Latitudinal biodiversity gradients of rocky intertidal assemblages: Spatial scales and complex associations with environmental factors
Abstract
Latitudinal biodiversity gradients vary across taxonomic groups and spatial scales, and various environmental factors have been associated with those patterns. However, it remains unknown whether taxonomic groups with strong ecological associations have similar or different latitudinal patterns. Macroalgae are foundation assemblages on rocky intertidal shores and are often inhabited by invertebrates, predominantly polychaetes. This study analysed latitudinal patterns of macroalgae and their associated polychaetes at different spatial scales and determined how environmental factors influenced those patterns. Macroalgae and polychaetes were collected from transects within 38 rocky intertidal shores of Western Australia at 14 latitudes between 18° S and 34° S. Latitudinal gradients in species richness, diversity (Simpson's diversity index) and abundance were evaluated at transect, site and latitudinal scales. Relationships between environmental factors and rocky intertidal assemblages were analysed using piecewise structural equation modelling based on direct, indirect and complex models. Macroalgae showed increases in species richness, diversity and abundance at transect and site scales towards high latitudes, but species richness and diversity patterns were unclear at the latitudinal scale where transect and site data were pooled. In contrast, polychaete diversity decreased towards high latitudes, although this pattern was unclear at the transect scale. Polychaete richness and abundance tended to follow parabolic patterns that peaked at 27° S. Relationships between environmental factors and rocky intertidal assemblages were best described by a complex model, with significant relationships more often at transect and site scales. Sea surface temperatures showed the strongest relationship with macroalgal and polychaete distributions.
1 INTRODUCTION
Latitudinal diversity gradients are an obvious pattern in ecology, especially during the Palaeozoic and the past 30 million years (Mannion et al., 2014). Those patterns are evident in both the Northern and Southern Hemispheres but tend to be stronger in the Western Hemisphere (Kinlock et al., 2018). In general, species diversity decreases from the lower to higher latitudes, but reverse trends have also been observed for few groups, such as parasites and aquatic plants (Willig et al., 2003). The maximum diversity of marine taxa is located between 10° N and 20° N (Powell et al., 2012), but a dip is also found near the equator resulting in a bimodality of latitudinal gradients in marine biodiversity (Chaudhary et al., 2016). Ecological, evolutionary, historical and stochastic processes are suggested to be the principal causes of those patterns (Willig & Presley, 2018). Understanding those drivers, especially environmental factors, provides insight into processes underlying the distribution patterns and helps us to predict present and future distribution trends and improve biodiversity conservation. Latitudinal gradients in species diversity and their drivers often vary depending on the spatial scale of analyses. The distribution patterns of local richness are predominantly affected by fine-scale abiotic and biotic interactions, while those of continental richness tend to be determined by larger scale processes, such as ocean currents (Wernberg, Thomsen, Connell, et al., 2013) and Continental Drifts (Poulin et al., 2014).
Rocky intertidal assemblages are composed of various taxonomic groups with different latitudinal patterns of species diversity (Iken et al., 2010; Konar et al., 2010; Miloslavich et al., 2013; Pohle et al., 2011), yet evaluation of spatial scale effects on those patterns is limited for herbivores (Rivadeneira et al., 2002) and combined taxa (Okuda et al., 2004). Macroalgae are foundation assemblages on rocky intertidal shores that typically show latitudinal increases in species diversity (Konar et al., 2010; Liuzzi et al., 2011; Santelices & Marquet, 1998; Smale et al., 2011). However, reverse patterns have also been observed at the site scale in Brazil and Uruguay, from 26° S to 37° S (Steigleder et al., 2019) and unclear patterns have been found at the regional scale in Indian Ocean (Price et al., 2006) and a global scale (Bolton, 1994). Small invertebrates (<10 mm), predominantly polychaetes, often inhabit the macroalgal thalli (Gallucci et al., 2020; Gestoso et al., 2012; Torres et al., 2015), yet analysis of the latitudinal patterns of associated invertebrates is limited to the echinoderms, which are similar to the general trends of the macroalgae they inhabit, i.e., diversity and abundance increase towards high latitudes (Iken et al., 2010). Latitudinal gradients in species diversity of polychaetes are currently known from softbottom habitats (e.g. estuaries, continental shelf and deep sea) and those patterns vary across locations (Ellingsen et al., 2007; Garza, 2008; Glover et al., 2001; Hernández-Alcántara et al., 2013; Martins et al., 2013; Moreno et al., 2021; Quiroz-Martinez et al., 2011; Saeedi et al., 2022; Shields & Blanco-Perez, 2013). However, we do not know whether macroalgae and their associated polychaetes have similar or different latitudinal patterns and how spatial scale affects those patterns.
Latitudinal patterns of rocky intertidal assemblages at different spatial scales may be influenced by environmental factors, including physicochemical water and substrate profiles (Bessey et al., 2019; Fenberg et al., 2015; Hadiyanto et al., 2020; Ibanez-Erquiaga et al., 2018; Meager et al., 2011). The distribution of invertebrates on rocky intertidal shores is also influenced by macroalgae (Hirst, 2008; Parker et al., 2001; Tano et al., 2016). It suggests that environmental factors may affect invertebrate distribution both directly and indirectly through changes in macroalgal distribution, making relationships between environmental factors and rocky intertidal assemblages more complex. Indeed, previous studies have found complex associations between environmental factors and the local distribution of rocky shore assemblages on the coast of Santa Barbara Channel, USA (Byrnes et al., 2011; Lamy et al., 2020; Miller et al., 2018), yet it remains unknown whether those associations will also be observed for latitudinal patterns. At a global scale, complex relationships between environmental factors and marine biodiversity are currently studied for inshore reef assemblages (Edgar et al., 2017), but that study does not account for different spatial scales of species diversity.
Rocky shores of Western Australia extending from 13° S to 35° S are one of the biodiversity hotspots and centres of endemism for marine benthic algae (Kerswell, 2006; Phillips, 2001; Vieira et al., 2021) and home to diverse invertebrates (Kendrick & Rule, 2014; Slack-Smith & Bryce, 2004). However, the latitudinal patterns of rocky intertidal assemblages along this coast still remain unknown as most studies of those assemblages are conducted within small scales (Bessey et al., 2019; Black et al., 1979; Scheibling, 1994; Wells, 1977). Hence, it gives us an opportunity to analyse latitudinal gradients in diversity and abundance of macroalgae and polychaetes on rocky intertidal shores at different spatial scales and determine how environmental factors influence those patterns.
2 MATERIALS AND METHODS
2.1 Study area
The coastline of Western Australia extends from 13° S to 35° S, with about 10°C difference in sea surface temperature between those latitudes (Wijffels et al., 2018). The coast is macrotidal in the north (13° S to 20° S) and microtidal in the south, with a difference in tidal range of over 2.5 m (Harker et al., 2019). Coastal waters of Western Australia are oligotrophic predominantly due to the influence of the Leeuwin Current that transports low nutrient waters from the tropics (Lourey et al., 2006; McLaughlin et al., 2019). Rocky intertidal shores constitute about 19% of the coastline that stretch from tropical to temperate regions (Edyvane, 2005), with different substrate profiles and erosional features (Semeniuk & Johnson, 1985).
2.2 Sampling
Samples were collected from 14 latitudes between 18° S and 34° S during low tide from September 2020 (spring) to January 2021 (summer). We could not collect samples between 23° S and 27° S due to high rock cliffs and remoteness. At each latitude, one to three sites (beaches with horizontal limestone rock platforms) were selected, depending on the availability and accessibility of rock platforms. In total, there were 38 sites across 14 latitudes (Figure 1, Table S1). At each site, we set out three line transects separated by 25–50 m, perpendicularly to the shoreline, then haphazardly placed three quadrats of 1 m × 1 m at the inner, middle and outer of the rock platform along each transect to determine substrate profiles. A quadrat of 0.2 m × 0.2 m was placed on the densest macroalgae inside the 1 m × 1 m quadrat. Previous studies have also used the same size (Lathlean et al., 2015; Pereira et al., 2006) and placement (Wieters et al., 2012) of quadrats to capture rocky intertidal assemblage variations across the platform.
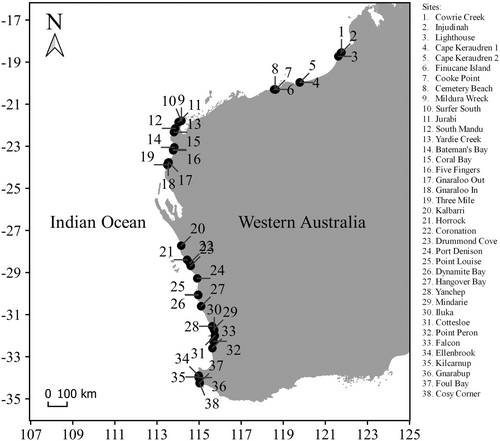
We scraped macroalgae inside the 0.2 m × 0.2 m quadrats and washed gently the samples using fresh seawater to detach fauna. Macroalgal samples were preserved in 95% ethanol and identified to species level. Open Nomenclature qualifiers (e.g. sp. 1, sp. 2, etc.) were used for unknown species names (Sigovini et al., 2016). The wet weight of each species was measured. We sieved the seawater used to wash macroalgae through 0.5 mm mesh to retain polychaetes. Polychaete samples were preserved in 95% ethanol, identified to species level (non-clitellate annelids excluding sipunculans and echiurans) and counted individually. We also used Open Nomenclature qualifiers (e.g. sp. 1, sp. 2, etc.) for unknown species names (Sigovini et al., 2016). Polychaete abundance was expressed as the number of individuals per 1 g of macroalgae.
2.3 Environmental factors
We selected substrate profiles (heterogeneity and rugosity) and physicochemical water (sea surface temperature, tidal range and total suspended matter) rather than properties of the air even though we appreciate that these may have some influences. Substrate heterogeneity was determined by calculating Simpson's reciprocal index of the individual proportion of rock platform components (macroalgae, barnacles, boulders, rubble, crevices, seagrass, pits, sand and bare) within 1 m × 1 m quadrats (the total percentage within a quadrat was 100%) (Meager et al., 2011). Substrate rugosity was determined by measuring the length of the actual platform surface within 1 m × 1 m quadrats using a metal chain (Risk, 1972). We downloaded mean sea surface temperature at the resolution of about 1 km from MARSPEC (Sbrocco & Barber, 2013), mean tidal range and mean total suspended matter at the resolution of about 9.2 km from Global Marine Environment Datasets (http://gmed.auckland.ac.nz). We matched coordinates of sites to the nearest available physicochemical data.
2.4 Data analysis
Latitudinal gradients in species richness, diversity (Simpson's diversity index) and abundance of macroalgae and polychaetes were evaluated at transect, site and latitudinal scales using first- and second-order regression models. The most parsimonious model was selected based on the lowest Akaike information criterion (AIC).
Relationships between environmental factors and rocky intertidal assemblages at each spatial scale were analysed using piecewise structural equation modelling (SEM). Piecewise SEM is a path analysis to resolve complex multivariate relationships among variables using linear, generalized linear, least-square or mixed effect models (Lefcheck, 2016). This approach has also been used in previous studies to analyse complex associations between marine environment and assemblages (Kuczynski et al., 2017; Miller et al., 2018; Paczkowska et al., 2019). In the present study, piecewise SEM was performed using R-package piecewiseSEM version 2.1.2 (Lefcheck, 2016).
Three conceptual models were tested: (1) direct model (environmental factors influence macroalgae and fauna independently); (2) indirect model (environmental factors influence fauna through changes in macroalgal distribution) and (3) complex model (environmental factors influence both macroalgae and fauna, but at the same time macroalgae also determine faunal distribution). Thus, the complex model suggests that environmental factors affect fauna both directly and indirectly through changes in macroalgal distribution (Figure 2, Table S2).
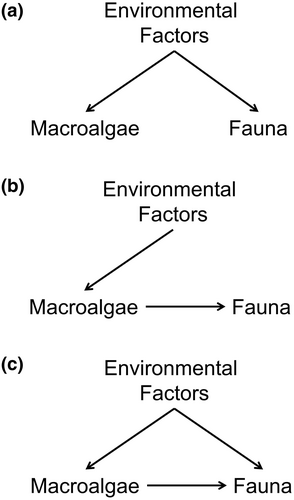
Assemblage and environmental data were normalized using logarithmic transformation before piecewise SEM analysis. Overall fit of the conceptual models was evaluated using Shipley's test of directed separation, including Fisher's C statistic (Shipley, 2009) and AIC (Shipley, 2013). Shipley's test of directed separation assesses whether there are missing non-hypothesized relationships, or we can exclude them in the model (Shipley, 2000). The most parsimonious model should have non-significant Fisher's C (p > .05) and the lowest AIC. The model was bootstrapped with 1000 randomization to measure the uncertainty of parameter estimates. Those estimates were standardized to make them comparable between associations. The total estimates of each environmental factor were then calculated to determine the most influential predictor (Table S3).
3 RESULTS
3.1 Latitudinal patterns
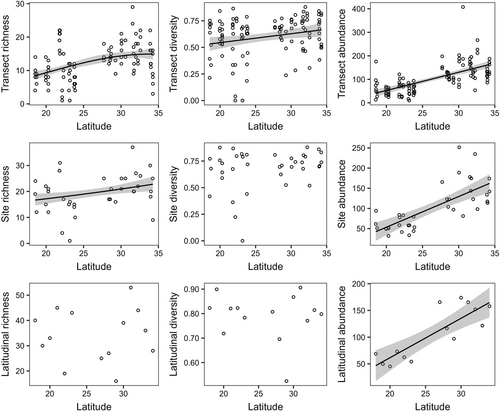
We identified 187 species of macroalgae (Table S4) and 126 species of polychaetes (Table S5). The latitudinal patterns of macroalgae varied depending on the spatial scale (Figure 3, Table S6). Species richness increased significantly with latitude by 85% (from 8.07 species at 18° S to 14.91 species at 34° S) at the transect scale (p < .05) and 37% (from 16.51 species at 18° S to 22.67 species at 34° S) at the site scale (p < .05) but not at the latitudinal scale (p > .05). Similarly, species diversity increased significantly by 25% (from 0.53 at 18° S to 0.66 at 34° S) at the transect scale (p < .05), but this trend was unclear at site and latitudinal scales (p > .05). A significant increase was also found for abundance by more than 250% (from 37.08–46.79 g/transect at 18° S to 160.97–164.85 g/transect at 34° S) at all spatial scales (p < .05).
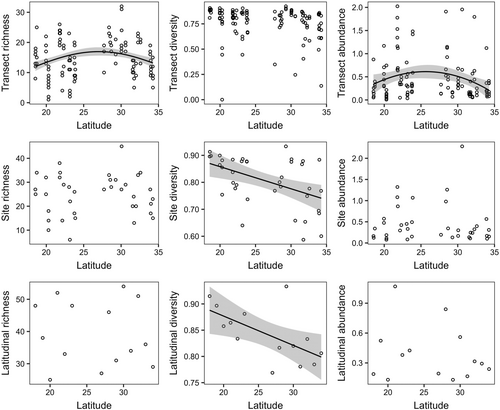
Polychaetes showed different latitudinal patterns from macroalgae, and those patterns also varied depending on the spatial scale (Figure 4, Table S7). At the transect scale, species richness reached the maximum number at 27° S (16.95 species), decreasing by 32% (11.48 species) towards 18° S and 21% (13.41 species) towards 34° S (p < .05). Abundance was also highest at 27° S (0.60 individuals/g macroalgae), then it decreased by 52% (0.29 individuals/g macroalgae) at 18° S and 62% (0.23 individuals/g macroalgae) at 34° S (p < .05). However, the parabolic pattern of species richness and abundance was not apparent at site and latitudinal scales (p > .05). Species diversity decreased significantly by 15% (from 0.87 at 18° S to 0.74 at 34° S) at the site scale (p < .05) and 10% (from 0.89 at 18° S to 0.80 at 34° S) at the latitudinal scale (p < .05) but not at the transect scale (p > .05).
3.2 Associations with environmental factors
Relationships between environmental factors and rocky intertidal assemblages were best described by a complex model for transect and site scales (Fisher's C p > .05, AIC = 102.00) and an indirect model for the latitudinal scale (Fisher's C p > .05, AIC = 99.51) (Table 1). Based on those models, environmental factors explained 15%–54% of the macroalgal and 20%–45% of the polychaete diversity metrics at the transect scale, 19%–65% of the macroalgal and 35%–54% of the polychaete diversity metrics at the site scale and 37%–80% of the macroalgal and 7%–61% of the polychaete diversity metrics at the latitudinal scale (Figure 5). Correlations between metrics were also found for both macroalgae and polychaetes.
| Spatial scales | Assemblages | Metrices | Sea surface temperature | Tidal range | Total suspended matter | Substrate heterogeneity | Substrate rugosity |
|---|---|---|---|---|---|---|---|
| Transect | Macroalgae | Species richness | −0.36 | −0.02 | 0.18 | 0.18 | 0.06 |
| Species diversity | −0.31 | 0.13 | 0.04 | 0.18 | 0.08 | ||
| Abundance | −0.55 | 0.14 | −0.09 | 0.12 | 0.13 | ||
| Polychaetes | Species richness | 0.04 | 0.02 | −0.19 | 0.13 | 0.04 | |
| Species diversity | 0.13 | 0.04 | −0.18 | 0.11 | 0.04 | ||
| Abundance | 0.32 | −0.23 | −0.05 | 0.02 | −0.09 | ||
| Site | Macroalgae | Species richness | −0.38 | 0.11 | 0.12 | 0.19 | 0.05 |
| Species diversity | −0.35 | 0.16 | 0.01 | 0.20 | −0.16 | ||
| Abundance | −0.59 | 0.13 | −0.10 | 0.10 | −0.13 | ||
| Polychaetes | Species richness | 0.03 | 0.01 | −0.13 | 0.18 | 0.09 | |
| Species diversity | 0.22 | −0.01 | −0.01 | 0.16 | 0.06 | ||
| Abundance | 0.28 | −0.28 | −0.10 | 0.13 | −0.07 | ||
| Latitude | Macroalgae | Species richness | 0.08 | −0.17 | 0.20 | 0.31 | 0.59 |
| Species diversity | −0.06 | 0.39 | −0.37 | 0.00 | 0.17 | ||
| Abundance | −0.50 | 0.07 | −0.08 | 0.06 | 0.17 | ||
| Polychaetes | Species richness | 0.05 | −0.12 | 0.13 | 0.15 | 0.25 | |
| Species diversity | 0.36 | −0.21 | 0.21 | 0.00 | −0.18 | ||
| Abundance | 0.07 | −0.01 | 0.01 | −0.01 | −0.02 |
- Note: Estimates were standardized and calculated after 1000 bootstrapping the model.
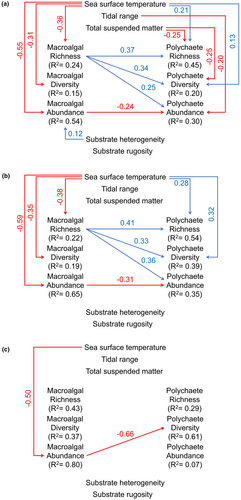
Environmental factors showed different relationships with macroalgae and polychaetes. The significant relationships for both assemblages more often at transect (three for macroalgae and five for polychaetes) and site scales (three for macroalgae and two for polychaetes) than at latitudinal scale (one for macroalgae and none for polychaetes) (Figure 5, Tables S8–S10).
Sea surface temperature had negative relationships with macroalgal richness (−0.36), diversity (−0.31) and abundance (−0.55) at the transect scale (p < .05). Those relationships were stronger at the site scale (−0.38 for species richness, −0.35 for species diversity and −0.59 for abundance) (p < .05). Nevertheless, sea surface temperature had a negative relationship with only abundance (−0.50) at the latitudinal scale (p < .05). In contrast, sea surface temperature showed positive associations with polychaete richness (0.21) and diversity (0.13) at the transect scale. Those associations were also stronger at the site scale (0.28 for species richness and 0.32 for species diversity) (p < .05) but not significant at the latitudinal scale (p > .05).
Tidal range and total suspended matter had non-significant relationships with macroalgal richness, diversity and abundance at all spatial scales (p > .05). In contrast, total suspended matter showed negative relationships with polychaete richness (−0.25) and diversity (−0.25) (p < .05) at the transect scale, and tidal range had a negative association with only polychaete abundance (−0.20) (p < .05) at that scale. However, those relationships were not significant at site and latitudinal scales (p > .05).
Substrate rugosity tended to show non-significant relationships with both macroalgal and polychaete richness, diversity and abundance at all spatial scales (p > .05). Substrate heterogeneity was associated positively with macroalgal abundance (0.12) at the transect scale (p < .05) but not significantly with macroalgal richness, diversity and abundance at site and latitudinal scales (p > .05). Non-significant relationships were also found between substrate heterogeneity and polychaete richness, diversity and abundance at all spatial scales (p > .05).
In addition to environmental factors, polychaetes showed significant relationships with macroalgae (p < .05) (Figure 5). Macroalgal richness was positively associated with polychaete richness (0.37), diversity (0.34) and abundance (0.25) at the transect scale. Those associations were stronger at the site scale (0.41 for species richness and 0.36 for abundance), except for species diversity (0.33). However, macroalgal abundance tended to have negative associations with polychaete abundance at transect (−0.24) and site scales (−0.31), and with polychaete diversity at the latitudinal scale (−0.66).
Based on the total estimate of relationship paths, sea surface temperature showed the highest estimates in four relationships at transect (−0.31 to −0.55 for macroalgae and 0.32 for polychaetes), five at site (−0.35 to −0.59 for macroalgae and 0.22 to 0.28 for polychaetes) and three at latitudinal scales (−0.50 for macroalgae and 0.07 to 0.36 for polychaetes). Other environmental factors that had the highest estimates were total suspended matter on transect richness (−0.19) and diversity of polychaetes (−0.18), substrate heterogeneity on the site richness of polychaetes (0.18), tidal range on the latitudinal diversity of macroalgae (0.39) and substrate rugosity on the latitudinal richness of macroalgae (0.59) and polychaetes (0.25) (Table 1).
4 DISCUSSION
Latitudinal gradients in the species diversity of rocky intertidal assemblages are known to vary across taxonomic groups (Iken et al., 2010; Konar et al., 2010; Miloslavich et al., 2013; Pohle et al., 2011) and spatial scales (Okuda et al., 2004; Rivadeneira et al., 2002). Here, we show that two associated rocky intertidal assemblages (macroalgae and polychaetes) have different latitudinal patterns in species richness, species diversity and abundance. Those patterns vary depending on the spatial scale of analyses, supporting that the latitudinal patterns of species diversity are scale dependent (Lyons & Willig, 2002; Willis & Whittaker, 2002). Our study also reveals that physicochemical water and substrate profiles can explain the latitudinal patterns of rocky intertidal assemblages in a complex model which involves both direct and indirect associations.
Macroalgae showed latitudinal increases in local richness, diversity and abundance. Latitudinal increases in macroalgal diversity and abundance have also been found on rocky shores in the northern hemisphere (Konar et al., 2010), Atlantic coasts (Liuzzi et al., 2011; Smale et al., 2020), temperate Pacific of South America (Santelices & Marquet, 1998) and South-West Australia (Smale et al., 2011). We could not identify the latitudes with the highest diversity and abundance possibly due to the narrower latitudinal range. Macroalgae reach the maximum diversity at the latitude of 23–40° (Fragkopoulou et al., 2022; Kerswell, 2006; Silva, 1992) and the maximum abundance at the latitude of 45–60° (Konar et al., 2010). Nevertheless, we found no clear latitudinal gradients in species richness and diversity of macroalgae at the latitudinal scale. The number of rare species in low latitudes is probably higher than that in high latitudes as reported by Hadiyanto et al. (2021), thus species turnover between sites will be higher at low latitudes than at high latitudes. Only when sites are combined at a latitudinal scale, increases in species richness and diversity become apparent and serve to equalize differences between high and low latitudes.
Unlike echinoderms (Iken et al., 2010), polychaetes show the classical trend of a decrease in species diversity towards high latitudes. Similar patterns also occur for polychaetes across other marine habitats, such as rocky intertidal shores of the Red and Mediterranean Seas (Ben-Eliahu & Safriel, 1982), estuaries of the western United States (Garza, 2008) and the continental shelf of Portugal (13–195 m depth) (Martins et al., 2013). However, the latitudinal trend in the species diversity of polychaetes was not clear at the transect scale. Unclear latitudinal patterns of local diversity have also been found for soft-bottom community in the Norwegian continental shelf (Ellingsen & Gray, 2002), rocky intertidal herbivores on the Chilean coast (Rivadeneira et al., 2002) and sessile assemblages on the northwestern Pacific coast (Okuda et al., 2004). Polychaete assemblages are probably saturated at the transect scale due to local species interactions, resource limitation and/or abundance compensation (Srivastava, 1999). We also found that species richness and abundance of polychaetes tended to follow parabolic patterns, with the highest numbers predicted to be at 27° S. The hump-shaped latitudinal patterns of species richness have also been found for other invertebrates although their maximum richness is located at different latitudes. Maximum species richness was observed at 15–25° S for mangrove crabs and razor clams in the southern hemisphere (Saeedi et al., 2017; Sharifian et al., 2020), 29–32° S for intertidal herbivores on the Chilean coast (Rivadeneira et al., 2002), 30° S for polychaetes in the southern hemisphere (Pamungkas et al., 2021) and 42° S for benthic polychaetes in the Southern Pacific coast (Moreno et al., 2021).
Environmental factors have been suggested to have direct effects on latitudinal gradients in the species diversity of rocky shore assemblages (Keith et al., 2014; Navarrete et al., 2014). However, our model suggests that environmental factors could influence rocky intertidal assemblages both directly and indirectly through changes in macroalgal distribution as foundation assemblages. Direct and indirect effects of environmental factors on rocky shore assemblages have also been observed on the coast of Santa Barbara Channel, USA, although these studies were done within a small spatial extent (Byrnes et al., 2011; Lamy et al., 2020; Miller et al., 2018). At a global scale, complex associations between the marine environment and species richness have been found for inshore reef assemblages (Edgar et al., 2017).
Environmental effects on rocky intertidal assemblages varied across spatial scales, supporting the scale dependence of environmental drivers of species richness (Whittaker et al., 2001; Willis & Whittaker, 2002). Environmental factors showed non-significant relationships with macroalgal and polychaete diversity at the latitudinal scale. Regional assemblages are composed of species with different distribution ranges, and distribution patterns at this scale are often determined by larger processes (e.g. geological processes) (Willis & Whittaker, 2002) or the evolutionary history of those assemblages (Moreno et al., 2021). Nevertheless, environmental factors tended to have more significant associations with macroalgal and polychaete diversity and abundance at transect and site scales, suggesting that environmental factors are apparently more important in determining the latitudinal patterns of local assemblages. Similar results have been found for fish diversity in Micronesia (Taylor et al., 2015) and Southern Brazil (Camara et al., 2019). Hence, we agree that environmental factors tend to be a filter of a regional species pool for the local assemblages, which is not possible for larger processes (Cornell, 2014).
In our model, sea surface temperature was the most influential factor on the distribution of macroalgae and polychaetes, supporting that temperature is a main environmental driver of the latitudinal distribution of rocky intertidal assemblages (Anderson et al., 2012; Blanchette et al., 2008; Fenberg et al., 2015; Ibanez-Erquiaga et al., 2018) and a global predictor for marine species distributions (Belanger et al., 2012; Tittensor et al., 2010). However, our model results reveal that associated taxonomic groups within rocky intertidal assemblages can have opposing influence of sea surface temperature on their diversity. Species richness and diversity of polychaetes increased with sea surface temperature, but those of macroalgae showed opposite trends. Similar findings have also been recorded in previous studies (Keith et al., 2014; Roy et al., 1998; Tittensor et al., 2010). Warm temperature possibly reduces physiological costs of polychaetes (Willig et al., 2003), but it is perhaps outside thermal boundaries (survival, growth and reproduction) of macroalgae (Bartsch et al., 2012; Rinde & Sjøtun, 2005; Rothäusler et al., 2009).
In contrast to sea surface temperature, tidal range and total suspended matter showed negative associations with polychaetes. Tidal action influences the distribution of rocky intertidal assemblages through physical (e.g. water motion, desiccation, high temperature) and biological pressures (e.g. predation) (Kunze et al., 2021) and changes in nutrients, sediment and turbidity (Lewis, 1968). Therefore, effects of tide and total suspended matter on those assemblages can be collinear. The species diversity of benthic macrofauna is often low in turbid waters (Andrew et al., 2006; Fernández-Romero et al., 2019; Melo et al., 2013) as high total suspended matter clogs feeding structures and reduces the feeding efficiency of suspension feeders (Newcombe & Macdonald, 1991). However, tidal range and total suspended matter had little influence on macroalgal diversity. This assemblage is perhaps able to deal with the physical pressures of tidal cycles (Clark et al., 2018; Holzinger & Karsten, 2013; Kregting et al., 2013) and take advantage of nutrient enrichment during those processes (Ladah et al., 2012; Pérez-Mayorga et al., 2011).
Habitat structures could also play important roles in determining the distribution of rocky intertidal assemblages. Substrate heterogeneity provided a positive effect on macroalgal abundance. Habitat heterogeneity represents the variability of resources for community development (Hanlon et al., 2018; Smith et al., 2014) and protection from predators (Jaimie et al., 2005). Therefore, increases in substrate heterogeneity often support diversity and abundance of rocky shore assemblages (Díaz-Tapia et al., 2013; Griffin et al., 2009; Littler et al., 1983; McQuaid & Dower, 1990; Munguia et al., 2011). However, the effect of habitat structures on rocky intertidal assemblages at large scales is smaller than that of physicochemical waters as also found on the coast of southern Brazil and Uruguay (Steigleder et al., 2019). Habitat structures are probably more important in explaining the distribution of rocky intertidal assemblages at small scales across which differences in physicochemical waters (e.g. sea surface temperature) are minimal.
Relationships between environmental factors and rocky intertidal assemblages become more complex as polychaetes also appear to be associated with macroalgal richness, as occurs for other invertebrates (Hirst, 2008; Parker et al., 2001; Tano et al., 2016). Polychaete richness, diversity and abundance increased with the species richness of macroalgae. Species-rich macroalgae perhaps enhance microhabitat heterogeneity and complexity reducing physical and predator pressures (Graham et al., 2016; Umanzor et al., 2017; Wernberg, Thomsen, & Kotta, 2013), allowing more species and individuals of polychaetes to co-occur. High abundances of macroalgae may also reduce space competition between associated fauna (Duffy & Harvilicz, 2001; Edgar, 1990) and spatially homogenize abiotic stressors (Beermann et al., 2013; Ørberg et al., 2018). This perhaps explains a negative association between macroalgal abundance and polychaete abundance in the present study.
Overall, we revealed that two associated rocky intertidal assemblages had different latitudinal patterns in species richness, species diversity and abundance, and those patterns varied depending on the spatial scale. Environmental factors, i.e., physicochemical water and substrate profiles, could explain the latitudinal patterns of rocky intertidal assemblages in a complex model. This model enhances our understanding on how environmental factors determine latitudinal gradients in species diversity. Sea surface temperature, the most influential factor for rocky intertidal assemblages, is projected to increase by 1–3°C by 2100 (Collins et al., 2013), and this change possibly will alter diversity and abundance of those assemblages in the future climate. Changes in local drivers, e.g., substrate profiles and water quality, could also influence the distribution of rocky intertidal assemblages. Therefore, we suggest that protecting rocky intertidal assemblages should need both global (e.g. climate mitigation) and local actions (e.g. habitat protection).
AUTHOR CONTRIBUTIONS
HH, JP and RKH conceived the ideas; HH, JP and RKH collected the samples; HH identified macroalgae and polychaetes; HH analysed the data with significant suggestions from JP and RKH; HH led the writing with assistance from JP and RKH; HH, JP and RKH reviewed and edited.
ACKNOWLEDGEMENTS
This paper is a part of PhD study of the first author at the University of Western Australia. The study was sponsored by Indonesia Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan), the Ministry of Finance, Republic of Indonesia, and the period of 2019-2023 (No. 201901220213791). Open-access publishing was supported by the University of Western Australia, as part of the Wiley-University of Western Australia agreement via the Council of Australian University Librarians. The authors would like to thank: the Department of Biodiversity, Conservation and Attractions (DBCA) and the Department of Primary Industries and Regional Development (DPIRD) of Western Australia for permits of macroalgal and polychaete collections (Regulation 4 No. CE006192, Regulation 25 No. FO25000309, Regulation 61 No. FT61000627, and Exemption Number 3547); Karajarri Rangers for the permit and help while collecting samples in Bidyadanga; and volunteers (Matilda Murley, Jessi Walker, Andri Irawan, Ni Made Indira Santi, Ni Luh Gede Rai Ayu Saraswati and Putriana Indah Lestari) for the help during the fieldwork. Open access publishing facilitated by The University of Western Australia, as part of the Wiley - The University of Western Australia agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
Macroalgal, polychaete and substrate data are available in figshare (https://doi.org/10.6084/m9.figshare.24912396). Mean sea surface temperature at the resolution of about 1 km is available at the MARSPEC (https://www.esapubs.org/archive/ecol/E094/086/#data), mean tidal range, and mean total suspended matter at the resolution of about 9.2 km are available at the Global Marine Environment Datasets (http://gmed.auckland.ac.nz).




