Investigating a possible role for the bacterial signal molecules N-acylhomoserine lactones in Balanus improvisus cyprid settlement
Abstract
Increased settlement on bacterial biofilms has been demonstrated for a number of marine invertebrate larvae, but the nature of the cue(s) responsible is not well understood. We tested the hypothesis that the bay barnacle Balanus improvisus utilizes the bacterial signal molecules N-acylhomoserine lactones (AHLs) as a cue for the selection of sites for permanent attachment. Single species biofilms of the AHL-producing bacteria Vibrio anguillarum, Aeromonas hydrophila and Sulfitobacter sp. BR1 were attractive to settling cypris larvae of B. improvisus. However, when AHL production was inactivated, either by mutation of the AHL synthetic genes or by expression of an AHL-degrading gene (aiiA), the ability of the bacteria to attract cyprids was abolished. In addition, cyprids actively explored biofilms of E. coli expressing the recombinant AHL synthase genes luxI from Vibrio fischeri (3-oxo-C6-HSL), rhlI from Pseudomonas aeruginosa (C4-HSL/C6-HSL), vanI from V. anguillarum (3-oxo-C10-HSL) and sulI from Sulfitobacter sp. BR1 (C4-HSL, 3-hydroxy-C6-HSL, C8-HSL and 3-hydroxy-C10-HSL), but not E. coli that did not produce AHLs. Finally, synthetic AHLs (C8-HSL, 3-oxo-C10-HSL and C12-HSL) at concentrations similar to those found within natural biofilms (5 μm) resulted in increased cyprid settlement. Thus, B. improvisus cypris exploration of and settlement on biofilms appears to be mediated by AHL-signalling bacteria in the laboratory. This adds to our understanding of how quorum sensing inhibition may be used as for biofouling control. Nonetheless, the significance of our results for larvae settling naturally in the field, and the mechanisms that underlay the observed responses to AHLs, is as yet unknown.
Introduction
Many reports have described enhanced settlement of algal spores and invertebrate larvae on bacterial biofilms, (e.g. ascidians, barnacles, bryozoans, corals, echinoderms, polychaetes, molluscs and sponges; reviewed by Wieczorek & Todd 1998; Hadfield & Paul 2001; Hadfield 2011). The microbially derived agents that mediate this induction are not only important for selection of surfaces, but can also trigger metamorphological events in certain species (Wieczorek & Todd 1998; Hadfield & Paul 2001; Hadfield 2011). Reports of both surface-attached and water-bourne attractants derived from microbial films have been described (Leitz and Wagner, 1993; Wieczorek & Todd 1998; Harder et al. 2002), but until recently, very few have identified the cue responsible (reviewed in Hadfield 2011). There is evidence to suggest that for larvae of some marine invertebrates, the receptor that detects the presence of a biofilm is a lectin. This includes the spirorbid polychaete Janua brasiliensis (Maki & Mitchell 1985), the ascidians Herdmania curvata (Woods et al. 2004) and Boltenia villosa (Roberts et al. 2007), and the barnacle Balanus amphitrite (Khandeparker et al. 2006). In addition, Grasso et al. (2008) found high levels of transcripts for a protein that includes a C-type lectin domain in the anterior tip of larvae of the coral Acropora millepora. In the polychaete, Hydroides elegans, inhibiting the activity of a p38 mitogen-activated protein kinase inhibited the biofilm-induced larval settlement (Wang & Qian 2010), and a similar protein has been shown to regulate settlement of the barnacle Balanus amphitrite (He et al. 2012).
An alternative settlement cue has been described for zoospores of the problematic biofouling macro-algae Ulva: N-acylhomoserine lactone signal molecules (AHLs) (Joint et al. 2002). Production of AHLs by biofilms affect swimming behaviour of the zoospores through a process of chemokinesis, which brings about decreased swimming speed (Wheeler et al. 2006) and increased settlement within areas of high AHL production, such as dense biofilm micro-colonies (Tait et al. 2005). These AHL signal molecules are used by bacteria to co-ordinate their behaviour on a population level: a process known as ‘quorum sensing’ (QS). QS links the concentration of signal molecule to the expression of multiple genes, including those involved in secondary metabolism, virulence and biofilm development in a variety of bacteria (Swift et al. 1997). Along with Proteobacteria, AHL production has been reported in Bacteroidetes and Cyanobacteria (Huang et al. 2008; Sharif et al. 2008), indicating AHL-mediated signalling is particularly widespread amongst marine bacteria. Specialist niches, such as biofilms, promote the growth of dense microbial populations in which AHL signalling can be detected (Huang et al. 2009), and concentrations of AHLs of ~600 pmol/cm2 can be detected within natural rocky shore biofilms (Tait et al. 2009).
Since the initial discovery of the involvement of AHLs in Ulva zoospore settlement, N-butanoyl-L-homoserine lactone (C4-HSL) has been shown to up regulate sporulation in the red algae Acrochaetium sp. (Weinberger et al. 2007) and a possible role for QS has also been suggested in the settlement of invertebrate larvae: using the QS blockers 5-hydroxy-3[1(R)-1-hydroxypropyl]-4-methylfuran-2(5H)-one, (5R)-3,4-dihydroxy-5-[(1S)-1,2-dihydroxyethyl]furan-2(5H)-one and triclosan Dobretsov et al. (2007) inhibited the establishment of a bacterial biofilm, and thereby decreased the settlement of larvae of the polychaete H. elegans and the bryozoan Bugula neritina. However, although synthetic AHLs (>100 μm) induced crawling behaviour in H. elegans (a prerequisite to larval settlement) none of the AHLs tested induced larval settlement to the same extent as natural biofilms (Huang et al. 2007). Dobrestov et al. (2009) also refer to similar but unpublished results for the barnacle Balanus amphitrite.
The response of B. amphitrite to bacterial biofilms has been the most widely studied, but several other barnacle species are known to settle preferentially on bacterial biofilms, including Balanus improvisus (O'Connor & Richardson 1998), Balanus trigonus (Thiyagarajan et al. 2006), Semibalanus balanoides (Thompson et al. 1998) and Elminius modestus (Neal & Yule 1994). Although the response of barnacles to a glycoprotein termed ‘settlement-inducing complex’ (SIPC), isolated from adult shells has been well documented (Matsumura et al. 1998; Dreanno et al. 2007), the nature of the cue derived from biofilms is not well understood. It is possible that marine biofilms produce a compound similar to SIPC or that they are likely to be responding to multiple cues (Hadfield 2011) such as a component of biofilm EPS (Khandeparker et al. 2006) or alternative, currently undetermined biofilm properties. Interestingly, for B. amphitrite, it is known that settling cypris larvae can distinguish between biofilms of varying community composition, preferring to settle on biofilms characteristic of their adult habitat (Lau et al. 2005).
The aim of the present study was to assess the impact of AHL signals on settlement of cypris larvae of the bay barnacle B. improvisus. This invasive species is thought to have originated in North America, but now has a world-wide distribution as a result of dispersal as a biofouling agent on the hulls of ships. Similar to the more widely studied B. amphitrite (Harder et al. 2001; Qian et al. 2003; Hadfield 2011), B. improvisus has been shown to settle preferentially on bacterial biofilms (O'Connor & Richardson 1998). There are, however, key differences: B. amphitrite has a preference for hydrophilic surfaces, but the presence of older biofilms enhance larval attachment, irrespective of the type of substrate (Hung et al. 2008). In contrast, B. improvisus has shown a clear preference for hydrophobic substrates (Dahlström et al. 2004) and smooth substrata (Berntsson et al. 2000), and the presence of a biofilm can alter the response of B. improvisus cyprids to particular surfaces, decreasing detachment to hydrophobic polystyrene but increasing attachment to hydrophilic glass (O'Connor & Richardson 1998). This indicates that the nature of the biofilm and perhaps also the B. improvisus cyprid-settlement cue may be altered by properties of the underlying substratum.
To investigate the role of AHL signal molecules on the settlement of cyprid larvae of B. improvisus, we adapted methodologies used to investigate the role of AHLs in Ulva zoospore settlement (Joint et al. 2002; Tait et al. 2005). Live single species biofilms of the marine bacteria Vibrio anguillarum, Aeromonas hydrophila and Sulfitobacter sp. BR1 were used to provide a natural supply of AHL signal, and the response of B. improvisus cyprids compared with AHL-deficient variants of the three strains. Attempts were also made to assess cyprid responses to biofilms of E. coli expressing recombinant AHL synthases, as well as to synthetic AHLs.
Materials and methods
Bacterial strains
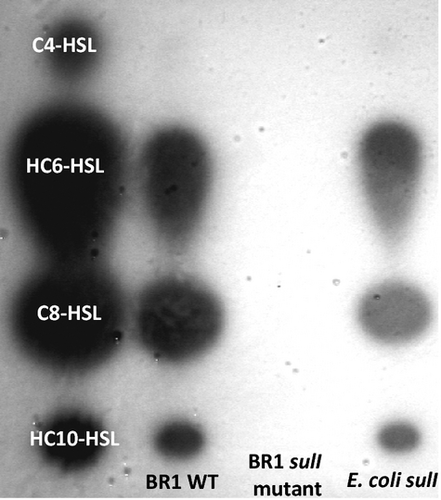
All bacterial strains and plasmids are described in Table 1. The influence of AHL signal molecules on cyprid settlement was assessed using three AHL-producing strains and their signal-deficient mutants Vibrio anguillarum and Aeromonas hydrophila each contain a mutation to the AHL synthases: vanM in V. anguillarum (Tait et al. 2005) and ahyI in A. hydrophila (Lynch et al. 2002). In addition, we also used a strain of V. anguillarum that expresses an inducible copy of aiiA, a lactonase enzyme that has been shown to degrade AHLs (Tait et al. 2005). An AHL-deficient variant of Sulfitobacter sp. BR1 was constructed first by transforming with the luxR::luxI' Gfp-based AHL reporter plasmid pRK-C12 (Reidel et al. 2001) to produce a strain that self-reported AHL production (BR1 pRK-C12). Transposon mutagenesis of BR1 pRK-C12 with the EZ-Tn5™ <R6Kγori/KAN-2 > Tnp Transposome kit (Epicentre Biotechnology) was used to randomly mutate the genome of BR1. The transformants were plated onto marine agar containing both gentamicin and kanamycin, and the colonies were then screened for the lack of Gfp production. The absence of AHL production was confirmed in dark colonies. As EZ-Tn5™ contains its own origin of replication, the insertion site was located by extracting the DNA (DNeasy extraction kit; Qiagen), partially digesting the DNA with EcoRV and self-ligating to form mini-plasmids. E. coli pir+ was transformed with the ligated DNA fragments and kanamycin-resistant colonies selected. An insertion in a gene with homology to luxI genes was located and designated sulI. This gene was amplified from BR1 using the primers sulIF (AGTTGCGATCATGGCAGAACC) and sulIR (TACAAGGATATCGACCAGCA), cloned into pGEM to generate pKT11 and transformed into chemically competent JM109. Using thin layer chromatrography (TLC) plates overlaid with the AHL biosensor Agrobacterium tumefaciens NTL4 (pCF218) (pCF372) (Fuqua & Winans 1996), AHL production by wild-type BR1 and Escherichia coli pKT11 was clearly visible, but there was no AHL production in BR1 with the mini-Tn5 insertion in the sulI gene (Fig. 1). Culture supernatants of the BR1 WT, the sulI mutant and E. coli pKT11 were extracted with dichloromethane and evaporated to dryness. The extracts were applied to RP18 F245 TLC plates (20 × 20 cm; VWR International) and a mobile phase of 60% (v/v) methanol used to separate the extracts. TLC plates were overlaid with the biosensor NTL4 (pCF218; pCF372) (Fuqua & Winans 1996) following the methodology of Mohamed et al. (2008). Following incubation at 30 °C overnight, the TLC plates were examined for the presence of blue spots, indicative of AHL production. The same AHLs produced by the BR1 WT were also produced by the E. coli expressing the recombinant sulI. No AHLs were detected in the presence of the BR1 sulI mutant, confirming the disruption to the AHL synthases in this bacterium.
| Strain or Plasmid | Description | References |
|---|---|---|
| E. coli | ||
| JM109 | recA1 supE44 endA1 hsdR17 gypA96 relA1 thi Δ (lac-proAB) | Schaefer et al. (1996) |
| A. hydrophila | ||
| AH-IN | Spontaneous mutation of A. hydrophila AH-1 lacking S-layer and O-antigen | Swift et al. (1997) |
| AhyI − | AHL-deficient variant:AH-IN with an in frame deletion of ahyI | Lynch et al. (2002) |
| V. anguillarum | ||
| NB10 | Wild type, serotype 01, clinical isolate from the Gulf of Bothnia | Norqvist et al. (1989) |
| DM28 | AHL-deficient variant: In-frame deletion of vanM | Milton et al. (2001) |
| NB10 Gfp | Gfp-labelled WT: contains mini-Tn7 PA1/04/03gfp (GentR) | This study |
| DM28 Gfp | Gfp-labelled AHL-deficient variant: DM28 containing mini-Tn7 PA1/04/03gfp (GentR) | This study |
| NB10/pDM44 | Wildtype carrying Autoinducer Inactivation protein (AiiA): contains a PA1/04/03::aiiA gene fusion (CmR) | Tait et al. (2005) |
| NB10/pDM42 | Wildtype carrying a Gfp-based AHL reporting construct: contains luxR-PluxI-RBSII::gfpmut3*-T0; (CmR) | Tait et al. (2005) |
| DM27/pDM42 | AHL-deficient variant containing a Gfp-based AHL reporting contruct: DM28 containing luxR-PluxI-RBSII::gfpmut3*-T0 (CmR) | Tait et al. (2005) |
| Sulfitobacter sp. | ||
| BR1 | Wild type, isolated from rocky shore | Tait et al. (2005) |
| SulI − | Mini-Tn5 insertion into sulI (KanR) | This study |
| Agrobacterium tumefaciens NTL4 (pCF218) (pCF372) | AHL reporter: produces a blue colour in the presence of 5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside (X-Gal) in response to a wide range of AHLs | Fuqua & Winans (1996) |
| Plasmids | ||
| pUX-BF13 | mob+ori-R6K; helper plasmid; providing Tn7 transposition functions in trans (AmpR) | Bao et al. (1991) |
| pRK600 | ori-ColE1 RK2-mob+ RK2−tra+ helper plasmid in matings (CmR) | Kessler et al. (1992) |
| pMiniTn7(Gm)PrrnB1 –gfp-a | PrrnB1-gfp cloned into NotI site of pBK-miniTn7-ΩGm | Lambertsen et al. (2004) |
| pSB401 | AHL reporter plasmid; luxR′′::luxCDABE (AmpR) | Winson et al. (1998) |
| pRK-C12 | AHL reporter plasmid; pBBR1MCS-5 carrying PlasB– gfp(ASV) Plac- lasR | Reidel et al. (2001) |
| pUCP18 | pUC18 containing 1.8-kb fragment for maintenance in Pseudomonas sp. (AmpR) | Shweizer (1991) |
| pMW47.1 | 2-kb PstI Pseudomonas aeruginosa PAO1 DNA insert (rhlRI) in pUCP18 | Latifi et al. (1995) |
| pT7T3 | General cloning vector derived from pUC18 (AmpR) | Pharmacia |
| pT7T3luxI | pT7T3 expressing luxI from Vibrio fischeri 7744 | Tait et al. (2005) |
| pET3a | Overexpression vector (AmpR), T7 promoter, pBR ori | Novagen |
| PETVanI2 | pET3a expressing vanI from Vibrio anguillarum NB10 | Tait et al. (2005) |
| pGEM | General cloning vector derived from pUC18 (AmpR) | Promega |
| pKT11 | pGEM expressing sulI from Sulfitobacter sp. BR1 (AmpR) | This study |
The miniTn7 system developed by Lambertsen et al. (2004) was used to make Gfp-tagged varients of V.anguillarum WT and the vanM mutant. A four parental mating between V. anguillarum NB10 or DM28 (recipients), E. coli pRK6000 (conjugation helper), E. coli pMiniTn7(Gm)PrrnB1gfp.-a (donor) and E. coli pUX-BF13 (transposition helper) was carried out, and transconjugants selected on TSB supplemented with 50 μg/mL gentamycin. Site-specific insertion of Tn7 downstream of the glmS gene was verified by PCR (Lambertsen et al. 2004).
Escherichia coli JM109 biofilms expressing vanI from V. anguillarum (producing 3-oxo-C10-HSL), luxI from Vibrio fischeri (3-oxo-C6-HSL), rhlI from Pseudomonas aeruginosa (C4-HSL/C6-HSL) and sulI from Sulfitobacter sp. BR1 [C4-HSL, 3-hydroxy-C6-HSL, C8-HSL and 3-hydroxy-C10 (Fig. 1)] were compared to biofilms containing the vector plasmids without the luxI homologues (Table 1).
Sulfitobacter sp. BR1 was routinely grown in Difco Marine Broth. V. anguillarum strains were grown in Tryptic Soy Broth (TSB), and A. hydrophila strains and E. coli strains in Luria Broth. Temperatures for incubation were 37 °C for E. coli and 25 °C for V. anguillarum, A. hydrophila and Sulfitobacter sp. BR1.
Preparation of biofilms
Biofilms were prepared as previously described (Tait et al. 2005). Briefly, cultures were grown overnight in rich media, the cells harvested by centrifugation, washed and resuspended in sterile, filtered seawater (0.2 μm, salinity 15‰) to an OD of 1.0. Varying volumes of cell suspension (50–100 μL) were used to inoculate biofilm culture vessels which contained 10-mL sterile, filtered seawater (0.2 μm, salinity 15‰) and sterile microscope cover glasses, and the vessel incubated for 24 h at room temperature. By adjusting the volume of the inocula, similar densities of signal-producing and non-producing biofilms were achieved.
Preparation of Balanus improvisus cyprids and settlement assays
Balanus improvisus cyprids were reared in a laboratory culture system at the Sven Lovén Centre for Marine Sciences in Tjärnö, Sweden as described by Berntsson et al. (2000). Settlement assays were performed by placing cover glass biofilms, synthetic AHLs plus clean cover glasses, or clean cover glasses only (controls) into each well of six-well culture plates (Corning Costar Cell Culture Plates) containing 10-mL sterile, filtered seawater (0.2 μm, salinity 15‰). Between 10 and 12 cyprids were added to each vessel (a minimum of 12 replicates) and incubated at 18°C with a light/dark cycle of 9:15 h for a period of 7 days. The vessels were monitored daily using a dissecting microscope (×10 magnification), and the numbers of (i) permanently settled cyprid larvae (following expulsion of cement), (ii) exploratory cyprids (non-permanent settlement or active crawling on vessel surface) and (iii) dead cyprids were recorded daily. Experiments with V. anguillarum were repeated with three separate batches of cyprids and experiments with Sulfitobacter sp., E. coli or synthetic AHLs repeated with two separate batches of cyprids. Due to varying quantities of cyprids within the different batches, experiments with A. hydrophila were conducted only once. As the E. coli died during the long incubations in seawater, biofilms were only monitored for 2 days. Each cyprid batch was derived from different multiple barnacle parents.
As biofilm density influences AHL production, care was taken to ensure biofilms of signal-producing and signal-deficient strains were of similar densities. The proportion of the surface area covered by bacteria was determined with microscope image analysis, using an Image ProPlus imaging system attached to a Reichert-Jung Polyvar microscope and a Optronics Magna Fire SP camera. Biofilm material was stained with crystal violet 1% aqueous solution, and counts were made of 20 random fields of view from each of four replicates. Measurements revealed similar per cent coverage for signal-producing and signal-deficient mutants of all three bacteria. The per cent coverage for V. anguillarum WT biofilms was 26.04 ± 1.54%, for V. anguillarum vanM mutant biofilms, 25.43 ± 1.14%, and for V. anguillarum expressing the recombinant AiiA lactonase, 25.33 ± 1.59%. A. hydrophila WT biofilm densities were 21.8 ± 1.3% and the ahyI- mutant, 23.77 ± 1.18%. For Sulfitobacter sp. BR1, biofilm densities were 42.42 ± 2.21% for the WT and 37.29 ± 3.67% for the signal-deficient mutant.
Quantification of introduced bacteria during cyprid settlement assays
To calculate the numbers of bacteria introduced to the biofilm along with the cyprids during the long experiments, Gfp variants of V. anguillarum and the vanM mutant were used. Similarly, to detect whether any of the introduced bacteria were making AHLs, a V. anguillarum vanM mutant carrying a gfp-based AHL biosensor luxR-PluxI-RBSII::gfpmut3*-T0 was used (Tait et al. 2005). This strain does not produce any AHLs, but expresses Gfp when an exogenous source of AHL is detected. This was compared to the number of Gfp-producing bacteria within biofilms of the V. anguillarum wild-type strains containing the same construct. Biofilms were counterstained with 1 mg/mL DAPI and viewed using a Reichert-Jung Polyvar microscope. A blue light filter (excitation, 450–495 nm; emission, 510 nm; dichroic, 510 nm) was used for Gfp fluorescence and an ultraviolet filter (excitation, 330–380 nm; emission 420 nm; dichroic 420 nm) for DAPI. Image Pro+ 5 (Media Cybernetics) was used to estimate the percentage of cells expressing Gfp. Counts were made of 10 random fields of view from each of four replicates.
Settlement assays using synthetic AHLs
To quantify cyprid response to synthetic AHLs, 0.5, 5 and 50 μm C6-HSL (N-hexanoyl-L-homoserine lactone), C8-HSL (N-octanoyl-L-homoserine lactone), C12, (N-dodecanoyl-L-homoserine lactone) and OC10-HSL (N-(3-oxodecanoyl)-L-homoserine lactone) (Sigma-Aldrich) were embedded in a 1% agarose/distilled water matrix (Tait et al. 2005). A consistent thin coating of agarose/AHL was applied to cover glasses using a mould. This agarose film was used in cyprid settlement assays. For each AHL concentration, 12 replicates were used and agarose films without AHLs were included as controls.
Given the rapid diffusion of AHLs from surfaces, which can occur within minutes for very short chain AHLs (Tait et al. 2005), and the long incubation times of these experiments, AHLs were also added directly to seawater. AHL concentrations of 0.5, 5 and 50 μm were maintained throughout the incubation. This first required measurement of the rate of degradation of AHLs in natural seawater. AHL degradation varies with temperature, acyl side chain length and also the presence of substitutions on the acyl chain (Tait et al. 2005; Hmelo & van Mooy 2009), and is much higher in natural, unsterilized seawater than in artificial seawater (Hmelo & van Mooy 2009). To measure the rate of degradation during incubation, natural seawater containing AHLs was incubated for 3 h and residual AHLs extracted with ethyl acetate and evaporated to dryness. Extracts were then resuspended in acetonitrile, added to white/clear-bottomed microtitre plate wells (Corning, UK) and 200 μL of the lux-based E. coli pSB401 AHL biosensor added. The microplates were incubated at 37 °C and the luminescence and absorbance (600 nm) monitored for a period of 8 h using a Berthold Mithras plate reader. Measurements of the areas under each curve were made, and a standard curve of relative light units (RLU)/OD600 as a function of AHL concentration constructed for each of the four AHLs. For each sample, five values were obtained and the mean determined. The per cent degradation of each AHL in the seawater was calculated as 1.54 ± 0.23% per h for C6-HSL, 1.02 ± 0.49% per h for C8-HSL, 4.57 ± 1.21% per h for OC10-HSL and 0.68 ± 0.24% per h for C12-HSL with reference to the calibration curve. Using these values, AHLs were replenished in the cyprid settlement assays every 8 h to maintain the desired concentration. For each AHL concentration, 12 replicates were used and agarose films without AHLs were included as controls.
Statistical analysis
Data are reported as a means with 95% confidence intervals. The software package PRIMER 6 (Clarke & Gorley 2006) with PERMANOVA+ (Anderson et al. 2008) was used for all statistical analysis. Multivariate permuational analysis of variance (PERMANOVA) based on Euclidean distance was used for analyses of the cyprid exploratory behaviour (see above) and settlement responses on V. anguillarum, A. hydrophila and Sulfitobacter sp. BR1 biofilms. Daily measurements of cyprid behaviour were used as response variables and the different treatments and their replicates used as samples. The multivariate nature of this analysis readily accounts for the non-independence of the daily measurements. For experiments using batch 1 and 3 cyprids, at least 18 replicates were analysed for every experiment. For batch two cyprids, at least 30 replicates were used. Significant terms were investigated further using pairwise comparisons with 999 permutations (Anderson et al. 2008). Tests for V. anguillarum biofilms were carried out with the two different signal-deficient mutants as separate treatments and also as a single, combined treatment with no differences between the conclusions made. Differences in the response of cyprid batch 2 to each of the 3 bacteria studied and also differences in the behaviour of the three separate cyprid batches in vessels containing V. anguillarum signalling and non-signalling biofilms were investigated by creating combined factors of ‘Bacterium × Biofilm Type’ and ‘Batch × Biofilm Type’ respectively. To clearly visualize differences within treatments, replicates were averaged and shown as MDS plots.
For experiments using E. coli and synthetic AHLs, where analyses typically used data collected on day 2 or day 7, anova was also used to test for differences in cyprid exploratory behaviour between no-biofilm controls and control E. coli biofilms, E. coli controls and E. coli strains expressing AHLs, and also in vessels with and without synthetic AHLs.
Results
Increased settlement of Balanus improvisus cyprids in the presence of AHL-producing biofilms
Substantially higher numbers of cyprids settled in treatments containing signal-producing bacteria than in non-signalling biofilm and no-biofilm controls (Fig. 2). These differences were statistically highly significant for all bacteria tested and for each of three batches of cyprids (PERMANOVA, Table 2, Fig. 2). Pairwise comparisons indicated that the AHL-producing wild-type biofilm caused significantly more settlement than the signalling-deficient mutant biofilms and the no-biofilm controls (Table 2). Settlement on the signalling-deficient biofilms was not statistically different from that on the no-biofilm controls (P > 0.12, Table 2), except in one case (larvae from Batch 1 on Sulfitobacter sp. BR1 biofilms settled significantly less on no-biofilm controls than on the AHL-deficient biofilms; Fig. 2, Table 2). Although more cyprids were recorded crawling on the AHL producing biofilms (with the exception of Vibrio anguillarum, batch 3; Fig. 2, day 2 data), most settlement occurred on the sides of the culture dishes. This behaviour is typical for this species under static laboratory conditions (Berntsson et al. 2000).
| Batch | Bacterium | PERMANOVA | PAIRWISE TESTS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | SS | MS | F | P (perm) | Unique perms | Groups | t | P (perm) | Unique perms | ||
| 1 | A. hydrophila | Biofilm | 2 | 5369.6 | 2684.8 | 33.35 | 0.001*** | 998 | WT, Mutant | 2.83 | 0.003*** | 998 |
| Res | 234 | 18838 | 80.505 | WT, No biofilm | 2.62 | 0.007*** | 997 | |||||
| Total | 251 | 28612 | Mutant, No biofilm | 0.47 | 0.875 | 998 | ||||||
| 1 | Sulfitobacter | Biofilm | 2 | 28059 | 14030 | 116.59 | 0.001*** | 998 | WT, Mutant | 4 | 0.001*** | 997 |
| Res | 234 | 28164 | 120.36 | WT, No biofilm | 5.58 | 0.001*** | 999 | |||||
| Total | 251 | 69209 | Mutant, No biofilm | 1.83 | 0.031** | 997 | ||||||
| 2 | Sulfitobacter | Biofilm | 2 | 20875 | 10438 | 105.79 | 0.001*** | 999 | WT, Mutant | 6.55 | 0.001*** | 998 |
| Res | 469 | 46273 | 98.662 | WT, No biofilm | 6.43 | 0.001*** | 997 | |||||
| Total | 485 | 140010 | Mutant, No biofilm | 0.65 | 0.714 | 999 | ||||||
| 1 | V. anguillarum | Biofilm | 2 | 11999 | 5999.5 | 12.631 | 0.001*** | 999 | WT, Mutant | 3.8 | 0.001*** | 999 |
| Res | 51 | 24224 | 474.98 | WT, No biofilm | 4.81 | 0.001*** | 999 | |||||
| Total | 53 | 36223 | Mutant, No biofilm | 149 | 0.121 | 998 | ||||||
| 2 | V. anguillarum | Biofilm | 2 | 34534 | 17267 | 31.724 | 0.001*** | 999 | WT, Mutant | 7.21 | 0.001*** | 999 |
| Res | 106 | 57695 | 544.29 | WT, No biofilm | 5.13 | 0.001*** | 999 | |||||
| Total | 108 | 92229 | Mutant, No biofilm | 0.77 | 0.569 | 999 | ||||||
| 3 | V. anguillarum | Biofilm | 2 | 8129.3 | 4064.6 | 10.665 | 0.001*** | 999 | WT, Mutant | 4.21 | 0.001*** | 999 |
| Res | 69 | 26298 | 381.13 | WT, No biofilm | 3.25 | 0.001*** | 993 | |||||
| Total | 71 | 34427 | Mutant, No biofilm | 0.59 | 0.675 | 993 | ||||||
- Asterisks indicate significant P values (*P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001).
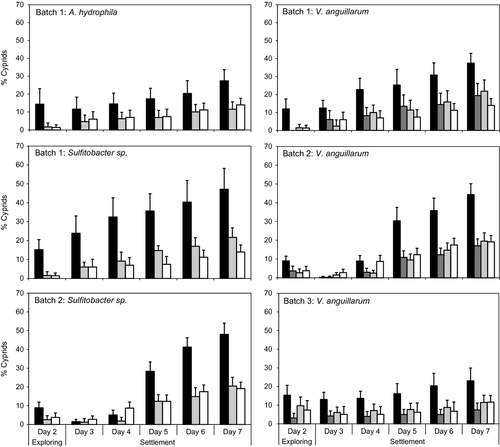
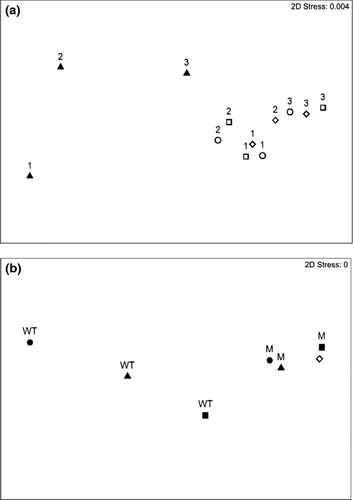
Overall levels of larval settlement varied between different batches of larvae (data for Sulfitobacter sp and V. anguillarum; Fig. 2). The possibility that larvae from different batches (genotypes) may have also responded differently to the different biofilm treatments was tested using data for settlement on V. anguillarum (the only bacteria species that was tested using three different larval batches). A significant Batch × Biofilm interaction was detected (Pseudo-F = 1.88; P = 0.036, Table 3). Further investigation of this interaction using multidimensional scaling (MDS) showed clear separation of settlement of the AHL signal-producing (WT) biofilms from that in the non-signalling controls (vanM mutant and V. anguillarum expressing the recombinant AiiA lactonase; Fig. 3A), and that responses in the non-signalling controls grouped much more closely together (Fig. 3A). Similar broad separation between AHL-producing WT strains and relatively tight grouping of non-signalling biofilms was also seen for all three bacteria species when compared using batch 1 cyprids (the only batch for which all three species and biofilm types were compared; Fig. 3B, Table 2).
| Source | df | SS | MS | F | P (perm) | Unique perms |
|---|---|---|---|---|---|---|
| PERMANOVA | ||||||
| Batch | 2 | 97829 | 48915 | 9.2449 | 0.001*** | 999 |
| Biofilm | 2 | 43689 | 21844 | 4.1286 | 0.002*** | 998 |
| Batch × Biofilm | 4 | 39877 | 9969.1 | 1.8842 | 0.036** | 999 |
| Res | 162 | 857140 | 5291 | |||
| Total | 170 | 1038500 | ||||
| Groups | t | P (perm) | Unique perms | |||
| PAIRWISE TESTS | ||||||
| WT, Mutant | 1.9139 | 0.017** | 999 | |||
| WT, No biofilm | 2.8794 | 0.002*** | 998 | |||
| Mutant, No biofilm | 1.1039 | 0.286 | 999 | |||
- Asterisks indicate significant P values (*P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001).
After 7 days incubation, the WT and the vanM mutant biofilms still contained similar bacterial coverage (WT biofilms: 24.67 ± 2.24%; vanM mutant biofilms: 26.19 ± 2.19%). However, addition of cyprids to the biofilm unavoidably introduced additional bacteria to the culture vessels, and this was assessed using Gfp variants of V. anguillarum WT and the vanM mutant. In control, axenic biofilms, the numbers of V. anguillarum still expressing Gfp were 97.6% for the WT and 98.1% for the vanM mutant after 7 days. Within the biofilms exposed to cyprids, 91.5 ± 0.98% bacteria within the WT vessels and 89.13 ± 1.23% bacteria within the vanM mutant biofilm were producing Gfp after the 7-day incubation period. Very few cells expressing Gfp were detected within the V. anguillarum vanM mutant carrying a gfp-based AHL biosensor (2.14 ± 1.24%). This shows that despite the relatively high number of introduced bacteria, very few of these were actively releasing AHLs. In contrast, biofilms of the V. anguillarum WT containing the same construct comprised 93.65 ± 5.12% Gfp-producing bacteria.
Experiments using AHL synthase-producing E. coli and synthetic AHLs also show an increase to cyprid exploratory behaviour and settlement
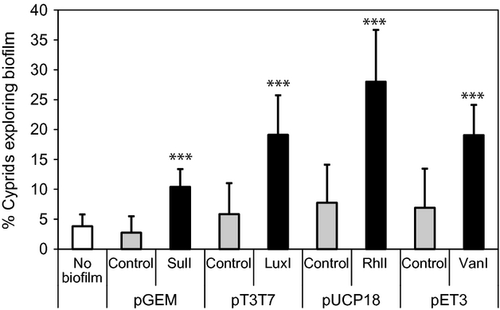
After 2 days, significantly higher numbers of cyprids were actively exploring the AHL synthase-producing E. coli biofilms than the control biofilms (Fig. 4). In contrast, there were no significant differences in cyprid exploration between the E. coli control plasmids and the no-biofilm controls (anova P = 0.683). This experiment was repeated with two batches of cyprids, with similar results each time.
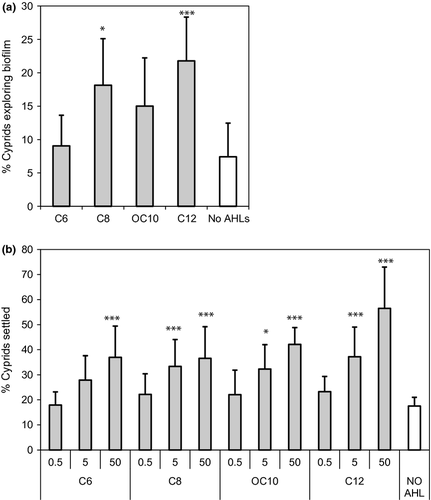
Assays using the synthetic AHLs C6-HSL, C8-HSL, OC10-HSL and C12-HSL in agarose films showed that only C8-HSL and C12-HSL elicited an increase in the number of cyprids actively crawling on the surface of the vessel after 2 days incubation (Fig. 5A; anova P = 0.037 and P = 0.001, for C8-HSL and C12-HSL, respectively). After 7 days incubation, there was no difference in cyprid responses between vessels containing AHLs and the AHL-free controls (results not shown). When AHLs were added directly to the seawater, there was increased settlement within vessels containing 50 μm of all four AHLs compared to controls (Fig. 5B). Using concentrations of AHLs close to those found in natural biofilms (5 μm), C8-HSL, OC10-HSL and C12-HSL, but not C6-HSL, increased cyprid settlement. The response towards OC10-HSL was marginally less significant than the response towards C8-HSL and C12-HSL (anova P = 0.023 for OC10-HSL and P = 0.001 for both C8-HSL and C12-HSL).
Discussion
Our results clearly demonstrate that AHL-producing biofilms influence settlement of cypris larvae of the barnacle, Balanus improvisus: AHL-producing variants of the marine bacteria Vibrio anguillarum, Aeromonas hydrophila and Sulfitobacter sp. BR1 all significantly increased settlement of B. improvisus cyprids in comparison with non-AHL-producing biofilms and controls (Figs 2 and 3); cyprids actively investigated biofilms of E. coli expressing recombinant AHL synthase genes significantly more than biofilms of E. coli not producing AHLs (Fig. 4); and synthetic AHLs at environmentally relevant concentrations increased the numbers of settling cyprids (Fig. 5B). In the majority of cases, there were no differences between settlement within vessels containing no biofilms and biofilms of the signal-deficient mutants. Taken together this evidence suggests that cyprid settlement in response to biofilms is either mediated directly by an AHL signal or mediated indirectly, for example, the AHL signal may control the production of an unknown biofilm-derived settlement cue.
Mutation to an AHL synthase is likely to impact other phenotypes, other than AHL production in the bacteria used in this study: quorum sensing is thought to constitute a global regulatory system for many bacteria. For example, transcriptomic studies of P. aeruginosa revealed over 500 genes regulated by LasRI and RhlI dispersed throughout the chromosome (Hentzer et al. 2003; Schuster et al. 2003; Wagner et al. 2003). It is, therefore, not surprising to find a link between quorum sensing and regulation of biofilm formation and development in many bacteria, including V. anguillarum and A. hydrophila. Biofilms of the AHL-deficient mutants in both these bacteria are less differentiated with no microcolonies (Lynch et al. 2002; Tait et al. 2005). Given the differences in structure for V. anguillarum and A. hydrophila biofilms, it is possible that the cyprid responses we observed were responses to changes in biofilm architecture rather than the presence or absence of an AHL signal. Conversely, under the conditions used to produce the Sulfitobacter sp. BR1 biofilms, there are no visible differences between the wild-type and signal-deficient mutant (data not shown). Nonetheless, our treatments may have caused unintended (and uncharacterized) changes to biofilm phenotypes that influenced in cyprid settlement. For example, EPS production has been linked to AHL production in certain bacteria (Sakuragi & Kolter 2007), and it has been shown that for some invertebrate larvae, the settlement cue involves recognition of biofilm EPS by lectin receptors (Maki & Mitchell 1985; Woods et al. 2004; Khandeparker et al. 2006; Roberts et al. 2007).
The possibility that additional unidentified features of the AHL-deficient variants of V. anguillarum, A. hydrophila and Sulfitobacter BR1 affected cyprid settlement were investigated using assays with E. coli expressing recombinant AHL synthases. As would be expected, the long incubation period of the experiments resulted in the death of the E. coli biofilms, and consequently after day 7, there was no difference in the numbers of cyprids settling within vessels containing signalling or non-signalling E. coli strains (data not shown). Exploratory behaviour precedes permanent attachment for B. improvisus cyprids (Berntsson et al. 2000), and therefore, the finding that significantly more cyprids were actively exploring the E. coli biofilms that expressed the recombinant AHL synthases (after 2 days) than the control biofilms corroborates the results from our settlement experiments using AHL-producing and AHL-deficient strains.
Finally, we assessed the biofilm-independent effects of AHLs on cyprid settlement with a range of synthetic AHLs. C8- and C12-HSL produced significantly more searching by cyprids after 2 days incubation than other AHLs (Fig. 5B). After this time, there were no differences between the numbers of cyprids settling in chambers with or without the presence of AHLs. These findings may be partially explained by the instability of AHLs in seawater (Tait et al. 2005; Hmelo & van Mooy 2009). AHLs consist of five-membered homoserine lactone rings with varied amide-linked acyl side chains. These acyl side chains can range from 4 to 18 carbons in length and may be saturated or unsaturated, with or without a substituent (usually an oxo or hydroxy) on the C3 carbon of the N-linked acyl side chain (Chhabra et al. 2005). The alkaline pH of seawater (typically pH 8.1) causes rapid hydrolysis of the lactone ring, and this increases with increasing temperature (Tait et al. 2005) Shorter acyl chain length AHLs, and those with substitutions on the acyl chain are also more susceptible. In addition, AHLs diffuse rapidly from surfaces (Tait et al. 2005): for short chain AHLs such as C6-HSL almost complete diffusion from the agarose matrix could be expected within <1 h. Thus, AHLs have an extremely short half-life in seawater and would only be expected to be biologically active within micro-niches such as biofilms. Given the long exposure times required for cyprid settlement within these laboratory experiments (days), it is unlikely that any synthetic AHLs, whether in seawater or within the agarose matrix, would still be biologically active. This may also explain why previous studies using synthetic AHLs within larval settlement assays (Dobretsov et al. 2007; Huang et al. 2007) have yielded ambiguous results. By calculating the rate of degradation of each AHL within the experimental vessels and replenishing regularly throughout the course of the experiment, we ensured that AHLs remained close to the target concentration and mimicked the natural release of AHLs from live biofilms. This methodology yielded significant results for seawater containing synthetic AHLs at biologically relevant concentrations (Fig. 5B). The response to a synthetic AHL suggests that cyprids can respond to the AHL signal directly. Note that this does not exclude the possibility that cyprids also used other biofilm-derived cues during our experiments with bacteria.
The long incubation period before cyprid settlement in our experiments (7 days) produced several potential problems, not least the introduction of ‘foreign’ bacteria along with the cyprids. By using V. anguillarum labelled with Gfp, we found the extent of colonization by non-Gfp bacteria after 7 days was as high as 10% of the biofilm. The identities of the introduced bacteria are not known. Neither is it known if there was a difference between those colonizing the signal-deficient or signal-producing biofilms, nor if there were differences in ‘foreign’ colonization between the three marine bacteria used. All these factors may have influenced cyprid settlement in our assays. Our attempts to determine the level of AHL signal produced by these marine bacteria using a V. anguillarum vanM mutant carrying a Gfp-based AHL reporter did, however, indicate that few of these were actively producing AHL signal: very low numbers of the V. anguillarum reporter bacteria were detecting an AHL signal produced by neighbouring, introduced bacteria (2.14 ± 1.24%). Consequently, while the biofilms of the signal-deficient strains may not have been entirely AHL-free through the course of the experiment, the concentration of AHLs in these treatments in comparison were the signal-producing strains was extremely low.
We found statistically significant differences in cyprid settlement behaviour from different larval batches (Table 3). Variability in larval response is well known (Raimondi & Keough 1990). Rearing conditions (Holm 1990), larval age (Holm et al. 2000) and type of microalgae used to feed the developing larvae (Clare et al. 1994) have all been shown to influence the attachment and metamorphosis of B. amphitrite. Consequently, offspring of the same parents raised at different times can respond differently to the same surface (Holm 1990). Therefore, care was taken to ensure that larvae used within these studies were reared using identical conditions in each case. Nonetheless, the number (and genetic identity) of parents that contributed to the larvae within each cyprid batch is unknown. The clear differences between larval responses we observed (Table 3) indicate the potential for larval selection and adaptation to different biofilms.
Although the number of cyprids exploring the biofilms of signal-producing bacteria was higher than those exploring the non-signalling biofilms and no-biofilm controls (with the exception of V. anguillarum, batch 3; Fig. 2, Day 2 data), many cyprids chose to settle on the sides of the vessel and not directly on the biofilms. This settlement behaviour is typical of B. improvisus within laboratory experiments (Berntsson et al. 2000). It is known that B. improvisus actively explores a large area before settling: the likelihood of final settlement at a particular site is directly related to searching behaviour that occurs over the entire surface of the dish prior to settlement (Havenhand, unpublished data). While the mechanism behind B. improvisus cyprid settlement may still be unclear, the critical point here is that without the presence of the AHL-producing biofilms, settlement was reduced (Fig. 2).
The series of experiments described here indicates AHL-signalling biofilms may be used by B. improvisus as a settlement cue under laboratory conditions and certainly highlights the need for further research, particularly using conditions more closely mimicking field conditions. Hydrodynamics and surface properties are known to have a significant impact on B. improvisus settlement (Berntsson et al. 2000; Jonsson et al. 2004) and will also influence the rate of diffusion of AHLs from surfaces. This is essential to clarify the importance of AHLs and AHL-signalling biofilms for larval settlement in the field. It is also not clear whether the cyprids are chemotactically attracted to the AHL signal, or whether the cyprid response is chemokinetic behaviour as shown to be the case with Ulva (Wheeler et al. 2006). Yet, it is becoming increasingly apparent that AHLs have biologically important properties beyond their role in cell-to-cell communication within species of bacteria. In the marine environment, there is now evidence that algae (Joint et al. 2002; Weinberger et al. 2007), polychaetes and bryozoans (Dobretsov et al. 2007; Huang et al. 2007) respond to the presence of a bacterial-derived signal. The effect of AHLs on other plant (Mathesius et al. 2003; Ortíz-Castro et al. 2008; von Rad et al. 2008; Bai et al. 2012), animal (Telford et al. 1998; Smith et al. 2002; Pritchard et al. 2005) and fungal cells (Hogan et al. 2004) has also been well documented. These findings show that AHL signals molecules can modify the behaviour of a wide range of evolutionarily diverse organisms. Studies of the underlying mechanism in each of these organisms are needed to reveal the origin and scale of this interaction.
Enhanced understanding of the role of AHL signalling within marine biofouling communities (Tait et al. 2005; Dobretsov et al. 2007; Huang et al. 2007, 2008, 2009) increases the importance of research into technologies that specifically disrupt AHL-mediated QS for biofouling control, as well as for disease control within aquaculture (Natrah et al. 2011). Screens for AHL inhibitory compounds from compounds obtained from the marine environment have already shown promising results (Dobretsov et al. 2011). Further investigations of the role of AHLs in mediating settlement responses, chemical defence and inter-specific communication of barnacles and other marine invertebrates are warranted.
Acknowledgements
This work forms part of the core strategic research activity of Plymouth Marine Laboratory, a collaborative centre of Natural Environmental Research Council. It was also supported by the EU-FP7 ASSEMBLE programme and was performed partly within the Linnaeus Centre for Marine Evolutionary Biology at the University of Gothenburg (http://www.cemeb.science.gu.se/), supported by a Linnaeus-grant from the Swedish Research Councils VR and Formas. We would like to express our heartfelt thanks to Martin Ogemark for providing the cyprid cultures used for these experiments, the Sven Lovén Centre—Tjärnö Laboratory for hosting the visit of KT in June 2010 and to Bob Clarke and Paul Somerfield for statistical guidance.
References
K.T. and J.H. designed the experiments, K.T. performed the research, and K.T. and J.H. analysed the data and wrote the paper.
Data accessibility
Data from all experiments (assays with biofilms of V. anguillarum, A. hydrophila, Sulfitobacter sp. BR1 and E. coli and assays with synthetic AHLs) have been stored under the Dryad Digital Data repository (http://datadryad.org/) : doi:10.5061/dryad.c3b75.




