Transcriptomic basis for reinforcement of elm antiherbivore defence mediated by insect egg deposition
Funding information
This work was supported by a grant from the German Research Foundation (DFG) within the Collaborative Research Centre 973 “Priming and Memory of Organismic Responses to Stress,” project B1
Abstract
Plant responses to insect egg depositions are known to shape subsequent defensive responses to larvae hatching from the eggs. Elm (Ulmus minor) leaves, on which elm leaf beetles laid their eggs, mount a more efficient defence against larvae hatching from the eggs. However, the molecular mechanisms of this egg-mediated, improved defence are insufficiently understood and have so far only been studied in annual plants. We analysed the dynamics of transcriptomic changes in larval feeding-damaged elm leaves with and without prior egg deposition using de novo assembled RNA-seq data. Compared to egg-free leaves, egg deposition-treated leaves showed earlier and/or faster transcriptional regulations, as well as slightly enhanced differential transcriptional regulation after the onset of larval feeding. These early responding transcripts were overrepresented in gene ontology terms associated with post-translational protein modification, signalling and stress (defence) responses. We found evidence of transcriptional memory in initially egg deposition-induced transcripts whose differential expression was reset prior to larval hatching, but was more rapidly induced again by subsequent larval feeding. This potential memory effect of prior egg deposition, as well as the earlier/faster and enhanced feeding-induced differential regulation of transcripts in egg deposition-treated leaves, may contribute to the egg-mediated reinforcing effect on the elm's defence against larvae. Hence, our study shows that a plant's experience of a stress-indicating environmental cue (here: insect eggs) can push the dynamics of the plant's transcriptomic response to subsequent stress (here: larval feeding). Such experience-mediated acceleration of a stress-induced plant response may result in improved stress resistance.
1 INTRODUCTION
Defoliation of trees by herbivorous insects poses a major threat to many forest ecosystems (Dajoz, 2000; Rullan-Silva, Olthoff, Delgado de la Mata, & Pajares-Alonso, 2013). Trees have evolved a wide range of strategies to cope with insect herbivory, thus limiting and surviving the attack. In addition to their constitutive defence, trees can activate a broad set of inducible defence responses to insect attack. Herbivory on trees is well known to induce changes in leaf morphology and chemistry (Eyles, Bonello, Ganley, & Mohammed, 2009; Haukioja, 1990; Karban & Baldwin, 1997; Schaller, 2008; Wu & Baldwin, 2010). Furthermore, trees significantly change their transcriptome when mounting wound-induced defences (Philippe, Ralph, Mansfield, & Bohlmann, 2010; Ralph, Jancsik, & Bohlmann, 2007; Ralph et al., 2006). Herbivore-inducible defences offer cost-effectiveness and great flexibility in unpredictable environments (Cipollini, Purrington, & Bergelson, 2003; Zangerl, 2003). However, a plant relying on infestation-inducible defence is especially vulnerable to the onset of larval feeding damage because in this early phase of attack, an inducible defence is not yet fully mounted (Frost, Mescher, Carlson, & De Moraes, 2008; Frost, Mescher, Dervinis, et al., 2008; Karban, 2011).
A plant's response to an environmental cue that precedes and reliably indicates impending herbivory can help a plant to cope with the drawback of induced defence, that is, the delay between being attacked and mounting a defence (Conrath, Beckers, Langenbach, & Jaskiewicz, 2015; Hilker et al., 2016; Martinez-Medina et al., 2016; Reimer-Michalski & Conrath, 2016). Both herbaceous plants and trees can respond to wound-induced volatiles from neighbouring plants or to insect egg depositions. Plants responding to these cues mobilize a more efficient defence against the herbivore, often resulting in reduced feeding damage and/or higher mortality among the herbivores (Arimura, Shiojiri, & Karban, 2010; Baldwin, Halitschke, Paschold, von Dahl, & Preston, 2006; Beyaert et al., 2012; Dicke, Agrawal, & Bruin, 2003; Frost, Mescher, Dervinis, et al., 2008; Heil & Karban, 2010; Hilker & Fatouros, 2015, 2016; Karban, Yang, & Edwards, 2014).
Molecular analyses revealed that plants show transcriptional changes in response to wound-induced leaf odour (Engelberth, Contreras, Dalvi, & Engelberth, 2013; Scala, Allmann, Mirabella, Haring, & Schuurink, 2013). Additionally, prior exposure to the odour of wounded plant tissue allows plants to mobilize an earlier (Ton et al., 2007) and stronger (Ali, Sugimoto, Ramadan, & Arimura, 2013; Vos et al., 2013) induction of defence genes in response to larval feeding damage.
Plants on which insects deposited their eggs showed numerous transcriptional changes in response to this highly reliable herbivore-indicating cue (Bonnet et al., 2017; Bruessow, Gouhier-Darimont, Buchala, Metraux, & Reymond, 2010; Firtzlaff, Oberländer, Geiselhardt, Hilker, & Kunze, 2016; Little, Gouhier-Darimont, Bruessow, & Reymond, 2007; Reymond, 2013). Furthermore, plants on which eggs had previously been deposited—such as Arabidopsis, tomato and tobacco plants—showed a stronger induction of defence-related genes upon damage by feeding larvae (Bandoly, Grichnik, Hilker, & Steppuhn, 2016; Bandoly, Hilker, & Steppuhn, 2015; Kim, Tooker, Luthe, De Moraes, & Felton, 2012; Lortzing et al., 2018). The studies available so far suggest that the egg-mediated, enhanced efficiency of feeding-induced direct defence against insect larvae is linked to changes in phytohormone levels (Kim et al., 2012; Lortzing et al., 2018), increased activity of proteinase inhibitors (Bandoly et al., 2015) and to increased levels of leaf phenylpropanoid derivatives taken up by the larvae (Austel, Eilers, Meiners, & Hilker, 2016; Bandoly et al., 2015, 2016; Lortzing et al., 2018).
In addition to the above-mentioned herbaceous plant species, elm trees (Ulmus minor) also mount a more efficient defence against herbivory when having received insect egg depositions on their leaves prior to feeding damage. Elm leaf beetle larvae (Xanthogaleruca luteola) feeding upon elm leaves which received eggs of this beetle species suffer higher mortality than larvae feeding upon egg-free leaves (Austel et al., 2016). A statistical comparison of the metabolite patterns of feeding-damaged elm leaves with and without prior egg deposition revealed, in particular, differences in the levels of several phenylpropanoids (flavonoids). Among them, high levels of the phenylpropanoid derivative robinin (= kaempferol-3-O-robinoside-7-O-rhamnoside, a flavone glycoside) were shown to increase larval mortality; larvae feeding on leaves with previous egg depositions need to cope with enhanced robinin levels (Austel et al., 2016). Hence, previously egg-deposited elm leaves can more efficiently limit the abundance of a specialized herbivore due to an egg-mediated change of the feeding-induced metabolite pattern.
Little is known about how insect egg deposition changes the transcriptome of trees. We do know that the pine (Pinus sylvestris) enhances expression of terpene synthases in response to egg deposition by pine sawflies (Köpke, Beyaert, Gershenzon, Hilker, & Schmidt, 2010; Köpke et al., 2008). We also know that the elm transcriptome undergoes significant changes in response to egg deposition by the elm leaf beetle (Büchel et al., 2012). Both of these egg-induced transcriptional changes are related to indirect defence in the form of induced leaf volatile emission that attracts parasitoids that kill the eggs (Meiners & Hilker, 2000; Mumm & Hilker, 2006; Mumm, Tiemann, Varama, & Hilker, 2005).
However, we possess only a limited understanding of how prior insect egg deposition affects transcriptomic changes in a tree in response to larval feeding damage. While it is well known that insect egg deposition induces transcriptional changes in plants (Hilker & Fatouros, 2016; Reymond, 2013), the dynamics of these changes have not been studied before in the course of the entire egg incubation period until larval hatching. Neither is it known how insect egg deposition on a tree affects transcriptomic changes induced by feeding damage caused by the hatching larvae.
In this study, we addressed these gaps in the current knowledge by analysing the transcriptomic responses of U. minor to elm leaf beetle (X. luteola) egg deposition and larval feeding at multiple points in time. Our aim in this study was to elucidate the molecular footprints that are associated with the effects of insect egg deposition on the defence of a tree against subsequent larval herbivory.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
Ulmus minor trees were propagated in an in vitro shoot culture that was established from a single elm tree originating from the Berlin Dahlem region. Shoots were grown as described by Büchel et al. (2012). For rooting, the shoots were transferred to half-strength DKW medium containing 3 mg/L IBA (Fenning, Gartland, & Brasier, 1993). After 5 days, the shoots were transferred to DKW medium without any hormone. About two-thirds of the transferred shoots started developing roots after 2 weeks. The rooting culture was kept under the same growth conditions as the shoot culture. Rooted shoots were transferred to soil and kept at 23°C and 16-hr light:8-hr darkness. After 3 weeks, trees were planted to larger pots and grown in the glasshouse until they were to be used in experiments. Plants were watered three times per week and fertilized once per week (Wuxal NPK fertilizer).
2.2 Insect culturing
Eggs, larvae and adults of the elm leaf beetle (X. luteola) were collected in the surroundings of Montpellier, France, where the elm leaf beetle occurs in high abundance due to the rich availability of elm trees and appropriate abiotic environmental conditions. We kept the field-collected individuals on glasshouse-grown elm trees for 2 weeks in the laboratory at room temperature until they were to be used for plant treatments. The conditions for elm leaf beetle maintenance in the laboratory are described by Austel et al. (2016).
2.3 Plant treatments
An overview of all treatments, sampling time points and abbreviations of treatments is provided in Figure 1. All experiments were conducted in a climate chamber (25°C, 70% RH, 250 μmol m−2 s−1 PFD, 16-hr light:8-hr darkness). Experiments were performed using 13-week-old (qRT–PCR experiment) and 15- to 19-week-old (RNA-seq experiment) elm trees. To avoid positional effects, the eighth leaf—counting from the tip of the main axis—was chosen for all treatments. The harvested leaf samples (approximately 100 mg per sample) were transferred into 2-ml screw-cap tubes that contained 1 g of Zirconox beads (2.8–3.3 mm; Mühlmeier Mahltechnik, Bärnau, Germany). Samples were immediately frozen in liquid nitrogen after harvesting. All treatments were performed in triplicate (N = 3).
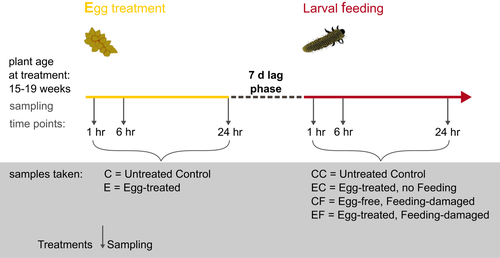
To ensure standardized treatment of the elm leaves with egg deposition, we simulated the beetle's natural egg deposition. In nature, the female beetle removes a small strip of the leaf epidermis with her mouthparts prior to egg deposition. This behaviour produces a small “scratch” in the leaf surface at the site where she will deposit her eggs in an elongated clutch. To mimic this egg deposition behaviour, we removed a 1 mm × 15 mm piece of the epidermal layer of the lower surface of an elm leaf with a scalpel. We then applied oviduct secretion that had been freshly dissected from a gravid female beetle to this “scratch.” Each leaf was treated with oviduct secretion obtained from one gravid female. The secretion functions as glue that attaches elm leaf beetle eggs to the leaf. The egg glue, but not the egg, is in direct contact with the scratched leaf surface (Hilker & Meiners, 2006). This leaf treatment—removal of a small piece of leaf epidermis plus application of the sticky oviduct secretion—has also been used by Austel et al. (2016), who showed that mimicking egg deposition in this way results in a reinforced defence against elm leaf beetle larvae. Furthermore, this leaf treatment is known to induce a so-called indirect defence involving egg parasitoids. Elm leaves subjected to this treatment emit an egg parasitoid-attracting odour, whereas “scratched” elm leaves without oviduct secretion do not (Meiners & Hilker, 2000). This study showed that simple leaf scratching triggers a different plant response than leaf scratching followed by treatment with the egg glue. In the study presented here, we were interested in how mimicking the natural egg deposition procedure, that is, leaf scratching plus application of egg glue, affects the direct plant defence against hatching larvae; leaf scratching does not occur in nature without being followed by the release of egg glue. Therefore, we chose not to treat leaves only with leaf scratching, although we expected the plant's response to leaf scratching plus application of oviduct secretion to show some overlap with a response to simple wounding. However, a pilot qPCR analysis revealed that levels of elm transcripts annotated to lipoxygenase (LOX), nonexpressor of PR genes (NPR1) and chalcone synthase (CHS) were highly variable, but not identical in “scratched only” leaves and “scratched leaves plus oviduct secretion” (N = 3 for each sample, harvested 6 hr after treatment; LOX, fold change in egg deposition-treated leaves vs. control: 2.51 ± 1.4, in “scratched only” leaves: 1.65 ± 0.3; NPR1, fold change in egg deposition-treated leaves vs. control: 5.93 ± 7.4, in “scratched only” leaves: 1.33 ± 0.3; CHS, fold change in egg deposition-treated leaves vs. control: 11.85 ± 19.0, in “scratched only” leaves: 1.08 ± 0.3). These results, as well as the aforementioned results from Meiners and Hilker (2000), indicated that we should analyse plant responses specific to egg deposition when treating leaves as described. Hence, we refer to “egg deposition-treated leaves” (E) as leaves treated by “leaf scratching” plus application of egg glue (= oviduct secretion) onto the scratch. Leaf tissue was sampled from egg deposition-treated leaves (E) around the treatment site at 1, 6 and 24 hr after the treatment. Untreated leaves (C) were sampled as control at equivalent time points (Figure 1).
Larval feeding treatments were applied to previously egg deposition-treated leaves and, for control purposes, to egg-free leaves (CF leaves: control and feeding-damaged, EF leaves: egg deposition-treated and feeding-damaged). For the feeding treatments, we placed five neonate elm leaf beetle larvae on the abaxial leaf surface 7 days after the initial egg deposition treatment (Figure 1). Leaf tissue was harvested from local, feeding-damaged leaves after removal of the larvae which had fed for 1, 6 or 24 hr upon a leaf (Figure 1). Neonate larvae on a previously egg deposition-treated tree were placed on the same leaf that had received the egg deposition treatment. We did not simulate larval feeding by mechanical damage and application of larval oral secretion because we aimed to analyse the dynamics of the transcriptomic elm responses over the course of continuous larval feeding. However, the dynamics of plant responses to continuous larval feeding damage cannot be perfectly simulated by a single artificial leaf wounding event and application of oral secretion (Bricchi et al., 2010; Lortzing et al., 2017). Furthermore, simulation of larval feeding by mechanical damage was not necessary for standardization of the extent of feeding damage because our previous studies showed no significant difference in larval feeding damage on previously egg deposition-treated and egg-free leaves (Austel et al., 2016).
Egg-free, untreated control samples (CC) for the feeding treatments were taken at equivalent time points. To control for effects of the egg deposition treatment after 7 days of egg incubation, we collected egg deposition-treated leaf samples without subsequent larval feeding treatment (EC) at the 1, 6 and 24 hr sampling time points (Figure 1).
2.4 Total RNA isolation
Frozen leaf tissue was ground three times in a Fast-Prep®-24 instrument (MP BIOMEDICALS) at a speed of 4.0 m/s for 10 s. Between the grinding steps, samples were again frozen in liquid nitrogen. Extraction of RNA from the ground material was performed in 15-ml Falcon tubes according to a phenol-based protocol modified from Ikoma et al. (1996) (for further details, see Supporting Information Appendix S1, RNA extraction method).
2.5 Library preparation and RNA sequencing
RNA integrity and quality were analysed with an Agilent 2100 Bioanalyzer on a Nano RNA 6000 chip (Agilent Technologies, Palo Alto, CA, USA) using the 2100 Expert software (version B.02.08.SI648; Agilent) at the facilities of BeGenDiv in Berlin. All samples showed an RNA integrity number (RIN) between 6.8 and 8.0.
Library preparation (Illumina TruSeq RNA Sample Prep Kit v2) and sequencing by synthesis using the Illumina HiSeq2000 (Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany) involved standard protocols from the manufacturer (Illumina, Inc., San Diego, CA, USA). Briefly, 1 μg total RNA was used for library construction. We obtained 54 libraries resulting from three biological replicates for each condition studied (Figure 1). The average insert size of the libraries was 220 ± 12 bp (Agilent 2100 Electrophoresis Bioanalyzer). Equimolar pools of six to seven libraries were quantified by qPCR and sequenced using eight lanes of one high-output run (paired-end, 2 × 100 cycles) as described by Mascher et al. (2013). The resulting sequence data can be accessed at the Gene Expression Omnibus (GEO) database under the Accession no. GSE77985.
2.6 De novo transcriptome assembly and differential expression analysis
Trimmomatic (version 0.33; compare Bolger, Lohse, & Usadel, 2014) was used to trim and filter low-quality sequenced reads. In particular, we trimmed the Illumina adapter sequences and then removed the first and last 3 bp of each read. Using a window size of 4 bp, we performed a sliding window trimming, cutting all reads starting from the 5′ end once the average quality score within the window fell below 15. Thereafter, reads with a length shorter than 50 bp were discarded. The final assembly was carried out with SOAPdenovo-Trans (Xie et al., 2014) by setting the kmer size parameter to 75 bp; kmers with a frequency no higher than two were eliminated (−d 2), minimum contig length was 200 bp (−L 200), and the length of difference allowed between the estimated and filled gap was 100 bp (−G 100). The elm leaf transcriptome assembly has been deposited as a Transcriptome Shotgun Assembly project at DDBJ/EMBL/GenBank under the Accession no. GFUU00000000.
Trimmed reads were mapped to the contigs with a length >1 kb in our assembly. Only uniquely mapped reads were retained, which resulted in an average of 49 million reads mapped per library.
Transcript expression analyses were performed with “R” (R Development Core Team, 2015) using the package DEseq2 (Love, Huber, & Anders, 2014), using its default parameters. To control for error rates of multiple testing, we adjusted the p-values using the false discovery rate (FDR) correction by Benjamini–Hochberg (Benjamini & Hochberg, 1995). Data shown in Figures 2-4 are based on significantly differentially expressed transcripts which we defined as those with a FDR ≤ 0.05 and absolute log2 fold change ≥1. These criteria provide best robustness for an analysis with a replicate number of N = 3 (see Schurch et al., 2016).

Regression analysis of the data shown in Figure 4 was carried out with R using the function lm. Function confint was used to calculate the confidence intervals for regression analysis. To compare differences between the slope parameter values of the regression, we used a nested ANOVA (anova function in R) and report the significance of the F test.
2.7 Gene ontology term enrichment analysis and pathway analysis
Gene ontology (GO) term enrichment analysis was performed using the BiNGO plugin (The Biological Networks Gene Ontology tool; Maere, Heymans, & Kuiper, 2005) of Cytoscape version 3.2.1 (Shannon et al., 2003). KEGG pathway analysis was performed using DAVID tools 6.8 (https://david.ncifcrf.gov; Huang et al., 2007). To extract putative functional annotations for the elm transcripts, we mapped the transcripts (with a length >1 kb) of our final assembly against the Arabidopsis transcriptome (TAIR10 annotation) using blast (BlastN, threshold E < 10−6). For 57.4% of transcripts from the full list of elm transcripts used for differential expression analysis, a similar sequence was described in Arabidopsis. This set of transcripts was used as the basis for our GO term enrichment and pathway analyses. Further annotations listed in the Supporting Information Tables S1–S4 are derived from Prunus persica and Malus domestica (both from Phytozome) and U. minor (from Büchel et al., 2012; elm2015 from Perdiguero, Venturas, Cervera, Gil, & Collada, 2015) based on blast queries (BlastN, threshold E < 10−6).
We identified statistically overrepresented GO terms from the “biological process” category by using a hypergeometric test and the Benjamini–Hochberg FDR correction (FDR smaller than or equal to 0.05) against the reference list containing all transcripts with a length >1 kb. Only GO terms to which more than two genes could be assigned were considered for the analyses. A heat map was built using the r package's ComplexHeatmap (Gu, Eils, & Schlesner, 2016) and circlize (Gu, Gu, Eils, Schlesner, & Brors, 2014). For clustering, we used the Euclidean distance and complete linkage method.
To functionally characterize transcripts differentially expressed in treated samples when compared to untreated controls, we subjected transcripts with a FDR ≤ 0.05 (adjusted p-values) and log2 fold change ≥1 to a GO term enrichment and pathway analysis. We compared egg deposition-treated samples without feeding damage (E samples, EC samples), egg deposition-treated samples with feeding damage (EF) and egg-free samples with feeding damage (CF) to untreated samples (C and CC samples; see Figure 1 for abbreviations). While a comparison of the expression ratios of EF samples versus controls and of CF samples versus controls revealed significant differences, the direct comparison of feeding-damaged leaves with prior egg deposition treatment versus those without prior egg deposition treatment (EF vs. CF, not normalized to untreated controls) revealed no transcripts which matched the criteria of a FDR ≤ 0.05, regardless of the fold change values. Therefore, in this specific comparison, we performed a GO term enrichment and pathway analysis of transcripts that showed a log2 fold expression difference ≥1 with a nonadjusted p-value ≤0.05 in order to detect modest transcriptional differences.
2.8 cDNA synthesis and qRT–PCR analysis
The purity and concentration of isolated RNA was determined spectrophotometrically at 230, 260 and 280 nm. The RNA (1 μg) was separated on a 1.2% agarose gel to assess the integrity of the isolated RNA. One microgram of the RNA was used for reverse transcription with the GoScript™ system (Promega, Mannheim, Germany) and according to the manufacturer's instructions, while 12.5 ng RNA was used in each 10 μl PCR. Real-time quantitative PCRs (qRT–PCRs) were performed in three technical replicates using the GoTaq® qPCR Master Mix (Promega) on a StratageneMX3005P machine with a two-step protocol (95°C for 30 s, 60°C for 60 s, 45 cycles in total) followed by a melting curve analysis (55–95°C).
Primers for the qRT–PCR were designed using Primer 3 plus (http://primer3plus.com/cgi-bin/dev/primer3plus.cgi). Each primer pair was tested for amplification efficiency and specificity, which was verified by melting curve analysis and gel electrophoresis on a 2.7% agarose gel. All primer sequences used in this study are listed in Supporting Information Appendix S1: Table S1. Details about the determination of reference genes and PCR efficiency are given in Supporting Information Appendix S1.
To determine valid reference genes for normalization of expression levels in our experimental data set, we tested different primer pairs that were based on a similarity search for published reference genes (Supporting Information Appendix S1: Table S2). This set of putative reference genes was first tested for expression stability on the different elm cDNAs, and later, a subset of them was used to determine the respective M-value for each gene according to Vandesompele et al. (2002) on the cDNA samples derived from RNA of the RNA-seq experiment. The summary of this analysis is provided in Supporting Information Appendix S1: Table S2; the expression values of SAND, UBQ and Splicing factor 3B subunit 5-like were most stable. For the quantification of transcripts with qRT–PCR, the reference gene index was calculated as the geometric mean of the expression levels of Splicing factor 3B subunit 5-like (transcript ID: scaffold20227) and UBQ (transcript ID: C579879). PCR efficiencies were determined with the program LinRegPCR (Ruijter et al., 2009). The relative expression levels of each gene were calculated according to a modified protocol of Livak and Schmittgen (2001) to determine the 2−ΔCt value for each sample and using the reference gene index for normalization.
3 RESULTS
3.1 Sequencing and de novo assembling of the elm leaf transcriptome
To gain insight into how elm leaf beetle egg deposition affects the elm's transcriptomic response to subsequently occurring larval herbivory, we sequenced the transcriptome of elm leaves subjected to different treatments: (a) simulated egg deposition (E), a treatment which allowed standardized egg deposition treatment and is known to improve the elm's defence against hatching larvae (Austel et al., 2016); (b) natural larval feeding damage, which is known to trigger a defence (CF); and (c) a simulated egg deposition treatment followed by a feeding treatment (EF). Samples were taken 1, 6 and 24 hr after the simulated egg deposition treatment and after the start of larval feeding. Control samples from untreated leaves were taken at equivalent time points (Figure 1).
We obtained 320 Gbases of raw sequence data (with an average of 59 million sequenced reads per library), with 92.6% of the bases having a sequence quality score >Q30. Our final assembly comprised 18,073 transcripts with a median size of 1.6 kb. We used the TRAPID online tool (Van Bel et al., 2013) for annotation of the transcriptome to gene families. This annotation is based on the similarity of elm sequences to those of the 25 plant species within the plaza2.5 reference database (Van Bel et al., 2012). Of the 18,073 transcripts analysed, 7,328 (40.5%) were annotated as full-length transcripts, with 74.4% of the annotated ORFs containing a start codon and 86.8% containing a stop codon. In total, 84.5% of the transcripts were assignable to a gene family within the TRAPID database.
3.2 Differential transcript expression after egg deposition treatment follows a pattern of fast and transient induction
To obtain an overview of the dynamics of transcriptional changes induced in elm leaves by egg deposition, we compared transcript levels in leaf tissue harvested at different time points after a simulated egg deposition with transcript levels in untreated leaves sampled at corresponding time points. The time points were chosen in order to examine immediate changes after treatment (1, 6 hr), changes after a short period of time (24 hr) and long-term changes detectable after the 7-day egg incubation period. Analysis of samples after the egg incubation period allowed us to check whether initial, egg-induced transcriptional changes were maintained even at time points when the larvae would have hatched.
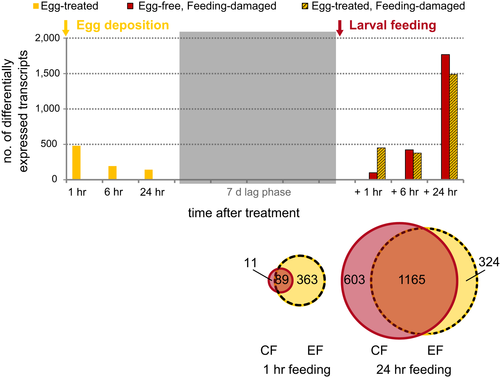
In total, 667 transcripts were significantly differentially expressed during at least one of the sampling time points after egg deposition treatment when compared to untreated leaf samples (Figure 2; Supporting Information Appendix S1: Table S3). The expression ratios range from approximately log2 fold 1–5 (absolute values, Supporting Information Appendix S2a, compare with Appendix S1: Figure S1, for data analysis disregarding any fold change cut-off). More than 90% of these transcripts were upregulated in the egg deposition-treated samples versus the untreated samples (Supporting Information Appendix S1: Table S3).
This initial transcriptional response to the egg deposition treatment rapidly declined. Only 7% of the transcripts differentially expressed 1 hr after treatment were still differentially expressed after 24 hr (Figure 2; Supporting Information Appendix S1: Table S3). Seven days after treatment, when the elm leaf beetle larvae were due to hatch, the transcriptional response to the egg deposition treatment had almost completely vanished (Supporting Information Appendix S1: Table S3; Appendix S2a): Only two transcripts were differentially expressed in the egg deposition-treated leaves (EC) at the 1-hr sampling time point, a scopoletin glucosyltransferase-like gene and another gene similar to the brassinosteroid-regulated protein, BRU1. Their potential involvement in antiherbivore defence of elm is unknown.
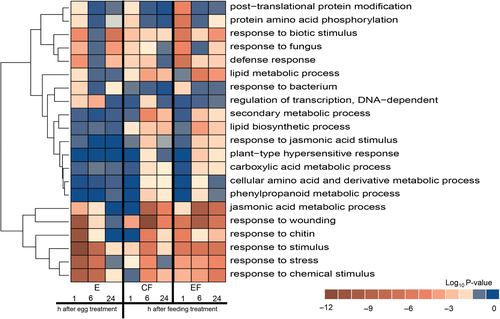
To gain a deeper understanding of the functional categories overrepresented in the egg deposition-treated samples (E), a GO term enrichment analysis for the category “biological process” was performed (Figure 3; Supporting Information Appendix S3). Overall, genes responding to egg deposition were overrepresented in GO terms associated with biotic stress responses such as “response to wounding” (GO: 9611), “response to chitin” (GO: 10200), “response to stimulus” (GO: 50896), “response to stress” (GO: 6950) and “response to chemical stimulus” (GO: 42221) when considering samples at the early time points after egg deposition treatment. Furthermore, the GO term “regulation of transcription, DNA-dependent” (GO: 6355) showed significant enrichment in the 1- and 6-hr egg deposition-treated samples (Figure 3). This category is interesting because it comprises transcripts annotated as AP2 domain/ERF, WRKY and MYB transcription factors, all of which have previously been implicated in the biotic and abiotic stress responses of plants (Buscaill & Rivas, 2014; Liu, Osbourn, & Ma, 2015; Müller & Munné-Bosch, 2015; Rushton, Somssich, Ringler, & Shen, 2010), and which also play a role in the regulation of secondary metabolism.
Hence, the egg deposition treatment induced a change in expression levels of transcripts involved in plant stress responses, but expression levels were quickly reset to the level of untreated leaves.
3.3 Previously egg deposition-treated leaves show a faster/earlier transcriptional response to larval feeding than egg-free leaves
As expected, the feeding stimulus itself elicited strong transcriptional responses in both the egg-free and previously egg deposition-treated leaves (Figure 2; Supporting Information Appendix S1: Table S3; Appendix S2b,c). The expression ratios of transcripts in feeding-damaged leaves ranged from log2 fold changes 1 to about 7 (absolute values; Supporting Information Appendix S2b; compare with Appendix S1: Figure S1 for data analysis disregarding any fold change cut-off).
To test the hypothesis that prior egg deposition affects the elm's feeding-induced transcriptome, we compared transcript expression profiles in feeding-damaged plants with and without prior egg deposition treatment (EF and CF plants, respectively) with those of untreated controls (CC plants). At the onset of larval feeding damage (1 hr), about 4.5 more transcripts were differentially expressed in the egg deposition-treated (EF) plants than in the egg-free, feeding-damaged (CF) plants. This result points to an earlier or faster response to larval feeding in egg deposition-treated plants than in egg-free plants (Figure 2). Later, after 6 and 24 hr of larval feeding, the numbers of differentially expressed transcripts were more similar in the egg deposition-treated and egg-free plants (Figure 2; Supporting Information Appendix S1: Table S3).
When comparing the log2 fold expression ratios of transcripts of feeding-damaged leaves with and without prior egg-deposition (Figure 4), we included all those transcripts in this comparison that were differentially regulated by feeding (in egg-free CF samples and/or egg deposition-treated EF samples when compared to untreated controls) during at least one of the sampling time points after onset of larval feeding (1-, 6- or 24-hr feeding-responsive transcripts). The expression ratios (CF/C:EF/C) were similar (close to 1) in egg-free and previously egg deposition-treated leaves after 6 hr (slope = 0.96) and 24 hr (slope = 0.98) of feeding damage. However, egg deposition-treated leaves exposed to 1 hr of feeding showed a slightly, but significantly stronger increase in expression ratios than egg-free leaves treated for 1 hr with larval feeding (slope = 0.67) (F test, nested ANOVA: p ≤ 2.2 × 10−16).
In summary, these results show that previously egg deposition-treated plants start responding to larval feeding damage not only with enhanced dynamics (more genes differentially expressed, Figure 2), but also with somewhat increased expression ratios (Figure 4).
3.4 Functional characterization of differentially expressed transcripts in feeding-damaged leaves with and without prior egg deposition treatment compared to controls
We further addressed the question of what functionally separates the differentially expressed transcripts in egg-free, feeding-damaged and egg deposition-treated, feeding-damaged samples. We compared these samples with control samples (EF/C and CF/C; Figure 3; Supporting Information Appendix S3). A GO enrichment analysis revealed that several terms of the category “biological process” were overrepresented to a higher significance level in the list of differentially expressed transcripts of egg deposition-treated samples (EF/C) when compared to egg-free, feeding-damaged samples (CF/C).
After 1 hr of feeding damage, these GO terms (enriched in EF samples) included especially “post-translational protein modification” (GO: 43687), “response to biotic stimulus” (GO: 9607), “response to fungus” (GO: 9620), “defence response” (GO: 6952), “jasmonic acid metabolic process” (GO: 9694), “response to chitin” (GO: 10200), “response to stimulus” (GO: 50896) and “response to stress” (GO: 6950), thus highlighting the possible function of these transcripts in pathways associated with signalling and defence (Figure 3; Supporting Information Appendix S3). A KEGG pathway analysis revealed that egg deposition-treated, 1-hr feeding-damaged plants show significant enrichment of differentially expressed transcripts in “ath04626: Plant-pathogen interaction” (Supporting Information Appendix S4).
At later time points after the onset of feeding, some of the GO terms that were more significantly enriched in egg deposition-treated, feeding-damaged plants (EF) than in the egg-free, feeding-damaged plants (CF) after 1-hr feeding became enriched to a higher significance level in the egg-free, feeding-damaged samples. This result provides further evidence for the delayed response described above for egg-free, feeding-damaged (CF) plants with respect to the number of differentially expressed transcripts. Delayed responses by egg-free plants to feeding damage are especially apparent for the GO terms “response to wounding” (GO: 9611), “response to chitin” (GO: 10200), “response to stimulus” (GO: 50896) and “response to stress” (GO: 6950) (Figure 3; Supporting Information Appendix S3).
We also addressed the question of what functionally characterizes the 603 transcripts that were differentially expressed in the egg-free (CF), but not in the previously egg deposition-treated leaves after a 24-hr feeding period (compare Figure 2). These 603 transcripts are enriched in GO terms related to translation, biosynthetic and photosynthetic processes (Supporting Information Appendix S5) and in the KEGG pathway “ath03010: Ribosome” (Supporting Information Appendix S5). The top three significantly enriched GO terms of the 324 transcripts uniquely expressed 24 hr after feeding in the previously egg deposition-treated (EF) leaves, but not in the egg-free (CF) leaves (Figure 2) are “response to water deprivation” (GO: 9414), “response to stress” (GO: 6950) and “response to water” (GO: 9415) (Supporting Information Appendix S5). These findings indicate that transcriptional regulation after 24 hr of larval feeding damage somewhat diverges in egg-free and previously egg deposition-treated elm leaves; however, both types of leaves have more than 1,000 transcripts in common, and these are differentially regulated to a similar extent and in the same direction (Figure 2; Supporting Information Appendix S2b,c).
According to this analysis, especially the enrichment of those transcripts in feeding-induced leaves with prior egg deposition treatment is interesting, which point to post-translational protein modifications, thus indicating that the egg-mediated effects on the feeding-induced transcriptome are also regulated at the post-translational level.
The RNA-seq-based expression patterns of feeding-damaged leaves with and without prior egg deposition treatment were validated by qRT–PCR measurements of the expression of a set of 15 transcripts. We chose these transcripts as representatives from GO terms which were enriched to a higher significance level 1 hr after the feeding stimulus in previously egg deposition-treated plants versus egg-free plants, namely “response to wounding” (GO: 9611), “defence response” (GO: 6952), “response to chitin” (GO: 10200) and “protein amino acid phosphorylation” (GO: 6468) (Figure 3). The samples analysed by qRT–PCR were harvested in an experiment independent of the one, which provided the samples for the RNA-seq analysis. Overall, the expression patterns measured by qRT–PCR largely corroborated the direction and expression levels detected previously in the RNA-seq analyses (Supporting Information Appendix S1: Table S4).
3.5 Functional characterization of differentially expressed transcripts in feeding-damaged leaves with and without prior egg deposition treatment compared with each other
The comparisons described above between feeding-damaged leaves with and without prior egg depositions focused on how transcripts of treated samples differed from untreated control samples (comparison EF/C and CF/C).
We also directly compared transcript levels of egg-free, feeding-damaged leaves and egg deposition-treated, feeding-damaged leaves (EF vs CF) without referring them to untreated controls. This direct comparison did not reveal differences in transcript levels with an adjusted p-value ≤0.05—regardless of the fold change threshold—at any of the analysed time points (Supporting Information Appendix S2d).
In the light of this, we then directly compared transcript levels of feeding-damaged plants with and without prior egg deposition treatment using the nonadjusted p-values ≤0.05 and a log2 fold change ≥1, in search of small biological differences. This analysis revealed differences between the two types of leaves for 223 transcripts after 1 hr of larval feeding, for 248 transcripts after 6 hr of larval feeding and for 85 transcripts after 24 hr of larval feeding (Supporting Information Appendix S2d, transcripts marked green or red; when considering these differences with a nonadjusted p-value ≤0.05 and no fold change cut-off, compare with Supporting Information Appendix S2d, transcripts marked light green or light red).
To gain insights in the biological function of the transcripts that differ in this direct comparison of egg deposition-treated, feeding-damaged leaves and egg-free, feeding-damaged leaves (log2 fold change ≥1 and a nonadjusted p-value of 0.05), we ran a GO term enrichment and pathway analysis. We detected no significantly enriched KEGG pathways or GO terms in the sets of transcripts of 1- and 24-hr feeding-damaged leaves (Supporting Information Appendices S4 and S6). However, the comparative analysis of transcripts of previously egg deposition-treated leaves and egg-free leaves after 6 hr of feeding damage identified enrichments in GO terms related to, among others, “cell wall organization” (GO: 71554) and “phenylpropanoid metabolic and biosynthetic processes” (GOs: 9698, 9699), including the suberin biosynthetic process (GO: 10345) and the “lignin metabolic process” (GO: 9808) (Supporting Information Appendix S6). A KEGG pathway analysis revealed significant overrepresentation of transcripts in the “ath00073: Cutin, suberin and wax biosynthesis” pathway after a 6-hr feeding period in egg deposition-treated, feeding-damaged leaves when directly compared with egg-free, feeding-damaged leaves (Supporting Information Appendix S4). These findings indicate enhanced activity in processes relating to cell wall structure and hardening.
Hence, the direct comparison of the feeding-induced transcriptome of leaves with and without prior egg deposition treatment revealed especially a differential enrichment of transcripts involved in cell wall organization and the phenylpropanoid pathway.
3.6 Profiling the role of early egg-induced transcription during later feeding challenges
As described above, only two of the transcripts that responded to the egg deposition treatment were differentially regulated by the end of the egg incubation and just prior to the onset of feeding damage. Hence, the higher number of differentially regulated transcripts at the onset of feeding damage was not due to maintained high levels of egg-responsive transcripts.
However, the previously egg deposition-treated leaves resumed differential expression of a considerable subset of these transcripts upon feeding damage. At the onset of feeding (1 hr) upon previously egg deposition-treated leaves, about 42% of the total 452 differentially regulated transcripts in egg deposition-treated, feeding-damaged leaves had also been differentially regulated by just the egg deposition treatment. This percentage fell to 18% after 24 hr of larval feeding upon previously egg deposition-treated plants. Thus, the longer the larvae fed upon previously egg deposition-treated leaves, the fewer transcripts that had also responded to the oviposition treatment were differentially regulated (compare with Supporting Information Appendix S1: Table S3).
We then asked what functionally characterizes the egg-responsive transcripts that resumed differential regulation after 1 hr of feeding. We subjected the transcripts that were found to be differentially regulated both in “only egg deposition-treated” (E samples) and in the egg deposition-treated, feeding-damaged (EF) samples after 1 hr of feeding damage to a GO term analysis. The analysis showed a similar distribution of GO terms in this set of transcripts (in total 189) as in the whole set of 452 transcripts differentially expressed after 1 hr of feeding on previously egg deposition-treated leaves: 75% of the top 20 GO terms of these two sets of transcripts overlapped (Supporting Information Appendix S3). However, only a few overrepresented GO terms were detected for those transcripts that were differentially regulated in egg deposition-treated, 1-hr feeding-damaged leaves, but that had not previously responded to the oviposition treatment (452−189, i.e., 263 transcripts). These GO terms essentially belonged to two groups, namely “protein amino acid phosphorylation” (GO: 6468, 21 transcripts) and “cell death” (GO: 8219, 7 transcripts), the latter of which were comprised almost exclusively of NB-LRR disease resistance proteins (Supporting Information Appendix S3).
4 DISCUSSION
Several studies have shown that a plant's experience of abiotic or biotic stress can affect the plant's transcriptomic responses to a subsequent, related stress (Avramova, 2015; Conrath et al., 2015; Crisp, Ganguly, Eichten, Borevitz, & Pogson, 2016). These effects are beneficial for a plant when they result in improved and more efficient responses to the subsequent stress (Hilker et al., 2016; Martinez-Medina et al., 2016).
While the dynamics of plant transcriptomic responses to subsequently occurring environmental stimuli have been well studied in annual model plant species (Ding et al., 2013, 2014; Voelckel & Baldwin, 2004), knowledge about the transcriptomic responses of tree species to subsequently occurring (stressful) environmental stimuli remains scarce (Frost, Mescher, Dervinis, et al., 2008). Here, we investigated the dynamics of transcriptomic changes of elm leaves to two naturally occurring, successive biotic stimuli, that is, insect egg deposition followed by feeding of the hatching insect larvae. Our results show that insect egg deposition upon a plant clearly affects the dynamics and somewhat the intensity of the plant's transcriptomic responses to the onset of larval feeding damage.
4.1 The rapidly discontinued response of the elm transcriptome to insect egg deposition differs from the response of other plant species to insect eggs
Considering the dynamics of the elm's transcriptomic responses to insect egg deposition, the majority of transcriptional changes occurred very soon after the oviposition treatment. Similarly, Arabidopsis thaliana changes gene expression of several defence-related genes very quickly (within 3 hr) in response to treatment with egg extracts of the butterfly Pieris brassicae (Gouhier-Darimont, Schmiesing, Bonnet, Lassueur, & Reymond, 2013).
However, while the elm transcriptomic response to elm leaf beetle oviposition treatment was transient and very quickly returned to the control level, other plant species maintain differential expression of a high number of egg-induced genes for several days (Brassicaceae: Bonnet et al., 2017; Firtzlaff et al., 2016; Little et al., 2007; Solanaceae: Geuss, Stelzer, Lortzing, & Steppuhn, 2017; Kim et al., 2012).
The dynamics of plant responses to insect eggs does not only vary among plant species, but may also depend on the insect species and its mode of egg deposition. While egg deposition by the elm leaf beetle and the treatment mimicking its natural egg laying are associated with leaf epidermal wounding, egg deposition by butterflies and moths does not involve such leaf wounding. Nevertheless, wound-inducible genes were not only found to respond to elm leaf beetle egg deposition (compare, e.g., GO terms 9611 or 9694), but also to egg depositions by lepidopteran species (Geuss et al., 2017; Little et al., 2007). These findings suggest that both wounding-associated insect egg deposition (by the elm leaf beetle) and wound-free egg deposition (by lepidopteran species) can affect the expression of sets of genes that show some overlap with respect to gene type, but which obviously differ with respect to the dynamics of expression.
A previous study by Büchel et al. (2012) on elm transcriptomic responses to elm leaf beetle eggs found several similar differentially expressed transcripts to those detected in our study. Their analysis revealed egg-responsive transcripts that were also assignable to the JA pathway (e.g., lipoxygenases), SA-mediated defence (e.g., PR proteins) and stress-responsive transcription factors (e.g., ethylene responsive transcription factor). However, Büchel et al. used a slightly different elm leaf beetle egg deposition treatment and harvested elm leaf samples at different time points (3, 48 and 72 hr) after treatment. They found most of the differentially expressed transcripts in leaves that received egg deposition and simultaneous feeding by the adult female beetles (EF samples in the Büchel study). Only 6.5% of the 667 egg-responsive transcripts detected in our study had a matching transcript (based on blast search) in the libraries of egg deposition-treated elm leaf material in the Büchel et al. (2012) study. We did not compare the transcriptomic data obtained from feeding-damaged elm leaves in our study against those of the Büchel et al. (2012) study because we analysed leaves damaged by larvae, whereas Büchel et al. studied leaves damaged by adults.
Consequently, the data available so far suggest that the plant genes determined as insect egg-responsive do not only depend on the plant and insect species, but also greatly depend on the sampling time point and the dynamics of response.
4.2 Rapid, but small plant transcriptomic changes mediated by insect egg deposition may exert significant ecological effects on feeding-induced plant defence
In general, efficient plant responses to stress are expected to require rapid transcriptional reprogramming of thousands of genes (Moore, Loake, & Spoel, 2011). However, our study indicates that an oviposition-mediated early boost in changes of expression levels of a small subset of feeding-induced genes can contribute to a more effective defence against hatching larvae. Similarly, insect larvae performed worse on previously egg extract-treated black mustard plants (B. nigra), in which <100 feeding-induced genes were differentially expressed when compared to feeding-induced plants without prior egg extract treatment (Bonnet et al., 2017). It is currently an open question whether treatment of B. nigra leaves with insect eggs also mediates an earlier and/or faster regulation of feeding-responsive genes (as detected in elms). Nevertheless, this example corroborates that egg-mediated transcriptional changes of a small subset of feeding-induced genes may result in significant ecological effects.
In addition to the number of differentially expressed genes, the time point of expression changes also impact on the ecological effects. So far, we do not know why prior egg deposition boosted the expression of feeding-induced genes only at the onset of damage, but not later. One explanation for this could be that the later feeding-induced transcriptional response of plants without prior oviposition treatment manages to catch up with the response of previously egg deposition-treated plants. Furthermore, we cannot exclude the possibility that larval feeding activity lasting for longer than 1 hr actively suppresses the egg-mediated, enhanced differential expression of elm transcripts observed at the onset of larval feeding. Several studies have shown that insect larvae can alter wound-induced plant defences by releasing secretions or bacteria into the plant wound. Depending on the plant and insect species studied, these releases were shown to either suppress or enhance plant defences (reviewed by Basu, Varsani, & Louis, 2018). However, a hypothetical suppression of the egg-mediated, enhanced differential expression of elm transcripts by elm leaf beetle larvae obviously does not negate the ecological effect of prior egg deposition, that is, the greater mortality of elm leaf beetle larvae feeding upon previously egg deposition-treated leaves (Austel et al., 2016).
Early expression of signalling and defence genes is known to significantly contribute to the effectiveness of plant defences against biotic stress. For example, earlier induction of defence-related genes in response to feeding damage has also been found in maize plants primed for improved antiherbivore defence by volatiles released from damaged neighbouring plants (Ton et al., 2007). The relevance of timely transcriptional changes for preparing stress responses is also well known for plant defence responses to pathogens (Ingle et al., 2015; Tao et al., 2003). However, prior to this study, egg deposition had not been shown to prompt earlier induction of defence genes upon feeding damage.
A further question is whether an early transcriptomic change can explain an ecological effect that is detectable only several days later. While prior egg deposition on elm leaves mediates an earlier and/or faster, and slightly stronger transcriptomic response to the onset of feeding damage, the ecological effects of prior egg deposition on larvae become evident only in the course of larval development; elm leaf beetle larvae suffer significantly higher mortality only after an 8-day feeding period, but not earlier (Austel et al., 2016). We suggest that the differences observed in the dynamics of the transcriptomic response of egg-free and egg deposition-treated elm leaves at the beginning of the feeding damage might cause slight differences in the leaf metabolome at later time points in the course of continuous larval feeding.
In addition to the number of genes responding to a stress and the dynamics of their expression, also the type of the stress-responsive genes matters with respect to the ecological effects. The enrichment of elm transcripts from GO categories related to cell wall organization processes and the phenylpropanoid pathway in egg deposition-treated leaves indicates subsequent changes in cell wall quality and phenylpropanoid patterns. Indeed, the metabolite pattern of egg deposition-treated and egg-free leaves has been shown to differ especially with respect to quantities of phenylpropanoid derivatives (among them the flavonoid robinin) after an 8-day larval feeding period (Austel et al., 2016). The finding that prior egg deposition results in enhanced levels of phenylpropanoid derivatives in feeding-induced leaves has also been shown in tobacco plants infested by moth eggs and larvae (Bandoly et al., 2015; in this case, caffeoylputrescine) and in A. thaliana infested with butterfly eggs and larvae (Lortzing et al., 2018; kaempferol derivatives).
Hence, even though an early stress-indicating environmental cue (here: insect egg deposition) modifies the transcriptional dynamics and expression levels of just a small subset of stress-responsive genes, this modification may be relevant to successfully counteract the stress.
4.3 Our results suggest that parts of the plant transcriptome can “remember” insect egg deposition and are primed for earlier/faster expression upon feeding damage
The egg-mediated reinforcement of the elm's defence against hatching larvae (Austel et al., 2016) may be due to either the early egg-induced changes in expression or to the egg-mediated, feeding-induced transcriptomic changes (priming) or to both types of changes.
Our study showed that the egg-induced transcriptome was reset in the end of the egg incubation time to the level of untreated leaves. Nevertheless, theoretically, the early egg-induced transcriptomic changes might have caused changes in primary and secondary leaf metabolite patterns, which persisted during the egg incubation time until larval hatching. If so, the neonate larvae had to cope with egg-induced changes in leaf nutritional quality. So far, no significant changes of elm leaf metabolite profiles have been detected in response to egg deposition at the time point examined (Austel et al., 2016). Future studies will need to elucidate the dynamics of the elm leaf metabolome in response to egg deposition.
Furthermore, the egg-mediated shift of the plant's transcriptome to earlier (and/or faster) differential regulation of a few hundreds of genes upon larval feeding damage may form a transcriptional basis for the reinforced defence of egg-experienced elm leaves towards feeding by elm leaf beetle larvae. In the light of the fact that the higher number of differentially expressed transcripts in feeding-damaged, previously egg deposition-treated leaves is not due to maintained egg-induced differential regulation of genes, our analysis suggests instead that (a) egg-responsive genes are also inducible by feeding damage and are highly responsive to the beginning of larval feeding and (b) feeding-responsive genes are induced earlier or faster in leaves that had a prior oviposition treatment. Our observation of an earlier or faster induction of a subset of transcripts in response to larval feeding, but only in plants that had been exposed to prior egg deposition, offers evidence for the concept of priming of inducible defence (Hilker et al., 2016; Martinez-Medina et al., 2016). We hypothesize that prior egg deposition shifts the transcriptome of elm leaves to a “primed state” in which it is sensitized to respond earlier or faster to larval feeding damage.
Transcriptional stress memory implies that transcript levels of a given locus display an altered response after a recurring stress stimulus that follows a recovery phase (Ding et al., 2013, 2014). Our data indicate that the elm has a transcriptional memory of an environmental stimulus, the egg deposition. Among the elm transcripts differentially expressed at the onset of feeding damage in egg deposition-treated samples, a subset (189 out of 452 transcripts) had initially responded to the egg deposition stimulus but did not show differential expression by the end of the egg incubation period. This subset of transcripts could be said to show “transcriptional memory,” as defined by Avramova (2015). Potential candidates for memory loci in the elm might include egg- and feeding-responsive WRKY transcription factors, which were differentially expressed in all types of treated samples. WRKY transcription factors, well known as key regulators of plant stress responses, have been shown to be among the genes with enhanced activation in plants primed for improved resistance against phytopathogens (Jaskiewicz, Conrath, & Peterhänsel, 2011). Furthermore, memory-conferring signalling components may accumulate after the priming stimulus and await re-activation by, for instance, phosphorylation upon a subsequent stress (Beckers et al., 2009; Galis, Gaquerel, Pandey, & Baldwin, 2009). Future studies will need to further elucidate the regulation of transcription of the signalling- and defence-related elm genes that are involved in the egg-mediated reinforcement of the elm's defence against leaf beetle larvae.
Taken together, our study suggests that a plant remembers its transient transcriptional response to insect egg deposition when insect larvae start feeding and that this transcriptional memory contributes to a reinforced response to larval herbivory.
5 CONCLUSION
Our analysis reveals that the dynamics of the larval feeding-induced transcriptome of a tree species is shaped by prior insect egg deposition on its leaves. Our data provide for the first time evidence for a more rapid, slightly enhanced induction of defence-related genes in feeding-damaged leaves that have received an egg deposition stimulus prior to larval feeding. Furthermore, our results hint at transcriptional memory in a set of feeding-induced genes after prior insect egg deposition. To gain a deeper understanding of the impact of the dynamics of plant transcriptome changes on the efficiency of plant defence against insects, more plant species with different life strategies (e.g., annual, perennial) and plants at different age need to be analysed with respect to their responses to insect egg deposition and larval feeding. Finally, our study underlines that not just transcriptional response intensity, but also response dynamics may shape a plant's resistance to subsequently occurring environmental stress.
ACKNOWLEDGEMENTS
We thank Professor Dr. Didier Morin, University Bordeaux, France, for collecting elm leaf beetles in France. Furthermore, we thank the technicians Gabriele Haberberger and Ute Braun, Freie Universität (FU) Berlin, for their help in growing the plants and elm leaf beetle rearing, as well as Sandra Drießlein and Ines Walde (Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany) for their assistance with the sequencing library preparation. Additional thanks are due to Leonhard Schmidt, a student helper at FU Berlin, for his assistance in conducting the RNA-seq study. We are grateful to the team from the Berlin Center for Genomics in Biodiversity Research (BeGenDiv), Berlin, and especially to Kirsten Richter, for valuable technical support.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization was carried out by S.A. and M.H.; methodology was carried out by S.A., J.M.M., R.B., A.H. and L.A.; investigation was conducted by S.A. and J.M.M.; original draft was written by S.A. and M.H.; further writing and editing were carried out by S.A., M.H., J.M.M., V.L., R.B., A.H. and L.A.; funding acquisition was led by M.H.
DATA ACCESSIBILITY
The data set is available at the Gene Expression Omnibus (GEO) database under the Accession no. GSE77985. The final elm leaf transcriptome assembly has been deposited as a Transcriptome Shotgun Assembly project at DDBJ/EMBL/GenBank under the Accession no. GFUU00000000. The version described in this paper is the first version, GFUU01000000.




