Question the Mark: A Review and Assessment of Bat Marking Practices
Funding: This paper was supported in part by the U.S. Department of Agriculture, Forest Service.
Susan C. Loeb and Joy M. O'Keefe are Co lead authors.
ABSTRACT
enBackground
It is often necessary to mark bats through tagging or other means to obtain essential information on their demography, movements and behaviour. However, marks may have lethal or sublethal effects and hence may bias study results. Understanding the effects of marks on bats will allow researchers and managers to develop guidelines to minimise effects.
Aims
Our aim was to review the effects and efficacy of marking techniques used on bats. Our objectives were to (1) describe marks currently used in bat research to identify motivations for marking, trends in commonly used types of marks and trends in the reporting of efficacy and injury rates in the recent literature, and (2) synthesise the body of literature on effects and efficacies of marking.
Methods
We conducted a targeted literature review and a systematic literature review. In the targeted review, we examined all papers on bat marking published from 2013 to 2022 in three bat- or mammal-focused journals to identify trends in bat marking over the past decade. The systematic review was a general review of papers that reported on the effects and efficacy of bat marking from the early 1900s to the present.
Results
Our targeted review found that researchers rarely report the effects of marks on bats and many papers fail to provide details of the marks and marking procedures. Our systematic review found that the effects of marks ranged from minor irritation and behavioural changes to potentially life-threatening injuries, such as changes in body condition; fewer deleterious effects have been reported from newer marking procedures such as passive integrated transponder (PIT) tags.
Conclusions
Further research on marking effects is needed, as well as more thorough reporting in the literature of marks and their effects so that useful guidelines can be developed.
RESUMEN
esA menudo es necesario marcar a los murciélagos mediante diversos medios para obtener información esencial sobre su demografía, movimientos y comportamiento. Sin embargo, las marcas pueden tener efectos letales o subletales y, por lo tanto, pueden sesgar los resultados de los estudios. Comprender los efectos de las marcas en los murciélagos permitirá a los investigadores y directivos desarrollar lineamientos para minimizarlos. El objetivo general de este trabajo fue revisar los efectos y la eficacia de las técnicas de marcaje utilizadas en murciélagos. Nuestros objetivos particulares fueron (1) describir las marcas utilizadas actualmente en la investigación con murciélagos para identificar las motivaciones actuales para el marcaje, las tendencias en las marcas comúnmente utilizadas y las tendencias en el reporte de la eficacia y las tasas de lesiones en la literatura reciente, y (2) sintetizar la literatura disponible sobre los efectos y la eficacia del marcaje en murciélagos. Se realizaron dos revisiones bibliográficas: una dirigida y una sistemática. En la revisión dirigida, se examinaron todos los artículos sobre el marcaje de murciélagos publicados entre 2013 y 2022 en tres revistas especializadas en murciélagos o mamíferos, para identificar tendencias en el marcaje de murciélagos durante la última década. La revisión sistemática fue una revisión general de los artículos con información sobre los efectos y la eficacia del marcaje de murciélagos desde principios del siglo XX hasta la actualidad. En la revisión dirigida se encontró que los investigadores rara vez reportan los efectos del marcaje en los murciélagos, y muchos artículos no brindan detalles de las marcas y los procedimientos de marcaje. En la revisión sistemática se encontró que los efectos de las marcas van desde irritaciones menores y cambios de comportamiento hasta lesiones potencialmente letales, como cambios en la condición corporal. Se han reportado menos efectos nocivos derivados de procedimientos de marcaje más recientes, como el uso de microchips de transpondedor integrado pasivo (PIT tags). Se necesita más investigación sobre los efectos del marcaje, así como informes más exhaustivos en la literatura sobre las marcas y sus efectos para que se puedan desarrollar lineamientos útiles.
1 Introduction
Bats play important roles in ecosystem functions worldwide yet are at risk from and suffering population declines due to disease, habitat loss, climate change, pollution, wind turbines and other anthropogenic stressors (O'Shea et al. 2016; Frick et al. 2020). There is a critical need to understand the impacts of these stressors, mitigate declines and support at-risk populations through research and monitoring. In light of the prevailing threats, it is imperative to gather demographic data to understand vital rates, ecology and population status. These data are needed to inform development and implementation of conservation strategies, even for species of least concern (O'Shea and Bogan 2003; Loeb et al. 2015). To obtain such essential data, it is often necessary to mark bats. Mark–recapture studies provide data on age, survival, roost fidelity and movements and can be used to estimate population size and trends at various spatial scales (Mohr 1953; Ellison 2008). As bats are typically small, cryptic and volant, there are limitations to the types of usable marks and their permanency (e.g., O'Mara et al. 2014; Meierhofer et al. 2025). Bat marking techniques include, but are not limited to, arm bands, PIT (passive integrated transponder) tags, collars, fluorescent powder, light tags, tattoos, wing punches, hair clipping and bee tags (Kunz and Weise 2009).
Marks have been applied to animals for centuries (Blancou 2001), but the effects of marks are not well understood, particularly for wild animals (Murray and Fuller 2000). Short-term effects range from skin irritation and behavioural changes such as overgrooming to more severe effects such as punctures, infections and deformations, which could be lethal or sublethal. Over longer time spans, marks may prompt behavioural, physiological, morphological and demographic changes (e.g., social isolation, mobility impairment, declines in reproduction and body condition), which could lead to mortality (Murray and Fuller 2000; Walker et al. 2012). Understanding the effects of marks on bats will allow us to refine our approaches and alert us to potential biases that may hinder the reliability of data gathered during marking studies.
It may be difficult for a researcher to select an appropriate marking method for their study objectives while minimising negative effects. Often, researchers employ marking methods used in similar studies or those recommended by university or institutional groups (e.g., Institutional Animal Care and Use Committees), professional organisations (American Society of Mammalogists; Sikes and Animal Care and Use Committee of the American Society of Mammalogists 2016) or by state, provincial or federal agencies that issue research permits. However, these recommendations can be vague, providing guidance for marking animals in general but not bats specifically, and may be outdated, not reflecting the best available science. Bat-specific recommendations exist (Aldridge and Brigham 1988; Barclay and Bell 1988; Kunz and Weise 2009), but researchers are still raising concerns over possible negative effects of some marks (Lobato-Bailón et al. 2023; Meierhofer et al. 2025). As the volume of bat research has escalated in recent decades, we are learning more about bat marking techniques that have proven effective, methods that are evolving (e.g., reduction in the size and mass of GPS tags; Kays et al. 2015) and emerging technologies not previously considered (e.g., using the unique patterns on bat wings to identify individuals; Amelon et al. 2017); however, descriptions and evaluations of these techniques are scattered among published literature and unpublished reports. Bats are unique in their morphological features, behaviour and energetics; therefore, a synthesis of information on all bat marking methods, their efficacy to yield study outcomes and their sublethal and lethal effects to bats would be useful to researchers and practitioners monitoring bats globally.
Our goal was to review the effects and efficacy (i.e., recapture or relocation) of marking techniques to inform future guidelines for marking bats. Our objectives were to (1) describe marks currently used in bat research and (2) review the entire body of literature on effects and efficacy of marking techniques. We reviewed recent bat literature (2013–2022) to identify current motivations for marking bats, trends in commonly used types of marks, the efficacy of different mark types and how often researchers report incidental injuries in studies not specifically focused on the effects of marking. We also synthesised the effects and efficacies (where possible) of various marks on bats. Our overall aim is to provide an unbiased review of the types of marks used and their effects on bats.
2 Methods
We conducted two types of literature review: targeted and systematic. For the former, we reviewed articles in three strategically selected journals. For the latter, we more generally searched databases for journal articles using key phrases. For both searches, we noted all reported potential effects of marks, including lethal and sublethal effects. Sublethal effects included (1) physical injuries ranging from small abrasions to infections, broken limbs and embedded bands, plus changes in body mass; (2) demographic effects such as changes in survival or reproductive success; and (3) behavioural effects such as observed social changes or changes to a bat's ability to capture prey. We focused on the most used types of marks (arm bands, radio transmitters, PIT tags and neck collars) but also reviewed papers on less common techniques such as tattoos and wing punches.
2.1 Targeted Literature Review
The overall goal of the targeted review was to identify the nature of bat marking methods over the course of the past decade (note: review was completed in 2023) and in particular, to quantify the number of papers that report recoveries, injuries or other effects of marks; determine what information is reported when marks are used; and identify important keywords for a systematic literature search. This review focused on journals likely to publish studies on many bat taxa from multiple geographic locations around the globe. We examined all issues of Journal of Mammalogy, Acta Chiropterologica and Australian Journal of Mammalogy published from January 2013 to December 2022. Although our targeted review does not follow the standard literature review methodology (e.g., Pullin and Stewart 2006), our approach followed methods commonly used by researchers to assess trends in the current published literature (e.g., Arnold 2010; Hurlbert 1984). While studies on bats are published in a wide number of journals, we reasoned these three journals would have the greatest number of studies on bats worldwide compared to other journals, thus allowing us to more efficiently reach our objectives. For each publication on bats, we recorded whether bats were marked or not. If bats were marked, we recorded species, study duration, purpose for marking, type and size or mass of mark, number of marked individuals, injury rate or other effects if reported, location of captures (roost or landscape) and recapture rates. We classified marks as permanent (duration expected to be > 2–3 months) or temporary based on expected longevity.
2.2 Systematic Literature Review
We also conducted a systematic literature review with the goal of identifying studies that specifically tested or described the effects of marks on bats (i.e., injuries, behavioural changes, death). We conducted a standard search using the Web of Science Core Collection. Keyword searches were [bat OR chirop* AND band* AND effect], [bat OR chirop* AND mark* AND effect], [bat OR chirop* AND PIT tag AND effect], [bat OR chirop* AND transmitter AND effect], [bat OR chirop* AND proximity sensor AND effect] and [bat OR chirop* AND collar or necklace AND effect]. We read titles and abstracts and further reviewed any papers that examined the effects of marks on bats. This was a broad approach that returned many papers to review, beginning in the early 1900s and extending to 2022, but many were irrelevant. Thus, we also inspected the literature cited sections of each relevant paper to find additional papers missed in our search. We did a second Web of Science search with [bat OR chirop* AND PIT tag] to examine trends for the use of this relatively new marking technique and extended our search date range to mid-January 2024. Articles indexed in Web of Science are primarily in English, and thus, papers published in non-English journals were likely missed. Some of these non-English papers were picked up through our searches of reference lists. Details of the marks used, species studied and effects from each paper in the systematic review are presented in Table S1.
3 Results
3.1 Motivations for Marking Bats and Marking Trends
We identified 421 papers published on bats in our targeted review from 2013 to 2022. Of those, 166 papers reported using some marking method (90 of 225 papers we reviewed in Acta Chiropterologica, 69 of 186 in Journal of Mammalogy and 7 of 10 in Australian Mammalogy). Bands, typically placed on the forearm, were the most frequently reported permanent marking method (Table 1). In three studies, bands were placed on the thumbs of large fruit bats (Rousettus madagascariensis and Pteropus natalis; Goodman et al. 2017; Todd et al. 2018; Noroalintseheno Lalarivoniaina et al. 2019). PIT tags were the second most reported permanent mark, followed by collars or necklaces (hereafter, ‘collars’; Table 1). We classified “wingerprints”, which identify individuals by unique collagen–elastin bundles in their wings (Amelon et al. 2017), as a permanent mark even though no external mark is applied, but this method appears to be uncommon. We did not detect any temporal trends in the number of studies using bands, PIT tags or collars over the 10-year period (Figure 1). Radio transmitters were the most reported temporary mark. Other common temporary marks were wing punches (often a by-product of genetic sampling), hair clipping and indelible ink marks. Less common temporary marking methods were nail polish, toenail clipping, miniature glue-on tags (bee tags), fluorescent powder and light tags (Table 1).
| Permanence | Mark type | Africa | Asia | Australia & New Zealand | Europe | Mexico | Central & South America | USA & Canada | Total |
|---|---|---|---|---|---|---|---|---|---|
| Permanent | Collars/necklaces | 2 | 4 | 2 | 5 | 13 | |||
| Forearm bands | 4 | 6 | 4 | 12 | 11 | 16 | 53 | ||
| PIT tags | 1 | 1 | 5 | 4 | 1 | 2 | 8 | 22 | |
| Tattoo | 1 | 1 | |||||||
| Wingerprints | 1 | 1 | |||||||
| Temporary | Bee tags | 1 | 1 | ||||||
| Fluorescent powder | 1 | 1 | |||||||
| Fluorescent tag glued to pinna | 1 | 1 | |||||||
| Geolocator | 1 | 1 | |||||||
| Hair clip | 1 | 6 | 7 | ||||||
| Light tags | 1 | 1 | 2 | ||||||
| Nail polish | 2 | 1 | 3 | ||||||
| Non-toxic paint | 1 | 1 | |||||||
| Pen mark | 1 | 2 | 2 | 4 | 1 | 10 | |||
| Radio transmitters | 4 | 2 | 7 | 16 | 2 | 3 | 23 | 57 | |
| Toenail clip | 2 | 2 | |||||||
| Wing punch | 5 | 2 | 1 | 4 | 5 | 17 | |||
| Total | 18 | 17 | 17 | 41 | 8 | 37 | 55 | 193 |
- Note: Marks are categorised as temporary (2–3 months or less) or permanent (> 3 months).
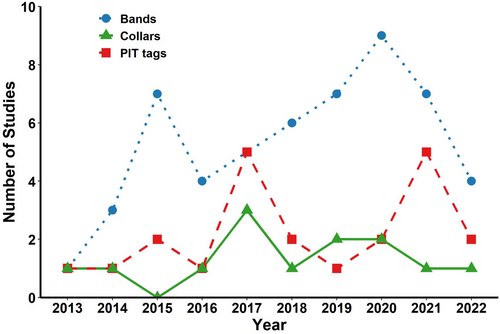
Most marks were used globally but the use of collars varied geographically. Although ball-chain collars and other types of collars have been used successfully on bats of varying sizes (Kunz and Weise 2009), they were used only in parts of the world where the largest bats occur—Africa, Asia and Central and South America. Collars were mainly placed on Phyllostomids or Pteropodids but also on small numbers of Vespertilionids, Molossids, Mormoopids and Rhinolophids (Tavares et al. 2017; Petersen et al. 2018; Palheta et al. 2021).
The goals of marking varied greatly; common reasons included studying population dynamics, community composition, migration, roosting and foraging habitat use, nightly activity patterns, roost fidelity, social organisation, population genetics, diet and parasites. One study examined the effects of bands and necklaces on Carollia perspicillata (Mellado et al. 2022), but only three of the other 165 studies in our targeted review reported injury rates caused by marking: transmitters (Threlfall et al. 2013), wing punches (Greville et al. 2018) and forearm bands (Law et al. 2020). Most studies did not report recovery rates (i.e., recapture or relocation), but 30%–40% of studies using the most common marking methods (banding, PIT tags and radio transmitters) did. Recovery rates were highly variable and varied with capture location (e.g., at a roost or on the landscape) and marking method. Mean (± SD) and median recovery rates were 15.6% ± 20.9% and 7.2% for bands (n = 25 studies), 57.4% ± 31.9% and 68.0% for PIT tags (n = 9 studies) and 78.9% ± 26.9% and 91.7% for radio transmitters (n = 16 studies), respectively.
3.2 Marking Effects and Efficacy
3.2.1 Bands
Researchers have been banding bats for over a century (Ellison 2008). Initially, bird bands were used on bats' legs, but injuries and scar tissue made reading them difficult (Griffin 1945). Forearm banding was introduced in 1940 (Trapido and Crowe 1946) and became standard. All nine researchers surveyed in North America by Hitchcock (1957) reported that aluminium bird bands (e.g., Figure 2a) caused injuries in most bats, and that some bands embedded into wing tissue were chewed and rendered illegible. Consequently, Hitchcock (1957) recommended rounded corner bands and upturned lipped bands to reduce injuries. Herreid et al. (1960) confirmed that rounded, lipped bands decreased embedded injuries in Tadarida brasiliensis (6% vs. 37% for bird bands), though 2-year injury rates were similar for the two band types. They suggested further modifications, leading to the adoption of the omega-shaped lipped bands (Figure 2b,c), now the standard for bat-specific bands, though coloured plastic bands are also used (Ellison 2008; García-Rawlins et al. 2021).
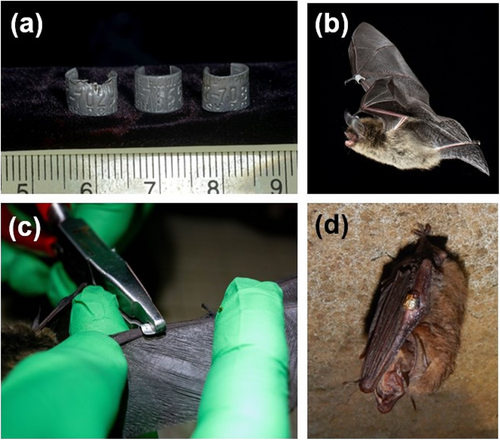
Since the early 1960s, varying efficacy issues and injuries related to bands have been documented across numerous studies. For example, Rousettus leschenaultii and Megaderma spp. either chewed off their bands (unspecified type) or died from infection (Brosset 1962). Corynorhinus townsendii banded with 2.8- or 3.0-mm diameter lipped-alloy bands experienced an 11.8% injury rate from 17 days to 1 year after banding (Pierson and Fellers 1993). There was no severe damage during the first year after banding 199 Nyctalus noctula with aluminium-lipped bands from the U.K. Mammal Society (size not specified; Cranbrook et al. 1965). However, during the following 2 years, six individuals (4% of 143 recaptures) had perforated wings from bands, and four individuals (3% of recaptures) had embedded and chewed bands. Cranbrook et al. (1965) reasoned the aluminium bands were inferior for this large bat and switched to harder magnesium-alloy bands; subsequently, they noted only one of six bats was injured and none chewed the band. Seven per cent of recaptured Myotis macropus banded with metal lipped bands issued by the Australian Bird and Bat Banding Scheme (size not reported) had significant injuries, including perforation of the wing, while 2% of recaptured Myotis macropus had minor injuries such as abrasions (Law et al. 2020). In another study of Australian vespertilionids (Chalinolobus morio, Vespadelus darlingtonia, Vespadelus regulus, Vespadelus pumilus) captured 4–11 years post-banding, injury rates were < 1% for the same types of bands (Law et al. 2023). Considerably higher rates of major injuries (> 20%) have been reported in other studies. Major injuries (e.g., forearm swelling, lack of band movement, lesions, tissue growing around the band, damage to patagium) occurred in 20% of individuals in 14 of 17 species from five families (Megadermatidae, Rhinolophidae, Emballonuridae, Molossidae and Vespertilionidae) recaptured in Australia (Baker et al. 2001). However, some bats were banded with bird bands and some with bat bands (see below and Table S1) and recapture rates were low. Similarly, 22% of Perimyotis subflavus experienced injuries from 2.4-mm diameter aluminium-lipped bands ranging from scarring to punctured wings over a 6-year period, despite standardised application with banding pliers (Perea et al. 2024). Based on a 12-year dataset on injury rates of multiple species of free-ranging bats in north-eastern Spain, Lobato-Bailón et al. (2023) concluded that 2.5–4 mm diameter lipped aluminium–magnesium bands can cause serious health effects ranging from skin thinning to embedded bands (e.g., Figure 2d). Of 790 banded bats in this dataset, 55% exhibited skin lesions near the band, but injury rates ranged from no injuries in Nyctalus lasiopterus (n = 12) to 66% of 259 banded Pipistrellus pygmaeus (Table S1).
In addition to injury rates, some studies examined the effects of bands on body condition. The Scaled Mass Index (SMI) of Pipistrellus kuhlii banded with 2.4-mm aluminium alloy bands did not differ from PIT-tagged bats (Locatelli et al. 2019). Banded and uninjured Carollia perspicillata had a similar SMI to unmarked individuals, but females with more severe lesions had lower SMI, amounting to ~1 g or 5% of body mass (Mellado et al. 2022).
Species are affected differently by bands. Carollia perspicillata exhibited a high rate (54.1%) of chewing on aluminium bands (Fleming 1988), whereas Nyctalus noctula showed considerable variation in chewing, with some aluminium-lipped bands showing no evidence of chewing for 2 years and others damaged to the point of illegibility (Cranbrook et al. 1965). Chewing often makes bands unreadable and may also deform the band (Figure 2a), leading to injury. Excessive chewing might also cause tooth wear (Reynolds et al. 2025). Some species, such as Phyllostomids, seem particularly injury prone. Eleven (50%) Carollia perspicillata in a captive colony banded with 4.2-mm diameter lipped aluminium–magnesium bands developed injuries, and 18.2% of injuries were severe (Lobato-Bailón et al. 2023). However, bats in captivity may be in poor condition due to not adapting well to the feeding regime or stress and not tolerate bands as well as individuals in the wild (Kravchenko et al. 2023). Mellado et al. (2022) also found relatively high injury rates in Carollia perspicillata. Of 531 bats banded with 3-mm anodised lipped aluminium bands, 25% had minor injuries and 10% had more severe wounds (e.g., embedded bands and inflammation). In contrast, only 13 of 3403 (0.4%) of bands of recaptured Pipistrellus pipistrellus (total banded = 15,839) needed to be removed due to injuries (Sendor and Simon 2003).
Age may influence injury rates and chewing rates. Carollia perspicillata and Myotis lucifugus marked as juveniles had lower injury rates and chewed bands less than bats marked as adults (Mellado et al. 2022; Reynolds et al. 2025), suggesting juveniles are more tolerant of bands than adults.
Multiple studies have assessed the effects of various factors such as band type, size and application method. For example, bat bands caused fewer injuries in Miniopteris schreibersii and Myotis macropus than did bird bands, whereas bird bands caused fewer injuries in Chalinolobus gouldii, Kerivoula papuensis, Nyctophilus geoffroyi, Vespadelus darlingtoni, Vespadelus regulus and Vespadelus vulturnus (Baker et al. 2001, see Table S1). Plastic split-ring bands caused injury rates in 80% of Myotis lucifugus, whereas injury rates from No. 2 U.S. Fish and Wildlife bird bands and lipped aluminium bat bands were lower, 15.7% and 2.5%–5.9% (depending on size), respectively (Reynolds et al. 2025).
Band size also affects injury rates. Because band size effects differ among species, Eurobats (2003) made species-specific recommendations for band diameters, generally recommending larger bands for larger bats. This recommendation was corroborated by Vlaschenko (2012) based on 10 years of data on bats. Intraspecies variation in injury rates has been reported for several species where different diameter bands were applied and effects compared. While most of 377 Nyctalus leisleri (forearm length 35–48 mm; GBIF 2023) marked with alloy bands showed no injuries from bands (197 recaptures; 11.2% overall injury rate), rates of injury were 40% for 2.4-mm bands versus only 9.6% for 2.9-mm bands (Zambelli et al. 2009). Likewise, injury rates were higher when Rhinolophus euryale (forearm 43–51 mm, GBIF 2023) and Rhinolophus mehelyi (forearm 48–56 mm, GBIF 2023) were marked with 2.9-mm bands (17.4% and 61.2%, respectively) versus 3.5-mm bands (7.1% and 9.1%, respectively; Dietz et al. 2006). For the smaller Myotis lucifugus (forearm length: 33–41 mm, Fenton and Barclay 1980), injury rates are lower when 2.9-mm bands are used (2.5%) versus 4.2-mm bands (5.9%; Reynolds et al. 2025). Among recaptured Chalinolobus morio (35–42 mm, GBIF 2023), 5.3% exhibited minor injuries and 31.6% exhibited major injuries when marked with 2.5-mm (No. 1), lipped copper–nickel alloy bat bands and no injuries when marked with 3-mm bands of the same material (Baker et al. 2001). Neonatal Tadarida brasiliensis (forearm length: 42–46 mm, Wilkins 1989) marked with No. 1 and No. 2 U.S. Fish and Wildlife Service bands incurred soft tissue injuries, skeletal damage and abnormal development of the forearm and digits (Perry and Beckett 1966); smaller No. 1 bands caused more frequent and severe injuries, but improper band application led to injuries for bats marked with larger bands (i.e., when squeezed too tightly).
Banding techniques, such as the amount of pressure applied to the band, and use of pliers can influence injury rates and body condition. For example, bands (0.15 g, unspecified type, Centro Inanellamento Pipistrelli) applied on Rhinolophus ferrumequinum (13–34 g) with excessive pressure were associated with a measurable loss in body mass (1–2 g) after 1 year, whereas properly tightened bands caused no such mass loss (Dinale 1965). In a 41-week study of the effects of bands on free-ranging Pipistrellus nanus in Malawi, Happold and Happold (1998) found that the degree of band closure was important to injury rates. Most recaptured bats (66% of 447 recaptures of 75 individuals) banded with 2.2-mm alloy flanged bat bands had a free-moving band and were uninjured, but 11% had irritation or healed wounds, 11% had bands immobilised by healed wounds and 11% had an active wound and immobilised band. Happold and Happold (1998) concluded that applying incremental pressure with a finger and thumb so that the flanges just barely closed prevented the band from falling off and resulted in fewer wounds and immobilised bands than squeezing the band until the gap was nearly closed. Banding pliers (Figure 2c) designed to standardise band application and maintain the shape of the band were used to apply 2.9-mm lipped aluminium alloy bands to 1460 Myotis lucifugus and 8 Myotis septentrionalis (Hicks et al. 2013). Half of the bats were banded on both wings to examine retention rates. Only 7 (0.8%) of 701 recaptured bats exhibited an injury 8–13 months after original banding, four of which were related to the band slipping proximally. Retention rates were high (99%), although 6.4% of bands showed signs of chewing. The authors concluded that bands applied with pliers had no effect on survival.
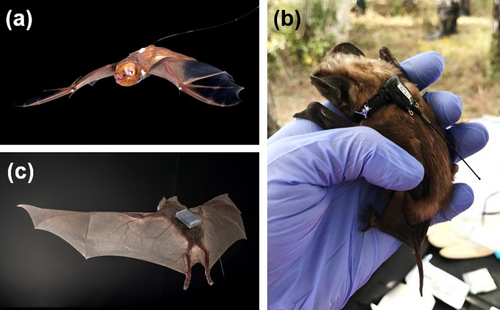
3.2.2 Radio Transmitters and Proximity Sensors
Radio transmitters (including GPS tags) and proximity sensors are temporary marks useful for studies on movement, behaviour and other aspects of bat ecology. Radio transmitters (Figure 3a,b) were first used on bats in the 1960s, becoming more common after the late 1980s (Clerc et al. 2021); proximity sensors (Figure 3c) have only recently been used on bats (Ripperger et al. 2016). Device mass, attachment type (e.g., glue, suture or collar), location on the body and novelty are factors that contribute to how these marks affect bats and impact their behaviour. All studies reported on sublethal effects except for one. Three Nyctophilus gouldi were found dead 7 days after radio tagging (Threlfall et al. 2013). However, the cause of death is unknown and could have been due to other factors as all three bats had high endoparasite loads.
Mass is an important factor influencing flight dynamics, with additional mass affecting manoeuvrability and foraging ability, particularly in cluttered habitats (Aldridge and Brigham 1988). Based on radio tracking studies of fruit bats, Bradbury et al. (1979) suggested a 10% rule (i.e., that the mass of the transmitter be ≤ 10% of body mass). However, they suggested that smaller insectivorous bats may need a lighter load. Aldridge and Brigham (1988) tested the manoeuvrability of Myotis yumanensis with disks weighing 5%–33% of their body mass in an obstacle course consisting of vertical twine of varying intertwine distances. Bats without disks could negotiate significantly smaller intertwine distances, and intertwine distance was negatively associated with body mass and wing loading for bats with and without disks. Because manoeuvrability should be inversely proportional to body mass, the authors assumed that a 5% increase in body mass would result in a 5% decrease in manoeuvrability. Consequently, they suggested radio transmitters should be < 5% of body mass for bats weighing < 70 g and that they should only be used when prey is abundant, as foraging ability may be affected.
Adherence to the 5% recommendation varies widely across studies and the American Society of Mammalogists currently recommends using transmitters that weigh 5%–10% of an animal's body mass (Sikes and Animal Care and Use Committee of the American Society of Mammalogists 2016). While 98% of 148 studies reviewed by O'Mara et al. (2014) used transmitters < 10% of bats' body mass, only 51% of studies used tags that weighed < 5%. More recently, Meierhofer et al. (2025) found that 55% of published studies from 1999 to 2022 reported transmitter weights > 5% of bats' body mass. However, effects of transmitter mass on bats have rarely been tested. A meta-analysis of transmitter effects on Nyctalus noctula found that although most individuals lost mass after tagging, this was not related to the mass of the transmitter but instead to the duration of tag attachment and initial body mass (Kelling et al. 2024). However, emergence time was delayed and foraging duration increased with tag mass. Radio-tagged Sturnira lilium emerged from their roosts about 25 min later than untagged individuals (Fenton et al. 2000). In contrast, activity time and behaviour of Myotis evotis did not differ between radio-tagged and untagged individuals despite transmitters weighing 5.9%–8.9% of body mass (Chruszcz and Barclay 2003). Tag loading (transmitter mass/body mass) did not affect Plecotus auritus foraging distance or time spent within 0.5 km of the roost (Entwistle et al. 1996) nor foraging time for Myotis moluccarum (Barclay et al. 2000). Furthermore, GPS tags weighing as much as 14% of body mass did not affect the ability of Leptonycteris yerbabuenae to hover at flowers or time spent foraging, compared to bats with much lighter transmitters (3.3% of body mass; Goldshtein et al. 2020).
While transmitter or sensor mass is important, the distribution of that weight on the bat's body relative to the bat's centre of mass (COM) must also be considered (Katzner and Young 2024). Based on weight-balanced calculations for aviation, devices placed at COM have little impact on flight control or stability, but the farther the device is from the bat's COM, the greater the effect of device mass on the bat's ability to maintain flight control and stability. For example, a device weighing 3% of body mass could substantially impact Lasiurus cinereus' ability to maintain stability if it was placed on the hip or tail, and even a collar could affect flight. The authors conclude that the 5% threshold only holds if the device is placed at COM. However, it should be noted that these calculations do not consider the energetic costs of the additional weight of the devices.
Some studies tested the effect of transmitters on foraging success, behaviour, body condition and survival. Foraging success rates of Lasiurus cinereus carrying radio transmitters weighing 2.5%–3.7% of their body mass were similar to individuals without transmitters (Hickey 1992). However, Lasiurus cinereus are relatively large and usually forage in open areas; closed-space foragers may be more affected due to a need for greater manoeuvrability. Measures of condition (body mass or body condition index) did not differ between radio-tagged and untagged hibernating Myotis lucifugus after 55 days (Jonasson and Willis 2012), tagged and untagged Nyctalus noctula (Roeleke et al. 2016; Voigt et al. 2020), nor tagged and untagged Rhinolophus ferrumequinum (Park et al. 2000). Apparent survival and fitness of Eptesicus fuscus with radio transmitters weighing 2.6%–5.7% of their body mass (mean = 3.7%) did not differ significantly from untagged bats; 37 of 40 bats were alive 1 year after radio-tagging (Neubaum et al. 2005). Based on a meta-analysis of 12 studies and 17 estimates of transmitter impacts, Meierhofer et al. (2025) concluded that transmitters affect bat behaviour and body condition, but that the overall magnitude of the effects may be negligible.
Transmitter attachment method greatly affects the efficacy and effects of this mark. Radio transmitters can be attached using adhesives, sutures or via collars (Amelon et al. 2009; Castle et al. 2015). Non-toxic, surgical adhesives are commonly used to attach transmitters to bats' dorsa (Figure 3a), with retention averaging < 10 days (O'Mara et al. 2014), but retention varies greatly depending on adhesive type (Carter et al. 2009). Use of adhesives can result in hair loss which could affect bats' thermal capabilities, particularly during hibernation. Because temperate zone bats usually moult during late spring or summer, a bald spot created during late summer may last throughout winter hibernation. For example, all 31 Myotis daubentonii banded and radio-tagged after moult and recaptured the following season had bald spots (Rolfes et al. 2021). However, nine of 11 females recaptured were reproductive, suggesting limited fitness impacts. Kurta and Murray (2002) made similar observations of bald spots and signs of reproduction for two radio-tagged Myotis sodalis recaptured the next summer, while nine other tagged bats had no bald spot upon recapture. Adhesive and solder on wires may also add to the device's mass and should be considered in the weight ratio.
Transmitters attached via collars (Figure 3b) are more common for larger bats. Three transmitter collar designs were tested on Pteropus alecto, Pteropus vampyrus and Pteropus neohibernicus in the field and on Pteropus alecto, Pteropus scapulatus and Pteropus conspicillatus in captivity (Smith et al. 2011). Wild bats tolerated the collars well, as did all five captive Pteropus alecto fitted with a leather collar with sheepskin glued to the inner surface, although three had some reddening under the collar to day 9 but not through day 28. In a second captive trial, one of eight Pteropus scapulatus and two Pteropus conspicillatus did not tolerate the collar, and it was removed. For the remaining animals, adverse effects were only observed 8 weeks post-collaring when issues such as wet dermatitis and ulceration, slipping of the collar and suppurative dermatitis began to appear. Several collar designs were tested on two neotropical fruit bats (Artibeus jamaicensis and Uroderma bilobatum), Mollossus mollossus and the European vespertilionid Nyctalus noctula (O'Mara et al. 2014). Collars made from shoelaces and tied with degradable suture material were the most effective and degraded within approximately 30 days. These collars worked well on Artibeus jamaicensis, Uroderma bilobatum and Nyctalus noctula and extended the effective transmitter life by ~21 days for Nyctalus noctula compared to glue. However, half of the Mollossus molossus removed the collars or continually scratched at them.
Suturing transmitters to the dorsum has also been tested on Eptesicus fuscus and Lasiurus cinereus (Castle et al. 2015). No irritation was observed on two Eptesicus fuscus captured 11–12 days after attachment, but the fur was worn away for one Eptesicus fuscus recaptured 227 days later, and there was loose skin near the caudal end of the transmitter, but no bleeding, bruising or inflammation. Of several Lasiurus cinereus captured 2–26 days post attachment, one showed signs of chafing despite the tag still being fully attached. Two Lasiurus cinereus were recaptured 213–224 days post-attachment; one had no fur loss, skin or wing injury or infection, whereas the other individual's tag had rotated and was attached only at one corner, but there was no skin damage. The authors concluded that, overall, there were no conspicuous adverse effects of suturing transmitters onto bats.
Whatever attachment method, the presence of a novel item may affect individual and colony behaviour. This has been most closely examined in the case of proximity sensors (Figure 3c), which log the amount of time animals are within a user-determined distance from each other. Proximity sensors placed on Myotis myotis, a species that roosts in tight clusters, appeared to have no behavioural effect based on videos (Ripperger et al. 2016). Desmodus rotundus appeared to habituate relatively quickly to proximity sensors attached with skin-bonding adhesive, with self-grooming decreasing after the first hour and remaining steady (Kline et al. 2021). However, two bats removed the tags and increased grooming rates, suggesting that secure attachment is important.
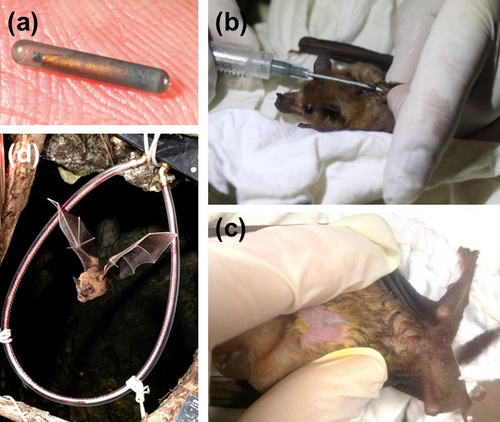
3.2.3 PIT Tags
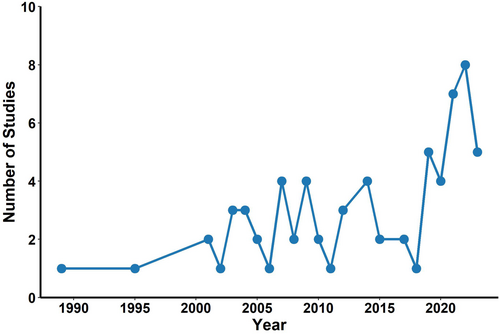
PIT tags are electromagnetic chips encoded with a unique number and injected under the skin (Figure 4a–c). When scanned with a specialised reader, the number is transmitted to a data logger and stored. PIT tags are a relatively new technology for bats; Barnard (1989) first published on their use in captive Eptesicus fuscus. While use is increasingly common, particularly over the last decade (Figure 5), the literature on effects is scant.
Possible effects of PIT tags include infection, irritation or hair loss at the injection site, movement of the tag within the body or undetected impacts to health. Body condition and recapture rates were similar for Myotis chiloensis and Tadarida brasiliensis with and without PIT tags in central Chile (Escobar et al. 2022). Similarly, body condition of Pipistrellus kuhlii PIT-tagged with 8.4-mm tags in southern Europe did not differ from banded individuals 2.6 years after marking (Locatelli et al. 2019). For Myotis daubentonii marked with 12-mm PIT tags and bands, there was no difference in recapture rate, body condition or reproductive success compared to individuals without PIT tags over a 3-year study (Rigby et al. 2012). No skin lesions were found in a captive colony of 21 Carollia perspicillata marked with 10-mm PIT tags, nor did video observations show evidence of discomfort or behavioural changes (Lobato-Bailón et al. 2023). These authors also reviewed data on recaptures of bats over a 12-year period in Catalonia, Spain, concluding that PIT tags did not cause undue impacts as no injuries were found for Myotis schreibersii (n = 116), Nyctalus lasiopterus (n = 5), Nyctalus leisleri (n = 79) or Pipistrellus pygmaeus (n = 246).
Efficacy of PIT tags, particularly their loss rate, is important in evaluating their use. Several studies used double marking methods to determine loss rates. Thirty-nine per cent of recaptured tagged and banded Myotis daubentonii either shed their PIT tags or the tags no longer scanned at the end of 3 years (Rigby et al. 2012). However, the success of PIT tagging increased as researchers gained more experience (e.g., correctly positioning tags). In contrast, PIT tag loss occurred in only 2.7% of 146 recaptured Chalinolobus gouldii (van Harten et al. 2021). The size of the PIT tag may affect efficacy. Smaller PIT tags (9-mm) implanted in Myotis lucifugus were detected significantly less often than 12-mm PIT tags, which the authors attributed to greater loss of the 9-mm tags and lower detectability by the antennas around each entrance to a roost in a building (Sandilands and Morningstar 2021).
Using adhesive to close the PIT tag injection site may cause hair loss. While fur loss was initially observed in 52 tagged Miniopterus orianae bassanii, 10 individuals captured 1–2 years after initial tagging had no external signs of tagging (van Harten et al. 2020). In three instances, thinner or atypically pigmented fur was observed at the injection site. All tags remained in their subcutaneous position between the scapulae, with no signs of tag migration. Hair loss < 10 mm in diameter was also observed in Chalinolobus gouldii at the injection site, which had received a small amount of glue, approximately 1 month after tagging, but usually regrew after 2 months (van Harten et al. 2021). However, one subadult female experienced more extensive hair and weight loss.
Many studies use permanent PIT tag readers within or outside roosts to monitor bat activity, social behaviour and survival (e.g., Ellison et al. 2007; Braun de Torrez et al. 2020; Waag et al. 2022; Rensel et al. 2023). Readers and antennas are arranged around entry and exit points of roosts and hibernacula to detect bats passing nearby (Figure 4d). Because bats need to be within ~50 cm of the antenna for the tag to be read, some access points may require extensive arrays. A novel item at a roost, no matter its size, may affect behaviour and could cause abandonment. However, no behavioural effects of PIT-tag antenna arrays were found for Myotis lucifugus, Eptesicus fuscus, Perimyotis subflavus and Myotis sodalis at two cave entrances during spring emergence (Britzke et al. 2014). The primary behaviour observed was circling, which is consistent with typical spring emergence behaviour. Contact with the reader, including landing, only accounted for 1%–3% of the behaviour during exiting. The authors concluded that careful placement of PIT tag antenna arrays does not limit bat movement and activity at cave entrances. However, site-specific configurations may be needed to limit behavioural impacts for some bat species (Adams and Ammerman 2015).
3.2.4 Collars
Multiple types of collars have been used, but ball-chain necklaces paired with coloured beads or bird or bat bands for individual identification are frequently used (Kunz and Weise 2009). Other collar types include plastic cable ties, coloured beads attached to nylon threads and colourless plastic tubes with identifying information inserted inside (Esbérard et al. 2011). Esbérard and Daemon (1999) did not observe significant issues with the plastic cable tie collars, including when applied on species with a gular gland, but Rodríguez-Posada and Santa-Sepúlveda (2013) observed deep skin abrasions in one Carollia brevicauda and one Sturnia ludovici. Kunz and Weise (2009) cautioned against using cable tie collars without vinyl tubing because bats can tighten them by chewing, resulting in injuries and choking. Injuries can also occur using ball-chain collars. Fifteen (2.2%) ball-chain necklaces were removed from 686 Carollia perspicillata recaptured over 2 years due to injuries (Fleming 1988). In contrast, no flesh injuries were observed in 411 Carollia perspicillata collared with ball-chain necklaces recaptured at least once (Mellado et al. 2022); however, 5% had alopecia which may or may not have been related to the collar. While collars may cause fewer injuries, collars may be lost more often than bands, resulting in biased estimates of survival (Mellado et al. 2022), and fading bead colour or rusting wire can occur (Alencar et al. 2020). Proper fit is essential to reduce both injury and loss rates. During an extensive study of the bats of Barro Colorado Island, thousands of bats were marked with ball-chain collars with low injury rates and a 6.6% loss rate (Handley et al. 1991). The authors suggest collars should be checked to assure that they are not too tight.
3.2.5 Marks to the Skin
Tattooing unique numbers or codes has been used on bats and was first reported by Griffin (1934). He used a 5-pointed tattoo needle, designed for marking rabbits, to apply numbers to the wings of Myotis lucifugus. The method proved unsatisfactory due to the lengthy application time, limited information that could be included in the mark and the need to recapture the individual in hand as the tattoo is generally on a body part not visible on a roosting bat. Bonaccorso and Smythe (1972) used a ‘tattoo outfit’ to punch numbers into the wing membrane (up to 5 digits applied at a time) and recaptured 12 of 91 tattooed Artibeus jamaicensis and 1 of 17 Saccopteryx bilineata with no injuries. Marks were legible after 32 days in wild-caught animals and 48 days in a captive animal. Patagium perforation was also tested in Myotis chiloensis and Tadarida brasiliensis and compared to hair-dye (Escobar et al. 2022). Maximum time that both types of marks were visible was similar (40–42 days), but a greater proportion of patagium perforation marks were visible at 40–42 days than hair dyes. A commercially available tattoo machine and black pigment, requiring marking time of < 20 s per bat, was used to mark 241 captive Rousettus aegyptiacus and 7771 wild-caught bats of 12 species in South Africa (Markotter et al. 2023). None of the captive Rousettus aegyptiacus showed signs of discomfort and 44 of the first captive bats to be tattooed still had legible tattoos after 927 days. Tattoos of some subadults and all neonates required reapplication due to fading. Of 439 recaptured wild bats over 256–2465 days, only 12 had illegible numbers, mostly on smaller species. The authors concluded that tattooing may be best for larger species, tattoos should be examined for legibility before release and training was essential.
Freeze-branding, that is, freezing the skin, destroys melanocytes in hair follicles and hair re-grows white. Sherwin et al. (2002) used branding irons (copper washer attached to a dowel) to freeze mark four species on their dorsa, observing them in captivity for up to 6 months prior to release. Although bats exhibited some discomfort during the processing, no long-lasting behavioural changes were observed. Four Corynorhinus townsendii were recaptured 3 years post-branding with still-readable marks. However, a limited number of symbols are available, and thus, this mark may not be suitable for large-scale studies.
Wing punches via sterilised biopsy tools create a short-term mark, as holes usually heal within 16–120 days, depending on punch size, location and species (Davis and Doster 1972; Faure et al. 2009; Weaver et al. 2009). Bleeding may occur if the punch is taken from an area of the wing with high vascularisation. Wounds created by wing punches may tear (Greville et al. 2018) and expand beyond the original punch size (Faure et al. 2009; Ceballos-Vasquez et al. 2015; Greville et al. 2018). The rate of wound healing can vary seasonally, with 8-mm diameter wounds healing faster during summer (4 weeks) than in winter (7 weeks; Ceballos-Vasquez et al. 2015). Biopsy punches in tail membranes of Eptesicus fuscus and Rousettus aegyptiacus healed significantly faster than those in the wings (Faure et al. 2009; Pollock et al. 2016; Greville et al. 2018). However, holes in tail membranes may put bats at risk of being entrapped on such objects as automobile antennas (Broders et al. 2013). It is not known if entrapment could also occur from holes in the wings.
3.2.6 Uncommonly Used Marking Methods
Other methods have been tested for short- and long-term marking of bats but are less common. Visible implant elastomers (VIE) are coloured polymers that are subcutaneously injected as a liquid into the forearm or leg of the animal, turning into a pliable solid. VIEs were tested on 650 bats of 22 species double marked with a collar in north-eastern Brazil (Alencar et al. 2020). The authors subsequently recaptured 54 individuals of seven species within 9 months, and the VIE was visible in all animals with no skin irritation or rejection of the VIE.
Small coloured and numbered disks such as bee tags or reflective tape glued to the backs or heads of bats have been used in hibernacula to track their movements (Kirkpatrick et al. 2019; Brown et al. 2025). Stickers have also been used on the bats' ear pinnae (Hooper and Amelon 2023). Tags can be applied to bats while they are in torpor without handling by using forceps or tweezers to place the tag on the bat. However, tactile disturbance, even just 15 s of rubbing, can cause a bat to increase its energy output and perhaps arouse (Speakman et al. 1991). Tag loss can also occur; Brown et al. (2025) recovered 4 of 39 tags (10.3%) on the floor of the hibernacula but 8 of 21 (38.1%) Perimyotis subflavus marked in the first month of the study retained their marks for at least 5 months, and 74.3% of bats that were marked for at least 3 months still had their tags at the end of the season.
Fluorescent powder is another marking method to study movements of bats both within hibernacula (Hoyt et al. 2018) and between maternity colonies and foraging areas (Medellin et al. 2018). The powder is dusted on the animal, and then, an ultraviolet light is used to either determine whether the animal has been dusted previously or to track trails of individual bats within a hibernaculum. To our knowledge, the effects of fluorescent powder on bats have not been tested but tests with deer mice (Peromyscus maniculatus; Stapp et al. 1994) showed that most (87%) had evidence of powder in their lungs, and 27% showed signs of pneumonia 3–27 days after marking. No mortality was observed, and the authors concluded that deleterious effects do not occur in most animals.
The collagen–elastin bundles in the wing, or wingerprints, are distinctive among individual bats and are another way to identify individuals (Amelon et al. 2017). This method requires no external marks or alteration of any body parts, although bat wings need to be photographed to obtain permanent records of individual wingerprints so that new and recaptured bats can be compared to known individuals. Thus, potential effects are limited to handling and time needed to photograph. This method has been used successfully to identify relatively small numbers of wild bats in flight cages and bat boxes (Fjelldal and van der Kooij 2024; Sørås et al. 2022, 2023). However, bats must be kept still long enough to get a good photograph, the wing must be held properly so that no parts are obscured, is time-consuming and would benefit from the development of pattern recognition algorithms that can automate identification (Amelon et al. 2017). Another technology, the p-Chip (p-Chip Corp.), consisting of a miniature laser-activated microtransponder semiconductor tag read with a red laser wand held in close proximity and containing a 9-digit alphanumeric code, has recently been tested in bats (Seheult et al. 2024). Thirty captive Eptesicus fuscus received chips dorsally over the right second metacarpal in the wing and dorsally over the right tibia. The wing location appears to be preferable due to shorter handling times, more consistent reading times, better visibility of the chip and zero instances of unreadable chips. Benefits over PIT tags include reduced costs and smaller needles, but they have more stringent proximity and operation requirements. These chips have also been tested in the field in 21 species in Peru and tags were detected 1–344 days after tagging with no signs of irritation at the injection site (Pellón et al. 2025).
4 Discussion
Marking bats makes it possible to answer an array of ecological and behavioural questions, but researchers need to balance appropriate marking type and method for the target bat species and study objectives to minimise the risk of harm. However, researchers should be aware that mark loss, injuries and behavioural changes related to mark application can affect study results and conclusions, thus negating the utility of the marking method (e.g., Mellado et al. 2022). We determined that frequently used marking techniques can have sublethal negative effects on bat health and behaviour; lethal effects are difficult to document for individuals in the wild. Rates of injury and severity of negative effects vary by bat species, maturity, behaviour, seasonal timing, mark permanency, application method and personnel experience and training. The impacts of marking within and across these factors are not well studied, and we lack standardised reporting of negative effects for marks used on bats. Collectively, this comprehensive literature review demonstrates that the bat researcher and management community should give more attention to how we mark bats, how bats are affected and how marking procedures affect study results because it is clear that commonly used marks are imperfect and there is room for improvement.
The effects of marks range from minor irritation and behavioural changes to potentially life-threatening injuries, such as changes in body condition. Usually injuries are superficial, such as skin irritation or hair loss. However, metal or plastic bat bands, a type of mark intended to be permanent, tend to cause more damage, sometimes becoming embedded in the skin, hindering bone development or slicing the wing membrane. Bats may be distracted by marks, spending time trying to dislodge them (e.g., by chewing, Fleming 1988) and this may prevent bats from engaging in normal behaviours (e.g., emerging from roosts later, Fenton et al. 2000). Trimming fur and the use of glue or sutures to apply radio tags or to close injection sites for PIT tags can lead to hair loss and skin irritation (e.g., Rolfes et al. 2021; van Harten et al. 2021); collars may have similar effects (Fleming 1988). Heavier marks, such as radio transmitters, may impose energetic costs and poorly placed marks may affect flight dynamics (Katzner and Young 2024); however, when researchers adhere to the 5% rule and place tags appropriately, effects seem to be negligible (Meierhofer et al. 2025). Injury rates from marking are likely underestimated from field studies. For example, injured animals may be less likely to be recaptured. The inherent difficulty of knowing the fates of animals in the wild means we do not understand the frequency of lethal or sublethal effects. Only when bats are marked with radio transmitters can we locate a marked bat after it has died (e.g., Threlfall et al. 2013).
More permanent marks, such as bands and collars, are most likely to negatively impact bats. However, PIT tags, also meant to be a permanent mark, may be an alternative to bands and collars. PIT tags may cause fewer injuries and are gaining in popularity in bat monitoring and research, although loss rates may be higher than for bands. We encourage more work to identify appropriate PIT tag sizes, increase detection ranges for antennas, report effects when using this marking method and promote more widespread use of PIT tag readers.
Bands have received the most study of the marks we considered; early bat researchers found that rounded and lipped bands (omega shape) are least likely to abrade or perforate bat wings (Herreid et al. 1960) and this omega shape has been adopted as the standard (Ellison 2008). Multiple studies demonstrated that band fit and how bands are applied affect the rate of injury. We encourage customising bands to the diameter appropriate for the target species and having trained personnel create an optimal gap with their fingers or banding pliers at the flanges to reduce the risk of injury (e.g., Hicks et al. 2013). Even with these improvements, bands remain problematic for some species and in some contexts (e.g., Lobato-Bailón et al. 2023), so careful consideration should be given to these factors. Furthermore, injury rates may increase over time, and thus, several years of study may be needed to fully assess the effects of bands on bats (Perea et al. 2024). Other factors may also affect injury and healing rates from bands but have not been examined to our knowledge. For example, microclimate, particularly humidity, roost structures (e.g., small crevices that may increase abrasion) and wing morphology may be important factors.
Because effects vary by bat species, age, time of year when the mark is applied and experience level of personnel for most marks, careful selection of the appropriate mark for the target species and age, plus more personnel training and standardisation of methods, should reduce injury rates and improve the efficacy of marks. As new techniques such as VIE, tattoos and wingerprints come into more widespread use, testing their effects and efficacy will be critical.
Our targeted review of papers recently published in mammal-focused journals showcased some of the difficulties inherent in assessing the effects of marks on bats and their efficacy. Only three of 166 papers reported injury rates associated with marking, and only 29%–38% of studies on bands, PIT tags and transmitters reported recovery rates (i.e., efficacy of the marking method). Furthermore, many studies failed to report critical data such as size or mass of the bands or transmitters, application or attachment method or details on the numbers of animals marked, their body masses or other morphometric information that might influence injury rate (e.g., the size of the propatagium or forearm width of bats with bands applied to the arm). Thus, we echo Meierhofer et al.'s (2025) call for more thorough reporting of information on the animals marked, the marks themselves, application methods and injury rates or other effects, including instances when no effects were found. Developing a standard injury scale that also describes the state of the mark (e.g., chewed, misshaped, out of place) would allow for comparisons across marking methods and allow practitioners to assess when injuries occur after the mark is applied as well as what types of injuries are most likely to heal. Considering both aspects might allow the researcher to make a decision about removing the mark or leaving it. For example, a scale documenting effects on bats could range from 0 to 4, where 0 = no lesion/injury or hair loss, 1 = minor irritation (should heal); 2 = major irritation (may not heal); 3 = permanent scar/bump, ingrown band or open wound; and 4 = wound extending beyond place of marking (see Law et al. 2020; Mellado et al. 2022; Perea et al. 2024, for examples). A scale related to the mark could range from 0 to 2, where 0 = good condition, identifiable, 1 = damaged/not working, partially identifiable, 2 = damaged/not working, unidentifiable. However, we note that each type of mark will have its own effects and will change in different ways over time, so mark-specific scales may be necessary.
Understanding and quantifying the effects of various marking techniques on bats as well as their efficacy is critical for weighing potential costs versus benefits of the information to be gained from marking bats (Perea et al. 2024). Furthermore, it will be necessary to assess how violation of assumptions regarding mark loss and changes in behaviour related to marking affect demographic parameters and bias our interpretation of data collected on marked animals (Lobato-Bailón et al. 2023). Marking effects can be assessed through several pathways including captive situations (e.g., Lobato-Bailón et al. 2023; Markotter et al. 2023), short- and long-term studies of known and accessible wild colonies (e.g., Neubaum et al. 2005; Mellado et al. 2022; Perea et al. 2024; Reynolds et al. 2025) and more thorough reporting of information in the published literature as well as to animal care regulators. Studies tend to be conducted on marks that are perceived to have greater effects (e.g., banding, transmitters), while studies showing no effects are less likely to be published (Murray and Fuller 2000). However, studies of all marks are necessary as our perception may be incorrect, and evidence of no effects is valuable. Robust experimental design is critical to testing the effects of marks, particularly selecting proper control or reference groups for lab and field studies (Murray and Fuller 2000). For example, understanding the effects of bands by comparing to PIT-tagged animals does not provide a true non-marked control. Furthermore, the power to detect statistically significant effects is often low in marking studies, particularly when the effect size is low (Cleasby et al. 2021). Thus, large sample sizes are often required. With a greater understanding of the effects of marks, we may be able to develop a decision tree to guide use of marks, enabling bat biologists to select the most appropriate mark for their research and monitoring objectives and creating standards across practitioners and guiding agencies.
It is crucial that we strive for more thorough reporting about marking in the published literature. When presenting work in which bats are marked, we encourage scientists to report, and reviewers and journal editors to critically evaluate, detailed information on the specifics of the marks and marking procedures, the number of animals marked, return rates, mark loss and any effects such as injuries (see potential scale above), changes in body condition (e.g., hair loss, weight change), demographic effects (survival and reproductive output) and direct mortality that are observed. Furthermore, storing data in centralised repositories (e.g., the Bat Band Centre at the Research Museum Koenig, the Bat Conservation Trust, North American Bat Monitoring Program) will increase the value of the data for regional assessments and provide greater resources for examining mark effects and efficacies. The authors' own experiences have been that data are often reported to agencies (state, federal, provincial) and not necessarily published or easily accessible to the scientific community.
Bat marking can be a very powerful tool to elucidate many aspects of bat ecology. However, even tools wielded with the best intentions can cause harm when used without complete understanding. More research, more thorough reporting and better training and standardisation will lead to better outcomes for marked bats while achieving research and monitoring objectives for bat researchers.
Author Contributions
Susan C. Loeb: conceptualization (co-lead), writing – original draft preparation (co-lead). Joy M. O'Keefe: conceptualization (co-lead), writing – original draft preparation (co-lead). Alyssa B. Bennett: writing – review and editing. Robert M. R. Barclay: writing – review and editing. Ashleigh B. Cable: writing – review and editing, visualization. Sarah M. Gaulke: writing – original draft preparation (supporting), writing – review and editing. Fernando Gual-Suárez: writing – original draft preparation (supporting), writing – review and editing. Samara Pérez-Harp: writing – review and editing. Vona Kuczynska: writing – review and editing, visualization. Cori L. Lausen: writing – review and editing. Bradford J. Westrich: writing – review and editing, visualization.
Acknowledgements
This paper was supported in part by the U.S. Department of Agriculture, Forest Service. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA, U.S. Fish and Wildlife Service or U.S. Government determination or policy. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. We thank Jennifer Kindel and Rodrigo Medellin for their input to various stages of this manuscript, Meghan O'Keefe for Russian translation and Brock Fenton, Richard Stark and Elizabeth Braun de Torrez for providing some images used in figures. F.G.-S. and S.P.-H. thank Dr. Claudia Moreno for her technical support at the LECVT.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




