Algal cover as a driver of diversity in communities associated with mussel assemblages across eastern Pacific ecoregions
Abstract
Research on intertidal mussel assemblages and associated communities has revealed that complexity and structure are influenced by environmental heterogeneity and local-scale factors affecting recruitment. Research in situ in eastern and western Pacific intertidal ecosystems has suggested drivers of species diversity and community structure encompassing large geographic scales, however, there are major gaps in geographic coverage. Our aim is to fill some of these gaps by analyzing macrofaunal functional group diversity and effects of environmental factors on intertidal mussel communities from three distinct marine ecoregions in the southern and northern hemispheres. We identified the effects of algal cover and environmental heterogeneity on species richness and evenness, and we modeled factors effecting mussel layer complexity from assemblages in three marine ecoregions. We analyzed macrofaunal species diversity within one of the austral ecoregions based on the width of the coastal shelf. Species richness was highest in samples from the northern hemisphere while evenness was highest in samples from the southern hemisphere. Similarity in functional group structure for all communities sampled was ≤55% (Bray–Curtis dissimilarity) and ≤35% (Chao–Jaccard dissimilarity). Wave exposure had a significant effect on shell length and complexity of mussel matrices on rocky bench platforms. The presence of algal cover had a strong effect on species richness in mussel matrices regardless of complexity, while algal canopies had no effect on species evenness. Overall, this study provides significant new insight on the community complexity of mussel beds in parts of the world which have been poorly studied in this regard.
1 INTRODUCTION
Mussels have been described as “bioengineer species” with significant linkages to species richness and evenness based on shell size, age-class, stratification of various age-classes of mussels, and the presence of algal epibionts (Alvarado & Castilla, 1996; Firstater et al., 2011; Guiñez & Castilla, 1999; Prado & Castilla, 2006). In dynamic marine ecosystems such as intertidal and shallow subtidal areas, algal cover functions as macro-scale habitat that defines community structure and provides a buffer from local climate extremes essential for a variety of intertidal species including mussels (Bulleri et al., 2002; Jenkins et al., 1999; Leite et al., 2021; Leonard, 1999; Stachowicz et al., 2008). It is in this extreme environment assemblages of intertidal mussels are exposed to the limits of their temperature thresholds (Helmuth et al., 2006; Szathmary et al., 2009; Williams et al., 2008). For example, during periods of extreme low tides when the intertidal zone is exposed for several hours, algal cover provides protection from desiccation and other effects from environmental extremes to under-story mussel communities (Prado & Castilla, 2006; Stachowicz et al., 2008; Storero et al., 2022; Valdivia, Aguilera, & Broitman, 2021; Valdivia, López, et al., 2021). Furthermore, there is evidence to suggest that the presence of canopy-forming algae influences the rates of recruitment and settlement of other algal species (Benedetti-Cecchi et al., 1996), barnacles (Leonard, 1999), and potentially, marine mussels as well. Bulleri et al. (2002) found that the experimental removal of canopies of Cystoseira resulted in an increase in branched algal and coralline species that corresponded with the disappearance of many other species. At the same time, potential interactions between algal cover and mussel species can be two-directional; Benedetti-Cecchi et al. (1996) demonstrated that the removal of mussels resulted in an increase in algal canopy likely due to the inhibitory effect of the mussels on algal recruitment.
The intertidal environment is highly variable in terms of temperature and radiation, and research on the physiological responses of mussels to environmental stress suggests that mussels in any given geographic range have developed adaptations that enable them to survive within certain limits (Helmuth et al., 2006; Szathmary et al., 2009). Due to their suitability as substrate in hard and soft bottom intertidal environments (Prado & Castilla, 2006; Thiel & Ullrich, 2002; Valdivia, Aguilera, & Broitman, 2021), mussel assemblages are ideal for measuring the diversity of macro-scale communities associated with them and thus the importance of addressing factors that mitigate the impacts from environmental stressors cannot be overstated, particularly when assessing mussel community diversity and hierarchical structure (Harley & Helmuth, 2003). The physiological adaptations attributed to various mussel species have explained temperature and desiccation tolerances to some degree, however, the wetting provided by ocean waves plays a significant role in mitigating heat stress when mussels living near their upper-temperature thresholds (Dahlhoff & Menge, 1996; Harley & Helmuth, 2003). For example, the orientation of mussel assemblages along the shore in a temperate region may serve to mitigate upper-temperature extremes brought about by small-scale changes in climate (Dahlhoff & Menge, 1996; Helmuth et al., 2006; Konar et al., 2010). Ocean waves play a role in the availability of nutrients along the shoreline as well as phytoplankton abundance and have been significantly linked to higher primary productivity, higher metabolic activity in mussels (Dahlhoff & Menge, 1996; Menge, Daley, Wheeler, Dahlhoff, et al., 1997; Menge, Daley, Wheeler, & Strub, 1997), and size distributions and recruitment of juvenile mussels (Alvarado & Castilla, 1996). Local-scale high primary productivity following nutrient delivery in shallow coastal areas has the potential to trigger bottom-up trophic cascades, which in turn facilitates the settlement of various planktotrophic macro-invertebrates onto suitable hard substrate in the intertidal zone such as the shells of mussels. Small-scale patterns in community structure may also be attributed to wave exposure while also taking into consideration other variables such as top-down effects (Dahlhoff & Menge, 1996; Menge, 1992; Menge, Daley, Wheeler, Dahlhoff, et al., 1997; Menge, Daley, Wheeler, & Strub, 1997).
Ocean waves also serve as a dispersal mechanism for marine algae and facilitate the transport of algal sporelings throughout their vertical range in the intertidal zone (Harley & Helmuth, 2003). The shells of intertidal mussels provide substrate for the thalli of seaweeds to anchor and where mussels are abundant, algae may form expansive upper-story canopies. Temperature and desiccation are the primary limiting factors for successful algal sporeling recruitment in the high intertidal zone while herbivore abundance and competition for space are primarily the limiting factors in the lower intertidal (Steneck & Dethier, 1994; Underwood & Jernakoff, 1984). Species of canopy-forming seaweeds found higher in the intertidal have been characterized as “drought resistant” as a result of surface to volume ratio of the blades, complex cellular structure, and lipid content; examples include the leathery macrophyte Fucus and the corticated macrophyte Ahnfeltia spp. (De Vogelaere & Foster, 1994; Schonbeck & Norton, 1979). Prado and Castilla (2006) found that algal canopies played a significant role in species richness in mussel assemblages on the Chilean coast.
Published work on broad-scale marine intertidal diversity has mostly been taken from reviews of the existing literature and mining of historical data from small-scale studies. Only in the past few decades has empirical research been undertaken in an effort to gain a fundamental understanding of species diversity, resilience, and stability in marine intertidal and shallow subtidal meta-communities on local and broad geographic scales (Bryson et al., 2014; Konar et al., 2010; Okuda et al., 2004; Thyrring & Peck, 2021; Valdivia, Aguilera, & Broitman, 2021; Valdivia, López, et al., 2021). Much of the research on mussel-dominated communities in the northern hemisphere has been somewhat limited in scale with a focus on physiological adaptations to heat stress and physical responses to environmental factors (Helmuth, 1998; Menge, Daley, Wheeler, Dahlhoff, et al., 1997), while in the southern hemisphere research has focused on local-scale nutrient uptake and structural complexity of intertidal mussel assemblages (Alvarado & Castilla, 1996; Firstater et al., 2011; Guiñez & Castilla, 1999), with more recent interest in broader-scale surveys (Ibanez-Erquiaga et al., 2018) and evaluation of multi-dimensional stability in intertidal communities associated with mussel assemblages (Valdivia, Aguilera, & Broitman, 2021; Valdivia, López, et al., 2021). Our analysis on the biodiversity of previously unexplored intertidal communities encompassed three distinct marine ecoregions on the Pacific coasts of North and South America and included multiple measures of alpha diversity to account for rare species and relative abundance (Chao et al., 2005; Colwell et al., 2012; Colwell & Elsensohn, 2014). We tested the effects of environmental variables on the complexity of mussel assemblages, and we tested the hypothesis that there were variations in macrofaunal species richness and evenness in assemblages of Perumytilus purpuratus based on environmental and regional factors. Lastly, we examined the degree to which algal cover and wave exposure explained variation in the species composition and diversity of intertidal mussel communities.
2 MATERIALS AND METHODS
2.1 The study sites
Marine ecoregions have been described in detail based on homogenous species composition and distinct oceanographic features (Spalding et al., 2007). We selected four sites within the North American Pacific Fjordland (NAPF) ecoregion, two sites within the Guayaquil (GUAY) ecoregion, and eight sites within the Humboldtian ecoregion based on the remoteness of the location, accessibility, relative lack of published research in the proposed field of study, suitable rocky intertidal substrate, and abundance of mussel species endemic to each ecoregion (Figure 1). Various efforts have been undertaken to compare species diversity and evenness in communities associated with mussel assemblages on bio-geographical scales regardless of oceanographic differences and the inter-specific nature of the mussel populations (Buschbaum et al., 2009; Menge et al., 2002). Thiel and Ullrich (2002) found variations in the abundances of specific taxonomic groups in assemblages of P. purpuratus at sites separated by 15 degrees of latitude on the Chilean coast, with a directional pattern of abundance in nemertean and polyclad worms. We have divided the Humboldtian ecoregion into two sub-ecoregions (HWS and HNS) based on the relative width of the continental shelf adjacent to the study sites, and similar to Thiel and Ullrich (2002) (see Appendix S1) for non-parametric summaries of mussel matrices in these sub-ecoregions. In order to magnify the spatial resolution of the Humboldtian ecoregion, two divisions are proposed based on the width of the continental margin: the Humboldtian wide-shelf (HWS) ecoregion is approximately 225 km from the edge of the continental shelf to the coastline; and the Humboldtian narrow-shelf (HNS) ecoregion. Three sites were chosen within the HWS sub-ecoregion and five sites were chosen within the HNS sub-ecoregion. Geographic coordinates for the sampling sites and ecoregions can be found in Table 1.
| Ecoregion/site | Latitude | Longitude |
|---|---|---|
| Guayaquil (GUAY) | ~0° S to 3° S | |
| Humboldtian | ~12° S to 26° S | |
| Humboltdian Wide-Shelf (HWS) | 12°37′ S to 12°58′ S | 75°11′ W to 76°40′ W |
| Playa Ensendada (PEN) | 12°38′ S | 76°40′ S |
| Playa Farallones (PFA) | 12°44′ S | 76°37′51 W |
| Playa Palmeras (PGA) | 12°57′56 S | 76°30′ W |
| Humboldtian Narrow-Shelf (HNS) | 15°21′ S to 23′ S | 70′ W to 75°11′ W |
| Reserva Punta San Juan (PSJ) | 15°18′ S | 75°11′ W |
| Universidad de Antofagasta (UOA) | 23°42′ S | 70°25′27 |
| North American Pacific Fjordland (NAPF) | ~50° N to 59° N | |
| Whale Park | 57°1′57.72″ N | 135°15′1.08″ W |
| Kayak Island | 57°0′30.42″ N | 135°21′11.16″ W |
| Sage Beach | 57°3′30.348″ N | 135°19′21.36″ W |
| Pirate's Cove | 56°59′13.2″ N | 135°22′42.96″ W |
2.2 Sampling mussel assemblages
We identified mussel species as the target assemblages (Engle, 2008) for each ecoregion. Two species of mussels (family Mytelidae) common to the eastern Pacific of the southern hemisphere are Perumytilus purpuratus (Lamarck 1819) and Semimytilus algosus (Gould 1850), and both have been studied in regard to species diversity in rocky intertidal communities in Chile (Prado & Castilla, 2006). Dense assemblages of P. purpuratus and S. algosus are typically found in the high to mid intertidal zone while S. algosus and P. purpuratus overlap in the mid zone, with S. algosus becoming the dominant species in the low zone (Tokeshi & Romero, 2000, L. Wilbur in review). Brachidontes adamsianus is the mussel species most commonly encountered in the southern tropical region (A. Pacheco, pers. comm.), and while P. purpuratus and S. algosus are similar in length at reproductive maturity (Torroglosa & Giménez, 2018), P. purpuratus and B. adamsianus share similar morphological features (scalloped ridges along the dorsal and ventral valves). Mytilus trossulus and Mytilus californianus are the two main species of mussels found in the rocky intertidal zone in the eastern Pacific of the northern hemisphere. Evidence of hybridization between the Mediterranean blue mussel Mytilus galloprovincialis and M. trossulus has been reported for Alaska, however, the distribution of pure stands of M. trossulus versus hybridized mussels is not well understood (Burger et al., 2006; McDonald et al., 1991). Another mussel species, M. californianus, is typically found in stands of one to a few individuals at or below mean lower low water (MLLW) (Wilbur et al., 2023). Here, we use Mytilus edulis/trossulus/galloprovincialis complex (abbr. MytCom) in reference to the mussel species in the NAPF ecoregion (we are following the suggestion of the editors at https://www.centralcoastbiodiversity.org/pacific-blue-mussel-bull-mytilus-trossulus.html).
In the mid to low zone, sessile and motile organisms such as the barnacle Cthamalus cirrata, the anemone Phymactis clematis, and the chitons Lucilinia nigropunctata and Chiton granosus recruit on mussel valves of P. purpuratus and S. algosus, while macro-algae (articulated red algae Corallina officinalis, foliose green algae Ulva rigida, Enteromorpha, and Colpomenia sinuosa, and corticated macrophytes belonging to the families Ahnfeltiaceae, Gigartinaceae, and Sarcodiaceae) also recruit as germlings on mussel valves. Sessile and motile invertebrates attach to the valves of these mussel species such as the barnacles Balanus glandula, Semibalanus cariosus, and the anemone Metridium senile, as well as filamentous seaweeds such as Pterosiphonia bipinnata and the canopy-forming leathery macrophyte Fucus gardneri.
Mussel assemblages were sampled during the austral and boreal summers for each ecoregion: in the NAPF ecoregion between June and July during the years 2017–2018; in the GUAY ecoregion in February 2017; in the HWS ecoregions in February 2017 and in the HNS ecoregion at Reserva Punta San Juan in March (late austral summer) of 2017. Additional mussel assemblage were sampled at Reserva Punta San Juan at N5s and at UOA in March 2018, and at S5 in March 2019. N5n was not sampled in 2018 or 2019 due to extinction of the communities there, and no additional mussel assemblages were sampled in order to avoid bias from re-sampling the same plot, permanent markers were installed as reference points at the highest point in the intertidal zone where biota were first encountered, and all mussels were sampled within the first 10 meters from the highest point in the intertidal zone and to the waterline where the mussels formed an extensive and continuous assemblage. Where mussel assemblages were patchy in distribution (not extensive and continuous), plots were selected where mussel coverage was at least one-third of a quadrat. Compass coordinates and distance from the reference marker to the quadrats were recorded in order to avoid re-sampling. Mussels were sampled by using a stainless-steel spatula to remove a 100 cm2 area of matrix from the rock within each of the quadrats. The samples were then placed in a shallow pan of seawater for 30 min to allow organisms to emerge from the mussel valves. Organisms ≥1 mm living within and on the mussels were identified and counted using a hand lens and Nikon field 20 × stereoscope (Arakaki et al., 2018, 2019; Dawson et al., 1964; Howe, 1914; Kozloff, 1996; Mendez, 2002; Romero, 2002). Mussel assemblages were sampled at sites within each of the four regions using a 70 cm × 50 cm quadrat strung to provide a grid with 100 intersections (Engle, 2008). Organisms were identified as species or the lowest taxonomic category using reference materials and dichotomous keys. All species were organized into functional groups according to the methods used in Steneck and Watling (1982), that is, invertebrates were categorized by subclass, and algae were categorized by a functional group number according to the level of cellular complexity and physical structure (for definitions of functional groups, see Steneck, 1988; Steneck & Dethier, 1994; Steneck & Watling, 1982).
2.3 Defining mussel layers
In the intertidal zone where predation and larval recruitment on hard substrates are the limiting factors of space, food web structure, diversity and relative abundance of species associated with these communities have been extensively described (Blanchette et al., 2009; Connell, 1961; Connolly & Roughgarden, 1999; Paine, 1966; Thyrring & Peck, 2021), Mussels in particular have become the focus of research, particularly for their characteristic as a “foundation species,” that is, once established, mussels function as biological substrate that increases the area suitable for the recruitment of additional species (Altieri et al., 2007; Alvarado & Castilla, 1996; Wilbur et al., 2023).
The results from the calculations from each of the raw shell lengths are summed using the formula.
S = 0.555 ⅀ni = 1 L1.44 * phi +0.1587 ⅀mi = n + 1 L2 * phi (Hosomi, 1985), where phi is a ratio used to measure body proportions in plants and animals, and phi = 1.6180339887495. The summed values were used to represent the variable stratum index (SI) for each corresponding plot (Guiñez & Castilla, 1999; Hosomi, 1987). The values for shell length (L), matrix depth (M), and stratum index (SI) for all plots in this study were evaluated for normal distribution of the frequencies with the Anderson-Darling test.
Each plot was categorized as mono-layered or multi-layered based on the median stratum index value calculated for that plot, that is, all plots with a stratum index <2.0 were categorized as mono-layered, and all plots with a stratum index ≧2.0 were categorized as multi-layered. Mono-layered matrices with a stratum index ≤1.00 were categorized as “characteristically mono-layered” according to the mussel assemblage self-thinning theory (Frechette et al., 1992; Guiñez & Castilla, 1999). For comparative analysis, the matrix depths recorded from the macro-faunal and algal sampling were used to define the layers using Prado and Castilla's (2006) classification of mussel matrix layers by measuring the depth of the matrix starting from the topmost mussel to the substrate, with mono-layered matrices defined as ≤2.0 cm in depth and multi-layered matrices defined as >2.0 cm in depth.
2.4 Scoring algal cover
- Cover A = a point on the grid where an alga covered primary rock substrate.
- Cover B = a point on the grid where an alga covered a secondary invertebrate substrate.
- Cover C = a point on the grid where an alga covered a secondary alga substrate.
The scores were then summed and converted to decimal percentages to give the total percent algal cover for each respective plot. When the sums for a mussel plot provided 25% or more coverage, the plot was categorized as “with cover”; plots with less than 25 percent coverage were categorized as “without cover” similar to the definition of cover used in Prado and Castilla (2006).
2.5 Defining wave exposure and platform angle
Wave exposure was determined qualitatively by assessing the orientation of the mussel plots to open ocean waves and swell similar to the methods used in Dahlhoff and Menge (1996), Menge, Daley, Wheeler, Dahlhoff, et al. (1997), and Menge, Daley, Wheeler, and Strub (1997). Mussel plots that were directly exposed to incoming ocean waves at sites that faced the open ocean were categorized as “wave exposed” and mussel plots that were exposed to waves at an oblique angle, for example on the leeward side of a bay, peninsula, or island, were categorized as “wave sheltered.”
The platforms where the mussel plots were sampled were arranged into two categories based on the slope of the substrate; a Häglof EC II D-R clinometer (Häglof Sweden) was used to measure the angle by standing some distance from the platform and sitting at the top of the platform through the clinometer. In general, platforms that were relatively level (the platform could be stood upon) (D'Antonio, 1986) had an angle <45° and were thus categorized as “bench,” and platforms that were relatively steep had an angle ≥45° and were thus categorized as “vertical.” Where it was difficult to measure the angle of the platform with the clinometer, photographs were used to assess the platform as “horizontal” or “vertical.”
2.6 Diversity analysis
For measuring mean gamma diversity for each ecoregion (for an explanation of mean diversity, please see Colwell et al., 2012, Colwell, 2013), we organized the samples according to species and functional groups and pooled the data by ecoregion. Because of the limited scope of available research on diversity in the mussel communities at the sites, we chose three measures of diversity in order to provide a spectrum of analysis: Shannon–Wiener Index (H) as a standard estimate of species richness and abundance in the samples (Shannon, 1948); Simpson's Inverse Index (1/D) (Simpson, 1949) to evaluate the probability encountering the same species in different samples; and Fisher's alpha (α) because it is sensitive to rare and unique species (Fisher et al., 1943).
One of the difficulties in using the point scoring design for incidence and abundance of species has been accounting for rare and unique species (Chao et al., 2014), thus, we attempted to predict the number of species including those that might be missed during sampling by extrapolating the species richness and then plotting the accumulation curves for each site (Colwell & Coddington, 1994; Peake & Quinn, 1993; Ugland et al., 2003). Two richness methods were used here; species richness (S), or the number of observed species within a given sample and the Chao 1 estimator (SChao1) for its sensitivity to rare and unique species (Chao, 1984). We used EstimateS software version 9.1 (Colwell, 2013) to graph the accumulation curves from calculated mean richness (Ssp) and mean functional group richness (Sfx) calculated from each sample using ±95% confidence intervals using randomized re-sampling (bootstrapped to 105 samples), with and without replacement (Chao, 1984; Colwell et al., 2012; Colwell & Elsensohn, 2014). To reduce bias among sample sizes, we rarified the incidence and abundance data for enumerating species richness (Chao, 1987; Colwell et al., 2004).
To compare communities across broad regional scales, we organized the species data into functional groups and evaluated using dissimilarity analyses in the vegan package (Oksanen et al., 2020), RStudio version 1.3.1in the R programming software version 4.0.2 (R Core Team, 2020; RStudio Team, 2020). The Bray–Curtis dissimilarity measure was chosen as a standard that accounts for the most abundant species in a sample (Bray & Curtis, 1957), and the Chao–Jaccard dissimilarity measure was chosen for its sensitivity to rare and unique species (Chao et al., 2005).
2.7 Statistical analysis
We used multivariate analysis to test the null hypothesis (Hθ) that environmental heterogeneity has no effect on the structural complexity of mussel assemblages on the broad geographic scale that we propose. We tested the response variables shell length (L), matrix depth (M), and stratum index (SI) with the pooled explanatory variables, that is, mussel matrices exposed to waves (n = 174) versus mussel matrices sheltered from waves (n = 104) and mussel matrices on bench substrate (n = 76) versus mussel matrices on vertical substrate (n = 202), and the combined effects of the variables. Exposure and substrate were analyzed as fixed effects. Akaike Information Criterion (AIC) scores were evaluated for models (1) using ecoregion as one random effect, and (2) site and ecoregion as nested random effects. For each respective dependent variable, the model with the lowest AIC score for each random effect or nested random effects was chosen as the best model (Akaike, 1974; Burnham et al., 2011). Ecoregion (n = 4) and site (n = 14) were analyzed as nested random effects on the response variables using linear mixed-effects modeling from the lmerTest package (Kuznetsova et al., 2017) in R. The pooled data for each response variable was assessed for normality using the Shapiro–Wilk test. The models with the lowest Akaike Information Criteria scores based on the random effects were chosen. Each linear effects model was then analyzed for significance of variance (Pr (>|f|), ⍺ = .05) using one-way ANOVA.
The presence of algae and the number of mussel layers as defined by the depth of the matrix has been suggested to play a significant local-scale role in the structure of macrofaunal communities in mussel assemblages (Prado & Castilla, 2006). In order to examine whether diversity could be predicted using the same variables on a broader geographical scale, we parsed the definition of mussel layers based on the method used to define them (stratum index and matrix depth), and we tested the null hypothesis (Hθ) that algal cover and mussel layers have no significant effect on species richness and evenness on mussel communities. We organized the mussel layer data into two sets as defined by stratum index or the matrix depth, and we nested the explanatory variables (mono-layered matrices with and without algal cover and multi-layered matrices with and without algal cover). We then pooled the data according to the nested variables, which resulted in non-parametric distributions and unequal populations among pairwise data sets. Methods in non-parametric analysis tend to be lower in power and accuracy for rejecting or accepting the null hypothesis (Siegel, 1957), nevertheless, we used the Wilcoxon rank sum test with 95% confidence intervals (CI) to compare the medians of the sample populations and bootstrapped the confidence intervals to check for variation from the sample CI (Zhou & Dinh, 2005). To reduce bias in species richness introduced by matrices with algal cover, we chose Menhinick's species richness (IMn = S/√(n)) for calculating species richness, where S is the number of species and n is the number of individuals, which effectively provides a standardized index of species richness (Menhinick, 1964). The Pielou's evenness index (J' = H′/ln(S) where H′ is the Shannon Weaver index value and S is the total number of species in the sample) measures equality in the sample population in terms of the number of individuals for each species sampled with the formula. The scale for Pielou's evenness index is from 0 to 1, with numbers closer to 1 indicating higher levels of evenness (Pielou, 1966).
3 RESULTS
3.1 Species richness and alpha diversity
A total of 64 species from 12 phyla were sampled from mussel plots in the NAPF ecoregion, with Fucus gardneri and Pterosiphonia bipinnata the most abundant algal taxa, and members of the genera Balanus, Semibalanus, Lottia, and Littorina among the most abundant invertebrate taxa. Thirteen species from six phyla were sampled in mussel plots from the GUAY ecoregion; Ulva was the most abundant algal taxon for this ecoregional group. Forty-four species from 11 phyla were sampled from the HWS ecoregion; the green foliose algae Ulva, the red crustose algae Lithothamnion spp., the polychaete Perinereis spp., and the limpet Scurria spp. were the most abundant taxa. Finally, 49 species from 11 phyla were sampled from the HNS ecoregion, where Chondracanthus spp. was the most abundant algal taxon and Perinereis spp., a spionid polychaete (presumed to be Proboscidia wellingtonensis), Echinolittorina sp., Scurria sp., and nemertean worms being the abundant taxa in the mussel communities (Table 2).
| North American Pacific Fjordland | ||||||
|---|---|---|---|---|---|---|
| Algal fx group # or taxonomic group | Kayak Island (KIS) | Sage Beach (SBE) | Pirate's cove (PCO) | Whale Park (WPA) | ||
| Chlorophyta | ||||||
| Chaetomorpha cartilaginea | 2 | M. Howe, 1914 | 5 | |||
| Chaetomorpha sp. | 2 | Kützing 1845 | 7 | 12 | 1 | |
| Cladophora sp. | 2 | Kützing 1843 | 5 | 3 | 38 | |
| Ulva intestinalis | 3 | Linnaeus 1753 | 2 | |||
| Ulvaria | 3 | Ruprecht 1850 | 9 | 5 | 18 | |
| Ulva lactuca | 3 | Linnaeus 1753 | 2 | 42 | 40 | |
| Ochrophyta | ||||||
| Alaria nana | 5 | H.F. Schrader 1903 | 2 | 10 | 1 | |
| Colpomenia peregrina | 3.5 | Savageau 1927 | 7 | |||
| Ectocarpus sp. | 2 | Lyngbye 1819 | 1 | 10 | ||
| Fucus gardneri | 5 | P.C. Silva 1953 | 34 | 134 | 13 | 103 |
| Ralfsia sp. | 7 | Berkeley 1843 | 3 | |||
| Scytosiphon lomentaria | 3.5 | (Lynbye) Link 1833 | 1 | 2 | ||
| Rhodophyta | ||||||
| Calliarthron tuberculosum | 2.5 | (Postels & Ruprecth) E.Y. Dawson 1964 | 4 | |||
| Ceramium sp. | 2.5 | Roth 1797 | 3 | 1 | ||
| Corallina frondescens | 6 | Postels and Ruprecth 1840 | 2 | |||
| Endocladia muricata | 4 | (Endlicher) J. Agardh 1847 | 13 | 16 | 2 | |
| Hildenbrandia sp. | 7 | Nardo 1834 | 1 | |||
| Mastocarpus sp. | 5 | Kützing 1843 | 1 | |||
| Mazzaella sp. | 5 | G. DeToni 1936 | 3 | |||
| Microcladia borealis | 2.5 | Ruprecht 1850 | 1 | |||
| Odonthalia floccosa | 2.5 | (Esper) Falkenberg 1901 | 14 | 5 | ||
| Pterosiphonia bipinnata | 2.5 | (Postels & Ruprecht) Falkenberg | 42 | 89 | 27 | 13 |
| Plocamium violacea | 2.5 | Farlow 1877 | 1 | |||
| Polysiphonia hendryi | 2.5 | N.L. Gardner 1927 | 7 | 1 | ||
| Polysiphonia hendryi var. compacta | 2.5 | Hollenberg (Hollenberg) 1961 | 6 | 1 | 2 | |
| Halosaccion glanduliforme | 3.5 | Kützing 1843 | 12 | 5 | ||
| Nemalion helminthoides | 3.5 | (Velley) Batters 1902 | 3 | |||
| Cnidaria | ||||||
| Anthopleura xanthogrammica | Hexacoralia | Brandt 1835 | 2 | |||
| Metridium senile | Hexacoralia | Linnaeus 1761 | 2 | |||
| Nematoda | ||||||
| Nematode sp. not det. | Nematoda | 9 | 4 | |||
| Platyhelminthes | ||||||
| Polyclad sp. not. det. | Polycladida | 3 | 1 | |||
| Nemertea | ||||||
| Emplectonema gracile | Nemertea | Johnston 1837 | 1 | 1 | 9 | 1 |
| Nemertea sp. not det. | Nemertea | 2 | 1 | |||
| Annelida | ||||||
| Glycera sp. | Errantia | Lamarck 1818 | 1 | |||
| Nereis sp. | Errantia | Linnaeus 1758 | 10 | 2 | 1 | 10 |
| Cirratulid sp. not det. | Errantia | 1 | ||||
| Mollusca | ||||||
| Hiatella arctica | Autobranchia | Linnaeus 1767 | 1 | |||
| Littorina scutulata | Caenogastropoda | Gould 1849 | 4 | 9 | 3 | 39 |
| Littorina sitkana | Caenogastropoda | Phillippi 1846 | 3 | 2 | 2 | |
| Lottia asmi | Patellogastrpoda | Middendorff 1848 | 2 | 6 | 2 | |
| Lottia digitalis | Patellogastrpoda | Rathke 1833 | 3 | 1 | 5 | |
| Lottia ochracea | Patellogastrpoda | Gould 1846 | 36 | 25 | 11 | |
| Lottia paradigitalis | Patellogastrpoda | Fritchman 1960 | 1 | 11 | 3 | 1 |
| Lottia pelta | Patellogastrpoda | Rathke 1833 | 2 | |||
| Lottia scutum | Patellogastrpoda | Rathke 1833 | 1 | |||
| Lottia sp. | Patellogastrpoda | 2 | ||||
| Nucella ostrina | Caenogastropoda | Gould 1852 | 2 | |||
| Mytilus species complex (MYTCOM) | Autobranchia | x | x | x | x | |
| Arthropoda | ||||||
| Balanus glandula | Cirripedia | Darwin 1854 | 10 | 66 | 19 | 94 |
| Amphibalanus sp. | Cirripedia | Darwin 1854 | 10 | 13 | 27 | 18 |
| Semibalanus cariosus | Cirripedia | Pallus 1788 | 7 | 108 | 26 | 65 |
| Chthamalus dalli | Cirripedia | Pilsbry 1916 | 10 | |||
| Balanus crenatus | Cirripedia | Bruguière 1789 | 2 | 2 | ||
| Chromopleustes oculatus | Eumalocostraca | Bousfield and Hendrycks 1995 | 1 | |||
| Amphipod sp. not det. | Eumalocostraca | 7 | ||||
| Idotea wosnesenskii | Eumalocostraca | Brandt 1851 | 2 | |||
| Isopod not. det. | Eumalocostraca | 2 | 1 | |||
| Crab, megalops phase | 1 | 1 | ||||
| Cirolana harfordi | Eumalocostraca | Lockinton 1877 | 1 | 9 | 1 | |
| Pagurus hirsutiusculus | Eumalocostraca | Dana 1851 | 1 | |||
| Neomolgus littoralis | Acari | Linnaeus 1758 | 1 | 1 | ||
| Mite sp. not det. | Acari | 2 | 1 | |||
| Echinodermata | ||||||
| Cucumaria sp. | Actinopoda | de Blainville 1830 | 10 | |||
| Chordata | ||||||
| Colonial tunicate sp. not det. | Urochordata | 1 | ||||
| Fx #/taxonomic grp. | Guayaquil | Humboldtian wide-shelf | Humboldtian narrow-shelf | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Playa Acapulco (ACA) | El Nuro (ENU) | Playa Ensenada (PEN) | Playa Farallones (PFA) | Playa Palmeras (PGA) | PSJ N5n | PSJ N5s | PSJ S4 | PSJ S5 | Univ. Antofagasta (UOA) | |||
| Chlorophyta | ||||||||||||
| Chaetomorpha firma | 2 | Levring 1941 | 10 | |||||||||
| Ulva rigida | 3 | C. Agardh 1823 | 18 | 1 | 15 | 58 | 28 | 3 | 7 | 7 | ||
| Ulva lactuca | 3 | Linnaeus 1753 | 51 | |||||||||
| Cladophora coelothrix | 2 | Kützing 1843 | 1 | 5 | ||||||||
| Blue-green algae | 1 | 1 | 15 | |||||||||
| Other green algae undet. sp. | 5 | |||||||||||
| Filamentous algae undet. sp. | 2 | 1 | ||||||||||
| Ochrophyta | ||||||||||||
| Petalonia fascia | 5 | (O.F. Müller) Kuntze 1898 | 8 | 1 | 4 | |||||||
| Brown micro-algae | 1 | 4 | 7 | |||||||||
| Rhodophyta | ||||||||||||
| Hildenbrandia sp. | 7 | Nardo 1834 | 5 | 5 | ||||||||
| Ahnfeltia duvillei var. implicata | 4 | (Kützing) M. Howe, 1914 | 9 | 19 | 1 | |||||||
| Ceramium rubrum | 2.5 | C. Agardh 1811 | 1 | |||||||||
| Chondracanthus glomeratus | 3.5 | (Howe) Guiry 1993 | 17 | 20 | 46 | |||||||
| Filamentous red algae undet. sp. | 2.5 | 9 | 3 | |||||||||
| Gigartina sp. | 4 | Stackhouse 1809 | 3 | |||||||||
| Lithothamnion sp. | 7 | Heydrich 1897 | 34 | 20 | 2 | |||||||
| Corticated red algae undet. sp. | 3.5 | 1 | ||||||||||
| Corallina officinalis | 6 | Linnaeus 1758 | 5 | |||||||||
| Iridaea tuberculosa | 4 | (J.D. Hooker & Harvey) Grunow 1886 | 16 | |||||||||
| Chondracanthus chammissoi | 3.5 | (C. Agardh) Kützing 1843 | 2 | |||||||||
| Red algae undet. sp. | 14 | |||||||||||
| Nitophyllum sp. | 3.5 | Greville 1830 | 1 | |||||||||
| Cnidaria | ||||||||||||
| Phymactis clematis | Hexacoralia | (Drayton in Dana 1846) | 87 | 70 | ||||||||
| Anemone undet. sp. | Hexacoralia | 13 | 2 | |||||||||
| Nematoda | ||||||||||||
| Nematode undet. sp. | Nematoda | 3 | 4 | 3 | 1 | 3 | ||||||
| Platyhelminthes | ||||||||||||
| Polyclad undet. sp. | Polycladia | 11 | 10 | 2 | 2 | 2 | 5 | |||||
| Nemertea | ||||||||||||
| Nemertean undet. sp. | Nemertea | 1 | 3 | 1 | 24 | 27 | ||||||
| Annelida | ||||||||||||
| Phyllodocidae | Errantia | Örsted 1843 | 2 | 8 | ||||||||
| Glycera sp. | Errantia | Haswell 1879 | 6 | 1 | 3 | 2 | 4 | 1 | ||||
| Nereis sp. | Errantia | Linnaeus 1758 | 4 | 3 | 1 | |||||||
| Perinereis sp. | Errantia | Kinberg 1865 | 15 | 8 | 17 | 50 | 4 | 7 | ||||
| Polynoidae | Errantia | Kinberg 1856 | 1 | 3 | 2 | |||||||
| Serpulidae | Sedentaria | Rafinesque 1815 | 1 | |||||||||
| Syllidae | Errantia | Grube 1850 | 3 | 21 | 1 | |||||||
| Oweniidae | Polychaeta | 2 | 1 | |||||||||
| Spionidae | Sedentaria | Grube 1850 | 9 | 6 | 15 | 29 | ||||||
| Hesionidae | Errantia | Grube 1850 | 1 | |||||||||
| Mollusca | ||||||||||||
| Argopecten sp. | Autobranchia | Monterosato 1889 | 1 | |||||||||
| Brachidontes sp.autobranchia | Autobranchia | Swainson 1840 | x | x | ||||||||
| Echinolittorina paytensis | Caenogastropoda | Phillipi 1847 | 6 | 4 | 11 | 16 | 2 | |||||
| Siphonaria lessoni | Heterobranchia | Blainville 1827 | 1 | 3 | 1 | 2 | ||||||
| Lottia orbignyi | Patellogastropoda | Dall 1909 | 4 | 34 | 18 | 6 | ||||||
| Echinolittorina peruviana | Caenogastropoda | Lamarck 1822 | 1 | 4 | 13 | 23 | 29 | 1 | 57 | 45 | ||
| Perumytilus purpuratus | Autobranchia | Lamarck 1819 | x | x | x | x | x | x | x | x | ||
| Scurria viridula | Patellogastropoda | Lamarck 1819 | 7 | 62 | 20 | 5 | 4 | 56 | 114 | 4 | ||
| Semimytilus algosus | Autobranchia | Gould 1850 | 20 | 13 | 20 | 1 | 2 | |||||
| Stramonita haemostoma | Caenogastropoda | Linnaeus 1767 | 15 | 3 | ||||||||
| Gastropod undet. sp. | 1 | 2 | ||||||||||
| Chiton granosus | Neoloricata | Frembly 1827 | 14 | 4 | 9 | 2 | 2 | |||||
| Fissurella sp. | Vetigastropoda | Brugiére 1789 | 2 | |||||||||
| Tegula atra | Vetigastropoda | Lesson 1830 | 1 | 2 | 2 | |||||||
| Prisogaster niger | Vetigastropoda | 1 | 1 | 5 | ||||||||
| Mussel undet. sp. | Autobranchia | 3 | 25 | |||||||||
| Chiton undet. sp. | Neoloricata | 1 | ||||||||||
| Incatella cingulata | Caenotgastropoda | G.B. Sowerby I 1825 | 2 | |||||||||
| Scurria ceciliana | Patellogastropoda | c'Orbigny 1841 | 3 | |||||||||
| Scurria parasitica | Patellogastropoda | c'Orbigny 1841 | 4 | |||||||||
| Arthropoda | ||||||||||||
| Grapsidae | Eumalocostraca | 1 | 6 | |||||||||
| Cancridae | Eumalocostraca | Latreille 1802 | 1 | 1 | 1 | |||||||
| Isopod | Eumalocostraca | Latreille 1817 | 1 | 2 | 8 | 6 | 1 | |||||
| Decapod undet. sp. | 1 | |||||||||||
| Decapod (megalops phase) | 1 | |||||||||||
| Amphipod | 3 | 1 | ||||||||||
| Balanus trigonus | Cirripedia | Darwin 1854 | 1 | 7 | ||||||||
| Chthamalus cirratus | Cirripedia | Darwin 1854 | 90 | x | 24 | 24 | 19 | 20 | 24 | |||
| Barnacle undet. sp. | Cirripedia | 7 | 11 | |||||||||
| Ostracod | Ostracoda | Latreille 1802 | 1 | |||||||||
| Porcellanidae | Eumalocostraca | Haworth 1825 | 4 | |||||||||
| Notochthamalus scabrosus | Cirripedia | Darwin 1854 | 1 | 18 | 47 | 1 | 1 | |||||
| Bryozoa | ||||||||||||
| Orange bryozoan undet. sp. | 1 | |||||||||||
| Porifera | ||||||||||||
| Encrusting sponge undet. sp. | 3 | 1 | ||||||||||
Plots of the means for the three measures of diversity computed from randomized resampling of the species and functional group data are shown in Figure 2a–c. Overall, the lowest mean indices were found for the sites sampled within the GUAY ecoregion. When diversity was measured by the proportion of species and functional groups (the Shannon–Wiener Index), the computed indices for communities sampled from the NAPF, HWS, and HNS ecoregions were all within one unit of index, with the highest Shannon–Wiener diversity found for sites within the HWS ecoregion. The probability of encountering similar species and functional groups across samples (the Simpson's Inverse Index) was approximately 1 ½ to 3 times higher in the HWS ecoregion, indicating that species richness and evenness are characteristic of the mussel communities sampled there (Table 3). Mean functional group diversity indices across all measures tended to be lower overall compared to mean species diversity indices, however, the order of ecoregional diversity from lowest to highest remained the same between the two groups. Mean Fisher's alpha diversity, which is sensitive to rare species, was highest for the pooled NAPF sites while Shannon–Wiener and Simpson's inverse was highest for the pooled HWS sites; these rankings may indicate that rare or unique functional groups are an underlying feature of mussel communities at the NAPF sites (Hubbell, 2015), while the higher Shannon–Wiener value for the HWS sites may suggest that functional group richness and evenness is higher in mussel communities there (Strong, 2016). Here, we consider Fisher's α to be useful in this study for approximating diversity at sites that may not have been sampled to completeness given the finite boundaries of the sampling design.

| Method | Species | Functional groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Bootstrapped SD | Lower 95% CI | Upper 95% CI | Mean | Bootstrapped SD | Lower 95% CI | Upper 95% CI | |
| GUAY | ||||||||
| Shannon–Wiener | 1.64 | 0.01 | 1.38 | 0.01 | ||||
| Simpsons Inverse | 4.18 | 0.01 | 3.28 | 0.01 | ||||
| Fishers' alpha | 2.35 | 0.28 | 1.71 | 0.22 | ||||
| S | 13.00 | 2.02 | 9.03 | 16.97 | 10 | 1.29 | 7.47 | 12.53 |
| SChao1 | 18.99 | 7.17 | 13.94 | 51.05 | 10.5 | 1.3 | 10.03 | 18.25 |
| Singletons | 3.98 | 0.14 | 1.99 | 0.1 | ||||
| Doubletons | 0.01 | 0.10 | 0.99 | 0.1 | ||||
| HWS | ||||||||
| Shannon–Wiener | 2.87 | 0.01 | 2.29 | 0.01 | ||||
| Simpsons Inverse | 12.21 | 0.01 | 7.15 | 0.01 | ||||
| Fishers' alpha | 10.41 | 0.70 | 3.77 | 0.33 | ||||
| S | 50.00 | 3.31 | 43.50 | 56.50 | 22 | 0.5 | 21.03 | 22.97 |
| SChao1 | 61.13 | 8.23 | 53.05 | 90.60 | 22 | 0.17 | 22.43 | 23.1 |
| Singletons | 13.02 | 0.14 | 1.02 | 0.14 | ||||
| Doubletons | 5.98 | 0.14 | 2 | 0.1 | ||||
| HNS | ||||||||
| Shannon–Wiener | 2.58 | 0.01 | 2.07 | 0.01 | ||||
| Simpsons Inverse | 7.00 | 0.01 | 5.45 | 0.01 | ||||
| Fishers' alpha | 10.35 | 0.66 | 3.46 | 0.5 | ||||
| S | 52.00 | 3.36 | 45.42 | 58.58 | 21 | 0.98 | 19.09 | 22.91 |
| SChao1 | 62.99 | 8.47 | 54.88 | 93.92 | 21.33 | 0.93 | 21.02 | 26.96 |
| Singletons | 12.00 | 0.14 | 2 | 0.14 | ||||
| Doubletons | 5.00 | 0.14 | 1.99 | 0.1 | ||||
| NAPF | ||||||||
| Shannon–Wiener | 2.71 | 0.01 | 2.16 | 0.01 | ||||
| Simpsons Inverse | 7.79 | 0.01 | 6.04 | 0.01 | ||||
| Fishers' alpha | 13.94 | 0.74 | 3.33 | 0.27 | ||||
| S | 72.00 | 2.82 | 66.48 | 77.52 | 22 | 0.98 | 20.08 | 23.92 |
| SChao1 | 79.58 | 5.45 | 74.14 | 98.85 | 23 | 2.31 | 22.07 | 36.32 |
| Singletons | 14.02 | 0.14 | 1.99 | 0.1 | ||||
| Doubletons | 10.99 | 0.17 | 0.01 | 0.1 | ||||
- Note: Mean index values with ±95% confidence intervals for the two species richness measures (Sest and Schao1) along with mean values for singletons and doubletons were calculated using randomized re-sampling with rarefaction in EstimateS v. 9.1 software.
Species accumulation curves for richness SSp, SFx, SSpChao1, and SFxChao1 estimators are shown in Figure 3a–d. Rarefaction compensates for varying levels of sampling frequency and was employed for both of the species richness estimators used in this study. The re-sampling with replacement design of the SChao1 estimator resulted in higher mean species richness across all ecoregions compared to mean species richness (S), although the SChao1 curve demonstrated a tendency for overestimation of the upper confidence intervals (Chao, 1984). The higher SChao1 values suggested that to a varying degree, rare and unique species were a characteristic among all ecoregions. The SSP and SFX accumulation curves were similar, respectively, in terms of highest to lowest mean values among the ecoregions, with the SSP accumulation curve for GUAY nearing an asymptote at n = 500 and the SFx increasing past n = 500. The species richness (SSp) and functional richness (SFX) curves for the HWS and HNS ecoregions were similar in terms of the richness values and curvilinear feature; Ssp continued to increase past n = 1000 while the curves representative of the HWS, HNS, and NAPF ecoregions for SFX reached asymptote ~n = 1000.
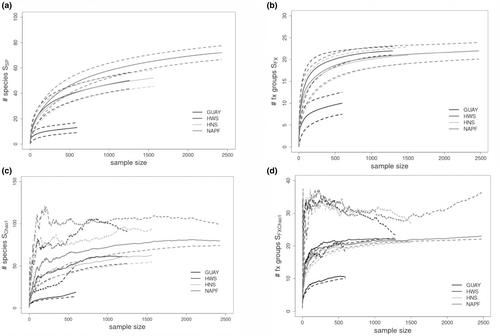
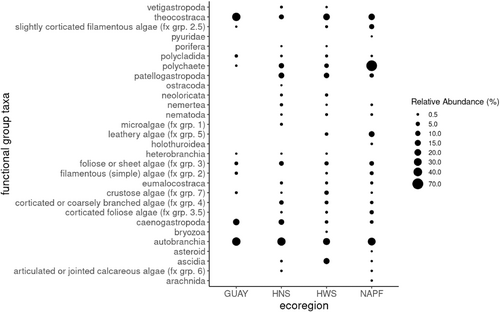
The highest mean values for singletons (species with only one individual in the sample) and doubletons (species with only two individuals in the sample) were calculated for mussel communities sampled at sites within the NAPF ecoregion, while the mussel communities sampled at sites within the HWS ecoregion had the second highest mean singletons (Table 2). Unique and rarely encountered species and functional group taxa at sites sampled in the NAPF ecoregion included the plate limpet Lottia scutum, the black and white amphipod Chromopleustes oculatus, the polychaete bloodworm Glyera spp., the plumose anemone Metridium senile, arachnids, corticated macrophytes (fx grp. 5), nemerteans, flatworms, and pyurids; in the HWS ecoregion, the red algae Ceramium rubrum and Gelidium crispum were rare and unique. In the GUAY ecoregion, rare species and fx group taxa included Siphonaria sp. (Dayrat et al., 2014; Güller et al., 2016), two Phyllodocid polychaetes color morphs, red filamentous algae (fx grp. 2.5), heterobranchs, and polychaetes; in the HNS ecoregion, the red alga Chondracanthus chammissoi, an Owenid polychaete, an encrusting sponge, green filamentous algae (fx grp. 2), crustose algae (fx group 7), nematodes, nemerteans, and vetigastropods were rarely encountered; and in the HWS ecoregion, the red algae Ceramium rubrum and Gelidium crispum were rare. In terms of the abundance of herbivorous functional groups in the mussel assemblages, Caenogastropods were abundant in the GUAY ecoregional sites, while caenogastropods, and patellogastropods were equally abundant in the HNS ecoregional sites. Patellogastropods were the most abundant grazers at sites in the HWS ecoregion, while grazers were relatively less abundant at the sites in the NAPF ecoregion (Figure 4).
3.2 Multivariate analysis of beta diversity
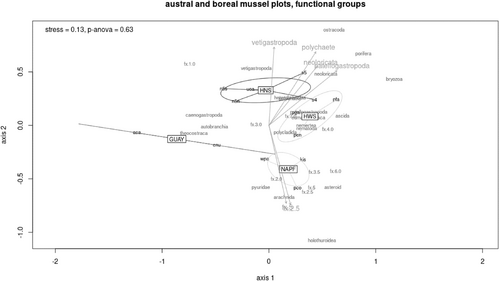
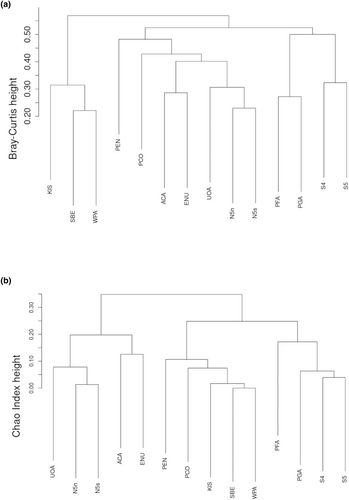
The mussel Brachidontes sp. and the periwinkle Echinolittorina paytensis were unique to the GUAY ecoregion, explaining the main dissimilarity among the two sites sampled within that ecoregion and sites within the HWS and HNS ecoregions. Red algae, amphipods, and the limpet Scurria were the species that distinguished the sites within the HWS ecoregion, and the spionid worm (proposed Proboscidia wellingtonensis) was a species characteristic of the sites only within the HNS ecoregion. Members of the vetigastropods (turban snails), neoloricata (chitons), and patellogastropoda (limpets), as well as polychaetes were significant functional taxa that differentiated the mussel assemblages within the HNS ecoregion, while functional algal groups 5 (leathery macrophytes) and 2.5 (filamentous red algae) were significant functional taxa that differentiated the mussel assemblages within the NAPF ecoregion. The raw data from all mussel plots were scaled for one nMDS ordination at the functional group level (Figure 5). The general rule for goodness-of-fit between observed and fitted distances and interpreting similarities among communities in an nMDS ordination is to achieve a stress value <0.20 (Rabinowitz, 1975), and in this case, we are confident that the ordination was a fair representation of similarity among sites (stress = 0.13, k = 2, p-ANOVA = 0.63). Although there was no overlap among the HNS, HWS, and NAPF clusters, the closeness of the ovoids drawn by the ordination suggests that although the ecoregions are distinct, functional group similarity exists between the HWS and HNS, and to a lesser extent the NAPF ecoregion. Interestingly, site S4 at PSJ shared more similarity with site PFA within the HWS ecoregion than it did within its own HNS cohort. The number of sites sampled within the GUAY ecoregion was insufficient to form a cluster but were included (demonstrated by a one-dimensional line in Figure 5); ACA and ENU were the least similar of all sites within an ecoregional cohort.
Functional algal groups have been ranked in terms of “grazing resistance,” with the presence of “structurally tougher” groups linked to increased functional group complexity (Steneck & Watling, 1982). Of the invertebrate functional groups sampled in this study, the caenogastropods (which includes littorinids and dovesnails) were the smallest of the herbivorous grazers in terms of general body size, followed by the patellogastropods (limpets), vetigastropods, (keyhole limpets and turban snails), and neoloricata (chitons). Errant polychaetes, patellogastropods (Scurria spp.), vetigastropods (Fissurella spp., Tegula atra, and Prisogaster niger), and the neoloricata (chitons) were functional groups that were well represented in the mussel communities in the HNS, although errant polychaetes and patellogastropods were also represented to a lesser degree in the HWS. Foliose, crustose, and corticated macrophytes were all well represented in mussel communities within the HWS ecoregion compared to communities within the HNS ecoregion, and in general, mature sporophytes were a common feature in mussel communities within the HWS ecoregion while algal sporelings or small filamentous structures were typical within the HNS ecoregion.
The cluster groups in the dendrograms were similar among all sites from all ecoregions at the functional group level of organization, although dissimilarity was greater among clades for the Bray–Curtis dendrogram compared to the Chao–Jaccard dendrogram. For example, the Chao–Jaccard method revealed that dissimilarity was highest at ~0.35, while the maximum dissimilarity for the Bray–Curtis method was ≤0.55 (Figure 6a,b). Only in the NAPF ecoregion were all eight of the functional algal groups found, which would explain the highest estimated functional richness SFX and SFXChao1 index values. Functional group taxa that distinguished this region included leathery macrophytes (fx grp. 5) and filamentous red algae (fx grp. 2.5). One of the most abundant and ubiquitous species belonging to algal fx. group 5 was Fucus gardneri, the primary cover forming alga on mussel assemblages throughout the Pacific Northwest. F. gardneri is a perennial brown seaweed that thrives in temperate, moist environments with a high level of resistance to grazing by herbivores and may completely dominate the mid intertidal zone, which is indicative of its low disturbance potential in the ecosystem (De Vogelaere & Foster, 1994; Steneck & Dethier, 1994). Of the algae representing functional group 2.5, Pterosiphonia bipinnata was the most abundant at sites within the NAPF and was significant in the nMDS analysis for differentiating this region at the functional group scale. P. bipinnata is the least resistant to grazing by Littorina spp. (Steneck & Watling, 1982) and standing stock of this alga were associated with caenogastropod and amphipod groups at most of the sites, which underscores its importance in the food web at the functional group-scale (Steneck & Dethier, 1994).
3.3 The effect of wave exposure and substrate angle on mussel matrices
In all cases, the models that combined site and ecoregion as nested random effects provided the best AIC scores for explaining variation due to random effects. Shell length and matrix depth were found to be significantly smaller in wave-sheltered locations, while much of the variation in mussel shell length, matrix depth, and stratum index was explained by the combination of wave exposure and bench substrate (L [β = 22.41, Pr (>|t|) < .001], MD [β = 26.87, Pr (>|t|) < .001] and SI [β = 2.70, Pr(>|t|) = .02]), with both fixed and random effects (ecoregion and site, R2C (L) = 0.82, R2C (M) = 0.45 R2C (SI) = 0.71) explaining up to 90 times the variance in the models than the variance that was explained by the fixed effects alone (R2M (L) = 0.08, R2M (M) = 0.04 R2M (SI) = 0.14). The standard errors in the models for stratum index were lowest overall, which suggests that stratum index is the best predictor of complexity in mussel matrices (Table 4). The characteristically mono-layered mussel matrices (Guiñez & Castilla, 1999) sampled at Playa Acapulco were likely responsible for the higher standard errors in the models for shell length and matrix depth (the assemblages of Brachidontes sp. sampled at Playa Acapulco) were consistently mono-layered across all plots sampled, and the smaller shell sizes may be due to the age-class of the mussels (Torroglosa & Giménez, 2018). In other words, median shell lengths of the Brachidontes sp. assemblages at Acapulco were equal to the matrix depths of the assemblages, which skewed the data when fit with the shell length data from the other ecoregional groups. Although shell length is used in the formula to calculate stratum index, the calculation's formula includes measuring the area of substrate occupied by the mussel matrix as well as calculating a coefficient based on mussel size (Hosomi, 1985). The formula for the calculation of the stratum index of mussel matrices provides a standardized the dataset and in this case, the distribution of the residuals was relatively homogeneous with most of the variation in the model explained by the random effects (sites within ecoregional groups).
| Fixed effects | Shell length (L) | Matrix depth (M) | Stratum index (SI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Pr (>|t|) | R2M, R2C | F, Pr (>|f|) | Estimate | SE | Pr (>|t|) | R2M, R2C | F, Pr (>|f|) | Estimate | SE | Pr (>|t|) | R2M, R2C | F, Pr (>|f|) | |
| Exposed | 22.41 | 3.92 | <0.001 | 0.10, 0.84 | 44.62, <0.001 | 26.87 | 5.33 | <0.001 | 0.03,0.45 | 5.40, 0.03 | 2.70 | 0.54 | 0.02 | 0.004,0.65 | 0.26, 0.63 |
| Sheltered | −7.28 | 1.09 | <0.001 | −7.36 | 3.17 | 0.02 | −0.19 | 0.38 | 0.63 | ||||||
| Bench | 19.02 | 4.56 | <0.001 | 0.004, 0.83 | 1.23, 0.27 | 24.38 | 5.99 | <0.001 | 0.001, 0.43 | 0.07, 0.80 | 2.45 | 0.59 | 0.01 | 0.014,0.65 | 0.65, 0.43 |
| Vertical | 1.24 | 1.12 | 0.27 | −0.87 | 3.36 | 0.80 | 0.34 | 0.42 | 0.43 | ||||||
| Exposed*bench | 20.91 | 3.89 | <0.001 | 0.08, 0.82 | 15.83, < 0.001 | 26.91 | 5.38 | <0.001 | 0.04, 0.45 | 0.07 | 2.73 | 0.43 | < 0.001 | 0.14, 0.71 | 2.57, 0.09 |
| Exposed*vertical | 2.01 | 1.31 | 0.13 | 1.24 | 4.24 | 0.77 | 0.07 | 1.01 | 0.61 | 0.11 | |||||
| Sheltered*bench | 1.93 | 5.13 | 0.71 | 2.11 | 9.91 | 0.83 | 0.59 | −0.11 | 1.25 | 0.11 | |||||
| Sheltered *vertical | −5.46 | 1.64 | 0.001 | −8.43 | 4.85 | 0.08 | 0.07 | −0.63 | 0.66 | 0.35 | |||||
- Note: Marginal coefficients of determination for fixed effects (R2M) and conditional coefficient of determination for fixed and random effects (R2C) were calculated for each model. F-statistics from analysis of variance with significance at Pr(|f|) < .05.
3.4 Richness (IMn) and evenness (J) versus the nested variables
Differences in Menhinick's species richness (IMn) and Pielou's evenness (J) were tested with the Wilcoxen rank-sum test (Table 5) using the pooled data from all ecoregions and the nested explanatory variables mono-layered matrices with cover versus mono-layered matrices without cover, and multi-layered matrices with cover versus mono-layered matrices without cover (Table 5). Multi-layered matrices defined by stratum index and matrix depth were significantly higher for IMn, with the greatest variances in median values found between multi-layered canopied matrices and mono-layered matrices without cover (SI |M1 − M2| = 1.22, p < .001; MD |M1 − M2| = 1.09, p < .001) (Figure 7). Rankings for all but one of the pairwise comparisons (mono-layered canopied matrices versus multi-layered matrices without cover as defined by SI) were significant for differences in median IMn (p < .05) for matrices defined both by SI and MD. Post hoc analysis of variance was performed on pooled canopied matrices (n = 44) versus pooled matrices without cover (n = 42) for both stratum index and matrix depth; for a large effect size (d = 0.40, n = 86) the power of the analysis 1−β = 0.96 (Cohen, 1988). Mean Menhinick's richness (D) was 1.5 times greater for canopied matrices (F = 38.99, Pr(>F) < .001) compared to (D) in matrices without cover, regardless of the complexity of the matrix.
| Menhinick's species richness (IMn)-stratum index | Menhinick's species richness (IMm)-matrix depth | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |M1 − M2| | CI (2.5 pctl) | CI (98 pctl) | CI (bootstrapped 2.5 pctl) | CI (bootstrapped 98 pctl) | p-Value | |M1 – M2| | CI (2.5 pctl) | CI (98 pctl) | CI (bootstrapped 2.5 pctl) | CI (bootstrapped 98 pctl) | p-Value | |
| Multi-layer with canopy v. mono-layer with canopy | 0.35 | −0.08 | −0.47 | −0.02 | −0.44 | .02 | 0.29 | −0.1 | −0.46 | −0.16 | −0.42 | <.001 |
| Mono-layer with canopy v. mono-layer no canopy | 0.87 | 0.67 | 0.99 | 1.14 | 1.41 | <.001 | 0.8 | 0.39 | 0.97 | 0.67 | 1.03 | <.001 |
| Mono-layer with canopy v. multi-layer no canopy | 0.18 | 0.01 | 0.04 | −0.03 | 0.38 | .05 | 0.27 | 0.03 | 0.44 | 0.06 | 0.46 | .03 |
| Multi-layer with canopy v. mono-layer without canopy | 1.22 | −1.3 | −0.97 | −1.04 | −1.36 | <.001 | 1.09 | −1.24 | −0.69 | 0.06 | 0.46 | <.001 |
| Multi-layer with canopy v. multi-layer no canopy | 0.53 | 0.31 | 0.65 | 0.27 | 0.61 | <.001 | 0.56 | 0.28 | 0.7 | 0.39 | 0.71 | <.001 |
| Mono-layer without canopy v. multi-layer without canopy | 0.69 | −4.3 | −0.85 | −0.45 | −0.87 | <.001 | 0.53 | −0.69 | −0.22 | −0.82 | −0.09 | .01 |
| Pielou's evennes (J) stratum index | Pielou's evenness (J) matrix depth | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |M1 − M2| | CI (2.5 pctl) | CI (98 pctl) | CI (bootstrapped 2.5 pctl) | CI (bootstrapped 98 pctl) | p-Value | |M1 − M2| | CI (2.5 pctl) | CI (98 pctl) | CI (bootstrapped 2.5 pctl) | CI (bootstrapped 98 pctl) | p-Value | |
| Multi-layer with canopy v. mono-layer with canopy | 0.04 | −0.08 | 0.04 | −0.09 | 0.05 | .45 | 0.41 | −0.05 | 0.06 | −0.07 | 0.07 | .97 |
| Mono-layer with canopy v. mono-layer without canopy | 0.08 | −0.13 | 8.63e−5 | −0.13 | 0.04 | .06 | 0 | −0.08 | 0.03 | −0.1 | 0.05 | .38 |
| Mono-layer with canopy v. multi-layer without canopy | 0.45 | −0.05 | 0.07 | −0.06 | 0.08 | 0.59 | 0.06 | −0.02 | 0.10 | −0.02 | 0.12 | .15 |
| Multi-layer with canopy v. mono-layer without canopy | 0.04 | −0.02 | 0.09 | −0.04 | 0.08 | .28 | 0.04 | −0.02 | 0.08 | −0.02 | 0.08 | .29 |
| Multi-layer with canopy v. multi-layer without canopy | 0.59 | 4.09e−5 | 7.0e−2 | −0.02 | 0.08 | .08 | 0.03 | −0.01 | 0.08 | −0.01 | 0.09 | .10 |
| Mono-layer without canopy v. multi-layer without canopy | 0.09 | 0.02 | 0.14 | 0 | 0.11 | .02 | 0.06 | 0.02 | 0.12 | 0.02 | 0.12 | .01 |
- Note: Confidence intervals from from the bootstrapped data (104 re-samples) for the test are within 2.5 and 98 percentiles. Absolute values of the difference in medians |M1 – M2 | and significance of test at p < .05.
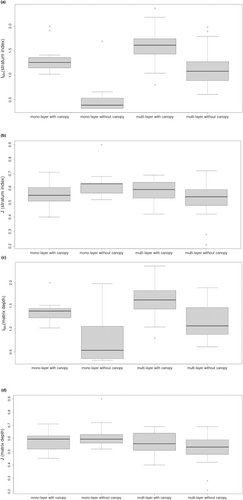
Variations in median Pielou's evenness J were generally much smaller than the variations found for Imn. Only one of the pairwise comparisons (mono-layered matrices versus mono-layered matrices without cover) was significant for differences in median J, although the variation was quite small (SI |M1 − M2| = 0.08, p = .04). There were no significant differences in the medians of Imn and J when the methods for defining mussel matrices SI and MD were compared according to each respective level of mussel layering and the presence or absence of an algal cover.
4 DISCUSSION
4.1 Mussel layers, algal cover, and variations in species richness and evenness
In this study, we demonstrate the value of macroalgal cover in driving patterns of macrofaunal diversity intertidal rocky systems, from the northern to the southern Pacific, specifically, algal cover and the interaction between wave-exposure and bench type substrate were the strongest factors shaping intertidal communities. The structure of mussel assemblages at wave-exposed sites showed more complexity than did mussel assemblages at wave-sheltered locations, algal cover was significantly associated with higher levels of species richness regardless of the complexity of the matrix, while canopied multi-layered matrices were highest in species richness and accounted for the highest variance in species richness index when compared to mono-layered matrices with no algal cover. The relationship between stratum index and species richness was stronger than the relationship between species richness and matrix depth, therefore we suggest that the stratum index of a mussel matrix is a better predictor of species richness. In general, mono-layered matrices had significantly lower indices of species evenness than did multi-layered matrices, in a very similar manner to Prado and Castilla (2006). Furthermore, there was no significant difference between the presence of an algal cover and the level of species evenness in the mussel assemblages sampled on the broad ecoregional scale that our samples were based on.
There is an underlying factor differentiating species richness and evenness between mussel communities in the HWS ecoregion and the HNS ecoregion. The removal of an apex predator has the potential to cause changes in trophic hierarchies (Castilla & Durán, 1985; Moreno et al., 1986), and the abundances of P. purpuratus at Playa Ensenada and Playa Farallones may have been linked to the unregulated harvesting of mussel predators that was observed during each field season. The asteroid Heliaster helianthus was present to some degree at UOA and was abundant at PSJ in the HNS ecoregion, was observed foraging on stands of P. purpuratus, but was not seen at any of the sites within the HWS ecoregion (Wilbur, in review). Concholepas concholepas (locally known as “loco”), a slow-growing carnivorous muricid snail ranging from 5 to 7 cm in length, was also seen foraging on mussels in the high to mid-intertidal zone at PSJ. The objective when selecting our study sites was to sample from areas of limited or low human impact, however, there was some level of human activity at all of the sites and PSJ was no exception. The waters surrounding PSJ have been historically subject to illegal fishing pressure (divers could be seen using hookah to fish for invertebrates only meters from shore) and thus the exists potential to drastically change species richness and trophic structures in the intertidal communities at PSJ (Harley & Rogers-Bennett, 2004; Kunze et al., 2021; Steneck et al., 2004). Nevertheless, PSJ provided a unique opportunity to survey mussel assemblages in a nearly pristine environment, and we suggest that the P. purpuratus assemblages at PSJ possess a trophic structure distinct from the P. purpuratus assemblages at the sites at UOA and within the HWS ecoregion.
4.2 Biodiversity patterns
Mussels from assemblages sampled throughout all ecoregional groups shared a similar characteristic of hosting sessile epibionts such as barnacles and algal sporelings on their shells. Of significance was the presence of species that were rare and unique in the quadrats such as the motile actinids Metridium senile in the NAPF ecoregion and Phymactis clematis in the HWS ecoregion. Species typically found as later-stage adults in the lower intertidal or subtidal zones may recruit in higher zones during larval or early life stages, which is an advantageous strategy in terms of benefiting from the filter-feeding strategy of the mussels as well as avoiding competition with conspecifics as well as other species (Caro et al., 2010; Dahlhoff & Menge, 1996).
Steneck et al. (2004) have suggested that fishing pressure can cause an effect of trophic cascades in marine environments, with species from one functional trophic group replacing species from an existing group. It has been established historically that the removal of a top predator has the potential to cause trophic level shifts and significant changes to the food web in localized ecosystems (Paine, 1966; Steneck et al., 2004; Whittaker, 1972). As mentioned previously, we observed an active artisanal fishing effort for “loco” during the surveys in the HWS ecoregion. This fishery required breath-hold divers in heavy surf to hand pick “loco” from rocky substrate. At sites within the HWS, we observed fishermen returning to the beach with large pouches full of “loco” and discarding the shucked shells in the supratidal zone. The only sites where living C. concholepas were counted during our surveys were at sites inside the marine protected reserve at PSJ where fishing of any kind is prohibited, but as mentioned previously, illegal fishing does occur on occasion. C. concholepas has a wide regional distribution and vertical distribution and because of its prolonged epineustonic phase, the dispersal potential for this species is considerable (Cárdenas et al., 2015; DiSalvo, 1988). There is strong evidence to suggest that C. concholepas plays a role as a keystone species in the southeast Pacific intertidal zone and while it is highly likely that a population of C. concholepas may be beyond reach of the fishermen in unregulated areas of the Peruvian coast.
The structural patterns for mussel communities at the algal and invertebrate functional group level in the warm temperate region of Peru and the cold temperate region of Alaska were very similar. This pattern has also been observed in the past in micro-biome communities associated with brown algae that show similar structure across different ecoregions (Capistrant-Fossa et al., 2021). Functional group turnover (beta diversity) was pronounced among ecoregional-groups, with significant overlap among most sites within each group. The greatest Bray–Curtis distance between two sites occurred between ACA and ENU in the GUAY ecoregion, likely due to the low number of species found in the mussel communities at ACA. The relatively low richness indices and the characteristically mono-layered matrices at ACA lend further evidence to our hypothesis that shell length can be used as a predictor of species richness in mussel communities (Wilbur et al., 2023).
4.3 The effect of wave exposure and substrate angle on mussel matrices
Prado and Castilla (2006) found that matrix depth had a significant effect on species evenness in assemblages of P. purpuratus on a very localized scale, with stratified matrices having significantly lower species evenness. The results of this study are consistent with the results of local scale research done by Prado and Castilla (2006) in mussel assemblages at Punta de Tralca in Chile, and our findings show that algal cover and mussel stratification can be used to predict species diversity and evenness in macro-scale communities associated with mussel assemblages throughout several marine ecoregions. Additionally, our results in relation to the effect of wave exposure on the homogeneity of mussel stratification are supported by Dahlhoff and Menge's (1996) findings that mussel physiology and growth are variable according to distinct environmental factors that drive food availability, as this too was consistent across mussel species on a broad regional scale. In summary, this study constitutes the first effort made to assess species diversity, compare functional group diversity, and measure the effects of biological and environmental factors on mussel assemblages and associated macro-communities using regionally distinct mussel species across a broad eco-regional scale. This is also the first effort to research macro-invertebrate and algal communities at previously unresearched areas of the Peruvian and Alaskan coasts, including a protected marine area in the south of Peru.
ACKNOWLEDGMENTS
Appreciations are due to Susana Cárdenas Alayza at the Center for Environmental Sustainability of Universidad de Cayetano Heredia in Lima Peru and the staff members of the Reserva Punta San Juan in Marcona Peru for their assistance with permitting and fieldwork, to Bruno Ibanez-Erquiaga at the Laboratorio de Ciencias del Mar, Universidad de Cayetano Heredia for his invaluable assistance with field guides and logistics, to the Ministero de la Producción de Peru for the permit to collect marine specimens, the Servicio Nacional de Áreas Naturales for permission to work in the Reserve, Shaleyla Kalez of EcoOceanica for her assistance with logistics in the northernmost ecoregion of Peru, and Prof. Aldo Pacheco from the Universidad de Cayetano for his assistance with logistics and species identification in Antofagasta Chile. This study was partially supported by the University of Aberdeen, School of Biological Sciences. We would also like to thank the MASTS pooling initiative and contributing institutions. The authors report no conflict of interest. Data is available upon request.
FUNDING INFORMATION
The Marine Alliance for Science and Technology for Scotland). MASTS is funded by the Scottish Funding Council (grant reference HR09011).
Open Research
DATA AVAILABILITY STATEMENT
Data available upon request.




