Deep-Sea Coral Abundance, Distribution, and Community Structure on Seafloor Features Across a Broad Depth Gradient in North-Central California National Marine Sanctuaries
Funding: This work was supported by National Oceanic and Atmospheric Administration.
ABSTRACT
Patterns in deep-sea coral (DSC) diversity and distribution are described for a range of benthic habitat features including offshore banks, continental shelf and slope, and submarine canyons in three national marine sanctuaries off the coast of North-central California. Sixteen visual datasets of DSC observations collected using underwater vehicles from 2010 to 2021 across a wide depth range of 35–3317 m were analyzed for zonation by depth and seafloor feature type. A total of 36,670 DSC from at least 20 families were documented in the study area. Taxa displayed patterns corresponding to depth and seafloor feature type, such as broad distribution across multiple depths and features or narrower depth range and fewer features. Significant divergence in DSC assemblage diversity and density occurred between banks and canyons, representing the shallowest and deepest depth extents in the study area. One species of Stylasteridae coral primarily inhabited banks and accounted for the highest density of all DSC taxa. Diversity of soft corals and gorgonians (Octocorallia) and black corals (Antipatharia) was greatest on the slope and canyons. Other octocorals such as sea pens (Pennatuloidea) collectively spanned the largest depth ranges throughout the study area on all features other than banks. Comprehensively describing DSC communities in relation to seafloor features throughout an extensive depth range may have applications to other regions globally where similar habitats and DSC families are found. Our growing understanding of taxonomic diversity and zonation adds to existing knowledge of depth and geographic distributions on the U.S. West Coast and provides a crucial foundation for effective management and conservation efforts for DSC communities.
1 Introduction
The unique combination of oceanographic patterns and undersea topography in the eastern Pacific, off the coast of North-central California, supports abundant and diverse protected marine communities within the National Oceanic and Atmospheric Administration's (NOAA) Greater Farallones National Marine Sanctuary (GFNMS), Cordell Bank National Marine Sanctuary (CBNMS), and the northern portion of Monterey Bay National Marine Sanctuary (MBNMS), which are administered by GFNMS and CBNMS. These three areas encompass 15,164 km2. The sanctuaries were designated under the authority of the National Marine Sanctuaries Act of 1972; GFNMS was designated in 1981, CBNMS in 1989, and MBNMS in 1992. The sanctuaries occur within the California Current Large Marine Ecosystem and are locally influenced by the Point Arena upwelling center, which contributes to the highly productive waters (Halle and Largier 2011) that sustain many resident and migrant animals as well as rich underwater biological communities of fishes and invertebrates (Office of National Marine Sanctuaries (ONMS) 2023, 2024).
Here, we focus on the sanctuaries' seafloor habitats, made up of different underwater landscapes including rocky banks, the continental shelf and slope, and deep submarine canyons. With the advancements in deep-water survey technologies, numerous benthic surveys have been conducted in the sanctuaries using remotely operated vehicles (ROV) and an autonomous underwater vehicle (AUV) since 2010 (Graiff et al. 2011, 2016, 2021; Fruh et al. 2013; Etnoyer et al. 2014; Roletto et al. 2017, 2020; Lipski et al. 2018; Graiff and Lipski 2023). Characterization and monitoring of seafloor habitats and their associated fauna provide information to resource managers that can be used for effective management of the sanctuaries. Particularly, there is an increased awareness of the ecological importance, vulnerability, and need for conservation of deep-sea corals (DSC).
Deep-sea corals, also known as cold-water corals, are defined as azooxanthellate cnidarians that generally occur at depths below 50 m (Roberts et al. 2009; NOAA 2010; Everett et al. 2022). These include both deep reef-building stony corals (e.g., Lophelia pertusa, recently synonymized with Desmophyllum pertusum) as well as branching colonies (e.g., bamboo and black corals) or single stalked corals (e.g., sea pens) that either occur individually or in aggregations that increase their habitat value (Hourigan et al. 2017). Deep-sea corals are a diverse group of marine organisms found worldwide, and research has revealed that they are often extremely long-lived and slow growing (Andrews et al. 2009; Roark et al. 2009; Prouty et al. 2017); characteristics that make them slow to recover from physical disturbance from bottom contact fishing gear such as trawling (Heifetz et al. 2009; Rooper et al. 2017; Yoklavich et al. 2018). The complex and three-dimensional structure of many DSC species provides protection and enhanced feeding opportunities for associated fishes and other invertebrates (Krieger and Wing 2002; Buhl-Mortensen et al. 2010; Baillon et al. 2012).
The importance of DSC ecosystems has become a major focus of international conservation efforts. The United Nations General Assembly Resolution 61/105 called upon States to take immediate actions to sustainably manage vulnerable marine ecosystems, such as cold-water corals, from adverse impacts of deep-sea fishing practices (UN 2007). In the United States, DSC conservation has been recognized by management agencies such as the Pacific Fisheries Management Council (PFMC), NOAA National Marine Fisheries Service (NMFS), and NOAA ONMS. Additionally, NOAA established the Deep-Sea Coral Research and Technology Program to increase scientific understanding of DSC and sponge ecosystems, as part of the 2007 reauthorization of the Magnuson-Stevens Fishery Conservation and Management Act.
Reviews by Roberts et al. (2009), Watling et al. (2011), and Cordes et al. (2016) highlight the growing knowledge of DSC diversity, abundance, and global distribution, in addition to the value of habitats they create and their vulnerability from anthropogenic impacts. On the U.S. West Coast, multiple efforts conducting visual surveys, sample collection, and seafloor mapping have contributed to new information on DSC in the region (Laidig et al. 2021, 2022), in the Olympic Coast National Marine Sanctuary (Fruh et al. 2010a; Bowlby et al. 2011), Northern California (Yoklavich et al. 2016, 2018), GFNMS and CBNMS (Graiff et al. 2011, 2016, 2021; Fruh et al. 2013; Etnoyer et al. 2014; Roletto et al. 2017, 2020; Lipski et al. 2018; Graiff and Lipski 2023), MBNMS (King and Brown 2019; King et al. 2021), Southern California, and Channel Islands National Marine Sanctuary (Fruh et al. 2010b; Lipski et al. 2011; Yoklavich et al. 2011, 2013).
Additionally, various studies have focused on the assemblage structure and distribution of DSC in the region. Watters et al. (2022) provided a biogeographical assessment of DSC off California using data from coastwide visual surveys. Community structure and diversity of DSC associated with seamounts off central and southern California have been described (Lundsten et al. 2009; McClain et al. 2009, 2010). Predicted distributions and habitat suitability of select DSC have been modeled for areas of the U.S. West Coast using presence or presence-absence data and associated environmental data (Etherington et al. 2011; Krigsman et al. 2012; Huff et al. 2013; Guinotte and Davies 2014).
These efforts have contributed toward the knowledge of DSC depth and geographic distributions along the U.S. West Coast (Clarke et al. 2017; Everett et al. 2022). Yet, there has not been a synthesized description of abundance, diversity, and distribution of DSC with respect to seafloor features: rocky banks, continental shelf and slope, and submarine canyons within the national marine sanctuaries of North-central California. In this study, we combine 16 datasets of visual DSC observations collected by ROV or AUV within a broad depth range, collected by various initiatives to meet specific research and management needs. Our objective is to provide a comprehensive description of DSC taxa-specific depth distributions in association with seafloor features off North-central California to inform management, monitoring, and protection of DSC communities. The approach we use to describe diversity and distribution patterns of DSC in relation to seafloor features could have applications beyond our area, as many of the coral families we observed are broadly distributed throughout the world's oceans.
2 Methods
2.1 Surveys
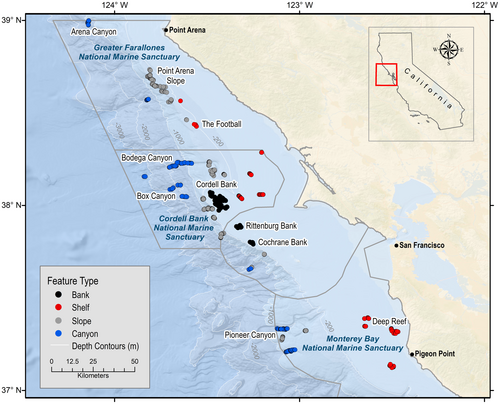
Sixteen visual DSC observation datasets collected from ROV and AUV expeditions conducted from 2010 to 2021 were combined for this study. Five ROVs and one AUV were used. The geographic extent of the study area was offshore Point Arena, California (38.95° N, 123.73° W) to offshore Pigeon Point, California (37.18° N, 122.39° W) at depths of 35–3317 m within GFNMS, CBNMS, and northern MBNMS (Figure 1). All expeditions were designed to survey the seafloor through quantitative visual transects in various substratum types identified from previously collected multibeam mapping data or, when possible, from predicted substratum maps interpreted from multibeam and backscatter data using methods described in Cochrane (2008).
The majority of the ROV surveys followed pre-planned routes to conduct multiple quantitative transects of a similar distance (150–200 linear meters of the seafloor) or duration (10–15 min) during each dive. The ROV pilot strived to maintain a consistent course along a depth contour or specific heading, speed (0.1–0.5 m/s, 0.25–1 kt) and height (0.5–1.5 m) above the seafloor. For a few surveys, dive video imagery of the seafloor was later subset into transects for analysis (e.g., some of the E/V Nautilus expeditions using the ROV Hercules). The sub-sampled transects met the criteria of moving forward in a consistent direction at a relatively consistent speed and height above the seafloor, and ROV navigation was plotted in GIS (ArcGIS; Esri, Redlands, CA) to ensure similar distances (~200 linear meters) of the seafloor were covered. All transects used for analyses were spaced at a minimum distance of 100 m apart to increase sample independence. The ROVs were equipped with LED lighting and an oblique forward-facing high-definition color video camera that continuously recorded video during the surveys. Paired parallel lasers were mounted 10 or 20 cm apart (vehicle specific) and positioned in the center of the video frame for sizing objects viewed in images. Details of each ROV's operating protocols during quantitative transects, camera specifications, and sizing lasers are provided in Graiff et al. (2011) (K2), Graiff et al. (2016) (Phantom), Raineault and Flanders (2020), and Graiff et al. (2021) (Hercules), Laidig et al. (2021) (Beagle), and Laidig et al. (2022) (Yogi). Specimens of DSC and other organisms of interest were collected in between quantitative transects using a manipulator arm and sample box on the ROV. Collections of biological specimens were identified and curated by the curator and collections staff at the California Academy of Sciences, Department of Invertebrate Zoology and Geology.
A bottom-tracking Seabed type AUV was used to collect still images of substratum and associated fauna. The AUV was pre-programmed before each dive to follow a specified dive track and to maintain a height of 3 m above the seafloor at a forward speed of 0.25 m/s (0.5 kt) for 4–6 h per dive. Images of the seafloor were collected using a stereo pair of downward-facing 5-megapixel color still cameras and a third 11-megapixel color still camera angled forward at approximately 35°. Lighting was provided by a strobe synced with the cameras. Still images were collected at a rate of one image every 10 s.
All vehicles had depth sensors, but other environmental sensors on vehicles differed. For example, not all vehicles had a conductivity, temperature, and depth (CTD) sensor, and a dissolved oxygen sensor; therefore, environmental data were not used as factors in the analyses. The ROVs and AUV were tracked using an ultra-short baseline acoustic positioning system that was integrated with the support vessel's GPS and provided bearing and range from the support vessel to the underwater vehicle. The width of the ROV transects was either provided with the navigation files by the ROV team or was calculated during post expedition video review by recording the ratio of the video monitor width to the laser spots on the video monitor (both measured with a ruler in cm) and multiplied by the actual laser width. The width measurements were taken approximately every 1 min and at the start and end of each transect. Transect length was determined from the navigation data in ArcGIS by summing the distances between successive UTM points. The total area surveyed on each ROV transect was calculated by multiplying the average transect width estimates by transect length. Specifications for each ROV's navigation system, methods for smoothing tracking data, and estimating transect length and width are detailed for each ROV in Graiff et al. (2011) (K2), Graiff et al. (2016) (Phantom), Raineault and Flanders (2020) and Graiff et al. (2021) (Hercules), Laidig et al. (2021) (Beagle), and Laidig et al. (2022) (Yogi). The AUV was equipped with several sensors to measure altitude and relative speed and direction over the sea floor, described in detail in Powell et al. (2018). The area of all nonoverlapping color-corrected digital stills from each AUV dive was calculated using camera optics and measured altitude off the seafloor (Clarke et al. 2020). The total area of each AUV dive was the sum of all nonoverlapping still images.
2.2 Image Processing
High-definition video collected on quantitative transects from the ROV's oblique forward video camera and non-overlapping digital stills from the AUV's downward looking camera were reviewed by expert analysts to identify and quantify DSC at least 5 cm in height to the lowest taxonomic level possible. Counts of DSC were made from video imagery collected during ROV transects when corals horizontally aligned with the two lasers visible in the center of the video frame and from the full field of view of the AUV's downward stills. Taxonomic guides, in situ photographs of voucher specimens, and assistance from taxonomic specialists were used to make identifications. If a DSC could not be identified to genus or species, it was assigned to a higher taxon (family or order). When possible, width and height of DSC were estimated to the nearest 5 cm using the ROV's paired lasers as a guide. No corals were measured in AUV surveys.
Substrate was classified by primary and secondary type based on particle size and vertical relief as described in Greene et al. (1999). The primary substrate type covered greater than 50% of the field of view, while the secondary substrate type covered between 20% and 50%. If the primary substrate coverage exceeded 80%, that was the only substrate defined. Distinct changes in substratum types greater than or equal to 10 s in duration along ROV transects were delineated with a time stamp. Substratum types were classified in each image per AUV dive. The substrate classifications were then aggregated into three classes based on primary and secondary type: hard rock (e.g., rock ridge, flat rock, boulder, cobble), mixed (hard substrata combined with mud or sand), and soft sediment (mud or sand). DSC observations were recorded with a time stamp that was linked to depth and latitude-longitude coordinates from the ROV or AUV tracking data.
2.3 Data Analysis
Each georeferenced DSC observation was assigned a substrate class determined from post expedition video analysis and plotted in ArcGIS for classification to a seafloor feature type using multibeam bathymetry and hillshade data layers. The seafloor feature types include: rocky banks (bank), continental shelf (shelf), continental slope (slope), and submarine canyons (canyon) (Figure 1). We developed definitions for each seafloor feature (Table 1) based on depth, seafloor topography, and substratum class representative of the seafloor feature, while acknowledging that in transitional zones, two feature types may overlap (e.g., slope and canyon feature types share some of the same depths).
| Seafloor feature type | Definition |
|---|---|
| Bank | Subsurface feature that rises from the continental shelf and is primarily made of rock substrate with high relief. Generally, banks are of continental origin, separated from the mainland during rifting, compression or some other geological event. |
| Shelf | The area of the seafloor that starts from shore and continues with a gentle slope to approximately 200 m in this study region. Substratum can be soft sediments or rocky habitat that are lower relief than a bank. |
| Slope | Area of the seafloor that descends from the edge of the continental shelf (~200 m) with depths rapidly increasing from the shelf break with a steep slope extending deep to the ocean floor. Substratum can be soft sediments and rocky outcrops or a mix of both. |
| Canyon | Distinct features that are steep-sided and have a V-shape cross section. Submarine canyons can cross the continental shelf and slope, cutting through the slope to the deep ocean floor. Canyon walls can be sheer rock with relatively no sediments or the walls can be steep soft sediment with no rock. |
We used densities of DSC (number of individuals per 100 m2) relative to seafloor feature type as the standardized metric for describing abundance patterns. Area per feature type was quantified by plotting transect navigation files in ArcGIS with the seafloor feature type data layers and taking the product of each transect area within the respective seafloor feature type. The overall density of DSC by feature type was estimated from all ROV transects and AUV dives combined by dividing the total count of DSC within each feature type by the total area of that feature type. Mean densities for each taxon by seafloor feature type were estimated by dividing the total number of individuals by the area of the associated ROV transects and AUV dives and averaged (including transects or dives with null taxon counts) within the respective seafloor feature type. We also calculated diversity indices (Primer 7, QUEST Research Ltd) such as species richness (Margalef, d), evenness (Pielou's, J), and diversity (Shannon-Wiener, H'loge) from counts of DSC identified to genus and/or species within each seafloor feature type. Corals were categorized into four taxonomic groups: hydrocorals (Anthoathecata), black corals (Antipatharia), soft corals (Octocorallia), and sea pens (Pennatuloidea). Observations of stony corals (Scleractinia) were not included in analyses because only solitary forms (i.e., cup corals) have been observed in the study area, and due to their small sizes, individuals were not consistently quantified over the years, and reef building Scleractinian corals (i.e., Desmophyllum pertusum) have not been observed in the study area. Records of dead corals were also excluded from the combined dataset.
Multivariate analyses were used to examine coral assemblages by seafloor feature type and depth in Primer 7. Transects (ROV) and dives (AUV) were binned into 100-m depth intervals from < 100 to 3400 m, and mean DSC densities/100 m2 within depth bins were calculated. The 100 m depth bins provided a sampling unit from the ROV transects and AUV dives, in lieu of oceanographic characteristics to determine water masses, that consisted of all DSC observations occurring within that 100 m interval, covering the full depth extent of the data and producing enough sampling units for statistical tests without overwhelming the analyses with an abundance of samples. There were no survey data collected, and therefore, no DSC observations in bins within 2200–2400 m and 2700–3200 m. A matrix of depth bins as samples and mean density of each DSC taxon identified to genus and/or species as variables was 4th-root transformed to reduce the influence of abundant species (Clarke and Warwick 2001). Bray–Curtis similarity coefficients (Bray and Curtis 1957) were calculated for taxa densities across all pairwise combinations of the depth bins. The resemblance matrix was analyzed with hierarchical cluster analysis using group average linkage. Similarity profile (SIMPROF) permutation tests (n = 999) were applied to the cluster analysis to find significant groups of depth samples at the 95% level or greater. We then applied nonmetric multidimensional scaling (nMDS) ordination to visualize groupings of depth samples. The associated seafloor features for each depth sample were included as factors in the cluster and nMDS analyses. A one-way analysis of similarities (ANOSIM), using 999 random permutations, was performed to test whether there were any significant differences between pairwise combinations of seafloor feature types. A similarity percentage (SIMPER) test was performed to determine the contribution of specific DSC taxa to the similarities and dissimilarities within and between seafloor feature types. We identified the taxa most important (≥ 50% cumulative contribution) in creating the observed patterns of dissimilarities between seafloor feature types.
3 Results
Seafloor imagery was collected on 332 ROV transects and 7 AUV dives, covering a total area of 308,176 m2 (Table S1). Areas surveyed ranged in depth from 35 to 3317 m, and mean depth increased from bank to shelf to slope and canyons (Table 2). Seafloor feature types crossed multiple depths; therefore, shared depth ranges occurred between bank and shelf features and slope and canyon features. The total transect area varied among feature types, with greater area surveyed on slope and canyon features than on bank and shelf features. Hard rock and mixed substrata dominated transects on bank features, totaling 96% of the total area surveyed, while soft mud-sand sediment comprised about half of the total substrata surveyed on the shelf and slope (47% and 54% respectively). Hard and mixed substrata on the shelf were from survey transects on low-relief rocky reefs assigned to the shelf based on our seafloor feature definitions. Areas of hard, mixed, and soft substrata types surveyed in canyons were somewhat evenly represented.
| Seafloor feature type | Mean depth (m) (range) | Area surveyed (m2) | Substratum | Total corals | Overall coral density (100 m2) | Total corals genus or species | Num. species (S) | Richness (d) | Evenness (J') | Diversity (H′ loge) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Hard | % Mixed | % Soft | ||||||||||
| Bank | 71 (42–111) | 26,016 | 73 | 23 | 4 | 10,058 | 39 | 10,058 | 4 | 0.33 | 0.44 | 0.62 |
| Shelf | 86 (35–199) | 30,598 | 32 | 21 | 47 | 10,524 | 34 | 7562 | 11 | 1.12 | 0.47 | 1.13 |
| Slope | 583 (202–1460) | 102,966 | 12 | 34 | 54 | 7161 | 7 | 6343 | 26 | 2.86 | 0.59 | 1.93 |
| Canyon | 1388 (692–3317) | 148,596 | 44 | 22 | 34 | 8927 | 6 | 7517 | 37 | 4.03 | 0.79 | 2.86 |
3.1 Coral Abundance and Distribution
A total of 36,670 DSC were documented in the study area, with 31,480 individuals identified to genus or species (Table 2). Diversity of DSC trended higher on deeper seafloor features (slope and canyons), that encompassed a broader depth range and larger survey area than shallower bank and shelf features. Conversely, overall coral density decreased from shallow to deep features, finding the greatest overall DSC densities on banks (39 individuals/100 m2) and the lowest in canyons (6 individuals/100 m2).
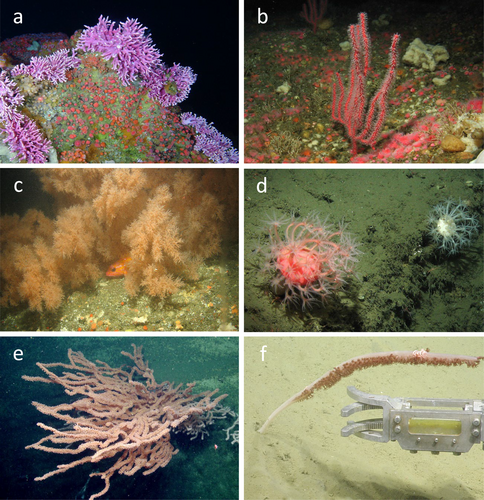
We identified DSC from at least 20 families and 39 genera, including 31 species (Figure 2, Table 3). We collected 105 specimens and were able to identify 63 individuals to 28 unique species, 40 individuals to a unique genus, 1 individual to family, and 1 to order. The coral group Anthoathecata, comprised of a single species of hydrocoral (Stylasteridae), Stylaster californicus, found on banks and the shelf accounted for 21.5% of total DSC counts in the study area (Figure 3). Mean densities of S. californicus found on rock substrata from 35 to 90 m depth were 148 individuals/100 m2 (SE = 37.1, n = 7793) on banks and 1 individual/100 m2 (SE = 0.7, n = 198) on the shelf.
| Coral group | Family | Scientific name | Count | Coll. | Depth (m) range | Depth (m) mean | Height (cm) range | Mean density/100 m2 (SE) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bank | Shelf | Slope | Canyon | ||||||||
| Anthoathecata | Stylasteridae | Stylaster californicus | 7991 | 2 | 35–90 | 61 | 5–25 | 147.8 (37.1) | 1.2 (0.7) | ||
| Antipatharia | Antipathidae | Antipathes dendrochristos | 1 | 1 | 96–96 | 96 | 100 | 0.003 (0.003) | |||
| Cladopathidae | Trissopathes sp. | 3 | 1207–1408 | 1286 | 35–50 | 0.002 (0.001) | |||||
| Schizopathidae | Alternatipathes alternata | 5 | 1 | 3296–3313 | 3306 | 25–35 | 0.004 (0.003) | ||||
| Alternatipathes bipinnata | 257 | 1 | 1082–3316 | 2492 | 5–60 | 0.21 (0.08) | |||||
| Bathypathes patula | 55 | 3 | 1718–2608 | 1921 | 15–50 | 0.05 (0.02) | |||||
| Bathypathes spp. | 46 | 1052–3317 | 2343 | 5–55 | 0.02 (0.01) | 0.03 (0.01) | |||||
| Lillipathes spp. | 19 | 3 | 1189–1823 | 1384 | 15–60 | 0.007 (0.005) | 0.004 (0.003) | ||||
| Parantipathes sp. | 308 | 3 | 1403–3305 | 1856 | 5–45 | 0.25 (0.09) | |||||
| Unknown | Antipatharia | 23 | 1 | 1195–3317 | 1892 | 10–40 | 0.006 (0.004) | 0.009 (0.006) | |||
| Octocorallia | Alcyoniidae | Gersemia juliepackardae | 114 | 2 | 829–1440 | 1026 | 5–35 | 0.02 (0.02) | 0.06 (0.02) | ||
| Gersemia spp. | 76 | 922–1180 | 1015 | 5–20 | 0.12 (0.07) | ||||||
| Chrysogorgiidae | Radicipes stonei | 74 | 3 | 1925–3308 | 2372 | 15–150 | 0.05 (0.02) | ||||
| Clavulariidae | Clavularia spp. | 25 | 1 | 354–1853 | 872 | 5–20 | 0.015 (0.008) | 0.011 (0.005) | |||
| Coralliidae | Bathyalcyon robustum | 1 | 1 | 2604–2604 | 2604 | 10–10 | 0.004 (0.004) | ||||
| Heteropolypus ritteri | 4166 | 95–3312 | 731 | 5–40 | 0.003 (0.002) | 9.7 (3.4) | 1.0 (0.2) | ||||
| Paragorgia arborea | 437 | 1 | 268–2451 | 1214 | 5–120 | 0.014 (0.009) | 0.25 (0.08) | ||||
| Paragorgia spp. 2 (white with pink polyps) | 233 | 415–1058 | 837 | 5–80 | 0.08 (0.05) | 0.18 (0.08) | |||||
| Paragorgia yutlinux | 180 | 5 | 750–987 | 879 | 5–70 | 0.06 (0.03) | 0.07 (0.07) | ||||
| Sibogagorgia sp. | 65 | 1 | 928–990 | 977 | 10–120 | 0.03 (0.02) | |||||
| Gorgoniidae | Callistephanus simplex | 294 | 3 | 751–1220 | 920 | 5–80 | 0.02 (0.01) | 0.20 (0.08) | |||
| Callistephanus spp. 1 (fan)a | 1313 | 15 | 157–2011 | 911 | 5–100 | 0.001 (0.001) | 2.7 (1.4) | 0.34 (0.09) | |||
| Callistephanus spp. 2 (stick) | 630 | 272–1100 | 355 | 5–35 | 0.21 (0.11) | 0.001 (0.001) | |||||
| Chromoplexaura cordellbankensis | 261 | 2 | 73–111 | 96 | 5–15 | 1.0 (0.4) | |||||
| Chromoplexaura marki | 5982 | 7 | 37–111 | 63 | 5–35 | 17.8 (3.5) | 21.8 (5.9) | ||||
| Eugorgia rubens | 1 | 1 | 75–75 | 75 | 40–40 | 0.007 (0.007) | |||||
| Keratoisididae | Acanella sp. | 41 | 3 | 1385–1944 | 1755 | 10–60 | 0.04 (0.02) | ||||
| Isidella tentaculum | 241 | 3 | 752–1481 | 986 | 15–200 | 0.004 (0.004) | 0.14 (0.05) | ||||
| Keratoisididae | 230 | 826–2651 | 1472 | 5–80 | 0.16 (0.03) | ||||||
| Keratoisis sp. 1 (thin branches) | 46 | 2 | 1748–2468 | 1888 | 10–80 | 0.04 (0.02) | |||||
| Keratoisis sp. 2 (thick branches) | 211 | 3 | 910–2069 | 1327 | 15–320 | 0.14 (0.06) | |||||
| Lepidisis sp. | 462 | 4 | 840–3314 | 1578 | 10–220 | 0.02 (0.02) | 0.27 (0.06) | ||||
| Paramuriceidae | Acanthogorgia sp. | 25 | 2 | 1051–1802 | 1366 | 10–40 | 0.02 (0.01) | ||||
| Plexauridae | Swiftia farallonesica | 50 | 1 | 185–190 | 188 | 5–20 | 0.09 (0.09) | ||||
| Primnoidae | Callogorgia kinoshitai | 365 | 3 | 1407–2011 | 1685 | 10–50 | 0.24 (0.09) | ||||
| Narella sp. | 11 | 1 | 3293–3315 | 3305 | 5–20 | 0.01 (0.01) | |||||
| Parastenella ramosa | 203 | 4 | 440–1828 | 1327 | 5–50 | 0.002 (0.002) | 0.13 (0.03) | ||||
| Plumarella williamsi | 108 | 3 | 294–486 | 360 | 5–25 | 0.58 (0.26) | |||||
| Primnoidae | 79 | 1 | 874–1544 | 1150 | 5–20 | 0.005 (0.004) | 0.04 (0.02) | ||||
| Unknown | Alcyonacea | 23 | 1169–1788 | 1476 | 5–25 | 0.02 (0.008) | |||||
| Pennatuloidea | Anthoptilidae | Anthoptilum grandiflorum | 9 | 1078–1272 | 1215 | 10–25 | 0.005 (0.004) | ||||
| Balticina californica | 3337 | 3 | 105–2002 | 368 | 5–150 | 11.1 (3.3) | 0.12 (0.04) | 0.51 (0.23) | |||
| Balticina-Funiculina complex | 955 | 457–1941 | 951 | 10–80 | 0.45 (0.33) | 0.51 (0.27) | |||||
| Funiculinidae | Funiculina quadrangularis | 165 | 4 | 976–1118 | 1067 | 5–25 | 0.11 (0.09) | ||||
| Funiculina spp. | 8 | 891–1053 | 979 | 5–25 | 0.01 (0.007) | ||||||
| Pennatulidae | Pennatula phosphorea | 4 | 330–330 | 330 | n/a | 0.007 (0.007) | |||||
| Pennatula spp. | 15 | 238–561 | 402 | n/a | 0.003 (0.002) | ||||||
| Pennatulidae | 485 | 231–1189 | 465 | 5–15 | 1.1 (0.08) | ||||||
| Ptilosarcus gurneyi | 6 | 1 | 54–290 | 198 | 10–15 | 0.03 (0.03) | 0.009 (0.007) | ||||
| Protoptilidae | Distichoptilum gracile | 28 | 1 | 1881–2007 | 1970 | 10–180 | 0.03 (0.02) | ||||
| Protoptilum nybakkeni | 35 | 1 | 3275–3303 | 3292 | 10–40 | 0.03 (0.02) | |||||
| Stachyptilidae | Stachyptilum superbum | 56 | 1 | 974–2650 | 1359 | 5–30 | 0.03 (0.03) | 0.01 (0.006) | |||
| Umbellulidae | Umbellula lindahli | 1166 | 2 | 705–2622 | 983 | 10–35 | 0.25 (0.14) | 0.40 (0.17) | |||
| Umbellula sp. 1 | 9 | 1 | 1076–3309 | 2744 | 15–25 | 0.006 (0.004) | |||||
| Unknown | Pennatuloidea | 4350 | 47–3302 | 583 | 5–140 | 10.6 (6.8) | 0.24 (0.13) | 0.94 (0.36) | |||
| Virgulariidae | Acanthoptilum gracile | 212 | 1 | 76–97 | 95 | 5–100 | 1.1 (0.6) | ||||
| Stylatula elongata | 401 | 2 | 41–97 | 58 | 5–45 | 3.0 (1.9) | |||||
| Virgularia sp. | 774 | 1 | 104–3302 | 594 | 5–15 | 0.25 (0.20) | 5.4 (2.1) | 0.02 (0.01) | |||
- a May include Callistephanus pacifica, Swiftia torreyi, and Swiftia kofoidi.
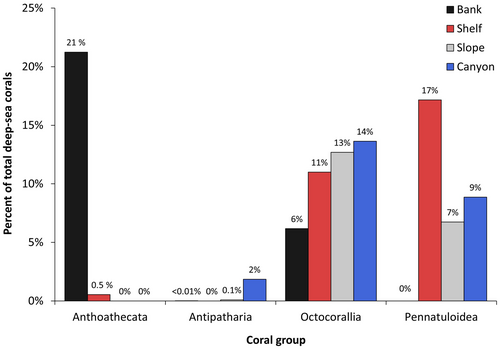
The most speciose group was Octocorallia with at least 27 taxa from 9 families that accounted for 44% of total DSC counts (Figure 3, Table 3). Octocorallia corals were found on all seafloor feature types (with most taxa in canyons) and across the full depth range of the study area (Figure 4). The Gorgoniidae accounted for 53% of total Octocorallia observations. The dominant species on both bank and shelf features was Chromoplexaura marki at a mean density of 18 individuals/100 m2 (SE = 3.5, n = 2003) and 22 individuals/100 m2 (SE = 5.9, n = 3979) respectively. Among the conspicuous red C. marki on Cordell Bank, a less common (1 individual/100 m2, SE = 0.4, n = 261) small-branching yellow gorgonian (height ≤ 15 cm) was collected in 2018 and later named Chromoplexaura cordellbankensis (Williams and Breedy 2019). Chromoplexaura cordellbankensis does not appear to be endemic to Cordell Bank. Since the description in 2018, researchers have confirmed observations of C. cordellbankensis at La Cruz Canyon off Big Sur, central California and Anacapa Island in southern California (Laidig et al. 2021). Red fan-shaped gorgonians with yellow polyps were grouped as Callistephanus spp. (fan) in this analysis but may include individuals of Callistephanus pacifica, Swiftia torreyi, and Swiftia kofoidi. These species appear very similar and could not be distinguished without genetic analysis (Meredith Everett, pers. comm.). The highest mean densities of Callistephanus spp. (fan) were observed on the slope (3 individuals/100 m2, SE = 1.4, n = 772). One individual of Eugorgia rubens was collected at 75 m from a low-relief rocky feature (Deep Reef) on the shelf in MBNMS (37.39° N, 122.63° W; ONMS 2018) and is the northernmost known locality for the species, approximately 109 km north of the previously known occurrence in Monterey Bay, California (Clinton Bauder, pers. comm.).
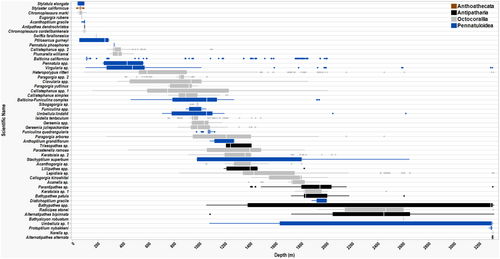
Taxa within the Coralliidae family accounted for some of the broadest depth distributions documented for the study area. Specifically, Heteropolypus ritteri was observed from 95 to 3312 m with the highest mean densities found on the slope (10 individuals/100 m2, SE = 3.4, n = 2828; Figure 4, Table 3). The two predominant paragorgid species found throughout the study area on the slope and canyons can be visually distinguished by skeletal structure and polyp color and were found to exhibit different depth distributions. Paragorgia arborea (pink structure and pink polyps) inhabited a greater depth range from 268 to 2451 m than Paragorgia yutlinux (white structure and pink polyps) found from 750 to 987 m. Individuals of Sibogagorgia sp. (n = 65) were only observed at the most southern range of the study area in Pioneer Canyon in MBNMS from 928 to 990 m.
Keratoisididae bamboo corals were predominantly observed in canyon features at relatively deep depths. Colonies of Lepidisis sp. displayed the greatest combined mean density (0.29 individuals/100 m2, SE = 0.08, n = 462) and depth range (840–3314 m) of the four bamboo genera observed in these surveys (Figure 4, Table 3). Two structural morphologies of Keratoisis spp. were seen. The most abundant were Keratoisis sp. 2 (n = 211), defined by robustly thick-branched colonies often reaching impressive sizes; 22 individuals measured over 2 m in width and height. The thin-branched morphology of Keratoisis sp. 1 was less abundant (n = 46) and inhabited lower relief rock substrata. The candelabra-shaped Isidella tentaculum inhabited the shallowest depth range (752–1481 m) of all the bamboo corals.
Primnoids were distributed among slope and canyon features. The highest densities observed were Plumarella williamsi on the slope, and Callogorgia kinoshitai and Parastenella ramosa in canyons (Table 3). The golden-colored gorgonian, Acanthogorgia sp., was not common, but the few observed were within the full geographic extent of canyon features in the study area. A new species of a white sea whip Plexauridae, Swiftia farallonesica, was collected at 182 m from a low-relief rocky feature on the shelf named “The Football” in GFNMS and is the only known location of this species to date (Williams and Breedy 2016). The only chrysogorgiid gorgonian documented in the area, Radicipes stonei, was observed in Box Canyon in CBNMS (1925–3308 m, Figure 4). This is an extraordinary range extension as it had only previously been found at two localities in the Aleutian Islands, Alaska (Ralf Cordeiro and Gary Williams, pers. comm.).
Sea pens, Pennatuloidea, comprised 33% of total DSC counts and were widespread from the shelf to canyons at depths 41–3309 m (Figures 3 and 4). We observed at least 16 taxa from seven families (Table 3). The most abundant were unknown Pennatuloidea that could not be identified to species, comprising 4350 individuals with the highest mean density on the shelf of 11 individuals/100 m2 (SE = 6.8, n = 2961). Densities of Balticina californica on the shelf were also 11 individuals/100 m2 (SE = 3.3, n = 2669). Visual identifications of sea pens benefited from voucher specimens collected for nine species. The Protoptilidae sea pen, Protoptilum nybakkeni (Williams and Lipski 2019) was found at the deepest mean depth of all sea pens in the study area (3292 m).
Antipatharia black corals were observed deeper than 1000 m with the exception of one large (1 m tall × 3 m wide) Christmas tree black coral, Antipathes dendrochristos, that could live to over a hundred years old (Love et al. 2007). A piece of the colony was collected from 95 m on Cochrane Bank in GFNMS (37.79° N, 123.25° W) representing the northernmost occurrence of this species to date (Etnoyer et al. 2014), approximately 432 km north of the previously known occurrence in southern California (Thomas Laidig, pers. comm). Although black corals were the least abundant group in the study area (2% of total DSC counts, Figure 3), at least eight taxa were identified, of which six were verified from specimen collections and five taxa were only found in canyons (Table 3). The deepest mean depth of all corals in the study area was from the black coral, Alternatipathes alternata, at 3306 m.
3.2 Coral Assemblage Patterns
Multivariate analyses identified patterns in the DSC community by depth and seafloor feature types. Hierarchical cluster analysis combined with SIMPROF found structure within the data, identifying 13 clusters that were not significantly different. Generally, these clusters included adjacent depth bins of two to four 100 m depth increments (Figure 5). Divergence in clusters of samples was most apparent between the shallowest and deepest depths, with relatively low dispersion of samples associated with slope and canyon features (Figure 6). The nMDS ordination overlaid with five clusters at the 20% similarity level defined assemblages of DSC taxa more closely associated with a seafloor feature type; however, depths associated with the shelf feature did not cluster together. Shelf sample < 100 m was more similar to bank depths, and shelf sample 100–200 m was more similar to slope samples 200–500 m. Samples 600–800 m in canyons were very dissimilar to other canyon depths, likely due to observations of two or fewer taxa for these depth samples.
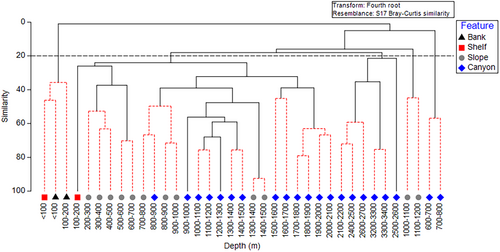
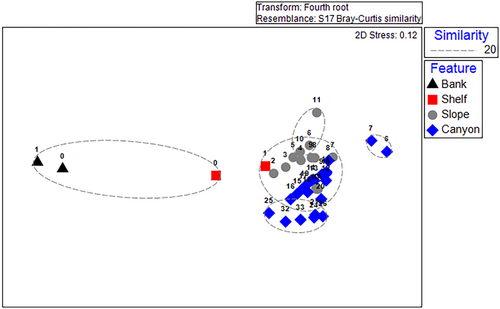
The results of the one-way ANOSIM (Global R = 0.411, p = 0.001) identified that the most dissimilar seafloor features based on coral assemblages are bank and canyon (R = 0.877, p = 0.004) and bank and slope (R = 0.94, p = 0.011). Shelf and canyon (R = 0.633, p = 0.016), shelf and slope (R = 0.669, p = 0.022), and slope and canyon (R = 0.231, p = 0.008) were significantly different, with a lower R statistic indicating that these seafloor features share similarities. Seafloor features that were not significantly dissimilar were bank and shelf (Table S2).
The SIMPER analysis also identified the greatest dissimilarity in DSC assemblages between bank and canyon (average dissimilarity = 100%). Taxa contributing most to the dissimilarity (those with 50% cumulative contribution) were gorgonians Chromoplexaura marki and C. cordellbankensis, hydrocoral Stylaster californicus on banks, and, in canyons, the soft coral Heteropolypus ritteri and the bamboo coral Lepidisis sp. The assemblage differences between bank and slope (average dissimilarity = 100%) were the result of the top three dominant species found on banks (C. marki, C. cordellbankensis, and S. californicus) and, on the slope, H. ritteri (Table S3).
4 Discussion
It is clear from our study that some DSC species have a broad distribution across multiple depths and seafloor features (e.g., sea pens) while other species correspond to a narrower depth range and fewer seafloor features (e.g., hydrocorals and bamboo corals). While this is not a novel concept, our assessment provides a straightforward description of DSC abundance and community structure corresponding to the different seafloor feature types and depth ranges within the national marine sanctuaries of North-central California.
Rocky bank features, like Cordell Bank (CBNMS), Rittenburg and Cochrane Banks (GFNMS), make up a small percentage (3.2%) of the total area of seafloor in the sanctuaries, yet supported the greatest densities of DSC in the study area likely due to the abundance of consolidated rock substrata compared to the other seafloor feature types. Of the four DSC species documented on banks, Stylaster californicus displayed the highest mean abundances on banks and of all DSC taxa in the study area. Stylasteridae corals are found worldwide and prefer rocky substrata with high current, stable salinity, and low sedimentation (Cairns 1992, 2011). Their reproductive traits result in limited larval distribution and high regional endemicity (Brooke and Stone 2007). We observed S. californicus localized to the shallowest depths < 90 m on high-relief hard substrata, primarily on Cordell Bank, which is located approximately 20 nautical miles west of Point Reyes, California and oriented into the prevailing current (Halle and Largier 2011) in relatively clear water, as evident by the presence of multiple species of Rhodophyta (red algae) more commonly associated with shallow nearshore habitats (Graiff and Lipski 2016). Dense aggregations of S. californicus colonies form on Cordell Bank's upper reefs, peaking in observations at 50–60 m, providing three-dimensional habitat and refuge for fish and other invertebrates. A survey of Farnsworth Bank in southern California found colonies of S. californicus between 31–66 m, and where the species was most abundant on the shallower reefs (30–40 m) colonies often covered all of the rock substrate to the point of excluding other structure-forming invertebrates (Love et al. 2010). Colonies of S. californicus can be relatively large in size, and most were 40 cm or less on Farnsworth Bank, while the majority of colonies on Cordell Bank were 20 cm wide or less.
As depth increased and rocky relief decreased, the dominant coral species on banks transitioned to the Gorgoniidae Chromoplexaura marki. Single or moderately branched individuals were observed, often creating additional vertical complexity to the relatively low-relief mixed substrata found in deeper waters on banks and reef features located on the shelf, which is similar to findings made by Stierhoff et al. (2011) of dense colonies of C. marki on low relief rock on Coquille Bank, off the coast of Oregon. Predictive models using presence-absence data and generalized linear models of Chromoplexaura sp. on Cordell Bank showed the taxa's preference for low sloping environments and distribution over a diversity of substratum types (Etherington et al. 2011).
Collectively, sea pens (Pennatuloidea) displayed the largest depth ranges throughout the study area on all seafloor features other than banks. Sea pens are adapted to anchor their peduncles into soft sediments and consequently inhabit large areas of uniform mud or sand (Williams 2011). As expected, the highest abundances of sea pens in the area occurred on the shelf where the primary substrate surveyed was soft sediment. Balticina californica was frequently documented in dense fields on the shelf and often reached heights of 1.5 m in vast expanses, adding vertical structure to a predominantly homogenous mud seafloor. Tissot et al. (2006) suggested that aggregations of sea pens may provide habitat and refuge for other invertebrates, influence prey availability, and alter water current flow. During an ROV survey on the CBNMS shelf, Graiff and Lipski (2023) observed dead pyrosome tunicates wrapped around the bases of B. californica sea pens. The collections of pyrosomes were preyed on by Ophiurina brittle stars. Also, basket stars (Gorgonocephalus eucnemis) were seen attached to B. californica as a likely feeding strategy extending them higher into the water column.
Deep-sea coral diversity increased on the slope and into canyons with varieties of Antipatharia, Octocorallia, and Pennatuloidea taxa. This is likely due to the expansive bathymetric range of these deep-water features, as well as various substratum types and water temperature ranges that are optimal for the colonization of various DSC species. The most conspicuous Octocorallia were bamboo corals (family Keratoisididae) and bubblegum corals (family Coralliidae). Large, habitat-forming Keratoisis spp. (up to 2 m in height and 3 m in width) and Paragorgia arborea colonies (up to 1.2 m in height and width) were often observed in groups growing perpendicular to walls in Arena, Bodega and Pioneer Canyons, creating “coral gardens” on an otherwise relatively barren vertical rock substrate. We hypothesize that this growing strategy is capitalizing on suspension feeding from the upward flowing water current. Ageing studies have determined the slow growth and longevity of bamboo corals to be in the hundreds of years (Roark et al. 2005; Andrews et al. 2009; Hill et al. 2011). Black corals have also been determined to be a long-lived species (Wagner et al. 2012). Bathypathes patula were commonly observed in Bodega Canyon, and this species can reach ages in excess of 385 years (Marriott et al. 2020). Therefore, recovery from disturbance may take decades to centuries, suggesting that canyons may serve as natural refuges for these long-lived species (van den Beld et al. 2017).
The soft coral, Heteropolypus ritteri, displayed the most widespread depth range for a single species throughout the study area and had the highest densities of all DSC taxa observed on the slope and canyons. Heteropolypus ritteri was often seen as solitary individuals and occasionally in large groups (> 50 individuals), inhabiting bare rock or rock that was covered with a thin mud veneer. Watters et al. (2022) documented H. ritteri across a wide deep range in three distinct geographic regions from northern to southern California. The success of this species could be attributed to its reproduction strategies, producing large larvae with high fecundity relative to other DSC species (Cordes et al. 2001) and the ability to recruit to mud-draped rock substratum, which is uncommon for other taxa of DSC (Roberts et al. 2009).
We observed other patterns in DSC assemblages due to variable composition of rock substrata that were sampled within a seafloor feature type. At the Point Arena Slope site, the majority of the consolidated flat rock was covered with a thick layer of mud. The mud veneer could be inhibiting coral recruitment and attachment, explaining the overall low density and diversity of corals observed in the area (Graiff et al. 2021). In Bodega Canyon at the base of a vertical rock wall, we noted a debris area of loose rock that had fallen from the wall above. Mixed among the rocks were dead and broken bamboo coral skeletons; often, their bases were still attached to a rock piece. Mortensen and Buhl-Mortensen (2005) have also documented unconsolidated rock no longer supporting the weight of the coral if the coral's size exceeds approximately two times the rock's diameter.
4.1 Data Considerations
We recognize the limitations of describing patterns in DSC communities without taking into account the complexity of variables other than depth and substrate. In addition to depth, substratum type, and reproductive and physical characteristics of DSC species, oceanographic variables such as temperature and salinity influence DSC distribution (Guinotte and Davies 2014). Auscavitch et al. (2020) identified that the community assemblies of central Pacific DSC varied within three bathyal water masses that differed in depth, temperature, salinity, or dissolved oxygen. Seafloor characteristics that can be derived from multibeam bathymetry data, such as slope, topographic position index, and rugosity, are also important (Etherington et al. 2011). The data collection methods for the 16 datasets we analyzed showed an evolution in later years to include temperature, salinity, and dissolved oxygen, which were not always collected in the earlier surveys.
Our present analysis allows us to describe general correlations between DSC distributions and oceanographic variables. For instance, in waters shallower than 200 m, the diversity of DSC is lower than observed in the deeper depths of the study area. Deeper and colder waters provide consistent thermal and salinity regimes to support diverse DSC communities (Roberts et al. 2009). Watters et al. (2022) found that DSC species that occurred over the broadest temperature ranges in their study area off California also occurred over the broadest depth ranges, while the species with the narrowest temperature ranges also had narrow depth ranges. The oxygen minimum zone (OMZ) may also be influencing DSC distribution. For the California Current, Moffitt et al. (2015) state the upper boundary of the OMZ (defined by O2 = 1.4 mL L−1) begins at approximately 600 m depth and the OMZ is thick (~1200–1500 m). We saw numerous DSC taxa spanning the OMZ, particularly bamboo corals beginning to appear at 750 m and black corals (with the exception of Antipathes dendrochristos) at 1000 m. There has been significant interest and research on reef building Scleractinian corals living in oxygen minimum zones (Lunden et al. 2014; Moctar et al. 2024). However, there is limited published data focused on DSC distributions (from taxonomic orders other than Scleractinia) within low-oxygen zones, particularly along the U.S. West Coast, supporting the need to collect and associate oceanographic variables to visual observations to strengthen the knowledge of DSC habitat preferences.
4.2 Management Applications
Often DSC distribution studies focus on a single seafloor feature type (i.e., seamount and canyon; Lundsten et al. 2009; McClain et al. 2010; Yoklavich et al. 2011; Baker et al. 2012) and selectively survey hard substrata. While these localized descriptions are useful, there is value in describing DSC communities in relation to multiple seafloor features and depths to assist resource managers in targeting conservation and management efforts. Such as the decision-making processes involved for spatial management of highly biodiverse and vulnerable areas, like offshore banks. Although these rocky features comprise a relatively small total area of the study area, they supported the highest densities on DSC and can also experience seafloor impacts from fishing activities like bottom contact gear (ONMS 2023, 2024). A similar scenario applies to the continental shelf, where vast expanses of sea pen fields (some of which have fallen over) have been observed near areas with anthropogenic tracks and significant seafloor scour presumably from bottom contact fishing gear (e.g., trawls or crab pots) (Graiff and Lipski 2023). Deep-sea coral communities and seafloor-specific datasets have been used by managers to address changes in seafloor protection and should be used in planned reviews of fishery regulations in future years. For example, on the U.S. West Coast, the PFMC recommended changes to Essential Fish Habitat Conservation Areas and Rockfish Conservation Areas, which are managed by NMFS (NOAA Fisheries 2020). These data can also be used to assist in identifying appropriate areas to establish offshore renewable energy sites where permitted.
In the event of an anthropogenic disturbance to marine resources (e.g., oil spills or vessel sinking) the response relies on community structure and diversity data of the most sensitive species expected in the area, like DSC, to guide the response and restoration efforts to the impacted seafloor. For example, DSC communities in the Northern Gulf of Mexico were damaged following the Deepwater Horizon oil spill (White et al. 2012; Etnoyer et al. 2016; Silva et al. 2016). The environmental response resulted in extensive research and damage assessments that have led to increased awareness among managers, and numerous regulatory measures have been put into place to prevent this kind of spill from happening again. Additionally, our approach could be used to track broad-scale changes in DSC communities over time as a result of climate change or other stressors. The global rise of ocean temperatures linked to numerous effects, like reduction in oxygenation, decreased pH, and inhibited food supply, is significantly impacting deep-sea communities (Sweetman et al. 2017) Because we report densities of DSC per unit area, additional data collection could allow a comparison to this analysis and indicate changes in species abundance and distribution patterns.
This study provides a unique view of deep-sea corals within the North-central California national marine sanctuaries. Differences in DSC community structure appear to be related to seafloor feature types, substrate, and depth. The large-scale approach of describing abundance and distribution patterns over a wide depth range offers a foundation for ecosystem management of areas with abundant and diverse coral communities. The heightened interest in the conservation of DSC as contributors to seafloor biodiversity and technological advances continues to expand our knowledge of sensitive DSC at regional and global scales. Future surveys focused on DSC in the sanctuaries and in other oceans of the world should prioritize characterizing poorly known regions, closing data gaps in depth ranges, and associating environmental variables to coral observations to detect oceanographic changes and responses due to changing ocean conditions.
Acknowledgments
We greatly appreciate the crews of R/V Fulmar, E/V Nautilus, NOAA Ships McArthur II, Bell M. Shimada, and Reuben Lasker and operators of the ROVs and AUV. Multiple lead NOAA scientists were instrumental for the success of the research expeditions including P. Etnoyer, J. Hyland, D. Watters and expedition leaders with Ocean Exploration Trust Inc. (E/V Nautilus) M. Brennan, D. Coleman, and N. Raineault. We thank M. Everett for taxonomic assistance. We greatly appreciate G. Cochrane for interpreting seafloor maps to select dive locations and interpolating ROV navigation data. We acknowledge numerous national marine sanctuary staff (M. Carver, J. de Marignac, D. Howard, K. Reyna, J. Stock, and S. Tezak) for their at-sea support and S. Gittings, S. Lyon, and M. Sugla for editorial reviews. We thank C. Piotrowski and J. Loacker for curation of collected material at the California Academy of Sciences. Diana Watters provided valuable input that greatly improved the manuscript. This manuscript incorporates multiple expeditions, and subsequent data analyses that were supported by numerous funders including NOAA's Deep-Sea Coral Research and Technology Program, Office of National Marine Sanctuaries, National Marine Fisheries Service, National Oceanographic Partnership Program, Ocean Exploration and Research Program, National Center for Coastal Ocean Sciences, Office of Marine and Aviation Operations, Marine Applied Research and Exploration, and Bureau of Ocean Energy Management.
Open Research
Data Availability Statement
Records of deep-sea coral observations used in this study can be accessed from NOAA's Deep-Sea Coral and Sponge Database (https://deepseacoraldata.noaa.gov/).




