampled are not directly comparable to a large cereal crop field. Our results may instead reflect how agricultural communities at field edges respond to climate change. However, because insect herbivory and parasitism rate are edge-dependent (Maron & Crone, 2006; Reeve & Cronin, 2010), our results might nevertheless be indicative of the direction of change for plant and animal populations and communities at larger spatial scales as a result of climate warming, although more research is necessary. Although all simulated climate warming methods have limitations (de Sassi et al., 2012), they are nevertheless one of the few tools available in empirically testing how ecosystems response to climate change and provide much-needed data for predictive network models (Staniczenko et al., 2017). In the future, complementary approaches including large-scale field experiments and small-scale mesocosms or laboratory experiments (see Romo & Tylianakis, 2013) might give a more comprehensive view of the ecosystem response to climate change. Second, we did not control non-crop plant or insect communities as we wished to quantify how they interact. Thus, conventionally managed cereal crops are likely to have responded differently to the experimental treatments. Third, we only examined the effects of treatment over a single growing season. Further temporal replicates would determine whether the response observed is year-dependent and the extent of interaction turnover (Kemp, Evans, Augustyn, & Ellis, 2017). Fourth, we did not consider other organisms potentially affecting the plant–aphid–parasitoid networks, such as ants interacting with aphids or predators consuming both aphids and parasitoids (Barton & Ives, 2014; Raso et al., 2014; Traugott, Bell, Raso, Sint, & Symondson, 2011). Future studies should examine a more exhaustive range of species interactions (e.g., following Pocock et al., 2012; Evans, Pocock, & Memmott, 2013), which are increasingly possible using the molecular approaches described here and/or next-generation sequencing technologies (Kitson et al., 2018). Fifth, we could not disentangle whether the insect responses were mainly due to foraging decisions of organisms (i.e., dispersal between the plots) or their demographic response to climatic manipulation (i.e., treatments affecting population growth rates), although it is likely that the observed aphid species responses were demographic. Further experimental manipulations at larger spatial scales (and including other important factors such as elevated CO2) are urgently required as well as more detailed observations of host–parasitoid and other predator–prey interactions, although this would need to involve much larger controlled enclosures than are currently available. Finally, we conducted a relatively exhausting sampling where all aphid mummies and more than 50% of aphids were collected for further molecular analyses. Such intensive sampling may certainly affect the aphid and parasitoid population dynamics at the plot level and could have potentially affected our results. However, adequate network analysis is very dependent on sampling completeness (Blüthgen, Menzel, & Blüthgen, 2006; Jordano, 2016; Rivera-Hutinel, Bustamante, Marín, & Medel, 2012). Consequently, such intensive sampling is well established in studying host–parasitoid interactions (see Traugott et al., 2008) and ecological networks more generally (e.g., Macfadyen et al., 2009) and was therefore necessary for the purposes of this study. Assessing the effect of climate change on aphid and parasitoid dynamics, while also a major issue, would then require a different experimental design.
4.2 Trophic level to network-level responses
When considering each trophic level separately, climate warming promoted weed growth (especially Chenopodium album and Cirsium arvense), which increased competition with the crop and contributed to a reduction in crop percentage cover. At the second trophic level, there was a fourfold increase in aphid abundance in the warmed plots, as we predicted, mostly driven by aphids associated with T. aestivum. At the third trophic level, contrary to our predictions, parasitism rates remained unchanged. However, a decrease in aphid–parasitoid interaction evenness in the warmed plots suggests that climate warming might benefit some parasitoid species at the expense of others. While both the reduction in crop yield and the aphid pest outbreak followed the general patterns observed in other recent studies (Bebber et al., 2014; Dong et al., 2013; Liu et al., 2016; Maxmen, 2013), the significant effects on network structure observed in this study provide new insights into how climate warming affects entire communities of interacting species. First, we found evidence that climate warming affects tripartite consumer–prey asymmetries, with significantly lower network vulnerability and linkage density. Second, connectance was not affected, most likely due to the high trophic specialization for both aphids and associated parasitoid wasps (Derocles et al., 2014; Le Ralec et al., 2011). Third, although there was no effect of treatment on tripartite interaction evenness, climate warming negatively affected bipartite aphid–parasitoid interaction evenness, suggesting that higher trophic interactions might be more sensitive in our system. Indeed, changes in tripartite network structure are essentially driven by aphid–parasitoid interactions: plant–aphid networks were not affected by simulated warming while aphid–parasitoid linkage density and vulnerability decreased. Overall, our results support the findings from de Sassi et al. (2012) showing that climate warming may have bottom-up effects (on host density and body size) which can in turn affect the structure of host–parasitoid networks.
4.3 Parasitism
We found no effect of climate warming on the parasitism rate and species richness of parasitoid wasps (although precipitation and warming treatment tended to decrease parasitoid richness), which are intimately linked to the ecosystem service of natural pest control (Derocles et al., 2014; Traugott et al., 2008). In Northern European agricultural habitats, the most abundant parasitoid species appear more specialized, with reduced attack rates on alternative hosts (Derocles et al., 2014). Macfadyen et al. (2009) showed significant differences in network structure between organic and conventional farms with more species at three trophic levels (plant, herbivore and parasitoid) on organic farms. Despite herbivores on organic farms being attacked by more parasitoid species, differences in network structure did not affect parasitism rate across a variety of host species. In our study, climate warming mainly influenced two parasitoid species, A. rhopalosiphi (the main natural enemy of Sitobion avenae) and A. ervi, driving a decrease in aphid–parasitoid interaction evenness. These species differ in their trophic specialization: A. rhopalosiphi is a specialist and A. ervi is a generalist (Kavallieratos et al., 2004; Starý, 2006). Previous work by Le Lann et al. (2014) under laboratory conditions showed a decrease in the attack rate of A. rhopalosiphi on S. avenae as a result of warming, whereas aphid defence rate increased. Under more realistic field-based scenarios, which include a greater range of interacting species, we found the opposite effect. This not only suggests that the degree of specialization may not necessarily explain which species will be more adaptable to environmental changes (as hypothesized by Rand & Tscharntke, 2007; Tylianakis et al., 2008; Jeffs & Lewis, 2013) but that other factors, such as changes in apparent competition (Morris, Lewis, & Godfray, 2004), might be important considerations within a food web context. Overall, an accurate assessment of natural pest control cannot be undertaken by the single measure of parasitism rate, but would require a careful examination of host and parasitoid population dynamics through further study and a different experimental design. These results, together with a recent study by Berthe et al. (2015) at the same study site that showed significant increases in Coleoptera activity densities but a reduction in community diversity as a result of climate warming, demonstrate the short-term impact of climate warming on higher trophic levels (i.e., predators and parasitoids) in particular. Given the potential top-down effects driven by these organisms, we expect that climate warming will result in long-term changes to the structure of the ecological network and consequently in natural pest control. Thus long-term climate-manipulation studies across spatial scales are necessary to better understand the effects of environmental change on agricultural plant–aphid–parasitoid interactions and the ecosystem service of natural pest control (Cardinale, Harvey, Gross, & Ives, 2003; Macfadyen, Craze, Polaszek, van Achterberg, & Memmott, 2011; Peralta, Frost, Rand, Didham, & Tylianakis, 2014; Tylianakis, Tscharntke, & Klein, 2006).
4.4 Impacts of climate warming on crop yields within an ecological network context
We found significant effects of climate warming not only on ecological network structure, but also on crop yield. Experimental warming has been shown to advance flowering and fruiting phenology for a range of plant species (Dong et al., 2013; Hovenden, Wills, Schoor, Williams, & Newton, 2008; Sherry et al., 2007), and in our study, first wheat leaves emerged three days earlier on average and ears emerged at least a week earlier in the warmed plots. Wheat grown under experimental warming produced fewer and lighter grain, resulting in a significant impact on crop yield. There was no significant effect of increased rainwater, which was perhaps surprising given the study was conducted during a very low rainfall season. The yield data from the experiment are nevertheless not directly comparable with commercial agricultural wheat yields. It should be emphasized that it was not our intention to simulate conventional farming methods, where routine spraying would have removed most of the weed species within our plots. Rather, we wished to study the community-wide response of interacting species across trophic levels. In this context, the reduction in crop yield was primarily driven by a combination of the wheat producing fewer, smaller grain as well as increased competition with weed species, rather than significant insect damage (aphid load was very low: 0.14 ± 0.09 aphids per wheat ear across all plots). Despite this, warming did trigger a fourfold increase in aphid abundance and this is likely to cause significant damage to crops in years when fluctuating aphid numbers are higher. As demonstrated recently by Gagic et al. (2016), attacks by several pests can have both positive and negative impacts on crop yield. In our study, crop yield models produced a better fit when pest aphid abundance was included as a covariate. However, it still remains unclear how crop (yield in particular) and non-crop plants are affected both directly and indirectly by changes in aphid–parasitoid interactions. By pioneering new molecular methods to construct highly resolved species interaction networks, we have provided new, cost-effective tools to examine the response of communities of interacting agricultural species to environmental change.
4.5 Merging DNA-based methods with ecological network analysis
Merging molecular methods and ecological network analysis (ENA) provides new tools for understanding ecology and evolution (Raimundo, Guimarães, & Evans, 2018). Here, we showed that the detection rate of aphid parasitism was more than three times higher using molecular assays than by conventional insect-rearing approaches. Plant–aphid–parasitoid networks, constructed using molecular methods, were more highly resolved than traditional rearing methods, with significant implications for host–parasitoid network-level analyses (Condon et al., 2014; Hrček & Godfray, 2015). Traditional approaches based on insect rearing and morphological identification would have failed to detect changes in species interactions mediated by the increase in temperature. Indeed, such approaches rely on the collection of aphid mummies and rearing adults for identification. In our study, only 181 aphid mummies were collected, while 709 parasitoids were detected and identified within their living aphid hosts. Parasitism cases using molecular methods were therefore able to capture a more exhaustively range of host–parasitoid interactions. These parasitism cases however still need to be considered cautiously: parasitoid eggs or larvae detected do not always achieve their development to the adult stage (Starý, 1989). Consequently, molecular tools could potentially overestimate parasitism (Traugott et al., 2008). Moreover, aphids collected on the same plant were placed together in a single tube. This may lead to a potential risk of contamination between aphids with parasitoid DNA. But this risk is low because in the same tubes we found both unparasitized and parasitized aphids (from different parasitoid species). Completely eliminating this risk would require each collected aphid to be separately stored but might also result in unrealistic, time-consuming sampling protocols.
In summary, this study provides the first evidence, to our knowledge, of the impact of climate change on farmland tripartite ecological networks, ecosystem services and agricultural output. In the short term, we highlight the potential winners (i.e., pests) and losers (i.e., pest natural enemies) of agroecosystems in a warmer world. Overall, our study provides insights into the potential threat of global warming on both farmland biodiversity and food production. Despite limited changes to biodiversity per se, climate warming affects the frequency of interactions between species, ultimately affecting network structure, although the long-term consequences of altered network structure on ecosystem functioning warrant further study. The detrimental impact of climate warming on wheat suggests the need for adapting future agricultural methods of cropping in response to a climate change (Asseng et al., 2013), cropping methods which in turn can also have cascading effects on agroecosystems and their networks of interactions. Considering the effects of environmental changes on ecological networks in dynamic models rather than snapshots of communities is essential (Säterberg, Sellman, & Ebenman, 2013) as well as taking into account a more complete range of interactions (i.e., “networks of ecological networks”; Pocock et al., 2012; Evans et al., 2013). Future studies should consider the combined effects of climate warming and elevated CO2 as the latter also affects wheat growth and grain yield in particular (O'Leary et al., 2015). Such changes in plants may also induce bottom-up effects on high trophic levels (e.g., pest arthropods feeding on the crop). Finally, increased rainwater did not affect the ecological networks and the crop yield in the present study, suggesting that extra water (either as increased precipitation or irrigation) might not mitigate the effects of increased temperature. Further considerations are nevertheless needed to understand the predicted changes in rainfall on agroecosystems. Consequently, future climate change experiments need to simulate more realistic climate change scenarios and consider increases in temperatures, precipitation and CO2 combined. A more exhaustive examination of climate change consequences on agricultural ecosystems through a combined approach using ENA and DNA-based methods is the fundamental first step to predict the impact of global changes on food production.
ACKNOWLEDGEMENTS
The project was funded by the University of Hull, with support from the Higher Education Innovation Fund (UK). We thank staff at Stockbridge Technology Centre for hosting the experiment and for additional help and support. We are grateful to Mike Dennett, Vic Swetez, Aifionn Evans, Stephen P. Moss, Jane Bunting and Lindsey Atkinson (all University of Hull, UK) and Bruce A. Kimball (Arid-Land Agricultural Research Center, USDA, Agricultural Research Service, USA) for their in help in setting up the experiment. We thank James J.N. Kitson (Newcastle University, UK) for his help with the Hive plots. We are grateful to David A. Bohan (INRA, Agroécologie, Dijon, France) for carefully reading and commenting on the manuscript.
AUTHOR CONTRIBUTION
D.M.E. designed the project. S.A.P.D. and S.C.F.B. performed the field sampling. S.A.P.D. and D.H.L. developed the molecular methodology. S.A.P.D., S.C.F.B. and P.C.N. performed the molecular work in the laboratory. E.D.M. processed the crop yield data. S.A.P.D. and D.M.E. analysed the data. S.A.P.D. and D.M.E. wrote the first draft of the manuscript. All authors contributed substantially to revisions.
DATA ACCESSIBILITY
DNA sequences were assigned GenBank Accession nos: MF154009–MF154409. Plot-level plant, insect and network data are available on Dryad: https://doi.org/10.5061/dryad.80vd7q6.




