Delimiting species of marine gastropods (Turridae, Conoidea) using RAD sequencing in an integrative taxonomy framework
Abstract
Species delimitation in poorly known and diverse taxa is usually performed based on monolocus, DNA-barcoding-like approaches, while multilocus data are often used to test alternative species hypotheses in well-studied groups. We combined both approaches to delimit species in the Xenuroturris/Iotyrris complex, a group of venomous marine gastropods from the Indo-Pacific. First, COI sequences were analysed using three methods of species delimitation to propose primary species hypotheses. Second, RAD sequencing data were also obtained and a maximum-likelihood phylogenetic tree produced. We tested the impact of the level of missing data on the robustness of the phylogenetic tree obtained with the RAD-seq data. Alternative species partitions revealed with the COI data set were also tested using the RAD-seq data and the Bayes factor species delimitation method. The congruence between the species hypotheses proposed with the mitochondrial nuclear data sets, together with the morphological variability of the shell and the radula and the distribution pattern, was used to turn the primary species hypotheses into secondary species hypotheses. Allopatric primary species hypotheses defined with the COI gene were interpreted to correspond to intraspecific structure. Most of the species are found sympatrically in the Philippines, and only one is confidently identified as a new species and described as Iotyrris conotaxis n. sp. The results obtained demonstrate the efficiency of the combined monolocus/multilocus approach to delimit species.
1 INTRODUCTION
The last decade has seen a burst of methods available to propose species hypotheses based on molecular data (Carstens, Pelletier, Reid, & Satler, 2013; Ence & Carstens, 2011; Fujisawa & Barraclough, 2013; Leaché, Fujita, Minin, & Bouckaert, 2014; Leavitt, Moreau, & Lumbsch, 2015; Puillandre, Lambert, Brouillet, & Achaz, 2012; Yang & Rannala, 2014; Zhang, Kapli, Pavlidis, & Stamatakis, 2013). Genetic data collection for species delimitation can be separated into two general approaches: (a) DNA barcoding, in which a high number of species/specimens are sequenced for one or a few markers, and (b) deep analyses of species complexes, where a limited number of species/specimens are analysed with larger genetic data sets (ranging from 5–6 markers to several thousand markers). These two approaches lend themselves to particular classes of methods. For example, species delimitation methods based on monolocus data, such as GMYC (General MixedYuleCoalescent method; Fujisawa & Barraclough, 2013; Monaghan et al., 2009; Pons et al., 2006), PTP (PoissonTree Process; Zhang et al., 2013) or ABGD (Automatic Barcode Gap Discovery; Puillandre, Lambert, et al., 2012) are typically applied to taxa with no or limited genomic data available (i.e., understudied and/or hyperdiverse groups). For such groups, sequencing many markers for many specimens and species can be problematic. For example, there may be financial constraints that limit genetic sequencing (but see e.g., Coissac, Hollingsworth, Lavergne, & Taberlet, 2016), but also because the lack of genomic information makes the identification of suitable markers for the species level difficult (but see e.g., Rutschmann, Detering, Simon, Fredslund, & Monaghan, 2017). Here, DNA-barcoding-like data can be used as a starting point to propose primary species hypotheses, in groups where such hypotheses do not exist or are highly questionable. Conversely, species delimitation methods based on multilocus data, such as SPEDESTEM (SPEcies Delimitation Using the Species Tree Estimation Method), BPP (Bayesian Phylogenetics and Phylogeography) or BFD (Bayes Factor Delimitation) (Ence & Carstens, 2011; Leaché et al., 2014; Yang & Rannala, 2014), are used to test alternative models of species delimitation. These methods are, generally, used for recently diverged lineages for which the speciation process may or may not be completely finalized (the “grey zone”—De Queiroz, 2007). Finally, the dichotomy between genetic sampling approaches can also be thought of in divergence times. For example, DNA-barcoding approaches are better suited for systems containing old lineages, where genotypes and phenotypes are distinct and have become fixed between lineages. In contrast, more recent lineages will suffer from contradictions between gene trees and species trees because not enough time has passed for lineages to completely sort. These systems will require finer methods based on population genetics concepts (Pante, Puillandre, et al., 2015).
RAD sequencing (restriction site-associated DNA markers) (Baird et al., 2008; Miller, Dunham, Amores, Cresko, & Johnson, 2006) is one relatively new approach that can be used to overcome the problem of collecting large multilocus genetic data sets in groups with traditionally poor genetic resources (Boucher, Casazza, Szövényi, & Conti, 2016; Herrera & Shank, 2016; Pante, Abdelkrim, et al., 2015). Here, we use a combination of DNA-barcoding-like approaches and RAD-seq data to delimit species. We initially apply DNA barcoding to define primary hypotheses of species delimitation and use RAD-seq data to verify and test the species hypotheses, in a poorly known group of marine gastropods, the Xenuroturris/Iotyrris complex. These species belong to the family Turridae, superfamily Conoidea, a hyperdiverse group of marine gastropods which developed a powerful venom apparatus to produce highly potent toxins used to capture their prey. The “turrids” are famous among malacologists for their highly variable shells, and this variability does not always coincide with species boundaries estimated using genetic data (e.g., Fedosov, Stahlschmidt, Puillandre, Aznar-Cormano, & Bouchet, 2017; Puillandre, Cruaud, & Kantor, 2010; Puillandre, Fedosov, Zaharias, Aznar-Cormano, & Kantor, 2017; Puillandre, Sysoev, Olivera, Couloux, & Bouchet, 2010). Several hypotheses (retention of ancestral polymorphism, convergence, phenotypic plasticity) have been proposed to explain how highly similar shells can actually correspond to species that diverged more than 60MYA or, conversely, how morphological variation among populations within species can exceed variation estimated at the species level (Duda, Bolin, Meyer, & Kohn, 2008; Puillandre, Baylac, Boisselier, Cruaud, & Samadi, 2009; Puillandre et al., 2011). In the Xenuroturris/Iotyrris complex, Kantor, Puillandre, Olivera, and Bouchet (2008) found that species with almost identical shells were easily distinguished with two genetic markers (COI—Cytochrome c Oxidase subunit I—and 28S) and by two very distinct radulae (i.e., teeth structure in gastropods). However, up until this study, only eight specimens spanning a small fraction of the known geographic area of this group (Vanuatu) were available.
The objective of the study is to revise the species delimitation in the Xenuroturris/Iotyrris complex using an increased number of specimens from a large geographic range, combining (a) the barcode fragment of the COI gene analysed using several species delimitation methods (ABGD, GMYC, mPTP) in order to propose primary species hypotheses (PSH); (b) a genomewide RAD sequencing approach, a method adapted to nonmodel organisms (Kess, Gross, Harper, & Boulding, 2015), analysed through tree reconstruction (using IQ-tree) and using the BFD species delimitation methods to test the alternative partitions of PSH proposed with the COI gene; and (c) morphological, anatomical and geographic data to propose secondary species hypotheses (SSH) in an integrative taxonomy context, where species, defined as definitely diverging lineages, are considered as hypotheses engaged in a process of validation or modification (Barberousse & Samadi, 2010; De Queiroz, 2007).
2 MATERIALS AND METHODS
2.1 Material
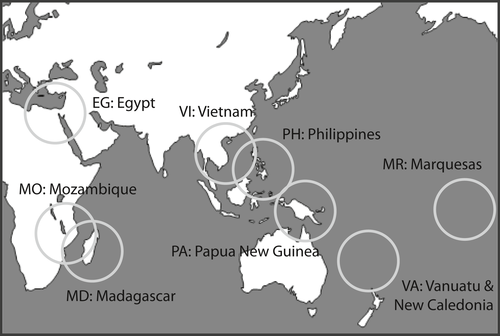
Specimens belonging to the Xenuroturris/Iotyrris species complex were collected during several field expeditions organized by the MNHN (Muséum National d'Histoire Naturelle): “Santo 2006” in Vanuatu, “Terrasses” in New Caledonia, “Inhaca 2011” and “Mainbaza” in Mozambique, “Atimo Vatae” in Madagascar, “Papua Niugini” in Papua New Guinea and “Pakaihi I Te Moana” in the Marquesas Islands); by joined Russian-Vietnamese tropical centre (Vietnam); by the University of Utah in collaboration with the University of the Philippines (Philippines); and by P. Stahlschmidt in Egypt (Figure 1). Specimens were routinely collected with use of SCUBA equipment, almost exclusively during night dives. Specimens were removed from the shell by using an isotonic solution of the magnesium chloride until relaxed (before 2012) or processed with the use of microwave (Galindo, Puillandre, Strong, & Bouchet, 2014). As part of another project, some shells were broken to access the venom glands; in these cases, the shells were photographed first. In all cases, a tissue-clip was preserved in ethanol. Voucher shells and the body of the molluscs were kept for future morphological studies; all vouchers were deposited in the MNHN collections and specimen data and COI sequences were submitted to BOLD and GenBank (Table 1). Because the monophyly of the species complex was demonstrated previously (see e.g., Puillandre, Modica, et al., 2012), only one closely related outgroup was included (Lophiotoma jickelli, Turridae).
| No MNHN | Country | Coordinates; depth | Genus | Species | BOLD ID | Genbank # for COI | Total reads | Reads passed | Total loci | Final loci |
|---|---|---|---|---|---|---|---|---|---|---|
| MNHN-IM-2007-17686 | Vanuatu | 15°33,6′S, 167°16,6′E; 8–9 m | Iotyrris | cingulifera | CONO514-08 | EU127880 | 694,199 | 630,308 | 3,617 | 2,311 |
| MNHN-IM-2009-17246 | Madagascar | 25°02.6′S, 47°01.2′E; 45–49 m | Xenuroturris | legitima | TEMPO044-18 | MH917863 | 3,600,990 | 3,374,050 | 17,905 | 12,615 |
| MNHN-IM-2009-24927 | Mozambique | 25°59.7′S, 32°54.5′E; −1 m | Iotyrris | cingulifera | TEMPO050-18 | MH917812 | 1,293,596 | 1,223,835 | 7,515 | 4,754 |
| MNHN-IM-2009-24928 | Mozambique | 25°59.0′S, 32°54.3′E; 3–5 m | Iotyrris | devoizei | TEMPO051-18 | MH917824 | 3,259,241 | 3,039,143 | 14,371 | 9,983 |
| MNHN-IM-2009-24929 | Mozambique | 25°59.7′S, 32°54.5′E; -1 m | Iotyrris | cingulifera | TEMPO052-18 | MH917809 | 1,981,423 | 1,866,477 | 11,077 | 7,354 |
| MNHN-IM-2009-24930 | Mozambique | 25°59.7′S, 32°54.5′E; 2–5 m | Xenuroturris | legitima | TEMPO053-18 | MH917868 | 3,740,707 | 3,504,351 | 17,562 | 12,488 |
| MNHN-IM-2009-24936 | Mozambique | 25°59.7′S, 32°54.5′E; 2–5 m | Xenuroturris | legitima | TEMPO054-18 | MH917867 | 2,707,631 | 2,537,846 | 15,716 | 8,064 |
| MNHN-IM-2009-24939 | Mozambique | 26°10.9′S, 32°57.2′E; −15 m | Iotyrris | devoizei | TEMPO103-18 | MH917821 | 1,487,639 | 1,425,098 | 7,175 | 4,774 |
| MNHN-IM-2009-24940 | Mozambique | 25°59.7′S, 32°54.5′E; 2–5 m | Xenuroturris | legitima | TEMPO055-18 | MH917866 | 2,115,504 | 1,999,266 | 9,503 | 6,312 |
| MNHN-IM-2009-24949 | Mozambique | 25°58.6′S, 32°54.1′E; −15 m | Iotyrris | devoizei | TEMPO056-18 | MH917823 | 2,673,240 | 2,487,408 | 11,010 | 7,162 |
| MNHN-IM-2009-24959 | New Caledonia | 23°19′S, 168°16′E; 180–220 m | Iotyrris | olangoensis | TEMPO057-18 | MH917843 | 1,147,331 | 1,073,660 | 6,083 | 3,242 |
| MNHN-IM-2009-24987 | Philippines | 10°17′35″S, 123°55′31″E; 15–25 m | Iotyrris | olangoensis | TEMPO058-18 | MH917848 | 621,317 | 590,364 | 3,927 | 2,687 |
| MNHN-IM-2009-24988 | Philippines | 10°17′35″S, 123°55′31″E; 15–25 m | Iotyrris | olangoensis | TEMPO059-18 | MH917842 | 2,547,012 | 2,401,207 | 13,361 | 9,616 |
| MNHN-IM-2009-24990 | Philippines | 10°17′35″S, 123°55′31″E; 15–25 m | Iotyrris | olangoensis | TEMPO060-18 | MH917847 | 4,790,989 | 4,454,342 | 22,688 | 16,716 |
| MNHN-IM-2009-24992 | Philippines | 10°17′35″S, 123°55′31″E; 15–25 m | Iotyrris | olangoensis | TEMPO061-18 | MH917846 | 2,904,170 | 2,709,792 | 15,444 | 11,259 |
| MNHN-IM-2009-24993 | Philippines | 10°17′35″S, 123°55′31″E; 15–25 m | Xenuroturris | legitima | TEMPO062-18 | MH917864 | 3,125,200 | 2,957,616 | 15,195 | 10,822 |
| MNHN-IM-2009-24997 | Philippines | 10°17′35″S, 123°55′31″E; 15–25 m | Iotyrris | musivum | TEMPO063-18 | MH917838 | 2,007,246 | 1,895,232 | 11,599 | 8,662 |
| MNHN-IM-2009-26289 | Vanuatu | 15°36,1′S, 166°58,5′E; 16 m | Iotyrris | devoizei | CONO512-08 | EU127879 | 1,253,969 | 1,143,822 | 6,011 | 3,804 |
| MNHN-IM-2009-29709 | Vietnam | 12°10,443′N, 109°16,298′E; 15–22 m | Iotyrris | cingulifera | TEMPO064-18 | MH917815 | 3,884,108 | 3,659,529 | 19,469 | 13,259 |
| MNHN-IM-2009-29714 | Vietnam | 12°10,443′N, 109°16,298′E; 15–22 m | Iotyrris | conotaxis n. sp. | TEMPO065-18 | MH917853 | 616,563 | 583,635 | 3,011 | 1,718 |
| MNHN-IM-2009-29715 | Vietnam | 12°10,443′N, 109°16,298′E; 15–22 m | Iotyrris | musivum | TEMPO066-18 | MH917837 | 2,800,010 | 2,680,824 | 15,992 | 12,044 |
| MNHN-IM-2009-29719 | Vietnam | 12°10,443′N, 109°16,298′E; 15–22 m | Xenuroturris | legitima | TEMPO067-18 | MH917862 | 1,188,373 | 1,098,161 | 4,985 | 3,181 |
| MNHN-IM-2009-29720 | Vietnam | 12°10,443′N, 109°16,298′E; 15–22 m | Iotyrris | notata | TEMPO068-18 | MH917840 | 9,135,778 | 8,614,984 | 32,432 | 21,909 |
| MNHN-IM-2009-29726 | Vietnam | 12°10,443′N, 109°16,298′E; 15–22 m | Iotyrris | notata | TEMPO069-18 | MH917839 | 2,968,648 | 2,785,558 | 13,687 | 9,024 |
| MNHN-IM-2009-33530 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Xenuroturris | legitima | TEMPO070-18 | MH917861 | 1,534,833 | 1,450,158 | 8,924 | 6,261 |
| MNHN-IM-2009-33531 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Xenuroturris | legitima | TEMPO071-18 | MH917860 | 286,377 | 2,484,120 | 1,1885 | 8,193 |
| MNHN-IM-2009-33533 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Xenuroturris | legitima | TEMPO072-18 | MH917859 | 8,601,257 | 8,172,565 | 35,030 | 2,4127 |
| MNHN-IM-2009-33534 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Xenuroturris | legitima | TEMPO073-18 | MH917858 | 2,988,491 | 2,765,920 | 1,3189 | 8,959 |
| MNHN-IM-2009-33536 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO074-18 | MH917826 | 3,377,363 | 3,242,368 | 16,184 | 12,077 |
| MNHN-IM-2009-33537 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO075-18 | MH917836 | 6,041,344 | 5,631,785 | 29,218 | 19,312 |
| MNHN-IM-2009-33538 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Xenuroturris | legitima | TEMPO076-18 | MH917856 | 5,710,015 | 5,371,853 | 25,099 | 17,868 |
| MNHN-IM-2009-33539 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | conotaxis n. sp. | TEMPO077-18 | MH917849 | 3,935,930 | 3,702,928 | 21,354 | 15,466 |
| MNHN-IM-2009-33540 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO078-18 | MH917835 | 2,165,240 | 1,989,705 | 10,988 | 7,999 |
| MNHN-IM-2009-33542 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | cingulifera | TEMPO079-18 | MH917817 | 2,718,353 | 2,551,693 | 12,902 | 8,822 |
| MNHN-IM-2009-33544 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | cingulifera | TEMPO080-18 | MH917820 | 2,713,372 | 2,543,124 | 15,457 | 1,1012 |
| MNHN-IM-2009-33545 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | cingulifera | TEMPO081-18 | MH917819 | 2,260,769 | 2,101,961 | 12,753 | 9,094 |
| MNHN-IM-2009-33546 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO082-18 | MH917834 | 2,615,219 | 2,453,002 | 13,097 | 9,545 |
| MNHN-IM-2009-33548 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | conotaxis n. sp. | TEMPO083-18 | MH917852 | 2,499,420 | 2,357,567 | 15,697 | 11,702 |
| MNHN-IM-2009-33549 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Xenuroturris | legitima | TEMPO084-18 | MH917857 | 739,942 | 698,742 | 3,750 | 2,132 |
| MNHN-IM-2009-33550 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | olangoensis | TEMPO085-18 | MH917841 | 1,967,315 | 1,823,804 | 8,611 | 5,820 |
| MNHN-IM-2009-33553 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO086-18 | MH917833 | 4,597,154 | 4,372,658 | 22,254 | 16,663 |
| MNHN-IM-2009-33554 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | kingae | TEMPO087-18 | MH917825 | 5,770,810 | 5,492,875 | 26,157 | 1,8567 |
| MNHN-IM-2009-33555 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Xenuroturris | legitima | TEMPO088-18 | MH917865 | 1,800,651 | 1,703,896 | 9,132 | 6,468 |
| MNHN-IM-2009-33561 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | olangoensis | TEMPO089-18 | MH917844 | 3,110,611 | 2,931,793 | 17,218 | 12,766 |
| MNHN-IM-2009-33562 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | olangoensis | TEMPO090-18 | MH917845 | 4,898,433 | 4,678,365 | 22,680 | 1,6345 |
| MNHN-IM-2009-33563 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | cingulifera | TEMPO091-18 | MH917814 | 4,990,679 | 4,612,622 | 2,1240 | 14,610 |
| MNHN-IM-2009-33564 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | conotaxis n. sp. | TEMPO104-18 | MH917854 | 6,835,600 | 6,491,429 | 29,470 | 22,148 |
| MNHN-IM-2009-33568 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO092-18 | MH917832 | 3383,055 | 3,228,317 | 18,860 | 13,367 |
| MNHN-IM-2009-33569 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Xenuroturris | legitima | TEMPO093-18 | MH917869 | 4,451,716 | 4,094,310 | 20,965 | 14,700 |
| MNHN-IM-2009-33570 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | conotaxis n. sp. | TEMPO094-18 | MH917851 | 3,232,203 | 3,038,981 | 15,976 | 1,1442 |
| MNHN-IM-2009-33572 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO095-18 | MH917831 | 4,993,521 | 4,816,966 | 23,634 | 18,023 |
| MNHN-IM-2009-33576 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO096-18 | MH917830 | 3,326,764 | 3,195,354 | 18,611 | 13,750 |
| MNHN-IM-2009-33577 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO097-18 | MH917829 | 3,392,736 | 3,076,448 | 17,071 | 12,471 |
| MNHN-IM-2009-33578 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO098-18 | MH917828 | 2,72,092 | 26,22,579 | 15,766 | 11,940 |
| MNHN-IM-2009-33579 | Philippines | 10°17′ S, 123°55′ E; 15–20 m | Iotyrris | musivum | TEMPO099-18 | MH917827 | 1,978,175 | 1,865,570 | 1,1715 | 8,576 |
| MNHN-IM-2009-6456 | Mozambique | 26°12′S, 35°03′E; 87–90 m | Iotyrris | conotaxis n. sp. | TEMPO043-18 | MH917850 | 612,170 | 548,856 | 1,571 | 779 |
| MNHN-IM-2009-7022 | Mozambique | 25°59.0′S, 32°54.5′E | Xenuroturris | legitima | TEMPO045-18 | MH917855 | 6,486,587 | 6,148,897 | 33,000 | 19,236 |
| MNHN-IM-2009-7023 | Mozambique | 25°59.0′S, 32°54.5′E | Iotyrris | cingulifera | TEMPO046-18 | MH917813 | 3,943,003 | 3,682,059 | 21,778 | 12,034 |
| MNHN-IM-2009-7024 | Mozambique | 25°59.0′S, 32°54.5′E | Iotyrris | cingulifera | TEMPO047-18 | MH917811 | 2,651,450 | 2,500,060 | 16,263 | 6,246 |
| MNHN-IM-2009-7025 | Mozambique | 25°59.0′S, 32°54.5′E | Xenuroturris | legitima | TEMPO048-18 | MH917871 | 8,045,196 | 7,655,973 | 40,943 | 14,177 |
| MNHN-IM-2009-7081 | Mozambique | 25°59.0′S, 32°54.5′E | Xenuroturris | legitima | TEMPO049-18 | MH917870 | 11,296,176 | 10,605,392 | 52,463 | 19,298 |
| MNHN-IM-2013-14888 | Papua New Guinea | 05°11′S, 145°49,5′E; 2–10 m | Iotyrris | cingulifera | TEMPO100-18 | MH917810 | 1,841,688 | 1,720,104 | 11,264 | 7,242 |
| MNHN-IM-2013-40060 | Marquesas Islands | 09°45,67′S, 138°50,69′W; 10–25 m | Iotyrris | devoizei | TEMPO101-18 | MH917822 | 9,016,668 | 8,525,416 | 33,530 | 23,998 |
| MNHN-IM-2013-52076 | Egypt | 26°48′48″N, 33°56′54″E; 1–2 m | Iotyrris | cingulifera | TEMPO102-18 | MH917818 | 4,929,978 | 4,693,937 | 30,097 | 16,529 |
| MNHN-IM-2013-52078 | Egypt | 26°48′48″N, 33°56′54″E; 1–2 m | Iotyrris | cingulifera | TEMPO105-18 | MH917816 | 1,894,537 | 1,730,192 | 10,657 | 5,192 |
| MNHN-IM-2009-29713 | Vietnam | 12°10,443′N, 109°16,298′E; 15–22 m | Lophiotoma | jickelii | CONO1878-17 | KY570852 | 23,237,420 | 22,117,193 | 46,894 | 3,348 |
A total of 95 samples were analysed; up to 12 samples were collected per locality and per morphospecies. However, most species in this complex are quite rare, and for some morphospecies and/or locality, only one sample was available. Furthermore, the RAD sequencing was not successful for several samples (see below), thus reducing the number of samples per morphospecies and locality, but still retaining at least one sample per locality and per morphospecies (Table 1).
2.2 Sanger sequencing and primary species hypotheses
DNA was extracted using the Epmotion 5075 robot (Eppendorf), following the manufacturers’ recommendations. A fragment of the COI gene was amplified using universal primers LCO1490/HCO2198 (Folmer, Black, Hoeh, Lutz, & Vrijenhoek, 1994). PCRs were performed in 25 μl, containing 3 ng of DNA, 1× reaction buffer, 2.5 mM MgCl2, 0.26 mM dNTP, 0.3 mM of each primer, 5% DMSO, and 1.5 units of Qbiogene Q-Bio Taq. Amplification consisted of an initial denaturation step at 94°C for 4 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 50°C for COI, followed by extension at 72°C for 1 min. The final extension was at 72°C for 5 min. PCR products were purified and sequenced by the Eurofins sequencing facility (France).
Chromatograms were edited using codoncode aligner v. 3.7.1.1 (CodonCode Corporation, Dedham, MA; www.codoncode.com). Sequences were aligned using MUSCLE (Edgar, 2004), and the accuracy of the alignment was checked by eye. Maximum-likelihood (ML) analyses were performed using raxml 7.0.4 (Stamatakis, 2006), with a GAMMA model applied independently to each codon position. Accuracy of the results was assessed by bootstrapping (1,000 replicates). Bayesian analyses (BA) were performed running two parallel analyses in mrbayes (Huelsenbeck, Ronquist, & Hall, 2001), consisting each of eight Markov chains of 200,000,000 generations each with a sampling frequency of one tree each 10,000 generations. The number of swaps was set to five, and the chain temperature at 0.02. Similar to the ML approach, unlinked models (each with six substitution categories, a gamma-distributed rate variation across sites approximated in four discrete categories and a proportion of invariable sites) were applied for each partition. For both mrbayes and beast (see below) analyses, convergence was evaluated using tracer 1.6 (Rambaut & Drummond, 2014), to confirm that the ESS values were > 200. A consensus tree was then calculated after omitting first 25% trees as burn-in. raxml, mrbayes and beast (see below) analyses were performed on the Cipres Science Gateway (http:// www.phylo.org/portal2/) using the RAxML-HPC2 on TG, mrbayes on XSEDE (3.2.6) and beast on XSEDE (1.8.2) tools, respectively.
Three methods of species delimitation were applied to propose PSH: (a) ABGD (Automatic Barcode Gap Discovery; Puillandre, Lambert, et al., 2012), which automatically detects a gap in the distribution of pairwise genetic distances, making the assumption that it corresponds to a threshold between intra- and interspecific distances; (b) GMYC (General Mixed Yule Coalescent model; (Fujisawa & Barraclough, 2013; Monaghan et al., 2009; Pons et al., 2006), which tests whether branching rates along an ultrametric tree fits better with a speciation model or a coalescent model, using the transition point between speciation and coalescence to delimit species hypotheses; and (c) PTP (bayesian Poisson Tree Processes; Zhang et al., 2013), which also compares speciation and coalescent models but relies on substitution rates calculated for each nodes instead of branching rates. The web server available at http://wwwabi.snv.jussieu.fr/public/abgd/ (version of March 2017) was used for ABGD, with the default parameters. The distance matrix was computed by ABGD, using the Jukes–Cantor substitution model. beast 1.8.1 (Drummond & Rambaut, 2007) was used to obtain a relative-rates ultrametric tree for the GMYC and PTP analyses, with a relaxed lognormal clock and a coalescent prior, determined as the best-fitting parameters to be used with the GMYC model (Monaghan et al., 2009). A GTR+I+G substitution model was applied, and the Metropolis coupled Markov chains (MCMC) were run for 100,000,000 generations. GMYC (both single and multiple versions), PTP and mPTP (Kapli et al., 2016; Monaghan et al., 2009; Pons et al., 2006; Zhang et al., 2013) were run using the web servers at species.h-its.org and mptp.h-its.org (versions of March 2017), respectively, using default parameters.
2.3 RAD sequencing
Single digest RAD sequencing (Baird et al., 2008) was conducted using the restriction enzyme SbfI on 95 samples. Barcoded Illumina library preparation and sequencing was subcontracted to Eurofins. Classical libraries were constructed and single-end sequenced on two lanes of Illumina HiSeq 2000. The sequencing resulted in a total of 293 million reads. Reads were demultiplexed according to the 10 base long barcodes with the allowance of one mismatch using fastx barcode splitter from the FASTX-Toolkit suite (http://hannonlab.cshl.edu/fastx_toolkit/). Seven million reads remained unassigned. The number of reads per sample varied from 6,700 to 23 million with a mean of 2.9 million.
Reads quality was checked using FastQC (http://www.bioinformatics.babraham.ac.uk) for each sample individually. Since part of the samples indicated quality drops after 70–80 bases, the ends of reads were cleaned-up following a sliding window approach using Fastq quality trimmer also from the FASTX-Toolkit suite. Reads were cleaned from the 3’ end using a window size of five, a step size of one and an average minimum score within the window of 20. Following clean-up, reads were checked again using FastQC to make sure quality profiles were satisfying.
Usable loci were produced from raw reads using pyRAD 3.01 (Eaton, 2014). The choice of this pipeline was made according to the fact that both (a) the presence of indels (which was anticipated between more distant samples) and (b) the trimming of reads result in variable reads length which is not allowed in the more commonly used pipeline STACKS (Catchen, Hohenlohe, Bassham, Amores, & Cresko, 2013). Several combinations of parameters were used and quickly revealed that samples with <1 million reads resulted in low numbers of usable loci leading to long branches artefacts. Such samples were discarded to obtain the final data set with the 66 remaining samples, including one outgroup (Table 1). Once discarding these samples, the outputs of pyRAD were relatively stable. Among the 66 samples treated here, 46 were micro-waved to remove the mollusc from its shell. The average number of reads obtained per sample was slightly higher for the micro-waved samples (3,971,187) than for the non-micro-waved ones (3,290,728), confirming than the micro-waves do not damage the DNA. The higher number of reads for the micro-waved samples could be explained by the fact that the micro-waved samples were also more recently collected.
A minimum coverage (Mindepth) of five reads was used, as well as a clustering threshold (Wclust) of 0.89, and a minimum of two samples shared by any locus (MinCov). A maximum-likelihood tree was produced using IQ-tree (Nguyen, Schmidt, von Haeseler, & Minh, 2014). We estimated the best substitution model for each locus with ModelFinder (Kalyaanamoorthy, Minh, Wong, von Haeseler, & Jermiin, 2017) following the BIC criterion. We then applied 1000 ultrafast bootstrap (UFBoot) (Hoang, Chernomor, von Haeseler, Minh, & Le, 2017) on each data set to obtain branch support. The same tree reconstruction was also conducted only keeping one SNP per locus and resulted in a similar topology. In order to test the effect of the parameter MinCov, which has a direct impact on the level of missing data in the final outputs, we also generated data sets corresponding to data missing for up to 30, 40, 50, 60, 70 and 80% of the samples (i.e., the maximum percentage of samples not having information for a specific locus to be kept, corresponding to MinCov values of 46, 40, 33, 26, 20 and 13, respectively). For each data set, the total number of loci, the total number of SNP and the percentage of nodes in the IQ-tree showing a bootstrap value above 75 and 95 were calculated.
The RAD-seq data set was also analysed with BFD to test alternative partition of species (see below) proposed by ABGD, GMYC or PTP with the COI data set. MLE (Marginal Likelihood Estimates) for each partition of species were obtained using the implementation of BFD∗ in the SNAPP (Bryant, Bouckaert, Felsenstein, Rosenberg, & RoyChoudhury, 2012) plug-in for beast v2.5 (Bouckaert et al., 2014). Given the high number of loci and the high level of missing data, we kept only the 10% of loci with the lowest level of missing data (corresponding to 470 loci) and performed multiple runs with various number of steps (20, 50, 100 and 200) and chain length (100,000 and 500,000) for the path sampling, with a preburnin of 50,000. Bayes factors (BF) were calculated from the MLE for each model pair. We followed Grummer, Bryson, and Reeder (2013) in recognizing a 2lnBf >10 as “decisive” support in distinguishing between competing species delimitation hypotheses.
2.4 Secondary species hypotheses
The PSH proposed with ABGD, GMYC and PTP were compared with the results obtained with the RAD-seq data. Because specimens from the same species are supposed to recombine on independent loci, contrary to specimens from different species, intraspecific relationships inferred from these two data sets are expected to be different; conversely, interspecific relationships are expected to be more congruent in both COI and RAD-seq trees (Pante, Puillandre, et al., 2015). Based on this property, we looked for PSH defined with the COI gene that corresponded to clades in the phylogenetic tree obtained with the RAD-seq data (i.e., all the specimens of a COI PSH cluster together in a single clade in the RAD-seq tree), a pattern in support of the hypothesis that these PSH are actually different SSHs. We applied the integrative taxonomy flowchart described in (Puillandre, Modica, et al., 2012) to add arguments in favour of one or two SSHs, when two PSHs are compared. In particular, we analysed species distribution patterns: an overlapping distribution between two sister-PSHs would support the hypothesis that they correspond to two different SSHs; an allopatric distribution is less informative, since although it demonstrates limited gene flow between populations, it does not allow judgments on whether the distant populations are two reproductively isolated species. All the analysed specimens exhibit a multispiral protoconch, indicative of planktotrophic development (Jablonski & Lutz, 1980) and suggestive of high dispersal capabilities.
A previous study (Kantor et al., 2008) demonstrated that in Xenuroturris/Iotyrris complex, the shell shape cannot be considered as a reliable character for delimitation of the species, with intraspecific variation exceeding the interspecific one. On the contrary, the spiral sculpture as well as coloration of teleoconch appeared to be important diagnostic characters. Therefore, we paid special attention to the sculpture of the subsutural zone (subsutural ramp) and the zone of anal sinus (sinus cords) as well as colour pattern of studied species. The teleconch characters were compared to the type specimens of known species in the group to link the species hypotheses to available species names. Because the radula has been shown to be variable within this complex, several specimens per PSHs (when possible, selected from the sequenced material) were dissected to identify the radula type. Radulae were prepared by standard methods (Kantor & Puillandre, 2012) and examined by scanning electron microscope TeScan TS5130MM in the Institute of Ecology and Evolution of Russian Academy of Sciences (IEE RAS). Terminology used for radula description follows Kantor (2006).
3 RESULTS
For clarity, the species names used from here onwards are attributed to either Xenuroturris or Iotyrris following the results of the phylogenetic analysis: X. legitima Iredale, 1929, I. cingulifera (Lamarck, 1822), I. notata (Sowerby, 1889), comb. nov., I. kingae (Powell, 1964), comb. nov. I. devoizei Kantor et al., 2008; I. olangoensis (Olivera, 2002), comb. nov., I. conotaxis n. sp., I. musivum Kantor et al., 2008.
3.1 Results of the exploratory approach
When ABGD is used with default parameters, two groups corresponding to the main lineages (identified as two different genera in Kantor et al., 2008) were detected. Therefore, we analysed each genus separately with ABGD. In the vicinity of the barcode gap (i.e., ~2%–6%), ABGD consistently defines two PSH within the first lineage (Xenuroturris) and 11 PSHs in the second (Iotyrris), with both the initial and recursive approaches (Figure 2). As shown in Figure 2, species names available in the literature were attributed to the PSH through the comparison of shell morphology to the known species, including the type specimens; one PSH, consistently defined by all the methods, could not be attributed to an available name and is thus described as a new species (I. conotaxis n. sp.—see below). Within X. legitima, I. cingulifera, I. devoizei and I. conotaxis n. sp., two allopatric lineages are recognized, one in the Indo-West Pacific Ocean (IWP—Vietnam, Philippines, Vanuatu, Chesterfield and Marquesas Islands), and the second in the West-Indian Ocean (WIO—Mozambique and Madagascar). Similar partitions are also found with the single version of GMYC, the PTP and the mPTP methods (the multiple version of GMYC returns an unrealistic number of PSHs—21, a result reported previously—for example (Fujisawa & Barraclough, 2013; Kekkonen & Hebert, 2014; Talavera, Dincă, & Vila, 2013). The exceptions are the following: with mPTP, the two lineages of I. devoizei and I. kingae are grouped in a single PSH; within I. olangoensis, two specimens, the unique one from Vanuatu, and one of the Philippines specimens, are considered as one or two PSH, in addition to a PSH, consistently found by all the methods, grouping the other specimens of I. olangoensis from the Philippines. Finally, the mPTP method groups in a single PSH the two allopatric lineages within I. conotaxis n. sp. Remarkably, the two specimens from Egypt do not cluster in the same PSH: one clusters in the IWP PSH of I. cingulifera, the other in the WIO PSH of I. cingulifera with all methods.
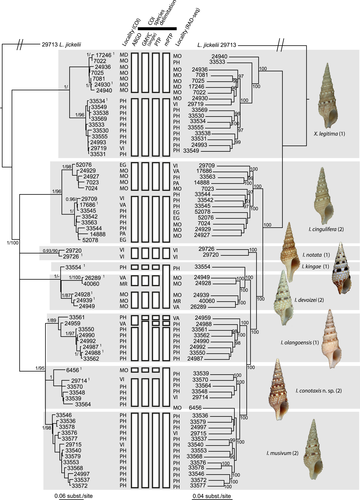
3.2 RAD-seq and secondary species hypotheses
Several sets of parameters were tested using the pipeline pyRAD for the generation of the alignment used for tree reconstructions. Unlike most other parameters tested, the parameter MinCov, corresponding to the minimum number of samples with information needed to keep a locus in the final data set, had a strong effect on the number of loci and SNP recovered from our RAD-seq data. The number of loci recovered varied from 2 to 17,060 and the number of total SNP from 22 to more than 97,393 when MinCov was changed in order to maintain 30 to 80% of the samples having information as the cut-off to keep a locus (Table 2). Nevertheless, even though adding more loci directly increases the percentage of missing data in a dramatic way, the topologies obtained were congruent with each other, when the supported nodes (Bootstraps values above 95) were considered (Figure 2, Appendix S1). Moreover, those obtained with more loci and thus more missing data were better resolved: nodes with bootstrap values more than 75 and 95 increase from 8 to 90% and from 3 to 65%, when MinCov is reduced from 46 to 13, respectively. This increase in number of supported nodes followed a linear function, with a higher correlation (R² = 0.95) for nodes with bootstraps values above 95 than for nodes with bootstraps values above 75 (R² = 0.79). This likely occurred because the number of nodes with bootstraps values above 75 reached a maximum with the data set containing 70% of missing data (92%), then decreasing to 90% with the data set containing 80% of missing data. With a MinCov value of 2, the data set included 103,060 loci after paralog removal.
| % of missing data | MinCov | # loci | # SNP | % nodes with bootstraps >75 | % nodes with bootstraps >95 |
|---|---|---|---|---|---|
| 30 | 46 | 2 | 22 | 7.93 | 3.17 |
| 40 | 40 | 39 | 251 | 28.57 | 12.7 |
| 50 | 33 | 474 | 3,415 | 80.95 | 44.44 |
| 60 | 26 | 2,639 | 17,248 | 87.30 | 50.79 |
| 70 | 20 | 7,013 | 43,828 | 92.06 | 61.9 |
| 80 | 13 | 17,060 | 97,393 | 90.48 | 65.08 |
The clades recovered with the RAD-seq data are congruent with the PSHs, or groups of PSHs, as defined with the COI (Figure 2). The PSHs X. legitima, I. cingulifera, I. notata, I. kingae, I. devoizei and I. olangoensis are found as independent lineages in the RAD-seq tree. In the more supported IQ-trees (obtained with 50 to 80% of missing data), these PSH are always recovered with high support, but the relationships within each of these PSH change from one tree to another. One exception is the clade that unites I. musivum and I. conotaxis n. sp.: these two PSHs are not always recovered because the position of the unique sample from the WIO, IM-2009-6456, constant moves from one PSH to the other. This pattern is probably artefactual, since this sample is the one with the lowest number of reads and loci. When this sample is removed, the pattern for I. musivum and I. conotaxis n. sp. is similar to the other species, with both PSH being always recovered as a fully supported clades in the IQ-trees, but with internal relationships changing from one tree to another.
The allopatric lineages within X. legitima, I. cingulifera and I. devoizei are not monophyletic in the RAD-seq tree. Furthermore, the monophyly of the WIO clade of X. legitima was not supported with the COI gene and the monophyly of the IWP clade of I. cingulifera was supported only in the Bayesian tree of the COI gene. The two specimens (MNHN-IM-2009-33561 and MNHN-IM-2009-24959) considered as separate PSH by some methods with the COI gene within I. olangoensis are embedded within the other I. olangoensis PSH in the RAD-seq tree.
For the two PSH that include allopatric lineages and sufficient number of samples, X. legitima and I. cingulifera, the BFD method was used to test the two alternative scenarios: one species with an Indo-Pacific distribution, or two allopatric (WIO vs. PO) species each. To reduce computation time, each data set was analysed separately, together with one sample (IM-2013-40060, the most complete sample in terms of reads and loci recovered) used as an outgroup. Whatever the number of steps and the chain length used for the path sampling, the MLE values recovered for the partition with only one species is always lower than for the partition with two allopatric species, with 2lnBf values all >100 (Table 3).
| Species | Number of species considered | Number of steps | Chain length | MLE |
|---|---|---|---|---|
| Iotyrris cingulifera | 1 | 20 | 100,000 | 1,188 |
| Iotyrris cingulifera | 1 | 50 | 100,000 | 889 |
| Iotyrris cingulifera | 1 | 100 | 100,000 | 559 |
| Iotyrris cingulifera | 1 | 200 | 100,000 | 771 |
| Iotyrris cingulifera | 1 | 200 | 500,000 | 1,038 |
| Iotyrris cingulifera | 2 | 20 | 100,000 | 1,336 |
| Iotyrris cingulifera | 2 | 50 | 100,000 | Infinity |
| Iotyrris cingulifera | 2 | 100 | 100,000 | 1,334 |
| Iotyrris cingulifera | 2 | 200 | 100,000 | Infinity |
| Xenuroturris legitima | 1 | 20 | 100,000 | 1,142 |
| Xenuroturris legitima | 1 | 50 | 100,000 | 1,109 |
| Xenuroturris legitima | 1 | 100 | 100,000 | 867 |
| Xenuroturris legitima | 1 | 200 | 100,000 | 535 |
| Xenuroturris legitima | 1 | 200 | 500,000 | 238 |
| Xenuroturris legitima | 2 | 20 | 100,000 | 1,498 |
| Xenuroturris legitima | 2 | 50 | 100,000 | 1,497 |
| Xenuroturris legitima | 2 | 100 | 100,000 | 1,402 |
| Xenuroturris legitima | 2 | 200 | 100,000 | 1,330 |
Given all these data, the PSHs were turned into eight SSHs, represented as grey boxes in Figure 2, as follows:
- the two allopatric lineages within X. legitima are not found in the RAD-seq data set and not supported as the best model in the BFD analysis, and they have the same radula type (see below) and similar shells: they are considered a single SSH, named X. legitima;
- the two allopatric lineages within I. cingulifera are not found in the RAD-seq data set and not supported as the best model in the BFD analysis, and they have the same radula type and similar shells: they are considered a single SSH, named I. cingulifera;
- I. notata is consistently recognized as an independent group with all data sets and methods, and the radula type and the shells are similar among studied specimens; it is considered as a SSH;
- I. kingae, although grouped with I. devoizei with mPTP, is morphologically (both shell and radula) and genetically different from I. devoizei; therefore, it is considered as an independent SSH;
- the two allopatric lineages within I. devoizei are not found in the RAD-seq data set, and they have the same radula type and similar shells: they are considered a single SSH, named I. devoizei;
- the two specimens (MNHN-IM-2009-33561 and MNHN-IM-2009-24959) that fall outside the main clade of I.olangoensis in the COI tree are not isolated with the RAD-seq data. The shell and radula are consistent among all the I. olangoensis specimens, and it is thus considered a single SSH;
- I. conotaxis n.sp. is consistently found separate from the other lineages, with both COI and RAD-seq data. Although radula type in specimens from this lineage is the same as in I. musivum, both species are genetically and morphologically (see species description below) distinct, and I. conotaxis n. sp. is considered a single SSH. The placement of the single specimen from the WIO remains uncertain, either as sister to I. conotaxis n. sp. or to I. musivum. The shell morphology of this single specimen is not conclusive as it is a subadult from deeper waters (80–90 m) compared to the IWP samples, and its morphology slightly differs from the adult shells of both I. conotaxis n. sp. and I. musivum. More specimens of this clade from the WIO are necessary to conclude.
- I. musivum is consistently found as a single PSH and is considered a single SSH.
Shell and radular morphology is congruent with the eight defined SSH. The examination of numerous specimens allowed concluding that the most informative characters of the shell are the spiral sculpture and colour pattern of the subsutural ramp and sinus cords. The subsutural ramp is the zone below the suture, which in Xenuroturris and Iotyrris is delimited by narrow but distinct abapical groove. It bears three to five cords, varying interspecifically in width and coloration: cords can have numerous dark speckles limited to cords or large subrectangular spots, that extend to the interspaces between cords (I. devoizei, I. kingae, I. olangoensis). Sinus cords are distinct elements of the spiral sculpture, originating at the posterior part of the anal sinus (Figure 4b). Originally paired, they can be subdivided by narrow longitudinal grooves. The pattern of this subdivision as well as the coloration of the cords is also important diagnostic characters. Although there is no single character that allows recognition of all studied species, each defined SSH has its own combination of characters states. The summary of diagnostically important shell characters is provided in Table 4.
| Characters/species | X. legitima | I. cingulifera | I. notata | I. kingae | I. devoizei | I. olangoensis | I. musivum | I. conotaxi |
|---|---|---|---|---|---|---|---|---|
| Subsutural ramp sculpture | 3–5 subequal cords | 3–5 subequal cords | 3 cords, central most prominent | 3 bulging cords, central very prominent | 3 bulging cords, central very prominent, rarely 2 cords | 3 cords, central most prominent | 3 cords, central most prominent | 3 cords, central most prominent |
| Subsutural ramp coloration | Background colour with speckles on cords | Background colour with speckles on cords | Background colour with speckles on cords | Large subrectangular brown spots | Large subrectangular brown spots | Large subrectangular brown spots | Background colour with speckles on cords | Speckles on cords, strong to weak brown spots |
| Sinus (=peripheral) cords morphology | Paired subequal, upper and lower ones subdivided in two in large specimens | Paired subequal, only upper one subdivided in two in large specimens | Paired subequal, sharp on top, not subdivided | Paired subequal rather weak | Paired subequal rather weak | Paired subequal rather weak, upper can be subdivided | Paired subequal, prominent, upper can be subdivided | Paired subequal, prominent, upper can be subdivided |
| Sinus (=peripheral) cords coloration | Large subrectangular brown spots and speckles on cords | Large subrectangular brown spots and very few speckles on cords (usually absent) | Large speckles on cords | Large aligned speckles on cords | Large aligned speckles on cords | Speckles on cords | Large subrectangular brown spots | Large subrectangular brown spots |
| Radular central formation | Spear-head shaped central cusp | Absent | Absent | Short blunt central cusp and indistinct lateral flaps | Broad with distinct lateral flaps and small central cusp | Narrow central cusp | Short central cusp, sometimes indistinct lateral flaps | Broad with distinct lateral flaps and small central cusp |
| Radular marginal teeth | Duplex | Semi-enrolled | Duplex | Duplex | Semi-enrolled | Duplex | Semi-enrolled | Semi-enrolled |
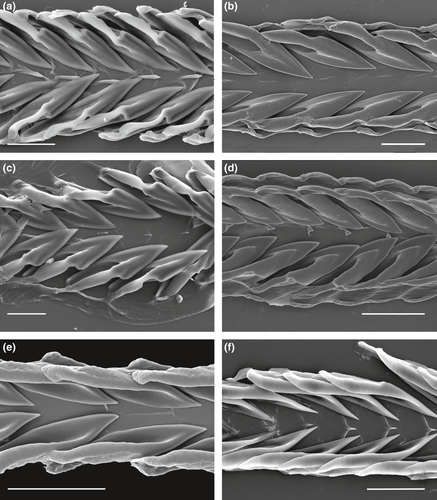
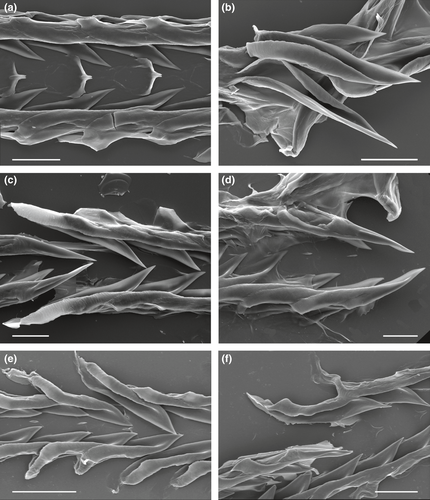
The radula in studied species of Xenuroturris/Iotyrris complex is rather variable; two major radular types can be recognized, differing in the morphology of the marginal teeth (Figure 3). In the first type, the marginal teeth have duplex shape, typical for Turridae and many other Conoidea. The anterior part of the tooth (closer to the tip), up to half of total tooth length, is solid, while the posterior part has two thickened edges, a thinner dorsal one (the accessory limb) and a thicker ventral one (the major limb) attached to the radular membrane (Figure 3a–d). The second radular type is characterized by “semi-enrolled” marginal teeth. Both edges of the marginal teeth are elevated and equally developed along the entire tooth length, and they delimit an intervening trough. The anterior solid part of the tooth is absent (Figures 3e,f; 5). This second type is unique in the family Turridae and is confined only to the genus Iotyrris. Nevertheless, we found that the radular types were not correlated with the phylogenetic relationships: X. legitima, I. notata, I. kingae and I. olangoensis have the duplex marginal radular teeth, while I. cingulifera, I. devoizei, I. conotaxis n. sp. and I. musivum have the semi-enrolled marginal teeth.
3.3 Species description
Superfamily CONOIDEA Fleming, 1822
Family TURRIDAE H. & A. Adams, 1853 (1838)
Genus Iotyrris Medinskaya & Sysoev, 2001
Iotyrris conotaxis n. sp. (Figures 4 and 5)
ZooBank registration: urn:lsid:zoobank.org:act:4FB76AA0-1519-4361-A3D9-4E47F002ABAA
Holotype: MNHN-IM-2009-33570. Paratypes, MNHN-IM-2009-33539, MNHN-IM-2009-33564, both from type locality.
Type locality: Philippines, Olango Island, off Cow-Oy, 15–20 m, collected during night dive.
DESCRIPTION (HOLOTYPE). Shell conical, consisting of 9.75 evenly convex teleoconch whorls, with high spire; shell diameter to shell height 0.34, aperture height (without canal) to shell height 0.28, spire height to shell height 0.49. Protoconch brown, partially eroded, remaining part of 4.25 convex whorls, sculptured with closely spaced axial arcuate threads, slightly prosocline, nearly orthocline on most whorls, but turning to strongly opisthocline on posteriormost part of last protoconch whorl. 26 threads on last protoconch whorl. 4–5 early teleoconch whorls nearly flat, slightly angulated at shoulder. Late teleoconch whorls convex in outline, particularly the last whorl. Last whorl sharply narrowing towards attenuated but short nearly straight siphonal canal. Suture deeply impressed, slightly wavy, nearly canaliculate due to raised subsutural cord. Subsutural ramp narrow, on last whorl with three distinct broadly spaced thin nodulose cords, with interspaces twice broader than cords. Abapical edge of subsutural ramp with very narrow but deep groove, sometimes obscured by nodulose edges of minor cords. This groove clearly seen on apertural lip, bordered by narrow cord. Two strongly raised subequal in width sinus cords, triangular in section abut subsutural ramp. Whorls portion below sinus cords sculptured with subequal narrow cords, from one on uppermost teleoconch whorls to four on penultimate whorl. On last whorl, seven subequal spiral cords below sinus before transition to canal, and 15 weaker cords on shell base and canal with narrow thread between some of them. Axial sculpture absent, except for inconspicuous growth lines. Aperture narrowly oval. Outer lip thin, evenly rounded. Anal sinus deep, U-shaped, situated on the shoulder. Inner lip slightly convex, columellar part straight, callus very narrow, not extending onto the parietal wall. Canal delimited from aperture by inconspicuous fold. Colour creamy white, with regularly spaced, light-brown spots covering cords of the entire shell surface and much more pronounced, strong, darker subrectangular spots on sinus cords. Subsutural ramp with light-brown irregularly shaped blurring spots, in some parts of shells merged, producing uniform brown subsutural ramp.
Shell height 32.8 mm, shell diameter 11.1 mm, last whorl height 16.8 mm, aperture height (without canal) 9.2 mm.
Radula was examined in three specimens: MNHN-IM-2009-29714 (Vietnam), MNHN-IM-2009-33539 and MNHN-IM-2009-33548 (Philippines) (Figure 5). It is very similar in all specimens, formed by semi-enrolled unbarbed marginal teeth, edges of the marginal teeth are elevated and equally developed along entire tooth length, and delimit a trough. The anterior solid part of the tooth is absent. The marginal teeth on both sides of the radular membrane are interlocked, so that tooth of one row is lying within the trough of a tooth of the subsequent row. Central formation is formed by a small and short central cusp and more or less developed lateral flaps.
Remarks. The species is represented in our material by six specimens, five from Olango Island, in the Philippines and one from Vietnam. Although most specimens were collected by hookah divers in the Philippines and the exact depth is not known, the usual operation depths during collecting in Olango Island is 15–20 m, while the specimen from Vietnam was collected by SCUBA at similar depths (15–22 m). The largest specimen reaches 36.7 mm in length.
All available specimens are rather similar in shell shape and coloration, most variable is the degree of development of light-brown spots on subsutural ramp, sometimes absent on most of whorl, but always present at least on some parts.
There is not a single pure diagnostic character for I. conotaxis n. sp. in the COI alignment.
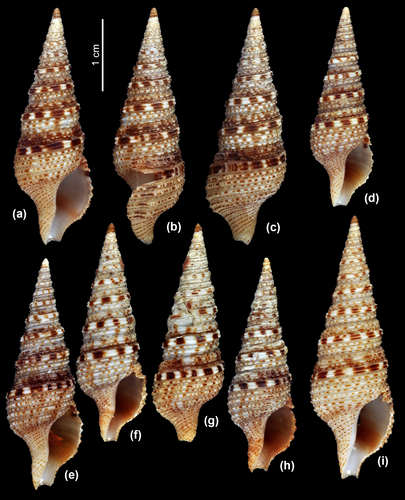
The species is most similar to the closely related Iotyrris musivum; the two species sympatric and probably syntopic in Olango Island and also occur syntopically in Nha-Trang Bay in Vietnam (Figures 6 and 7). Despite the strong similarity, the new species can be reliably distinguished from I. musivum by the presence of brown spots on, or sometimes nearly completely brown subsutural ramp, which is creamy in I. musivum. Both species have similar radulae with semi-enrolled marginal teeth. There is some similarity between the new species and I. olangoensis, also sympatric and syntopic in the Philippines and Vietnam, but I. conotaxis n. sp. can be readily distinguished by the presence of distinct brown spots on the sinus cords, which are only speckled in I. olangoensis. Besides the two species differ in the radular morphology as I. olangoensis has duplex-type marginal teeth.


4 DISCUSSION
4.1 Diversity in the Xenuroturris/Iotyrris complex
The combination of both exploratory (ABGD, GMYC and PTP) and hypothesis-testing (BFD) methods allowed us to delimit eight SSH in the Xenuroturris/Iotyrris complex, one of which being described as a new species. All these SSH are supported by both the mitochondrial and nuclear data sets, but also by diagnostic features of the shells and the radula. Although only two main types of radula morphology are recognized in the species complex, closely related species can possess different radula types (e.g., I. olangoensis vs. I. conotaxis n. sp. and I. musivum).
Indeed, the distribution of the two radula types in the phylogenetic tree, with species exhibiting the same radula type not clustering together, raises taxonomic and evolutionary questions. Previously the radular type (semi-enrolled vs. duplex marginal teeth) was considered as a reliable character to differentiate the genera Iotyrris Medinskaya & Sysoev, 2001 (type species I. marquesensis Sysoev, 2002) with semi-enrolled teeth and Xenuroturris Iredale, 1929 with the duplex ones (Kantor et al., 2008). The originally monotypical Iotyrris Medinskaya & Sysoev, 2001, was described on the presence of a semi-enrolled marginal radular teeth, unique—at that time—for the Turridae. The type species, I. marquesensis, was not and is still not sequenced. With the enlarged data set, the situation became more complex: three species with duplex marginal radular teeth were confidently placed into Iotyrris clade, namely Iotyrris olangoensis, I. notata and I. kingae, and the supposed congruence between the phylogeny and the radula type is thus no longer true. It may also appear that I. marquesensis does not belong to this clade and therefore the use of the name Iotyrris may not be appropriate, or the name itself may appear to be a synonym. Furthermore, within the same type (semi-enrolled or duplex teeth), the radular morphology can be quite different. Thus in I. marquesensis, the semi-enrolled marginal teeth have a distinct barb on the dorsal limb (Sysoev, 2002): Figure 2c,d), which is not pronounced in any species here assigned to Iotyrris. The central formation is absent in I. marquesensis and I. cingulifera, while being rather distinct in I. devoizei and I. conotaxis n. sp. Similarly the shape of duplex radular teeth in the first radular type is quite variable, as well as the degree of development of the central formation (Figure 3a–d).
Contrary to what Kantor et al. (2008) have concluded (“radular type is indeed reliable for revealing relationships”), the evolution of the radulae types does not follow the phylogeny, and multiple convergence and/or reversion would be needed to explain the observed pattern. Remarkable is the difference in the radula between sister species (nearly identical in shell morphology and coloration) I. kingae and I. devoizei (Figure 3d,f, correspondingly for radula and Figure 7 for shells). One hypothesis is that the radula is more labile than is commonly assumed and its morphology in Turridae may be related to diet, as it has been shown in the related family Conidae (Kohn, Nishi, & Pernet, 1999; Tucker & Tenorio, 2009, p. 200). This hypothesis would also suggest that diet itself is labile, with closely related species being characterized by different diets, a hypothesis already proposed for cone snails (Kohn & Orians, 1962). Identifying the preys would therefore be necessary to test a potential correlation between the radula type and the food habit, thus explaining the high lability of this anatomical structure.
There remains several nonsequenced species probably belonging to Xenuroturris/Iotyrris complex, some with known radular type (based on published and our unpublished data) – I. marquesensis (semi-enrolled) (Figure 7i), X. millepunctata (Sowerby, 1909) (duplex) (Figure 7j), X. gemmuloides Powell, 1964 (duplex), X. cerithiformis Powell, 1964 (duplex) (Figure 7h), as well as the more enigmatic X.? castanella Powell, 1964 and X.? emmae Bozetti, 1994. Their generic position can be clarified only based on molecular studies.
4.2 Species delimitation methodology
As emphasized in the introduction, DNA-based species delimitation methods are generally designed either for less-known taxa, using monolocus data, to propose de novo species hypotheses, or for more difficult to tackle species complexes, using multilocus data, to test predefined competing partitions of species. The two strategies, that we can term as “exploratory” and “hypothesis-testing,” respectively, are rarely combined. When predefined partitions of species are already available, and disagreements among taxonomists on species boundaries exist, exploratory methods will be mostly useless, and hypothesis-testing methods will be favoured to identify the most likely species partition. On the contrary, when dealing with a largely unknown group, exploratory methods will be favoured to propose PSH. Here, we combined both strategies, applying a monolocus, COI barcode, data set, and a multilocus, RAD-seq, data set, being used to test alternative partitions proposed by the former.
The methods applied to the COI data set, now widely accepted as robust and congruent (see e.g., Kekkonen, Mutanen, Kaila, Nieminen, & Hebert, 2015; Schwarzfeld & Sperling, 2015), all proposed similar partitions in the case of the Xenuroturris/Iotyrris complex. However, each method has their own limitations (Carstens et al., 2013; Miralles & Vences, 2013; Reid & Carstens, 2012) (e.g., linked to the substitution model used to calculate the genetic distances or to the quality of the input phylogenetic tree, sensitivities to the quality of the sampling (Ahrens et al., 2016; Hamilton, Hendrixson, Brewer, & Bond, 2014)), justifying the use of several methods.
More importantly, most of the PSH proposed with the COI gene were confirmed by the analysis of the RAD-seq data set. Interestingly, the phylogenetic reconstructions based on RAD-seq data sets exhibiting varying levels of missing data were quite congruent. Trees based on more loci, but consequently on higher levels of missing data, were better resolved, even for deeper relationships. This counter-intuitive result has been observed and discussed recently by several studies (see Eaton, Spriggs, Park, & Donoghue, 2017; Tripp, Tsai, Zhuang, & Dexter, 2017 for recent reviews). Consensus on the position to deal with missing data in RAD-seq analysis has not been reached and several studies strongly advocate for a case to case approach since in some situations, as selecting loci to decrease the proportion of missing data can possibly generate a strong bias (see for example Huang & Knowles, 2014).
In addition to the phylogenetic approach applied to the RAD-seq data set, the BFD method clearly identified, for both X. legitima and I. cingulifera, the hypothesis considering all Indo-Pacific samples as only one species as the more likely. This was in comparison with a partition in two species each, one in the WIO and the other in the IWP. Here again, the level of missing data probably led to unstable MLE when various number of steps and chain length for the path sampling are used, but the important difference between the MLE of the two competing partitions convinced us that the BF was decisive.
Thus, the successive use of exploratory and hypothesis-testing methods was necessary to reject the hypothesis that allopatric populations within X. legitima and I. cingulifera constitute different species. Indeed, analysing the COI data set only would have led to recognize these allopatric populations as species, as supported by all the exploratory methods (Figure 2). As exemplified here, the tree topology obtained with two distant populations within species can easily mimic the topology obtained with two sister species, even when considering the genetic distances: the genetic distances between the two allopatric pairs within X. legitima and I. cingulifera (K2P distances = 1.7%–3.6%) are actually similar to the genetic distances between I. devoizei and I kingae (1.5%–2.6%) and I. conotaxis n. sp., I. musivum and I. olangoensis (1.9%–3%). Applying various methods of species delimitation remains the best strategy to counter-balance the limitations of each, as discussed before, but more important is the joint analysis of independent genetic markers, since gene trees, taken independently, are not necessarily congruent with the species tree (Degnan & Rosenberg, 2009).
Consequently, and except the case of I. devoizei and I. notata, all the species are present in sympatry (in the Philippines). However, a scenario in which allopatric speciation with subsequent changes in the distribution areas cannot be ruled out (Berlocher, 1998; Chesser & Zink, 1994). Following the criteria of Coyne and Orr (2004), determining the age of the speciation events could help to test these two scenarios, but in the absence of calibration points (either biogeographic events or fossils), we were not able to reconstruct a dated tree. Similarly, exploring whether, if Xenuroturris species may have diverged in sympatry (e.g., by niche partitioning linked to the apparition of new toxins and prey shifts (Duda & Lee, 2009; Fedosov, Tiunov, Kiyashko, & Kantor, 2014; Nicolas Puillandre et al., 2014)) or not would require sequencing the transcriptomes of the venom glands and identifying the preys of different species, and in particular, in the only pair of sympatric sister species, I. musivum and I. conotaxis n. sp.
5 CONCLUSION
The combined use of classical barcoding, next generation sequencing and morphological observations enabled us to give new insight into the evolution of this species complex. Most defined SSH were linked to already described species and one new species is described. This study demonstrates the utility of combining both exploratory and hypothesis-testing methods. In the absence of primary species hypotheses, or, as it is the case here, when morphology-based species hypotheses are doubtful, analysing monolocus data with exploratory methods such as ABGD, GMYC and PTP rapidly produce PSH. However, such PSH had to be taken cautiously, since high levels of divergence can also be observed between populations within species (and conversely, low genetic distances can results from a lack of variability between species for a given marker). Evaluating these PSHs with independent markers using a hypothesis-testing method constitutes a desirable strategy to tell apart SSH from, for example, populations within species.
ACKNOWLEDGEMENTS
A large part of the molecular material in this paper originates from various shore-based expeditions and deep sea cruises, conducted respectively by MNHN (Inhaca 2011); by MNHN, Pro-Natura International (PNI) and Institut de Recherche pour le Développement (IRD) as part of the Our Planet Reviewed programme (Santo 2006, Atimo Vatae, Papua Niugini); and by MNHN and Institut de Recherche pour le Développement (IRD) as part of the Tropical Deep-Sea Benthos programme (Mainbaza, Terrasses, Pakaihi I Te Moana). In-country partners include the Maritime College, Luganville; Universidade Eduardo Mondlane, Maputo and University of Papua New Guinea, Port Moresby. Funders and sponsors include the Total Foundation, Prince Albert II of Monaco Foundation, Stavros Niarchos Foundation, Richard Lounsbery Foundation, Vinci Entrepose Contracting, Fondation EDF, the French Ministry of Foreign Affairs, the French Fonds Pacifique and the Government of New Caledonia. The specimens from the Philippines used in this study were obtained by AF in conjunction with a collection trip supported in part by the “Conus-Turrid” project (principal investigator B. M. Olivera, University of Utah, USA). Collection of material in Vietnam was supported by the Russian–Vietnamese Tropical Center. We are thankful to the staff of the Tropical Center for assistance in organization of the field sampling and loan of some laboratory equipment. Peter Stahlschmidt collected the specimens from Egypt, with a permit from the Hurghada Environmental Protection and Conservation Association (Hurghada, Egypt). All expeditions operated under the regulations then in force in the countries in question and satisfy the conditions set by the Nagoya Protocol for access to genetic resources. The authors also thank Virginie Héros, Julien Brisset, Philippe Maestrati and Manuel Caballer Gutierrez for their help in curating specimens, and Mark Phuong, Sarah Samadi and Guillaume Achaz for constructive comments on the manuscript. We are grateful to Dr. Norine Yeung from Bishop Museum, Honolulu Hawaii for providing the photos of the types of Xenuroturris kingae. This project was partly supported by the Service de Systématique Moléculaire (UMS 2700 CNRS-MNHN) and by the project CONOTAX, funded by the French ANR (grant number ANR-13-JSV7-0013-01). The contribution of Yu.I. Kantor and A.E. Fedosov was supported by the grant No. 16-14-10118 from the Russian Science Foundation (principal investigator Yu.I.Kantor). The scanning electron microscopy was conducted using Joint Usage Center “Instrumental methods in ecology” at the IEE RAS.
AUTHOR CONTRIBUTION
J.A. and L.A.C. performed the molecular experiments; B.B. vouchered and registered the samples; J.A., P.Z. and N.P. analysed the data; A.F. and Y.K. performed the morphological analyses and described the new species; N.P., B.B., A.F. and Y.K. participated in the field sampling; all authors participated in the research design and wrote the manuscript.
DATA ACCESSIBILITY
All samples are vouchered in the MNHN collection. Sample data and COI sequences will be uploaded in BOLD. COI sequences will be uploaded in GenBank. RAD-seq data and COI and RAD-seq trees have been uploaded in Dryad: https://doi.org/10.5061/dryad.k2q42




