A meta-analysis of the effects of anthropogenic disturbances on tropical mammal functional diversity
Editor: RG
Abstract
- Understanding the processes that shape biological communities under a variety of perturbations is a central challenge in ecology and conservation. Mammals contribute to critical functional processes of an ecosystem, such as seed dispersal to maintain forest carbon stocks.
- The analysis of functional diversity, which measures the range and value of the ecological traits of organisms, provides critical information to understanding the link between biodiversity and ecosystem functioning.
- A growing number of studies have investigated the effects of anthropogenic disturbances on mammalian functional diversity; however, their results are very heterogeneous.
- Here, we conduct a comprehensive meta-analysis of the effects of such anthropogenic disturbances on mammalian functional diversity in the tropics. We highlight major trends and analyse the influence of the type of anthropogenic disturbance, subgroups, and the functional diversity index used/applied.
- Our results indicate a negative effect of anthropogenic disturbances on mammalian functional diversity, particularly on functional richness (FRic) and functional dispersion (FDis). Habitat isolation was the stressor with the strongest effect, while agriculture and urbanisation showed a positive link with functional diversity.
- These results indicate that anthropogenic disturbances not only affect taxonomic diversity, but also reduce the functional diversity of mammals, which is likely to affect ecosystem functioning and possibly ecosystem service provision.
INTRODUCTION
Growing human populations and the increasing demand for natural resources have severely impacted biodiversity, with many natural habitats included in the mosaics of land converted to human use (Cisneros et al. 2015). Agriculture, hunting, logging, and especially habitat loss and fragmentation are known to be strong predictors of loss of mammalian diversity (Gonzalez-Suarez et al. 2013, Brodie et al. 2021). Several studies have documented the effects of anthropogenic disturbances on species loss (Chiarello 1999, Umetsu & Pardini 2006, Lyra-Jorge et al. 2009, Dotta & Verdade 2011), but other dimensions, such as phylogenetic and functional diversity, are still poorly addressed.
Knowledge of functional diversity provides essential information for understanding the relationship between organisms and their functions in the ecosystem. It is therefore considered important for assessing the anthropogenic impacts on ecosystem processes. The position and amount of functional space occupied by species, for example, can be related to the use of environmental resources, such as food, territory, and sexual partners, composing different dimensions of functional diversity. Each species has a unique set of traits that are associated with one or more functions. Thus, if a species is negatively affected by some disturbance, its functions may also be affected, providing a different response compared to traditional measurements such as species richness (Safi et al. 2011, Mouillot et al. 2013). Species extinction can be caused by a variety of factors. However, there is no consensus about which anthropogenic threats cause declines in functional diversity (Brodie et al. 2021). Changes in environmental conditions after disturbance can act as a filter, allowing only a narrow spectrum of traits to persist (Edwards et al. 2014).
A growing number of studies have identified functional diversity as a crucial tool for assessing ecosystem processes. The development of indices that measure functional traits and express species distribution in a multidimensional space (Petchey & Gaston 2006, Villéger et al. 2008) is driven by the need to quantify the facet of diversity related to the numerous complementary components of the functional structure of communities. However, the relationship between these indices is not well established. Some are highly correlated, and there is still no consensus on the ideal ones to use (Mouchet et al. 2010). The choice of the index can influence the outcome of the analysis. Ideally, the use of a combination of indices that address these complementary characteristics would allow a more complete assessment of the situation (Mouchet et al. 2010).
Despite the important ecological functions of mammals, such as seed dispersal, predation and population regulation, and their role as parasite reservoirs and disease vectors (Ahumada et al. 2011), studies relating mammalian functional diversity to anthropogenic disturbances are still scarce and reveal heterogeneous results (Edwards et al. 2014). This heterogeneity may be related to the fact there is a wide range of different anthropogenic disturbances that affect mammals in different regions (Ahumada et al. 2011, Edwards et al. 2014). Furthermore, distinct species are known to respond differently to disturbances, with some species being more affected than others. Changes in the environment can affect the abundance of distinct species. Those that perform unique functions in ecosystems are of particular interest in this context. If human-induced disturbances affect species with these characteristics or significantly reduce their abundance in the community, ecosystem function is more likely to be impaired (Mouillot et al. 2013).
- Functional diversity would be negatively related to anthropogenic disturbances (however, we predicted different responses depending on the particular disturbance).
- More negative impacts would occur on medium and large mammals because of their larger home ranges and higher vulnerability to anthropogenic disturbances, especially stressors associated with habitat loss and fragmentation (Magioli et al. 2021).
- Functional richness metrics would show stronger negative responses to anthropogenic disturbances compared to other metrics because they can efficiently discriminate assembly rules implicit in the community structure, independent of species richness (Mouchet et al. 2010, Luck et al. 2013).
- More negative effects would be associated with habitat loss and fragmentation, hunting, and logging than with other anthropogenic disturbances (e.g. urbanisation), which have already been shown to be detrimental to different dimensions of biodiversity (Ahumada et al. 2011). Conversely, agriculture has been found to have varying impacts, depending on the type of management (Flynn et al. 2009).
METHODS
Data collection
We conducted our literature search for studies that have examined the relationship between anthropogenic disturbances and any indices that denote mammalian functional diversity using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol to search for papers (Moher et al. 1999). We searched the Web of Science and Scopus databases for peer-reviewed scientific papers using the following search terms: (‘functional diversity’) AND (‘mammal*’) AND (‘disturbance’ OR ‘degradation’ OR ‘conversion’ OR ‘alteration’ OR ‘fragmentation’ OR ‘disturbance’ OR ‘habitat loss’ OR ‘agriculture’ OR ‘hunting’ OR ‘poaching’). A second search was conducted using only the terms ‘functional diversity’ AND ‘mammals’ to further increase the number of studies. The terms were searched in the title, abstract, keywords, and main text. To supplement our dataset, we performed a search of grey literature studies in Google Scholar, including thesis and dissertations, using the search terms ‘functional diversity’ AND ‘mammals’.
The inclusion criteria included studies that: 1) Reported effects of anthropogenic disturbances compared to control areas (areas that, according to the authors, have not suffered from anthropogenic disturbances, generally protected areas or continuous forests) or examined effects across a gradient of disturbances on functional diversity. 2) Provided sufficient data for meta-analysis (i.e. statistical test values and sample sizes). A total of 556 scientific articles were found (245 in Web of Science and 311 in Scopus). Of these, 229 were eliminated because they were duplicates and 290 because they did not address the topic of interest. Of the remaining 37 articles that related functional diversity to anthropogenic disturbances, 11 did not have the necessary data for meta-analysis. Subsequently, six thesis or dissertations and three articles were added to the references based on a Google Scholar search. Therefore, the final meta-analysis included 35 studies that assessed the effects of anthropogenic disturbances on mammalian functional diversity (Appendices S1 and S2).
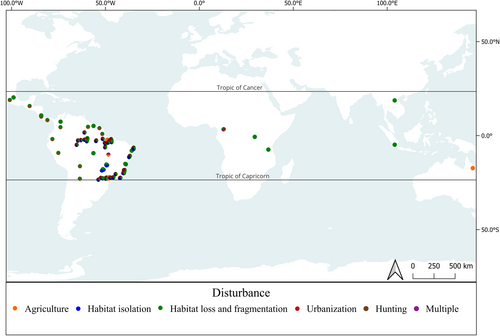
The studies were published between 2011 and 2022 (Fig. 1). In general, they used more than one index and/or examined the effects of more than one anthropogenic disturbance. We considered each outcome to be independent, resulting in 35 studies with 147 effect sizes. The effect size is a statistical parameter used to compare the results of different studies on the same scale (Koricheva et al. 2013); that is, the number of comparisons that measure the variable of interest (in this case, we had 147 comparisons that measured the anthropogenic impacts on mammalian functional diversity).
Data extraction
From each study that compared the disturbed and control areas, we extracted the sample size (sample sites in each study), mean functional diversity index, and error estimates (standard error or variance) for each treatment. For studies that evaluated anthropogenic disturbances (Table 1, e.g. agriculture, hunting, habitat loss, and fragmentation), we extracted the sample size and the correlation coefficient (r). If the authors reported the data only in figures, we used ImageJ or GetData Graph Digitizer to extract the values. We also collected information on the region where the study was conducted (tropical or temperate), the functional diversity index used (e.g. functional richness, functional dispersion), and the group of terrestrial mammals evaluated (bats, small, medium, and large mammals; or all mammals) (Chiarello 2000, Paglia et al. 2012).
| Subgroup | Categories (no. of studies/no. of comparisons) |
|---|---|
| 1. Group |
1.1 All mammals (6/30) All non-aquatic mammals, regardless of the group. 1.2 Bats (5/19) Volant mammals. 1.3 Medium and large (11/61) Mammals weighing more than 1 kg. 1.4 Small mammals (5/14) Mammals weighing less than 1 kg. |
| 2. Index |
2.1 Functional diversity (FD) (8/25) 2.2 Functional dispersion (FDis) (9/30) 2.3 Functional divergence (FDiv) (5/11) 2.4 Functional evenness (FEve) (7/15) 2.5 Functional richness (FRic) (8/11) 2.6 Rao's quadratic entropy (RaoQ) (6/18) |
| 3. Disturbance |
3.1 Agriculture (7/21) Studies evaluating the impact of agricultural production systems, pasture and agrosilvopastoral practices. 3.2 Habitat loss and fragmentation (18/77) Studies that evaluated the effects of the reduction in the amount of habitat in the landscape or the size of the fragments. 3.3 Habitat isolation (4/5) Studies that evaluated the effects of fragment isolation or connectivity metrics. 3.4 Hunting (3/8) Studies that evaluated the effect of hunting. 3.5 Urbanisation (3/4) Studies that evaluated the effects of urban sprawl. 3.6 Multiple (2/3) When the study considered more than one type of anthropogenic disturbance but did not distinguish the effects separately. |
Meta-analysis
Effect size
We used two approaches to calculate effect sizes, following common procedures in meta-analyses of diversity data (Hillebrand & Gurevitch 2016, Kinlock et al. 2018). When a study compared functional diversity between a control site and a degraded site, Hedge's d was calculated from the difference in values between the two categories. The d-value was obtained from a measure of the mean, deviation, or standard error provided in the article for the control and treatment groups. The d-value was then converted to a zr-value, which is a measure of the effect size for continuous data, using a web calculator (www.psychometrica.de/effect_size.html). If a study reported correlation or other basic statistical test results, such as Z-scores, Student's t, F, or R2 statistics assessing functional diversity and anthropogenic disturbance, the significance values and test statistics of these metrics were converted to correlation coefficients (Koricheva et al. 2013). Therefore, we converted the correlation coefficients to the zr-values.
Meta-analytical models and moderators
After computing individual effect sizes, we assessed the overall effect size along with the corresponding 95% confidence intervals utilising a multilevel meta-analytic model implemented with the ‘rma.mv’ function from the ‘metafor’ package (Viechtbauer 2010) in the R software (R Core Team 2023). This model is generally chosen when individual effect sizes are not independent. By relaxing the assumptions of independence inherent in fixed-effect and random-effect models, we could specify random effects, especially in scenarios involving multiple effect sizes from the same study (Koricheva et al. 2013, Nakagawa et al. 2017). Thus, we incorporated study identity and effect size within each study as random factors to estimate variations between and within studies, respectively [R syntax: random = list (~1|Study ID/individual effect ID)] (Felix et al. 2023). Positive z-values in this context denote an increase in functional diversity in a degraded environment, while negative values indicate a decrease. Effects were considered non-significant if the confidence intervals overlapped zero.
To investigate the effect of explanatory variables on effect sizes, we formed subgroups and used a meta-analysis of mixed-effects models to explore the heterogeneity of study responses to anthropogenic disturbance of mammal functional diversity with three moderators: 1) Taxonomic group (small mammals and bats; medium and large mammals; and a third subgroup with unspecific combinations including all types of non-aquatic mammals, which we called ‘all mammals’). We classified medium and large mammals as those weighing more than 1 kg (Chiarello 2000, Paglia et al. 2012). 2) Functional diversity index (functional diversity, functional dispersion, functional divergence, Rao quadratic entropy, functional richness, and functional uniformity). 3) The type of anthropogenic disturbance studied (agriculture, habitat isolation, habitat loss and fragmentation, urbanisation, hunting, and multiple if more than one anthropogenic disturbance was considered). We decided not to include comparisons from a single study in order to obtain more robust estimates (Table 1). Therefore, some indices (functional originality, mean closest taxon distance, standardised mean distance from the closest taxon, functional specialisation, mean paired distance, mean paired functional distance, mean closest taxon functional distance) and types of anthropogenic disturbances (defaunation, large dam construction, local extinction, logging) were not used in the analyses of the respective moderators.
Publication bias
We checked for possible publication bias as well as the strength of our results using funnel plots and Egger's regression test (statistical approach) (Egger et al. 1997). When the intercept of our regression model was greater than 0, this indicated asymmetry of the data. For significant results, we also used Rosenthal's fail-safe number (FSN) to calculate the number of studies with nonsignificant effect sizes that would need to be included in our analyses to make our results nonsignificant (Rosenthal 1979, Rosenberg 2005, Fragkos et al. 2014). To compare the zr between different groups of studies, we used heterogeneity analysis (Q statistic) to test the homogeneity of categorical groups with respect to effect sizes using a random effects model (Koricheva et al. 2013). In complementation, we estimated I2 to test heterogeneity across studies in the models. This statistic indicates the percentage of variation due to data heterogeneity rather than chance (Higgins et al. 2003). All analyses were performed in R (R Core Team 2023, version 4.3.2), using the Metafor package (Viechtbauer 2010).
RESULTS
Of the 147 effect sizes calculated, most were measured in the tropics (n = 124), while temperate regions were underrepresented (n = 23). Because we found few studies in temperate region that met our inclusion criteria, we decided to focus our analyses only on tropical regions, with 124 effect sizes. These studies investigated medium and large mammals (n = 61), small mammals (n = 14), bats (n = 19), and all mammals (n = 30). The functional diversity indexes that were most studied were functional dispersion (n = 30), functional diversity (n = 25), Rao quadratic entropy (n = 18), functional evenness (n = 15), functional richness, and functional divergence, both with (n = 11). Habitat loss and fragmentation (n = 77), agriculture (n = 21), multiple factors (n = 3), hunting (n = 8), habitat isolation (n = 5), and urbanisation (n = 4) were the most common anthropogenic disturbances.
The overall mean effect size for all 124 comparisons was (zr = −0.12 [95% CI: −0.28; 0.04], Fig. 2), which indicated that anthropogenic disturbances had a negative but not significant effect on the functional diversity of mammals when all metrics were combined. Total heterogeneity was high and significant (Q = 797.15; P < 0.001), and the heterogeneity due to random effect within and between studies was moderate (within study estimates = 0.08, square root = 0.29, I2 = 38%; between study estimates = 0.13, square root = 0.36, 57%).
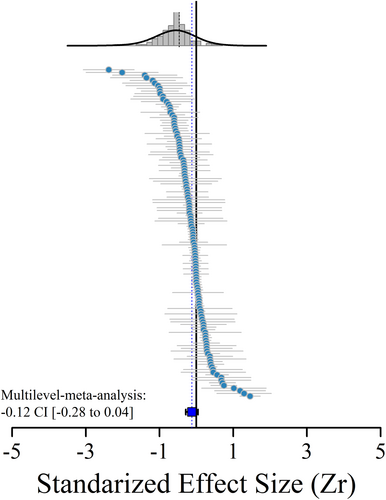
The effect size analysis based on different moderators revealed negative outcomes of mammalian functional diversity for some metrics. We found a significant negative effect of anthropogenic disturbances on functional diversity for studies performed for all mammals (−0.42 [95% CI: −0.76; −0.07]), and among studies that used functional richness (−0.40 [95% CI: −0.72; −0.08]) and functional dispersion (−0.31 [95% CI: −0.59; −0.03]). Finally, among the anthropogenic disturbance types investigated, only habitat isolation negatively affected functional diversity (−0.61 [95% CI: −1.03; −0.18]). For the remaining mammal groups and disturbance variables, there were no substantial differences between areas with more or less anthropogenic disturbance. We found non-significant differences in heterogeneity for most moderators, indicating homogeneity among the parameters used (Fig. 3).
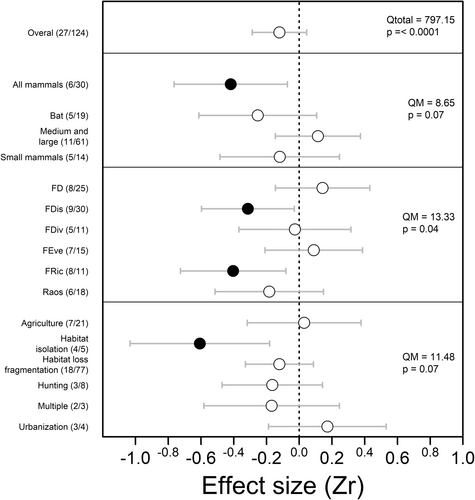
For the moderators, we found non-significant differences in heterogeneity, indicating homogeneity among the parameters used (Taxonomic group: QM = 8.65, P = 0.07; Functional diversity index: QM = 13.33, P = 0.04; Type of anthropogenic disturbance: QB = 11.48, P = 0.07). The Rosenthal fail-safe number (FSN) for all mammals (370 studies), functional richness (76 studies), functional dispersion (425 studies), and habitat isolation (39 studies) was large, relative to the number of independent comparisons included in the meta-analysis (30 studies, 11 studies, 30 studies, and 5 studies, respectively), indicating the strength of our results. We did not find evidence of publication bias from visual inspection of funnel plots (effect size vs. sample size). There was a lack of relationship between effect size and publication year (Appendix S3). Egger's test intercept was less than 0 (Appendix S4), which reinforces the absence of publication bias and indicates the strength of our results.
DISCUSSION
Based on our results, we accept predictions I and III. Our findings demonstrate that anthropogenic disturbances generally reduce the functional diversity of mammals, particularly when assessing functional richness (FRic). However, we did not find any specific results related to medium and large mammals, habitat loss and fragmentation, hunting, or logging. We will discuss each topic in detail below.
We found that anthropogenic disturbances tend to negatively affect the functional diversity of mammals. Given that similar patterns of diversity loss have already been observed for other facets of diversity (i.e. taxonomic, phylogenetic, and genetic diversity) (Lino et al. 2019, Brodie et al. 2021, Romero-Muñoz et al. 2021), demonstrating that in disturbed environments, not only are individual species lost, but also functional attributes and broader ecological functions.
Our research revealed that a significant number of studies have been conducted in tropical regions, with a relative gap in temperate regions. To improve the evaluation of this topic, it is necessary to conduct new studies, particularly in regions such as Europe and the United States, which have not yet been evaluated. There is a consensus that tropical regions are hosts to the greatest species richness on the planet, but the same pattern is not clear when observing functional diversity (Feng et al. 2020), although there is a correlation between species richness and functional diversity that is not linear. The pattern of functional diversity is related to the way species have colonised the planet over time, with biological traits shaped by processes such as speciation, extinction, and dispersal, among others, making it complex to understand (Davies et al. 2008, Safi et al. 2011). At a certain point, the increase in the number of species stabilises functional diversity because of the increase in redundant functions (Safi et al. 2011). Although numerous species perform redundant functions in tropical regions, they face greater anthropogenic threats, rendering these communities more susceptible to loss of functional diversity.
We found that the effects were most significant when all mammals were evaluated together. Contrary to our expectations, a positive trend was observed, but with non-significant effects for medium and large mammals when evaluated separately from the other groups. This result should be interpreted with caution, since some anthropogenic disturbances, such as hunting, which can have an important effect on reducing the functional diversity of mammals (Brodie et al. 2021), have been poorly studied because of the practical difficulty in quantifying these effects (Arévalo-Sandi et al. 2018, Laméris et al. 2020). In contrast, we found a trend towards a negative effect on the functional diversity of small mammals in the most degraded areas. In general, because of their smaller body size, these animals have a lower dispersal capacity, often depend only on the resources available in vegetation fragments, and are more affected by anthropogenic disturbances, which are mainly influenced by landscape characteristics such as the size of the natural vegetation fragments and reduced landscape connectivity (Bovendorp et al. 2019, de la Sancha et al. 2020, Palmeirim et al. 2021). As forest areas decline, small mammals gradually go extinct. However, this extinction may not necessarily reduce functional diversity because other species with redundant attributes can take over their ecological functions (Palmeirim et al. 2021). The presence of medium and large mammals can also influence the functional diversity of small mammals, as shown in a study in the Atlantic Forest, a highly fragmented biome where areas with greater richness of medium and large mammals had a lower functional diversity of small mammals, but this functional diversity increased according to forest patch size (Bovendorp et al. 2019).
The use of functional diversity indices along perturbation gradients can reveal the most direct effects on the community structure (Cornwell et al. 2006). Currently, there is a wide variety of functional diversity indices (Villéger et al. 2008), each of which captures different aspects of the functional space. We observed that the indices respond differently to anthropogenic disturbances. Consistent with our predictions, anthropogenic disturbances caused a significant reduction in functional richness, since this index measures the volume of functional space occupied by all species in the community (Villéger et al. 2008). Thus, in highly disturbed environments, there is a filtering of species through the filtering of traits, causing a reduction in this functional diversity dimension. In disturbed areas, the amount of functional space occupied by species is smaller, and existing resources are used inefficiently (Mouillot et al. 2013). Additionally, anthropogenic disturbance was found to have a negative impact on functional dispersion (FDis), which measures the abundance distribution in a species' characteristic functional space. This suggests that areas with greater anthropogenic disturbance have greater resource competition, potentially reducing the occurrence and abundance of species. Therefore, niche complementarity may play a role in mitigating these effects (Mason et al. 2013).
We also found that habitat isolation had a negative effect on mammalian functional diversity, with results similar to those found for birds (Matuoka et al. 2020), where continuous forests in more connected landscapes had greater functional diversity. Connectivity between areas also allows greater gene flow and increases the availability of habitats, increasing the number of available resources, whereas smaller and more isolated fragments have a limited number of available niches, often supporting a limited number of functional traits (Magioli et al. 2015, Ehlers Smith et al. 2020).
Although they showed a positive trend, the evaluation of agriculture and urbanisation did not yield significant results. It is important to mention that although there were no apparent negative effects, other factors like crop type and land use intensity (which were not assessed in this study) could impact these findings (Etard et al. 2022). Etard et al. (2022) also found a similar pattern with no significant results for mammals. However, there was a decrease in FRic in both intensely used areas and those with little use, but no reduction overall. Additionally, there was a trend of functional improvement in mammal communities in agricultural and urban regions, contrary to our expectations. Further research indicated that disturbed areas have a higher chance of non-native and functionally novel species colonisation, which could potentially offset any local functional decline. Furthermore, these areas can enhance the resource availability from crops, thereby increasing the likelihood of synanthropic species occupying them (Etard et al. 2022).
This meta-analysis provides valuable information on how anthropogenic threats are affecting mammalian functional diversity, demonstrating the importance of using complementary indices, and in particular, the effect of habitat isolation. Overall, we observed that mammalian functional diversity can change under anthropogenic pressures, allowing a better understanding of which threats are causing changes and where they are most intense. Understanding species responses and possible threat patterns can be a powerful basis for conservation planning and management. Future work should be based on direct measurements of organism characteristics in communities under different land use management practices at different landscape scales and should seek to assess characteristics of specific ecosystem processes. It is also important for studies to evaluate how these anthropogenic disturbances affect different groups of mammals, since they play important ecological roles, and even if there is a substitution of functions, this may be accompanied by a simplification of communities.
ACKNOWLEDGEMENTS
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the doctoral fellowship.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the conclusions of this study are openly available on ‘Mendeley Data’ at https://doi.org/10.17632/wrrwnkb4yk.3.




