Blackfin Seabass Utilize Small Estuarine Lagoons as Nurseries: Implications From Juvenile Sampling at Habitat and Seascape Scales
Funding: This work was supported by Miyazaki Prefectural Fisheries Research Institute; University of Miyazaki.
ABSTRACT
Estuarine lagoons provide nursery habitats for marine fishes; however, small lagoons (< 1 km2) have been overlooked. To evaluate the nursery function of small estuarine lagoons (SELs) for temperate marine fish, this study used seining on the coast of the northwestern Pacific (Kyushu, temperate Japan) during the juvenile seasons (winter and spring) to perform juvenile sampling at two scales and an abundance/size comparison of blackfin seabass (Lateolabrax latus). As a preliminary survey, habitat-scale (inside vs. outside lagoon habitats) sampling was attempted in two SELs during February and April. Subsequently, seascape-scale sampling was undertaken during the juvenile season (January–May). The seascape consisted of two types of estuaries (lagoons and rivers) and sandy beaches (embayed and exposed). A preliminary survey showed no clear difference in abundance among the habitats, but significantly larger juveniles were observed inside than outside the two SELs. In the seascape survey, peak juvenile abundance during the first half of the study period was concentrated in habitats other than the lagoon estuary, whereas no peak was recorded during the second half. Moreover, the lagoon estuary was significantly larger than the marine habitats, and the monthly occurrence of juveniles was continuous in the lagoon estuary but intermittent in the riverine estuary. These results imply that seabass juveniles utilize the SEL habitat as they grow, highlighting the potential nursery function of estuarine lagoons for marine fish, even at a small scale.
1 Introduction
Estuary is generally defined as an aquatic ecosystem with closed geomorphology and temporal saline variability with freshwater discharge and seawater mixing areas (Pritchard 1967; Reid and Wood 1976; McLusky and Elliott 2004; Wolanski and Elliott 2016). Although the estuarine area is quite small relative to other marine systems, the net productivity of estuaries may be maximal among all ecosystems (Day Jr. and Yáñez-Arancibia 1982; Costanza et al. 1997; McLusky and Elliott 2004; Day Jr. et al. 2013; Litvin et al. 2018). Indeed, estuaries have a high abundance of nekton (fish and invertebrates), as previously discussed (McHugh 1967; Elliott and Hemingway 2002; Cowan Jr. et al. 2013; Whitfield and Pattrick 2015; Litvin et al. 2018).
Among the many types of estuarine ecosystems, estuarine lagoons/lakes (sense Whitfield and Elliott 2011; Wolanski and Elliott 2016) are characterized by morphology; the open sea is interrupted by barrier beaches, dunes, or bars, and distinct small channels form relative to the surface area of the lagoon (Barnes 1980; Whitfield and Elliott 2011). According to Wolanski and Elliott (2016), “estuarine lagoon(s)” refers to a “lagoon-estuarine environment” (Day Jr. and Yáñez-Arancibia 1982) and “bar-built estuary” (Pinet 2016). “Coastal lagoon,” as explained by Nixon (1982) and Kjerfve (1994), is a broader term that also includes environments with a rigid salinity level (equal to and/or higher than the sea), such as a hypersaline lagoon. A common environmental characteristic of coastal lagoons is their shallow depth, generally 1–5 m on average and rarely deeper than 10 m (Barnes 1980; Little 2000; Newton et al. 2018). These areas provide diverse habitats for inhabitants (Barnes 1994; Yáñez-Arancibia et al. 1994; Rodrigues-Filho et al. 2023) and high fishing productivity (Pérez-Ruzafa et al. 2024).
Coastal lagoons should be regarded as one of the most threatened ecosystems worldwide due to pressures causing habitat degradation and loss (Kennish and Paerl 2010; Newton et al. 2014; Cataudella et al. 2015; Pérez-Ruzafa et al. 2019; Facca et al. 2020; Lacoste et al. 2023). The need for conservation has been emphasized more clearly by recent studies showing higher diversity and abundance of fish in coastal lagoons than in other coastal habitats (Azevedo et al. 2017; de Andrade-Tubino et al. 2020). On the other hand, the ecological functions of lagoons must be analyzed and evaluated in the context of the characteristics of a specific body of water (Pérez-Ruzafa et al. 2019). In this context, there has been bias regarding the size of lagoons studied. The coastal lagoons investigated and evaluated to date have been relatively large (> 1 km2 in surface area), as reported by Pérez-Ruzafa et al. (2007) and Newton et al. (2018). Thus, small coastal lagoons (< 1 km2) should also be evaluated to understand their ecological importance comprehensively and to link conservation practices to lagoon ecosystems. This perspective may be important, particularly for regions with primarily small lagoons, such as the Japanese Archipelago, where 82% of lagoons are < 1 km2 in surface area (Hayashi 2015). Among these coastal lagoons, the present study focused on small estuarine lagoons (SELs).
Estuarine lagoons have been regarded as nursery grounds for marine fish species, based on surveys of juvenile occurrence patterns inside each lagoon (Yáñez-Arancibia and Lara-Domínguez 1988; Cyrus and Forbes 1996; Hannan and Williams 1998; Maci and Basset 2009; Romero-Berny et al. 2020). However, identifying hotspots of juvenile density based on seascape-level (continuous multiple-habitat) comparisons is an initial process when evaluating the nursery function of particular habitats (Beck et al. 2001; Kimirei et al. 2015; Nagelkerken et al. 2015; Whitfield and Pattrick 2015; Murase et al. 2020). In this context, relevant studies of estuarine lagoons have been very limited. Several marine species were more abundant in an Australian estuarine lagoon than nearby marine habitats (Potter et al. 1997). Furthermore, recent studies involving otolith microchemistry analyses detected a major nursery contribution of estuarine lagoons to the adult population of marine fishes (Tournois et al. 2017; Currie et al. 2024). Thus, although studies comparing estuarine lagoons have been geographically and taxonomically limited, they have illustrated the importance of such environments as marine fish nurseries.
Both negative (Ambrose and Meffert 1999; Fodrie and Levin 2008) and positive results (Maci and Basset 2009) have been shown regarding the nursery function of SELs for marine fish. However, the importance of SELs for marine fish should not be ignored, because small and intermittently open estuaries contain juveniles of specific marine fish species (Blaber 1973). Moreover, when diadromous fish are considered, the results of multiple-habitat comparative studies imply that both anadromous and amphidromous fishes use SELs as extended nurseries (Hayes et al. 2008; Murase et al. 2020). Overall, evaluations of the nursery function of estuarine lagoons for marine fishes using objective/quantitative methods have been insufficient worldwide. In particular, estuarine lagoons with a small surface area have been ecologically underestimated.
Temperate seabass in the genus Lateolabrax, endemic to East Asia, are important constituents of commercial and sports fisheries (Konishi 1995; Shoji et al. 2002). These species function as predators of foraging fishes, benthic crustaceans, and zooplankton in estuarine and coastal ecosystems (Konishi 1995; Yamazaki 2002; Arakaki et al. 2014). Among these seabasses, the blackfin seabass, Lateolabrax latus, generally reaches a length of 80 cm; it is mainly distributed in Honshu, Shikoku, and Kyushu in southern Japan and on the southern coasts of Korea (Murase et al. 2012; Hatooka 2013). This seabass species is not often commercially distributed in Japan, but it occasionally trades at a higher price than L. japonicus (Yamasaki 2021; Nakayama 2022). In contrast to congeners that inhabit embayments, blackfin seabass prefer coastal waters facing the open sea and generally occur on exposed rocky shores (Konishi 1995; Hatooka 2013; Arakaki et al. 2014); subadult/adult individuals also inhabit riverine estuaries during the summer and autumn (Murase et al. 2012). The gonadosomatic indices and histological observation of gonads of specimens collected on the coast of southern Japan indicate that these fish spawn from January (winter) to March (early spring) (Kunishima et al. 2021). This spawning season is aligned with the fishing season on exposed rocky shores (Arakaki et al. 2014), and there is no evidence that adults occur in river or estuarine environments during this season, indicating that this seabass species spawns in marine environments similar to the congener (L. japonicus: Islam et al. 2011; Tamura et al. 2013). Additionally, the occurrence of the smallest size range of juveniles in marine areas (Kinoshita and Fujita 1988; Arayama et al. 2002; Suda et al. 2002; Fujita 2004) supports the marine spawning habit of blackfin seabass, but few studies have been performed on their nursery grounds.
Past seine surveys of juvenile seabass at surf-zone sites (Kinoshita 1984; Kinoshita and Fujita 1988; Fujita 2004) and a riverine estuary (Fujita et al. 1988; Fujita 2004) on the Pacific coast of the Shikoku mainland, southern Japan showed that the abundance of L. latus was lower in an estuary and higher in surf-zone sites than L. japonicus. Based on these results, Fujita et al. (1988) and Fujita (2004) concluded that blackfin seabass do not use estuaries as primary nursery grounds and that surf zones are important for their early life stages. However, when the numbers of individuals reported by Fujita (2004) were converted into numbers of individuals per haul, the numbers in estuaries and surf zones were quite similar (0.14 vs. 0.15, respectively) and there was a difference in size structure between the two habitats: larger in the estuary (11.2–49.6 mm in total length: Fujita et al. 1988; Fujita 2004) than in the surf zone (9.2–27.0 mm: Kinoshita and Fujita 1988; 10.2–36.0 mm: Fujita 2004). These data do not conclusively indicate a difference between the two habitats in terms of the relative importance of juvenile abundance, but they imply that such fish ingress into estuaries as they grow. Subsequently, Yube et al. (2006) performed seine sampling of blackfin seabass juveniles at two sites (a relatively closed embayment and a riverine estuary) in western Shikoku, revealing a difference in size structure between the sites: juveniles < 20 mm in body length were more abundant in the embayment than in the estuary. The results of previous studies have implied that blackfin seabass juveniles settle in open and embayment sites, then extend their nursery grounds to estuaries. However, previous studies did not clearly define the habitats or classify data from each habitat despite an understanding of fish habitat connectivity between different environments requires pattern identification using consistent methods (Able 2005; Kimirei et al. 2015; Bradley et al. 2019). Thus, the use of nursery grounds by blackfin seabass remains unknown.
Our preliminary surveys to reveal the SEL fish assemblage structure in southern Japan revealed juvenile blackfin seabass within SELs. We hypothesized that seabass juveniles utilize SELs as habitats during growth, and quantitatively seine-sampled two scales of juvenile seabass along the coast of Miyazaki Prefecture, Kyushu, southern Japan to evaluate the nursery function of SELs for marine fish: habitat-scale sampling (the preliminary survey mentioned above), which was implemented to compare juvenile seabass abundance and size between inside and outside the habitats of the two types of SELs; and seascape-scale sampling, which comprised an extended survey of temporal variation in juvenile densities and size structures among four environmentally distinct shallow habitats (including one SEL from the preliminary survey). The main focus of the present study was consistent spatiotemporal analysis of the habitat selectivity of seabass juveniles during growth.
2 Materials and Methods
We field-sampled at habitat and seascape scales using seines along the east coast of Kyushu (Miyazaki Prefecture), southern Japan (Figure 1). A habitat-scale survey was preliminarily conducted to determine juvenile seabass occurrence within two shallow SELs that were approximately 70 km apart in a straight line (the survey at this scale is hereafter referred to as the “preliminary survey”). Both lagoons were permanently open, soft-bottomed (nonvegetated with seagrass) lagoons with short and narrow entrance channels but different mechanisms of freshwater dilution. Myoken Bay is located on the northern coast of Miyazaki Prefecture (Figure 1A: Nobeoka City, 32°30′17.0″N 131°40′55.0″ E) and has a surface area of 0.136 km2 (Miura 2013). It is a typical estuarine lagoon with direct freshwater inputs (i.e., the presence of several river or stream inflows within the lagoon). The other SEL was Hitotsuba Lagoon, located on the south coast of Miyazaki Prefecture (Figure 1B: Miyazaki City, 31°55′18.0″N 131°27′50.0″E), with a surface area of 0.122 km2 (Miura 2013). This lagoon lost approximately one-tenth of its area in the 1980s due to the development of a commercial port; it is now a small cove with only a small area remaining at the northern end (Miura et al. 2005). At the same time, the direct flow of freshwater into this lagoon was lost; diluted water may be flowing into the lagoon indirectly from the river estuary located approximately 500 m southwest of the lagoon outlet. Although there is no direct freshwater influence, this lagoon is considered an estuarine lagoon based on the significantly lower salinity inside than outside (see results), as well as fish assemblage data regarding the dominance of typical estuarine fishes (based on estuaries being where most of their life cycle occurs) in Japan, such as Favonigobius gymnauchen, Gymnogobius uchidai, and Pseudogobius masago (Y. Ikehara unpubl data).
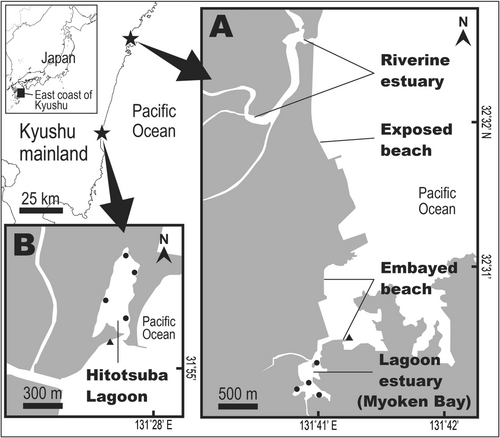
A seascape-scale survey (hereafter the “seascape survey”) was conducted along the northern coast of Miyazaki Prefecture (Figure 1A: southern Nobeoka City), which is a 4-km stretch of soft bottomed habitat (without any vegetation of seagrass) that is partially fragmented by commercial and fishery ports. The targeted habitats include riverine/lagoon estuaries (estuarine contexts; the lagoon estuary corresponds to Myoken Bay) and exposed/embayed beaches (marine contexts). Habitats for the seascape survey were selected considering topographical differences and the presence/absence of wave exposure (surf zone present only at the exposed beach) in each context. Detailed information about each sampling site is presented in the study by Murase et al. (2020). SEL is represented by “lagoon estuary” when discussing the seascape survey.
During the preliminary survey, blackfin seabass juveniles in the SELs (Myoken Bay and Hitotsuba Lagoon) were quantitatively sampled in February and April 2017, which are the core months of juvenile blackfin seabass occurrence (Kinoshita and Fujita 1988; Fujita 2004; Yube et al. 2006). Four sampling sites were set inside and another single site was set outside each lagoon (Figure 1) to compare the inside and outside habitats. To sample the juveniles, a small seine net (2-mm mesh), consisting of two 2.3-m-long/0.78-m-high wings and a 2.55-m-long/0.92-m-wide bunt, was towed along the shore for 10 m while holding the net open at a width of 3 m (i.e., 30 m2 per seine sampled). This sampling method was used four times per month at each site (40 m of seining at each site each month) during the day, at a depth of approximately 0.5 m. Water temperature and salinity were sampled once per haul. Water temperature was directly measured using a digital thermometer (TT-508, Tanita Co. Ltd., Tokyo, Japan) at the water surface. Salinity was measured by sampling water from the middle layer with a 2-mL tube using a digital salinity meter (PAL-06S, Atago Co. Ltd., Osaka, Japan).
A larger seine net was used for the seascape survey than for the preliminary survey, considering the more open/sea habitat (see Kanou et al. 2002 for details of the net); this was consistently used over targeted habitats to collect juvenile seabass. Seine sampling was conducted monthly during the day in each habitat from January to May 2018, which included the season for juvenile blackfin seabass in southern Japan (Kinoshita and Fujita 1988; Fujita 2004; Yube et al. 2006). The seine was laid parallel to the shore at a depth of approximately 0.8 m and towed 50 m at a width of 4 m (i.e., 200 m2 per seine sampled). Four rounds of sampling in this manner were conducted at each habitat, except in May 2018 at the exposed beach, where only two rounds were conducted due to poor conditions. Two seine samplings were conducted at each northern and southern embayed beach every month (four samplings at the embayed beach each month). Water temperature was measured directly once per haul using a digital dissolved oxygen meter (ID-150, Iijima Denshi Co. Ltd., Tokyo, Japan) in the middle layer of the water column; salinity was determined twice per haul using the same method as in the preliminary survey. The environmental data lacked two observations due to poor conditions at the exposed beach in May, as noted above. These missing data were imputed for the analysis by repeating values of the sampled data, as described by Murase et al. (2020).
All fish were euthanized immediately after sampling via placement in plastic bags filled with a slurry of crushed ice and seawater to minimize suffering (McCormick 2016; Grande et al. 2019). Thereafter, blackfin seabass juveniles were identified in the laboratory in accordance with the methods of Kinoshita and Fujita et al. (1988), Okiyama (2014), and Yokogawa (2019). After each sample had been sorted, specimens were fixed with 10% formalin and preserved in 70% alcohol. The size of specimens was measured to the nearest 0.1 mm standard length (SL, length from snout tip to the distal end of the hypural plate; Okiyama 2014) with needle-point calipers. Notochord length (snout tip to the posterior end of the notochord; Okiyama 2014) was considered the SL for juveniles without completed hypural plate. Size classes of examined juveniles for histogram were divided into level of 5 mm, noting that the fins of all observed individuals were completed at 15 mm SL. In addition, examined juveniles were classified as following two developmental stages by referring Kendall et al. (1984): L-stage (larval stage), having not completed the full fin ray; J-stage (juvenile stage), being with the full fin ray.
Differences in environmental/biological parameters between the inside and outside of lagoons in the preliminary survey were compared using the Wilcoxon signed-rank test because the normality assumption was violated after the data had been log10 (x +1.0)-transformed. However, normally distributed data with heterogeneous variance were subjected to Welch's t test after the data had been log10 (x +1.0)-transformed. Tests among seascape survey habitats were performed when the homogeneity of variance based on Bartlett's test was violated after the data had been log10 (x +1.0)-transformed, as follows: fish size was tested with the Kruskal-Wallis test, whereas monthly abundance and environmental parameters were tested with the Friedman-Wilcoxon signed-rank test. Monthly one-way repeated-measures analysis of variance (ANOVA) and the Tukey test were adopted to analyze fish abundance from January to March after the data had been log10 (x +1.0)-transformed. Each statistically significant difference was determined at a confidence level of p = 0.05. As described above, the exposed beach was seined only twice in May and no juvenile blackfin seabass (see Results) were captured; thus, the analysis was conducted with the assumption that no individuals were caught for the third and fourth seines in May. The coefficient of variation (CV) of the environmental parameters was calculated as the standard deviation divided by the average mean value for each month.
3 Results
Monthly variations in environmental parameters inside and outside the two SELs are shown in Figure 2. Mean water temperature and salinity values were significantly lower inside than outside each month at both lagoons, with the exception of salinity in Myoken Bay during February, which tended to be lower inside than outside.
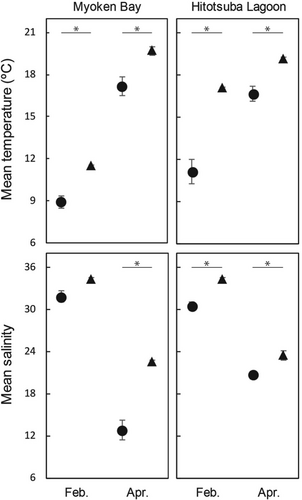
Seine collection of seabass juveniles from Myoken Bay during the 2 months resulted in a total of 24 individuals. Inside/outside comparisons of juvenile abundance resulted in 2/1 and 13/8 individuals in February and April, respectively; no significant difference was detected for either month (Wilcoxon rank-sum test, W = 28, n1 = 16, n2 = 4, p > 0.05 in February; W = 17.5, n1 = 16, n2 = 4, p > 0.05 in April). Spatial/monthly variations in juvenile size in the two lagoons are shown in Figure 3. Although no clear spatial difference in size was detected for February (inside vs. outside: 12.1–14.0 vs. 12.8 mm SL), the juveniles inside (range, mean ± SD 13.0–33.3, 22.2 ± 7.9 mm, n = 13) were significantly larger than those outside (10.6–15.3, 12.6 ± 1.5 mm, n = 8) in April (t test, t = 4.2629, p < 0.001). Composition of developmental stages differed among months and habitats: February, all individuals of juvenile collected inside and outside classified as L-stage; April, L-stage accounting for 15.4% and 87.5% of individuals inside and outside, respectively but J-stage 84.6% and 12.5% in each.
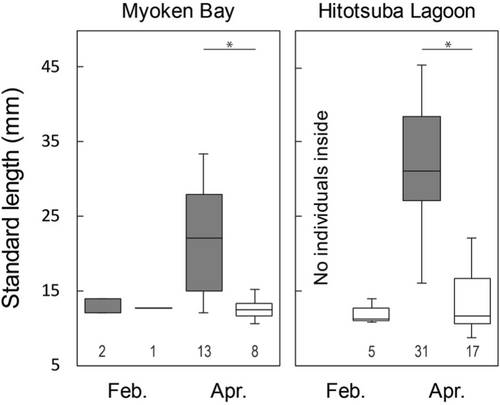
Seine collection of seabass juveniles from the Hitotsuba Lagoon over the 2 months resulted in a total of 53 individuals. Inside/outside comparisons of juvenile abundance resulted in 0/5 and 31/17 individuals in February and April, respectively, representing an absence of juveniles inside in February; no difference in juvenile abundance was detected in April (Wilcoxon rank-sum test, W = 18, n1 = 16, n2 = 4, p > 0.05). In February, juveniles outside were 10.9–14.0 mm (mean ± SD 12.0 ± 1.4 mm). Consistent with the Myoken Bay data, juveniles collected inside in April (16.1–45.4, 32.4 ± 7.9 mm, n = 31) were significantly larger than those collected outside (8.8–22.1, 13.6 ± 4.4 mm, n = 17) (Wilcoxon rank-sum test, W = 519.5, p < 0.001). As did in Myoken Bay, composition of developmental stages spatiotemporally differed: February, all individuals of juvenile classified as L-stage; April, all individuals inside being J-stage and L−/J-stages accounting for 70.6/29.4% of individuals outside.
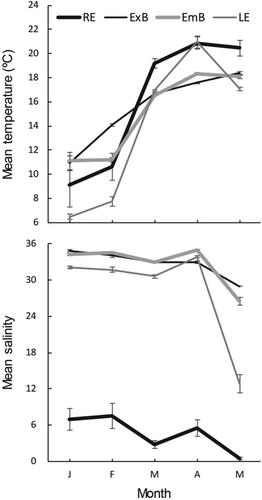
Monthly variations in environmental parameters across the four habitats in the seascape survey from January to May are shown in Figure 4. Mean water temperature did not vary significantly between the riverine estuary and marine habitats throughout the study period (Wilcoxon signed-rank test, n = 80, p > 0.05). However, the mean water temperature in the lagoon estuary was significantly lower than that in the riverine estuary (p < 0.05); it was lowest in January and February. The mean water temperature from March to May was significantly higher in the riverine estuary than in the other three habitats (n = 48, p < 0.05). The CV values for water temperatures from January to May ranged from highest to lowest as follows: lagoon estuary (0.46), riverine estuary (0.35), embayed beach (0.24), and exposed beach (0.20). These findings indicate greater temperature instability in estuarine habitats than in marine habitats. Mean salinity did not vary significantly among the marine habitats (Wilcoxon signed-rank test, n = 160, p > 0.05), but salinity was significantly lower in the estuarine habitats than in the marine habitats (p < 0.0001); the salinity of the riverine estuary was significantly lower than that of the lagoon estuary (p < 0.0001). Similar to water temperature, CV values for salinity were higher in estuarine habitats than in marine habitats, but CV values were highest in the riverine estuary. The specific values were riverine estuary (0.64), lagoon estuary (0.31), embayed beach (0.11), and exposed beach (0.07).
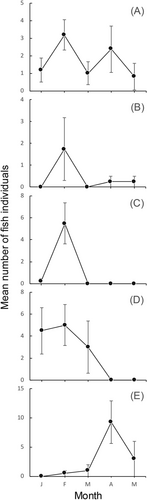
Juvenile seabass sampling across the four habitats between January and May resulted in a total of 137 individuals. The highest number of juveniles captured was in the lagoon estuary (55), followed by the embayed beach (48), exposed beach (23), and riverine estuary (9). The monthly changes in the mean numbers of individuals per haul at each habitat are shown in Figure 5. The combined value for the four habitats peaked twice in February and April; the value of each habitat peaked in February, except in the lagoon estuary where the peak month was April. These values did not vary significantly among the four habitats throughout the study (Friedman rank-sum test, X2 = 3.2286, df = 3, n = 80, p > 0.05). However, when the study period was divided into two halves, significant differences in the numbers of juveniles captured among habitats were detected for each period: between January and March, the values were significantly higher for the embayed beach than for the estuarine habitats (ANOVA, F [3, 44] = 6.295, p < 0.01; Tukey test, p < 0.01). Significant variations were detected among habitats in March–May (Friedman rank-sum test, X2 = 8.125, df = 3, n = 48, p < 0.05), reflecting higher values in the lagoon estuary and the absence of juveniles in marine habitats at the end of the study (April and May). No significant differences were found between any two pairs of habitats (Wilcoxon signed-rank test, n = 80, p > 0.3).
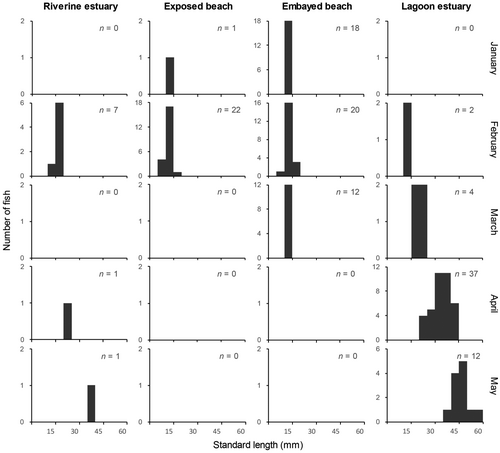
The frequency distributions of size classes in each habitat and month are shown in Figure 6. The juvenile sizes ranged from 8.5 to 56.0 mm SL (mean ± SD 21.6 ± 12.6 mm, n = 137); the histogram indicates an increase in body length over time and variation in size structure among habitats. Juveniles were captured in marine habitats only during the first month of the study (January), with a size range of 10.0–12.9 mm. In February, similar size classes (8.5–12.9 mm) were recorded relative to the previous month in all habitats—the < 12.0-mm class was restricted to marine habitats—as well as larger individuals (13.0–17.8 mm). In March, older juveniles (16.9–23.3 mm) were caught in the lagoon estuary, whereas a similar size class as the former months (11.1–14.8 mm) was recorded at the embayed beach. In April and May, when juveniles were restricted to estuarine habitats, the largest size classes (21.7–42.1 and 38.7–56.0 mm in April and May, respectively) were recorded (sizes recorded at the riverine estuary were 22.6 and 39.8 mm in April and May, respectively). Additionally, a significant difference in juvenile size was detected among habitats throughout the study period (Kruskal-Wallis test, X2 = 98.27, df = 3, n = 137, p < 0.0001), except for the pairing among marine habitats (Wilcoxon rank sum test, p = 1). The mean SLs increased significantly in the following order (p < 0.01): lagoon estuary (mean ± SD 34.6 ± 9.7 mm, n = 55), riverine estuary (19.4 ± 8.0 mm, n = 9), and marine habitats (12.2 ± 1.7 mm, n = 50 and 11.9 ± 2.0 mm, n = 23 in embayed and exposed beaches, respectively).
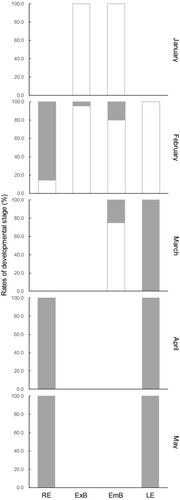
The occurrence rates of two developmental stages in each habitat and month are shown in Figure 7. All the juveniles collected in January were L-stage, but J-stage began to be collected from February, and all the individuals collected after April were J-stage. At the riverine estuary, all the individuals were J-stage, except for L-stage, which accounted for 14.3% of abundance in February. Similarly, at the lagoon estuary, all juveniles collected after March were J-stage, except for two L-stage individuals collected in February. In contrast, the majority of juveniles (75.0–100.0% of abundance) collected in marine contexts from January to March were L-stage.
4 Discussion
The results of the preliminary and seascape surveys revealed consistent values for the environmental variables inside and outside the SELs. Additionally, significantly larger juvenile blackfin seabass were observed inside than outside the SELs during the second half of the study in each survey (April in the preliminary survey; March–May in the seascape survey). This spatial difference in size structure was consistently reflected in the occurrence rates of each developmental stage, representing predominant J-stage occurrence inside the SELs. The sequential occurrence of juveniles in the lagoon estuary, rather than the riverine estuary, implies that juvenile seabass utilize SELs as nursery grounds to grow. These two habitats may play different roles for juveniles.
The patterns of occurrence (the smallest size class and predominant of L-stage juvenile recorded from January to March) at the exposed and embayed beaches were similar, but a pattern distinct from that in estuarine habitats. Because the spawning season in southern Japan extends from January to March (Kunishima et al. 2021), this result in the marine habitats implies that both of the marine habitats in the present study have roles as settlement grounds from the spawning grounds. Although no significant differences in temperature or salinity were detected between the marine habitats, juveniles were more abundant at the embayed beach than at the exposed beach; however, the duration of juvenile occurrence was longer at the embayed beach (until March) than at the exposed beach (until February). Embayed topography (Potter et al. 1997; Murase et al. 2020; Pursiainen et al. 2021) and/or less wave exposure (Romer 1990; Clark 1997; Layman 2000; Watt-Pringle and Strydom 2003; Inui et al. 2010; Nakane et al. 2013) at the coasts tended to increase fish abundance compared with the coasts under open and/or wave-exposed conditions. This increased abundance implies advantages for small fish regarding wave disturbance (Romer 1990), food availability (Clark 1997; Murase et al. 2020), and predator avoidance (Harvey 1998). Thus, quieter habitats may function better as settlement grounds during the early life stages of fish that use the coastal environment. Although more detailed investigations of several types of marine habitats are required, closed marine topography may be more important than open topography for juvenile seabass survival at times of settlement and recruitment toward nearshore habitats.
In contrast to the marine habitats, the duration of juvenile occurrence in the estuarine habitats was biased toward the latter half of the study period. Additionally, juveniles were significantly larger in estuarine habitats than in marine habitats, which was reflected as predominance of J-stage juveniles. This size structure difference between estuarine and marine habitats is consistent with the size range differences found in previous studies (Fujita et al. 1988; Kinoshita and Fujita 1988; Fujita 2004; Yube et al. 2006). The earlier occurrence of relatively small individuals in the sea and later occurrence of relatively large fish in estuaries is consistent with the distribution of the congener L. japonicus (Islam et al. 2011). L. japonicus remains in the marine environment, where it forages zooplankton, even after reaching the juvenile stage (Hibino et al. 2006). According to the general features of marine estuarine-dependent species juveniles occur mainly in estuaries and less commonly in the sea: Whitfield, Able, et al. (2023), the results of the present study and previous results imply that blackfin seabass are dependent on the estuarine environment, at least during the latter part of their early life history. Notably, Inoue et al. (2008) reported several size ranges (25–196 mm SL) for blackfin seabass in multiple surf-zone habitats near a river input. Although they did not report the size structure of each habitat, the wider size range of blackfin seabass recorded at various beach habitats adjacent to the estuary may represent habitat expansion along an estuary-sea ecotone during the juvenile to subadult stages. If blackfin seabass are regarded as an estuarine-dependent species, the following two points, based on the work of Whitfield, Able, et al. (2023), should be considered: it is unclear whether the species would become extinct if no estuaries were available as nurseries; however, if the blackfin seabass population decreased, environmental degradation of estuaries would presumably be involved.
Furthermore, the results of this study show clear differences in juvenile occurrence patterns among estuarine habitats, as well as between estuarine and marine habitats. The timing of juveniles in the riverine estuary was intermittent, rather than continuous; there were fewer fish in the riverine estuary than in the lagoon estuary. These results indicate that juveniles may not regularly use the riverine estuary as a habitat, together with the instability of this environment as a nursery ground. Notably, the lagoon estuary was distinct from the other three habitats: juveniles occurred continuously from February until the end of the study period, and they grew monthly. Considering previous reports (Baptista et al. 2020; Whitfield, Potter, et al. 2023), this finding implies that juvenile blackfin seabass utilize lagoon estuaries as they grow, and that these environments are nurseries that exhibit multiple survival benefits for juveniles compared with riverine estuaries. The present results with inter-habitat comparison across different environmental contexts support the conclusion of Maci and Basset (2009) that a Mediterranean SEL is important as a nursery for several marine species of fish. The role of SELs for marine fishes has been demonstrated in different regions [Mediterranean Sea (Maci and Basset 2009) and northwestern Pacific (the present study)], highlighting their ecological importance and the need to reconsider the function of SELs as marine fish nursery in various regions of the world.
Lagoons are shallow and have extremely narrow exits relative to their areas. They have several physical aspects that differ from those of general estuarine environments, such as riverine estuaries (Barnes 1980). The large surface area of a lagoon allows the water temperature to increase easily in the summer and to decrease dramatically in the winter. Salinity tends to be more stable in lagoons than in estuaries over short intervals, unless there are large changes in precipitation. The difference in CV values among estuarine habitats in the seascape survey was consistent with these differences in physical characteristics between lagoons and estuaries. The CV values for water temperature and salinity were larger in the lagoon and riverine estuaries, respectively; moreover, significantly higher mean water temperature and salinity values were detected in the riverine and lagoon estuaries, respectively. Higher CV values and significantly lower temperatures in the lagoon estuary match the inclusion of winter (when the water temperature is lowest) and spring (when the water temperature rises) during the survey period; they are also consistent with the inside of the lagoon being colder relative to the riverine estuary due to its larger surface area. Indeed, the decrease in water temperature, which was substantial during January and February (mean temperature for each month: < 8°C; Figure 4), affected the magnitude of the CV value. Preferable temperature and salinity conditions for juvenile blackfin seabass have not been investigated. However, higher temperatures may lead to a shorter period before upstream migration of L. japonicus into the estuary (Fuji et al. 2018). When the mean water temperature in the lagoon estuary was below 8°C in February, the mean number of juveniles was less than that in the riverine estuary, but numbers increased as the water temperature increased (Figure 5). Therefore, the low water temperature may restrict ingress of juvenile blackfin seabass into the estuary. Additionally, the monthly mean salinity values were lower in the riverine estuary (0.5–7.5) than in the lagoon estuary (12.9–33.9) or marine habitats (26.5–35.0) (Figure 4). Therefore, similar to other marine estuarine-dependent fish (e.g., Cynoscion acoupa: Whitfield, Able, et al. 2023), the difference in salinity conditions between the riverine estuary and marine habitats may restrict the use of riverine estuaries by juvenile blackfin seabass due to osmoregulatory stress. These results imply that juvenile blackfin seabass ingress into estuarine habitats while avoiding extremely low water temperatures and salinity.
The general shape of lagoons may be associated with the food supply of juvenile predatory fish. Most primary consumers are found in substrata due to the deposition of detritus, whereas zooplankton is less abundant in typical estuaries (i.e., estuaries not lagoons) than in the sea due to tidal and riverine runoff (McLusky and Elliott 2004). However, zooplankton and small crustacean nekton (e.g., mysids) are present year-round in temperate lagoons; the diversity of these animal species is low due to the instability of salinity and other physical characteristics, but a few species do reproduce and dominate (Espinosa et al. 2019; Karpowicz et al. 2023). This dominance is presumably due to the wide-flat surface area and narrow outlets of lagoons, which limit the outflow of surface/mesopelagic animal species with less swimming ability. Furthermore, David et al. (2016) used stable isotope analysis to show that the estuarine intertidal zone strongly participates in planktivorous fish production by providing feeding habitats for zooplankton species. Thus, the intertidal area, which constituted more than half of the SELs in the present study (85% of water surface area in Myoken Bay and 51% in Hitotsuba Lagoon; Miura 2013) serves as a rich food source for zooplanktivorous fishes. As a result, these lagoon environments offer a survival advantage by providing exclusive access to animal food resources for marine fishes that can adapt to brackish water environments.
Blackfin seabass are predatory, beginning in the juvenile stage, and their main prey items vary considerably depending on site and environment. They function as facultative predators; copepods dominate their stomach contents in the estuary (Fujita et al. 1988), but amphipods and isopods are prey items in other estuaries (Yube et al. 2006), while crustacean nekton (mysids) dominate in the surf zone near the estuary (Inoue et al. 2005) and copepods and cladocerans are predominant in the embayment area (Yube et al. 2006). Fish larvae constitute a relatively small proportion of stomach contents in each environment (Fujita et al. 1988; Inoue et al. 2005; Yube et al. 2006). Similar to the generally known prey–predator relationship between zooplankton and fish (Lomartire et al. 2021), the abundance of zooplankton and small nekton could be the main reason for juvenile seabass ingress and aggregation in estuarine lagoons. The abundance of zooplankton in some temperate lagoon systems tends to be higher during the spring (Alvarez-Borrego 1994; Karpowicz et al. 2024), and this pattern is consistent with the temperate climate in Japan (Godhantaraman and Uye 2003). The seasonal dynamics of temperate zooplankton correspond with blackfin seabass juvenile occurrence within the SELs. Therefore, juvenile blackfin seabass, which are adapted to brackish water, may ingress into estuarine lagoons when conditions are favorable and prey organisms are abundant.
Several species of marine fish (Nakajima et al. 2008) and juvenile aggregations of blackfin seabass (Y. Yamasaki unpubl data) have been confirmed in pools formed within rivers (i.e., floodplain pools; Halyk and Balon 1983, wando-pools; Nakajima et al. 2008), which have a shape similar to that of lagoons. Such environments may provide the same nursery function as the SELs investigated in this study. Indeed, previous studies have revealed juvenile blackfin seabass in riverine estuaries (Fujita et al. 1988; Fujita 2004; Yube et al. 2006). Because their study sites included several habitats within an estuary and/or that were located in the inner part of a bay, those sites may have had conditions similar to an estuarine lagoon. However, the shape of a river habitat is likely to change due to natural and anthropogenic effects (Zhang et al. 2022; Steinritz et al. 2024). Therefore, although the physical and biological processes of coastal lagoon ecosystems will be affected by future climate change (Newton et al. 2018), at short timescales, lagoons formed along the coast provide a more stable habitat for aquatic organisms than lagoon-like topography formed within rivers. Nevertheless, lagoons are landforms formed over long periods via coastal erosion and sedimentation (Barnes 1980; Martin and Dominguez 1994); when they are lost, they may be difficult to restore. It is important to determine the direct contribution of each habitat to adult fish populations when identifying true nurseries (Beck et al. 2001), but it is difficult to make a strict definition of a nursery and provide concrete solutions to this identification challenge (Able 2005; Layman et al. 2006). Therefore, it is essential to identify hotspots of juvenile abundance and manage the coastal environment in a manner that such habitats and the connectivity among them are maintained (Nagelkerken et al. 2015; Murase et al. 2020).
5 Conclusion
The present study provides comparative data regarding the patterns of juvenile temperate seabass occurrence in estuarine and marine habitats, showing that most larger juveniles occur at SELs. There are very few studies on the ecological functions of SELs for marine fish, but the results of the present and previous studies emphasize that even small lagoons may function as nurseries that support marine fishes, including local fisheries and recreationally targeted species. Although coastal lagoons face various alarming threats, such as global climate change (Pérez-Ruzafa et al. 2019), unique landforms of all sizes provide multiple ecosystem services [e.g., coastal lagoons (Rodrigues-Filho et al. 2023)]; their anthropogenic loss (i.e., through reclamation) should be avoided to ensure sustainable use of coastal environments. The present study will serve as basic information that calls for reducing such impacts on small-scale coastal landforms and for the accumulation of further knowledge.
Author Contributions
Atsunobu Murase: writing – review and editing; writing – original draft; methodology; investigation; formal analysis; conceptualization; funding acquisition. Yuta Yamasaki: writing – review and editing; investigation; data curation. Mika Mukai: writing – review and editing; investigation; data curation. Yuta Ikehara: writing – review and editing; investigation; data curation. Yukiya Ogata: writing – review and editing; investigation. Kaito Inoue: writing – review and editing; investigation.
Acknowledgments
We are grateful to the following for their support of this study: Hidenobu Kawano and Kenji Nakanishi, former Miyazaki Prefectural Fisheries Research Institute members, for their support, including field surveys and carrying out the research; members of the Nobeoka City Fishermen's Cooperative for permitting the field surveys; and all students who helped with the field surveys and laboratory work necessary for this study. We also thank Katsuhisa Uchida, Tamotsu Michishita, and Tsuyoshi Fujishiro of the Center for Innovative Agriculture, University of Miyazaki for their support at the center during the surveys. This research was partly supported by funding from the Miyazaki Prefectural Fisheries Research Institute and the University of Miyazaki.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




