Boring Pattern of Isopods in the Intertidal Hard Mud Substratum of Gulf of Khambhat, With Two New Records From Gujarat Coast
Funding: The authors received no specific funding for this work.
ABSTRACT
The study of the burrowing and boring pattern is an important bioturbatory behavioral display that provides information about interactions of animals with their surrounding factors and habitat selection preferences. Previous studies have explored the boring activities of sphaeromatid isopods in a variety of substrates, including wood, polystyrene, and rock, across different regions worldwide. However, no previous reports have been documented on the boring pattern of isopods in hard mud substratum. Present studies were carried out on the silt-clay rich hard substratum of coastal mudflats at Kamboi, the northern innermost region of the Gulf of Khambhat, Gujarat. This habitat is selectively preferred by the isopod Sphaeroma annandalei Stebbing 1911, which is indicated by its abundance. To decipher the boring patterns, we examined the sediment blocks during low tides, took multidirectional sections of the exposed regions, and photographed the boring patterns. It showed that there were incidences of the bores of different sizes crossing each other. We primarily interpreted it as the borings of younger and older isopods. However, to further confirm, resin casting was done, which gave us a complex network of interconnected borings. It clearly exposed, for the first time, the continuous interconnected burrows of different sizes of individuals (0.56 mm to 4.75 mm) of S. annandalei in hard mudflat habitat. The study has recorded the presence of two species, S. annandalei and Cirolana willeyi Stebbing 1904, for the first time from Gujarat.
1 Introduction
Bioerosion is a process in which organisms modify the substratum by different mechanical and chemical methods such as burrowing, boring, grazing, scraping, perforation, and removal of consolidated rocks (Ka'zmer and Taborosi 2012; Davidson et al. 2018; Dodge-Wan and Nagarajan 2020). Dorgan (2015) described various mechanisms of burrowing and boring in different types of substratum by different groups of organisms. The difference between burrowing and boring largely depends on the mechanical properties of the substrate. Borers penetrate hard materials like rock and wood through scraping or chemical secretions, while burrowers pass through softer substrates by displacing sediment grains (Dorgan 2015). In coastal habitats, bioeroders affect intertidal ecology by altering local environmental conditions in the affected area. Marine borers are ecosystem engineers, creating their habitat in hard and solid substrates such as wood, corals, shells, bones, rocks, and hard packed clay (Dharmaraj and Nair 1982; Dodge-Wan and Nagarajan 2020). Marine borers, including shipworms (bivalves) and isopods (crustaceans) have the potential to seriously damage marine structures (Neily 1927; Cragg et al. 1999; Davidson 2012). Thiri and Yang (2022) noted that an isopod, Sphaeroma terebrans Bate, 1866, caused severe damage to mangrove forests in China by boring through the aerial roots of trees. Sphaeromatid isopods are well known for their capacity to bore into different types of substrates such as timber, wooden structures, polystyrene floats, mangroves, rock substrates, hard packed clay, and other marine structures (Dharmaraj and Nair 1982; Davidson 2012; Mohammad 2014; Thiri and Yang 2022). Boring activity of sphaeromatid isopods has been reported in a variety of substrates throughout the world, reviewed and listed by Dodge-Wan and Nagarajan (2020). Sphaeromatid isopods can survive and grow in a wide range of salinity (Nair et al. 1989).
In India, only a few research articles have been published on the ecology of sphaeromatid isopods. A total of four species and a variety of sphaeromatid isopods: Sphaeroma walkeri Stebbing, 1905, Sphaeroma terebrans Bate, 1866, Sphaeroma annandalei Stebbing, 1911, Sphaeroma triste Heller, 1865, and Sphaeroma annandalei travancorensis Pillai, 1955 have been reported from India (Unni et al. 2020). Although S. annandalei is a severe pest that is destroying wood along the Kerala coast, detailed research on this species has not yet been done. This species is a highly dangerous wood borer that has been reported from the estuaries of Godavary, Vellar, Neendakara, and Cochin. It has also been documented from Visakhapatnam and Mumbai. Pillai (1961); Purushotham and Rao (1971), and Cheriyan (1973) have reported that this species is present in the sea as well as in the estuarine and brackish water localities. Dharmaraj and Nair (1982) gave a brief account of the feeding biology and boring activity of sphaeromatid isopods in the laterite and hard clay substrata along the southwest coast of India. Nair et al. (1989) described the effects of salinity on the boring activity of S. annandalei and also observed the interspecific relation between S. annandalei and the cirolanid isopod Cirolana willeyi. C. willeyi is a free-living fouler, widely distributed throughout the estuaries of the Kerala coast. They are often observed in the burrows of sphaeromatids and are often misidentified as actual borers. Santhakumari (1973) investigated the interspecific relationships (predators, commensals, and associates) of wood-burrowing isopods, including S. walkeri, S. annandalei, S. triste, S. terebrans, and S. annandalei travancorensis.
The systematics and biology of marine isopods are well documented. Many researchers have also done their work on the boring activity of different species of sphaeromatid isopods in different substrates (Dharmaraj and Nair 1982; Davidson and Rivera 2010, 2012; Davidson 2012; Mohammad 2014; Dodge-Wan and Nagarajan 2020; Thiri and Yang 2022). But the boring architecture/boring pattern for the entire population of any isopod is still missing and has not been reported so far. What type of interspecific relationships exist between adults and juveniles in a population is also a notable question. The present study reports the study of the extensive boring activity of Spheroma annandalei in the hard mud substratum of intertidal habitat in the Gulf of Khambhat, Gujarat. The boring pattern and burrow architecture of this species have also been described by physical tracing of the burrows and resin casting methods. This paper explored the interrelationships between the burrows of juveniles and adults of the same species, S. annandalei.
2 Material and Methods
2.1 Study Area
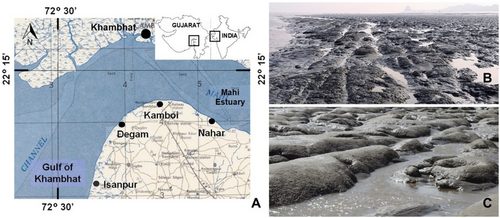
The Gulf of Khambhat is a tidal regime in the Arabian Sea on the western part of India between the Saurashtra peninsula and mainland Gujarat. It has a distinctive funnel-shaped geometry, with a wide southern mouth spanning over 200 km and tapering to a narrow band of about 30 km at its northernmost point. The Gulf of Khambhat shows extreme currents, high tidal amplitude (up to 12 m), and excessive churning of bed material. The tide is significantly amplified within the gulf due to its unique shape and varying bottom friction coefficients (Nayak and Shetye 2003), as well as the broad continental shelf off the northwestern coast of India (Joseph et al. 2009). The tidal range in the gulf reaches approximately 10 m at Bhavnagar, making it the highest along the Indian coast (Kumar et al. 2006). The currents in the gulf are predominantly tide-driven; during flood or spring tide, the currents flow north-northwest, and the velocity increases northward from about 0.8 m/s at the mouth of the gulf to a maximum of 3.4 m/s in the inner northern apex (Kumar and Kumar 2010). The tidal span is 1.5 to 5 km on the western coast and 2–8 km on the eastern coast of the gulf, increasing upstream. At Kamboi, the maximum tidal exposure was over 2 km. The sampling sites are located in the upper to mid intertidal area. Sediment inputs from major rivers like Narmada, Tapi, Mahisagar, Sabarmati, and Shetrunji contribute significantly to its hydrodynamics and high turbidity.
The study area was then confined to the lowermost estuarine region of Mahisagar at Nahar to Isanpur along the eastern bank of the Gulf. The area has soft as well as hard mud with a silty-clayey composition. The present study was conducted on the hard mudflat habitat of Kamboi (22°12′55.67″N, 72°36′25.42″ E) and Nahar (22°11′33.21″N, 72°41′23.75″ E) along the northwestern innermost coast of the Gulf of Khambhat, Gujarat, on the west coast of India (Figure 1). The tidal mudflats of the Gulf of Khambhat are a vital ecosystem along India's coastline, known for their significant potential in carbon sequestration and supporting high biodiversity. A comprehensive field survey was carried out along the eastern bank of the Gulf of Khambhat. Kamboi and Nahar were selected for the present study because the hard mud substratum is widely spread at both study sites. The intertidal mudflats of Kamboi exhibit distinct zones and microhabitats due to the different sediment compositions and hydrodynamic processes (Pandya 2011). From the upper intertidal zone to the lower intertidal zone, the mudflats of Kamboi were mainly divided into four distinct zones according to the variation in their sediment composition. It includes soft mudflats rich in varying proportions of sand, fine sand, and silt, and hard mudflat habitats rich in fine silt and clay. Zone 1 is the uppermost tidal region, submerged only during the highest high tides and remaining dry for the rest of the time. It spans approximately 10–20 m and is characterized by loose, silty, or clayey sand, eroded from nearby cliffs and wind activity. Due to significant vehicular and human disturbances, this zone lacks benthic fauna. The substrate composition includes sand (23%), silt (76%), and clay (1%). Zone 2 is covering an area of 80–130 m, this zone consists predominantly of silty-clayey soil and includes sand (11%), silt (80%), and clay (9%). Zone 3 shows a heterogeneous habitat due to the presence of hard mud substratum and tide pools. The spread of the hard mud substratum is about 20 to 100 m with minimal slope, which is mainly composed of a high amount of clay and silt (40%–45%), 20%–25% fine to medium sand, 30%–35% coarse sand, and 3%–5% gravel. Zone 4 is the lowermost intertidal zone, extending about 1 km from the high tide mark, featuring fine sandy substrate with the least slope. The sampling area, Zone 3, is fully exposed throughout the moon cycle and therefore can be considered as an upper to mid intertidal area. The hydrodynamic conditions, including current force, circulation, and water movement, vary significantly during high and low tides and across different lunar days, resulting in diverse ripple formations. The substrate composition comprises sand (19%), silt (80%), and clay (1%). The hard mud substratum is a part of Zone 3 where the present study was carried out. The hard mud substratum is relatively more compact than other areas, primarily due to its sediment composition, which consists of tightly packed clay with a high organic content. The hard mud substrata had tide pools of different sizes and depths and were filled with sediments of different compositions due probably to the varying depositional and erosional impacts of tides on different moon days. To study the boring activity of isopods, physical tracing and resin casting were conducted during fieldwork on the following dates: 12 June 2022, 04 April 2022, 02 May 2023, and 13 September 2023. The boring activity of isopods was observed only in the hard mud substratum.
2.2 Sediment Sampling and Soil Texture Analysis
To analyse sediment composition, soil samples were collected using scooping and coring methods. The collected samples were dried and powdered. To prevent clumping of sediment grains, the soil samples were initially treated with hydrogen peroxide, followed by sodium fluoride. Sediment composition analysis was performed using the mechanical dry sieving method (Chepil 1951; Kemper and Rosenau 1986; Skidmore and Powers 1982). Standard ASTM (American Society for Testing and Materials) sieves with mesh sizes of 2 mm, 1 mm, 0.50 mm, 0.25 mm, 0.150 mm, 0.125 mm, 0.09 mm, and 0.075 mm were used to determine the sand and silt/clay fractions based on the sediment characteristics.
2.3 Specimen Sampling, Abundance and Body Size Measurements
To assess the abundance of isopods, sediment blocks were collected from various locations during low tide. A total of ten blocks were sampled to evaluate isopod abundance. The isopods were extracted using the sediment sieving method, where sediment samples were passed through sieves with mesh sizes of 1 mm, 0.5 mm, and 0.25 mm to retain the specimens. The isopods from each sediment block were counted individually. Additionally, the body width of 400 specimens was measured using digital vernier calipers.
2.4 Burrow Architecture: Physical Tracing Method and Resin Casting
For the study of burrowing architecture, the physical tracing of the burrows and the resin casting were done in situ during the low tide, as well as the sediment cores collected from different locations of study sites that were transported to the laboratory. To examine the modified structures and boring activity in the hard mud substratum, burrows of isopods were traced physically with the help of forceps, scalpel, and needle and cleaned carefully with a brush to see the internal structures and interconnections between the burrows. Burrows were identified by the imprint of the isopod body wall as horizontal lines on the burrow walls, which are marked by the movement of isopods. In the in situ studies, the isopods were recovered from most of the burrows, which confirms that the burrows were constructed by isopods only. For further confirmation of internal burrow structures, the resin casting method was used. The unsaturated polymer resin was mixed with cobalt and a catalyst. After constant stirring, the mixture was poured into the burrows with the help of small beakers/syringes until the burrows were completely filled. When the burrow cast got solidified in around an hour, the sediment around the burrow cast blocks was removed and thoroughly cleaned. The opening diameter of the burrows was initially measured on-site during block collection using vernier calipers. Later, additional measurements were taken from resin casts of the burrows, also using vernier calipers.
3 Results
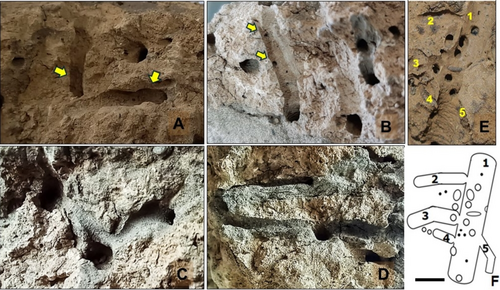
The hard mud substratum of Nahar and Kamboi shows excessive bioerosion due to the boring activity of isopods (Figure 2). The population of Sphaeroma annandalei was found in high density. The free-living isopod, Cirolana willeyi, was also observed during the excavation, but the number of individuals of C. willeyi collected was very low. Population density of isopods was observed by direct count of burrows and isopods in the similarly sized sediment blocks from different locations. The density of S. annandalei was observed to be a maximum of 3257 ± 200 and a minimum of 1500 ± 200 individuals per square meter at different locations of study sites. Individuals of C. willeyi were much less abundant compared to S. annandalei, with only about 5–10 individuals found in a few sediment blocks. They constructed a dense network of interconnected burrows in the hard mud substratum. Maximum boring intensity was observed on the exposed surfaces of the hard mud substratum. The number of burrows was higher on the upper surface and lateral side, and the number of burrows reduced with the increased depth of the sediment. The compactness of the hard mud substratum could be a reason behind this.

Animals were counted from ten sediment blocks, with specimens from various phyla/animal groups, such as isopods, amphipods, rove beetles, crabs, and polychaetes, collected from the sediment cores (Table 1). However, isopods were the most dominant group in the macrobenthic community of the hard mud substratum, comprising 150–450 individuals (74%–86% of the total burrowing macrobenthos) per sediment block (n = 10). Each block had a large number of adult and juvenile isopods, indicating successful reproductive rates and successful establishment of the isopod population. The size (width) of isopods from 400 individuals, including adults and juveniles, was recorded. No morphological traits were used to distinguish between adults and juveniles. Out of 400 individuals, the lengths of 252 individuals were measured at up to 3 mm, with a mean value of 2.8 ± 0.60 mm (Min: 1.3 mm, Max: 3 mm). The widths of these 252 individuals were measured at up to 2 mm, with a mean value of 1.27 ± 0.40 mm (Min: 0.56 mm, Max: 1.96 mm). The remaining 148 individuals had lengths above 3 mm, with a mean value of 5.2 ± 0.71 mm (Min: 3.2 mm, Max: 11 mm), and widths above 2 mm, with a mean value of 3.34 ± 0.78 mm (Min: 2.04 mm, Max: 4.72 mm). Juveniles accounted for approximately 63% of the total S. annandalei population.
| Total count | Isopods | Polychaete | Other arthropods | Isopod % | |
|---|---|---|---|---|---|
| Min. | 159 | 125 | 11 | 8 | 74.25 |
| Max. | 435 | 323 | 89 | 54 | 86.79 |
| Average | 266.4 | 206.2 | 35.6 | 24.6 | 77.61 |
The burrows created by isopods in harder substrates are actually more permanent compared to those in softer substrates due to the characteristic features of the substratum. Isopods are known for their burrowing behavior, and their burrows can be quite intricate and robust. Sphaeroma annandalei that burrow into hard, clayey mud substratum are basically filter feeders and most likely feed on the organic content and/or the algae and fungi lining their burrows (Rotramel 1975; Dharmaraj and Nair 1982).
3.1 Physical Tracing of Burrows
Sphaeroma annandalei is known as a marine borer, with earlier studies reporting boring activity in laterite and hard clay (Dharmaraj and Nair 1982). To see the boring pattern and architecture of burrows of isopods in the hard mud substratum, multidimensional sections of sediment cores were studied from different sediment blocks (Figure 2). Internal structures were studied by in situ cleaning and physically tracing the burrows. In the hard mud substratum, burrows of isopods were found in high density, with the burrows constructed in close proximity to one another (Figure 3A,B). The high sediment density, due to the presence of coarse sand, gravel, and the compact clayey composition of the hard mud substratum, limits the available space for these organisms to construct their burrows. When the isopods were displaced during the study, they moved on the soft mud or swam through the tide pools to locate the hard mud to form a burrow in it (Figure 3D,E). The smaller burrows of the juvenile isopods pass through the larger burrows of the adult isopods; Figure 4B is clear evidence of this. The opening of smaller burrows was present inside the larger burrows. These organisms usually freely occupied each other's burrows. In situ tracing of burrows exhibits different-sized burrows of isopods. During physical tracing, isopods emerging from burrows were captured, and their size was measured with the help of vernier calipers. The size of burrows depends mainly on the size of the individuals. Figure 4E,F shows very clearly that the large vertical burrow is intersected by smaller burrows of varying sizes. Adjacent burrows were found to be very close to each other, with only a thin wall of sediment separating these burrows (Figure 4A). In general, we did not observe any fusion of burrows, even when they were in close proximity.

3.2 Resin Cast of Burrows
To check and observe the burrow/boring pattern of isopods and confirm the observations of physical burrow tracing, resin casting of sediment blocks was done in situ as well as in the blocks brought to the laboratory (Figure 5). Several burrows of the juveniles could not be recovered through resin casting due to improper or incomplete penetration of resin into very thin burrows, and several such small, thin burrows were broken while cleaning the cast. However, still many connections could be retained, and therefore, the architectural pattern of juveniles and adult isopod burrows could be studied to draw out certain conclusions. After removing the sediments, the complex network of small tunnels of isopods was recovered (Figure 5B).

In the resin cast, a complex network of interconnected burrows of the Sphaeroma annandalei was found (Figure 6A,E). Identification of the burrows was mainly based on the size of organisms and in situ observations. S. annandalei constructed cylindrical burrows that were horizontal, vertical, oblique, and semicircular types of connected tube-like structures. Burrows of varying sizes were recovered, with a diameter of 1.5 mm to 7.2 mm and a length of 20 mm to 70 mm, depending on the size of the individuals (Figure 6B–D,G). The opening diameters of the burrows recorded on-site during collection and physical tracing using vernier calipers ranged from 3.7 to 10 mm (n = 50), with an average of 5.9 ± 0.20 mm. In contrast, the burrows recovered inside the blocks using resin casts had widths ranging from 0.7 to 6.2 mm (n = 64), with an average of 4.3 ± 0.56 mm. The number of burrows with a width of up to 3 mm was 21, while the remaining 43 measured above 3 mm. These observations suggest that juvenile burrows were fewer in number compared to adult burrows. However, juveniles were more abundant across all sediment blocks, indicating that juveniles utilized the spaces within adult burrows.
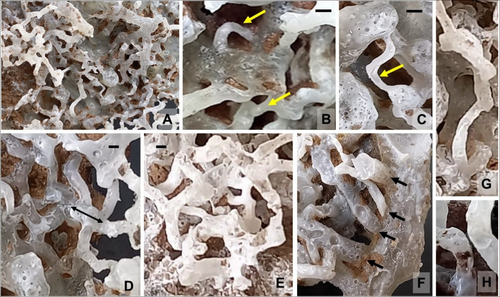
The count of the total number of burrows in the sediment blocks was not possible due to the interconnections and branching of the burrows. However, the data indicate that the number of burrows in each block was lower than the number of individuals, suggesting that multiple individuals shared the burrows. The burrow of each isopod is connected to the burrow of another isopod or at times, with the burrow of another animal; the interconnection between these burrows is clearly visible and has been confirmed by both physical tracing and the resin casting method (Figure 6E,F). Some of the burrows were very close to each other, but still, they were differentiated by a thin wall of sediment (Figure 6H). Hence, we did not find fusion of the closely arranged burrows or the breakage of the partition wall/burrow lining. It is difficult to determine the inhabitants of each burrow, but it is possible that the larger burrows belong to adult isopods and the smaller, thinner burrows belong to juveniles.
4 New Records of Isopods From Gujarat Coast
4.1 Systematics
Order Isopoda Latreille, 1816.
Suborder Sphaeromatidea Wägele, 1989.
Superfamily Sphaeromatoidea Latreille, 1825.
Family Sphaeromatidae Latreille, 1825.
Genus Sphaeroma Bosc, 1801.
4.2 Sphaeroma annandalei Stebbing, 1911 (Figure 7)

Sphaeroma annandalei Stebbing, 1911, 181, P1. X.; Barnard, 1936, 174; 1940, 405; Pillai, 1955, 134, P1. VII. figures 23–35; Joshi and Bal, 1959, 62; Kensley, 1978, 113.
Sphaeroma irakiensis irakiensis Ahmed, 1971, 77–79, fig 1.
Sphaeroma irakiensis bidentata Ahmed, 1971, 79–80, fig 2.
4.2.1 Type Locality
West Bengal, India (Stebbing 1911).
4.2.2 Material Examined
Twenty-five specimens (MSUB-ZL-AR-CR-107), Kamboi (22°12′53.61″N, 72°36′14.63″ E), Gujarat, 12 June 2022, hard mud substratum, coll. V. Prajapat. Specimens are deposited in the Department of Zoology Museum, The Maharaja Sayajirao University of Baroda, Vadodara, Gujarat.
4.2.3 Remarks
The body is oval-shaped, with a total length ranging from 1.3 mm to 11 mm (n = 25) and a width of 1.57 mm to 3.56 mm (n = 25). Species identification was done following the description of Stebbing (1911) and Pillai (1955). The diagnostic feature is the pleotelson dorsal surface with two pairs of large, prominent submedian tubercles followed by a single median tubercle, flanked by a longitudinal row of three small tubercles on each side. No new features were observed.
4.2.4 Habitat
Based on the original description of Stebbing 1911, the species was found in the larger canals of the sponge Spongilla alba bengalensis. The species has also been found in the laterite and hard clay blocks in estuaries and intertidal mudflats (Dharmaraj and Nair 1982). The present study describes hard clayey muddy substratum as a natural habitat of S. annandalei.
4.2.5 Distribution
The species is so far reported from the northern areas of the Indian Ocean, from the Persian Gulf to Malaysia (Khalaji-Pirbalouty and Wägele 2010).
In India, the species is reported from Cochin, Mumbai, Akathumuri backwaters, Kadinamkulam and Akathumuri Lakes, Shanghu mugham, Madras coast, and from several other localities both along the West and East coasts of India (Dharmaraj and Nair 1982; Unni et al. 2020).
The species is reported for the first time from Gujarat.
Suborder Cymothoida Wägele, 1989.
Superfamily Cymothooidea Leach, 1814.
Family Cirolanidae Dana, 1852.
Genus Cirolana Leach, 1818.
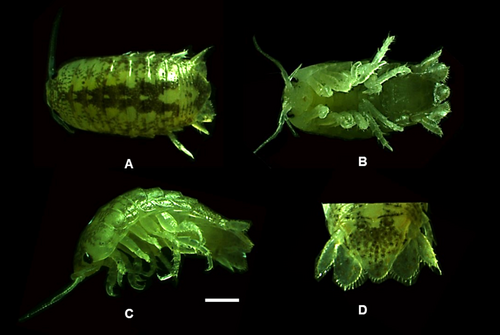
4.3 Cirolana willeyi Stebbing, 1904 (Figure 8)
Cirolana willeyi Stebbing, 1904, 11, pI. 3.-Nierstrasz, 1931, 58; Barnard, 1935, 312, figure 18b; Pillai 1954, 5; 1967: 270, figure 2a, b; Jones 1976, 216; Hamsa and Nammalwar, 1978, 517. Cirolana nigra Chilton, 1924, 884, pI. LX figures 3,6.
Anopsilana willeyi Bruce, 1981a, 955.
Anopsilana willeyi Bruce, 1986, figures 137, 138.
4.3.1 Type Locality
Ceylon (Sri Lanka) (Stebbing 1904).
4.3.2 Material Examined
Nine specimens (MSUB-ZL-AR-CR-106), Kamboi (22°12′53.61″N, 72°36′14.63″ E), Gujarat, 12 June 2022, hard mud substratum, coll. V. Prajapat. Specimens are deposited in the Department of Zoology Museum, The Maharaja Sayajirao University of Baroda, Vadodara, Gujarat.
4.3.3 Remarks
Body color yellowish to dark brown, and pale brown yellow colored chromatophores are present on the middorsal region of body. Total length ranging from 3.5 mm to 6.2 mm (n = 9) and a width of 1.8 mm to 3 mm (n = 9). Species identification was done following the description of Stebbing (1904). No new features were observed.
4.3.4 Habitat
The species has been found in estuarine and mangrove habitats and also in laterite and hard clay embankments in estuaries and intertidal mud flats of Kerala (Bruce 1981b and 1986; Nair et al. 1989).
4.3.5 Distribution
The species was originally described from Ceylon (Sri Lanka) (Stebbing 1904) and was later reported in estuarine and mangrove habitats from East Africa to Australia (Bruce 1981b & 1986), Singapore (Cai and Teo 2012).
In India, the species was so far reported from the southwest coast of India, Akathumuri, and Cochin backwaters (Nair et al. 1987 & 1989).
The species is first time reported from Gujarat.
5 Discussion
In the present study, two species of isopods, Sphaeroma annandalei and Cirolana willeyi, were reported for the first time from the Gujarat coast. Cirolana willeyi was previously recorded from Akathumuri and the Cochin backwaters (Nair et al. 1987, 1989) and is known only from the southwest coast of India. Sphaeroma annandalei has been documented from Mumbai, Kerala, Shanghumugham, the Madras coast, and both the west and east coasts of India (Unni et al. 2020). Additionally, C. willeyi has been frequently observed in association with S. annandalei (Nair et al. 1989). Nair et al. (1989) conducted an experiment on the boring activity of S. annandalei and its interspecific relationship with C. willeyi. For this, they prepared five artificial conditions to observe their burrowing behavior. Their study revealed that C. willeyi preferred to enter already constructed burrows of S. annandalei rather than construct new burrows for themselves. However, they occupy these burrows only in the absence of S. annandalei or when S. annandalei abandons their burrows. S. annandalei was observed excavating new burrows, and they showed no interest in occupying the burrows/bore holes left by C. willeyi. Sphaeroma is known as a habitual wood borer, but the study of Dharmaraj and Nair (1982) reported the presence of S. annandalei from the laterite blocks and hard clay embankments of the Astamudi and Kadinamkulam backwaters, southwest coast of India. Earlier, Nanda (2003) reported the occurrence of isopod larvae from the same study area of the Gulf of Khambhat and Mahi River estuary, suggesting successful reproduction of isopods in the coastal waters.
The boring pattern and burrow architecture of Sphaeroma annandalei in the hard mudflat habitat was reported here for the first time. The burrows created by S. annandalei in hard mud substratum show complexity, branching, and interconnections between the burrows of adults and juveniles. Detailed observations of burrow architecture were described here with the help of physical tracing and resin casting methods. A complex network of interconnected burrows reflects the spatial limitations imposed by the compactness of the substratum. Most of the burrows were interconnected and passed through each other. Intersecting burrows indicate that sometimes, but not always, one individual uses the space of another individual's burrow while coming in or out. It shows mutual cooperation among the individuals of S. annandalei. The dense population of S. annandalei indicates that the hard mud substratum provides a good habitat for this species to survive. In the present study, the abundance of S. annandalei, with a large number of individuals including juveniles and adults, indicated successful establishment of the population. In the hard mud substratum, the population of S. annandalei is sharing their habitat with other organisms like sea anemones, polychaetes, amphipods, rove beetles, crabs, and several meiobenthos, etc. The isopod burrow connectivity was seen with that of the crab burrow, but it could not be confirmed for the burrows of other benthic organisms sharing the same habitat.
Several studies have been conducted on the boring activity of different species of Sphaeroma, such as the rock boring activity of Sphaeroma triste, which was reported by Dodge-Wan and Nagarajan (2020). Their study revealed that S. triste creates simple, unbranched, and cylindrical perforations with circular cross-sections and hemispherical blind ends. On the contrary, S. quoianum shows branched and interconnected burrows in peat and mud (Talley et al. 2001). Similarly, in mangrove wood, the burrow complexity has also been observed for S. terebrans (Messana et al. 1994; Thiel 1999). Interconnections have been observed between the burrows of young and adult S. terebrans by Messana et al. (1994). Davidson and Rivera (2012) did lab experiments on burrow formation by Sphaeroma quoianum (Milne Edwards, 1840) in different substrates and reported that S. quoianum formed vermiform, straight burrows and they did not change the directions; however, long burrows created in Styrofoam and marsh bank substrata could be sinuous. The length of the burrows observed in the present study was comparable to previously reported burrow sizes. However, we found that only a few burrows were straight; most were sinuous, branched, and interconnected, forming a complex gallery network. The terminating end of the burrow could be found for only a few of them and in such cases it was the rounded, tapering end much like the bottom of a centrifuge tube. The orientation of the borings and the degree of curvature could not be determined. Despite extensive study of resin casts, no specific boring patterns were observed. The orientation, direction, and length of the burrows appear to depend on the composition of the substratum, which includes gravels as well and, probably, hinders burrowing.
Davidson and Rivera (2012) reported that the mean burrow length, total number of burrows, and per capita erosion of the substrate were reduced in the harder substrata. The hard mud substrata in the present study probably inhibited the long straight burrow formation, and therefore, small branched, interconnected/anastomosing burrows were made by isopods in the available substrata. The burrows of isopods are expected to collapse in soft substratum. They were found moving on the medium soft mud and swimming in the loose mud but cannot burrow there. Therefore, although there is more than 1 km of intertidal mudflat (soft and medium soft mudflat inclusive) exposed during low tides at Kamboi, the isopods were distributed only in about 40 to 100 m wide hard mud substratum, indicating their very strong microhabitat/substratum (sediment composition) preferences.
We have been studying this study site for over 20 years and did not find any significant change in the overall distribution of hard mud substratum at Kamboi and other nearby areas along the gulf. Therefore, it is necessary to review isopod population density in this habitat in the context of their erosional potential due to boring. The habitat where isopods were distributed is a barren area lacking algae or mangrove vegetation. However, they significantly contribute to the diversity of local communities by increasing landscape heterogeneity and offering shelter to commensal species. Further studies are also needed to understand the interactions of these isopod species with adjacent sediment-dwelling species of boring amphipods, polychaetes, rove beetles, and crabs and the impact of extensive boring of isopods on the mudflat habitat of Kamboi.
Acknowledgements
The authors are grateful to the Head, Department of Zoology, The Mahraja Sayajirao University of Baroda for providing lab facilities and support. V.P. is thankful to CSIR-HRDG for financial support under the CSIR NET JRF scheme.
Open Research
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.




