Zooplankton community and copepod carcasses and non-predatory mortality in six tropical estuarine systems (Northeast of Brazil)
Abstract
Copepod carcasses and non-predatory mortality can occur due to natural senescence, disease, pollution, and physicochemical stress. Our objective is to evaluate and characterize the rates of non-predatory mortality and the contribution of zooplankton community carcasses, represented by the copepods, in tropical estuarine ecosystems with different degrees of urbanization. During the study, there was a significant difference between environments and copepod carcasses, with Suape Bay (moderately urbanized) being the environment with the highest abundance of adult copepod carcasses (93.3%). The average non-predatory mortality rate of adult copepods was 0.15 day−1: Santa Cruz Channel (sparsely urbanized) contributed the lowest values, with a mortality rate of 0.01 day−1, and Suape (moderately urbanized) had the highest rate (2.80 day−1). The families Paracalanidae (0.554 day−1) and Oithonidae (0.122 day−1) had the highest values, with an average carcass decomposition of 4.7 days. Among the environments studied, there was little differentiation between carcass percentages and mortality rate, not supporting the hypothesis that the higher rates of mortality among non-predatory copepods were related to large urban centers. However, there was an almost proportional contribution of carcasses and non-predatory mortality in all but a few areas, agreeing with the hypothesis that there is spatial variation with respect to carcasses in tropical estuaries. With our work, we show that estuaries can provide the estuarine food web with a significant portion of copepod carcasses, which may vary by family, and that it is not necessarily environments with a higher degree of urbanization that will have higher mortality rates.
1 INTRODUCTION
The estuarine system is considered more productive than oceanic and continental waters, due to unique hydrodynamic characteristics, retention of nutrients, phytoplankton, algae plants, and detritus (Elliot & McLusky, 2002). The high input of inorganic nutrients and organic matter from terrestrial origin makes estuaries highly productive, and they are considered a natural breeding habitat for several animals (Attrill, 2002; Ketchum, 1983; Miranda et al., 2002). Some natural and geological features are attractive to the human population near these areas. Population growth and anthropic activities around these environments, such as the creation of harbors or the extraction of organisms for fishing, can modify the aquatic environment and create stressful conditions that cause biodiversity loss and affect ecosystem functioning and biological cycles, such as those of zooplankton organisms (Attrill, 2002; Karydis & Kitsiou, 2013; Nascimento et al., 2020).
Mortality is a natural process for all living things, which can affect everything from the population dynamics of one species to even the structure of a whole community, regardless of whether it is in aquatic or terrestrial ecosystems (Di Capua & Mazzocchi, 2017). Non-predatory factors are related to an estimated 25%–33% of marine copepod mortality (Hirst & Kiørboe, 2002). Ignoring this type of mortality can lead researchers to incorrect interpretations of the population dynamics of these groups (Diniz et al., 2021; Silva et al., 2020; Tang & Elliott, 2014). The causes of non-predatory mortality can be highly variable, and the most common are senescence, feeding, physicochemical stress and even marine pollution (Pavlova & Melnikova, 2006a, 2006b, 2011; Tang et al., 2014). This last factor is usually associated with urban estuaries, and, together with other anthropic impacts and the sensitivity of copepods, makes the group more susceptible to a higher mortality rate.
Carbon- and nutrient-rich carcasses from non-predatory mortality can be consumed in the water column or sink and decompose, contributing to nutrient regeneration, vertical flow of particulate matter and carbon flux, and also as a source of nitrogen and phosphorus for benthic primary producers, even more so than fecal pellets (Frangoulis et al., 2011; Gentleman & Head, 2017; Tang & Elliott, 2014). Data on non-predatory mortality can provide information on the function of copepods in structuring detritivorous pelagic webs and help in understanding how different environmental conditions influence population development (Carlotti et al., 2000; Martinez et al., 2013; Tang & Elliott, 2014). Understanding how factors that influence spatial distribution and variations in the proportion of the non-predatory mortality of organisms in the environment can provide information about trophic dynamics, considering herbivorous and detritivorous food webs, especially in dynamic environments such as the estuarine systems (Martinez et al., 2013; Tang & Elliott, 2014). This study aimed to evaluate and characterize the non-predatory mortality rates and the contribution of planktonic copepod carcasses in tropical estuarine ecosystems, with different degrees of urbanization, considering the following two main hypotheses: (i) estuarine ecosystems in tropical regions contribute different percentages of zooplankton carcasses, varying spatially; (ii) estuaries inserted in large urban centers present higher rates of non-predatory mortality in planktonic copepods, assuming higher rates of non-predatory mortality in a higher degree of urbanization.
2 MATERIALS AND METHODS
2.1 Study areas
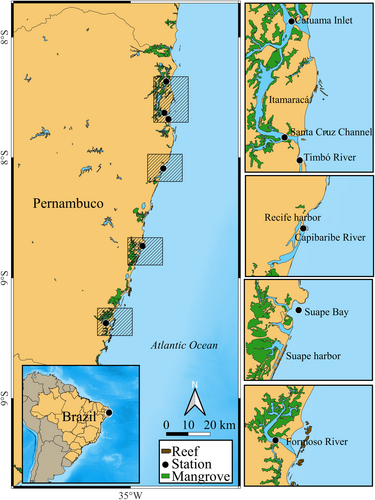
The study was carried out in six estuarine ecosystems located on the east coast of Northeastern Brazil, with different degrees of urbanization: estuaries of the Capibaribe River, Formoso River, Timbó River, Santa Cruz Channel, Suape Bay and Catuama Inlet (Figure 1, Table 1). All those have different geological features, availability and coverage of mangrove vegetation, as well as different anthropogenic impacts (Costa et al., 2015; Farrapeira et al., 2009; Vila Nova & Torres, 2012). These six estuarine environments were categorized into three impact categories (low urban, moderately urbanized, and strongly urbanized) from visual interpretation obtained during field visits, existing data, and by means of environmental indicators such as mangrove filling, sewage discharge, percentage of vegetation and urbanization, population abundance, economic activities, dissolved oxygen, solid waste, trophic state index of the basin in which the estuary lies, chlorophyll-a, diversity and abundance of zooplankton and cyanobacteria. For each indicator, a score was assigned, where 1 (low) was used when it was not very aggressive to the environment, 3 (moderate) when the impact was in specific places, and 5 (extreme) when there was a drastic influence (Table 1). In the end, summing up the scores, environments with 1–28 points were considered low urban (Timbó River, Catuama Inlet, Santa Cruz Channel), moderately urbanized (Suape Bay, Formoso River) from 29 to 39 points, and strongly urbanized (Capibaribe River), from 40 to 50 points (Tommasi, 1994; and author's adaptations).
| Timbó River | Capibaribe River | Formoso River | Suape Bay | Catuama inlet | Santa Cruz Channel | |
|---|---|---|---|---|---|---|
| Economic activity | 3 | 5 | 1 | 3 | 5 | 3 |
| Degree of urbanization | 1 | 5 | 3 | 3 | 1 | 3 |
| Degree of vegetation | 3 | 5 | 1 | 5 | 3 | 3 |
| Landfill of the mangrove | 3 | 1 | 5 | 1 | 5 | 5 |
| Solid residues | 1 | 5 | 1 | 1 | 1 | 1 |
| Effluent discharge | 3 | 5 | 3 | 5 | 3 | 3 |
| Population density | 3 | 5 | 3 | 5 | 3 | 3 |
| Trophic state indexa | 3 | 5 | 1 | 5 | 1 | 3 |
| Dissolved oxygen | 3 | 5 | 5 | 1 | 1 | 3 |
| Chlorophyll-a | 5 | 5 | 3 | 3 | 3 | 1 |
| Zooplankton density | 1 | 1 | 5 | 3 | 1 | 1 |
| Cyanobacteria densitya | 1 | 3 | 3 | 2 | 1 | 1 |
| Zooplankton diversity | 1 | 3 | 3 | 2 | 1 | 1 |
| Total | 28 | 48 | 36 | 34 | 28 | 28 |
- a In the absence of data on the indicator in estuaries, we use values from the river basins where they are located or from the same region. References: Figueiredo et al. (2006); Noronha et al. (2011); Maciel & Querino (2020); Bezerra et al. (2018); CPRH (2001); Vila Nova & Torres (2012); Barcellos et al. (2018); Steiner et al. (2015); APAC (2021).
2.2 Data collection
2.2.1 Environmental variables
The hydrological variables (temperature, pH, dissolved oxygen, total solids, turbidity, and salinity) were measured using a Horiba U-52 probe at each sampling point in each estuary. For chlorophyll-a determination, water samples (1 L) were collected at the surface of all points, then were filtered (between 100 and 400 mL) in a glass fiber filter GF52/C of 0.45 μm, with the aid of a vacuum pump, and stored in the freezer. The extraction of the chlorophyll-ɑ was carried out in a 90% acetone solution for 18 h at 4°C. The analysis was performed using a spectrophotometer, following Parson et al. (1984).
2.2.2 Plankton sampling
Two sample cruises were carried out for each estuarine environment. The campaigns were carried out between November 2020 and September 2021. A total of 36 samples were collected, six for each station. Among 36 samples, 18 samples were fixed for the qualitative-quantitative analysis, and the other 18 were stained with neutral red for the non-predatory mortality study. The samples were collected during the daytime (between 9:00 a.m. and 12:00 p.m.) and low tide of syzygy. Zooplankton samples were obtained through water filtration by simultaneous horizontal towing of two 64 μm mesh nets in the surface layer (~1 m), in three areas of each estuary: posterior to mouth, (ii) mouth, and (iii) anterior to mouth.
Samples for quali-quantitative analysis were carried out through trawls using a flowmeter coupled to the mouth of the net to estimate the filtered volume, for 3-min trawls, in the subsurface portion of the water column and at a speed of 1 knot. Samples were fixed in 4% neutral formalin. In non-predatory mortality characterization and experimentation samples, a blind cup was placed in the net to avoid the mortality of organisms during trawling. After each trawl, the technique was used to estimate non-predatory mortality by adding 1.5 mL of the 1% neutral red stock solution in deionized water for every 1000 mL of live zooplankton sample (Dressel et al., 1972; Elliott & Tang, 2009). The samples were then packed in a dark glass and kept for 15 min in a bucket with ambient water to maintain temperature and avoid animal mortality during this step in the field. Subsequently, the samples were concentrated in fine nylon mesh filters (45 μm) and carefully rinsed with filtered seawater to remove excess dye, placed in Petri dishes covered with aluminum foil, stored on ice and taken to the laboratory. They were then kept in the freezer until processing (a maximum of 90 days after collection).
2.2.3 Laboratory analysis
The identification and counting of zooplanktonic groups from qualitative and quantitative samples took place with the aid of a stereomicroscope and microscope, with support from the specialized literature: Boltovskoy (1981, 1999), Trégouboff and Rose (1957), and Björnberg (1981). Each sample was diluted to a variable volume according to the concentration of organisms and then homogenized. Three 2 mL subsamples (totaling 6 mL) were taken and analysed in a Sedgewick-Rafter chamber. Copepods were analysed in their naupliar and adult stages. Adults were identified at the species level, and nauplii and early copepodites to the family level. The other organisms were classified at high taxonomic levels. For the mortality experiment, neutral red samples were thawed and diluted in filtered seawater and acidified to pH <7 by adding 1 mL HCl per 10 mL sample to enhance the neutral red coloration in the animals (Elliott & Tang, 2009). According to these authors, organisms were classified into live (colored, deep red) and dead (not colored, cream or transparent). Organisms were counted using the stereomicroscope and microscope with reflected light, with the laboratory in low light (to avoid the loss of staining of the material under analysis). The first 100 individuals of each sample were sorted and identified at the family level.
2.2.4 Carcass decomposition experiment and non-predatory mortality rate
In each environment, an additional trawl was executed in the intermediate station to collect live copepods and to perform the carcass decomposition experiments. The zooplankton collected had been previously processed. For each experimental series (per estuary) 40 organisms were separated according to the typical families of the three main groups of estuarine copepods of the region: Cyclopoida (Oithonidae), Calanoida (Acartiidae) and Harpacticoida (Tachidiidae). After this sorting, the individuals were placed in the freezer for 30 min at −20°C. Subsequently, the organisms were distributed in 12-cavity culture plates, incubated in seawater filtered on a 5.00 μm cellulose nitrate filter, in a germination chamber, with temperature ranging from 25 to 27°C (simulating the ambient temperature at the time of collection) and 12-h photoperiod, identified with initial and local time. The experiment was observed daily, and the organisms were considered decomposed when they reached the advanced stage, with most of the tissues having disappeared, following the study of Tang et al. (2006).
Non-predatory mortality rates were estimated using the formula: m = D/[t(1 − D)], where D is the fraction of carcasses in the neutral red sample (dead copepods) and t is the time (days) required for complete decomposition, obtained from the decomposition experiment (Tang et al., 2006). The combination of the technique and the experiment allows the estimation of non-predatory mortality based on the abundance data of dead and live copepods obtained during the field study (Di Capua & Mazzocchi, 2017). Data generated by the decomposition experiments with the model families were also used to estimate the mortality rate of other families belonging to the same orders (Cyclopoida, Calanoida, and Harpacticoida).
2.2.5 Data analysis
The analyses of the zooplankton community structure were performed based on calculations of total abundance (traditional method; ind. m−3), abundance of living copepods, done in the same way as the traditional method, but disregarding the dead plot obtained through the non-predatory mortality experiment (ind. m−3), relative abundance (%), percentage of the dead (carcasses) (%) and non-predatory mortality rate (day−1). The analyses to compare the non-predatory mortality rate, carcasses, total abundance, and live copepods among the estuaries were performed using the unidirectional Permutation Analysis of Variance (PERMANOVA).
The dissimilarity matrix was used to create the non-metric multidimensional scale ordering (nMDS; Clarke & Warnick, 2001). A multivariate PERMANOVA (Vegan package, version 2.4–6; Oksanen et al., 2018) was used to detect differences in community structure according to spatial variability, comparing the six estuarine regions. The Bray–Curtis similarity and dissimilarity matrix were used. We used the residuals under the complete model with 9999 repetitions (Anderson, 2001).
Potential linear regressions were performed to observe possible relationships among the non-predatory mortality rate per day (independent variable), the carcass rate, and the non-predatory mortality with environmental factors (Chlorophyll-a, pH, and temperature). The biological attributes were log-transformed into (x + 1) to improve the normality and homoscedasticity of the data. Statistical analyses were performed using R software (version 3.2.2, R Core Team, 2015), using the RStudio interface (version 0.99.473, RStudio Team, 2020), Past 4 and Primer following the assumptions of each test and considering p-values <.05 as significant.
3 RESULTS
3.1 Environmental characterization
Hydrological parameters were very similar in all tropical environments during the study period, regardless of the degree of urbanization (Table 2). The averages of temperature, salinity, dissolved solids, and turbidity were 27.8 ± 1.6°C; 24.3 ± 13.7; 22.8 ± 10.1 mg L−1; and, 18.3 ± 24.7 NTU, respectively. On the other hand, Chlorophyll-ɑ, pH, and dissolved oxygen showed large differences between some areas. The Chlorophyll-ɑ (mean = 11.6 ± 9.6 mg m−3) had a minimum value of 2.8 mg m−3 in Suape (moderately urbanized, according to Table 1), and a maximum value of 61.06 mg m−3 for the Capibaribe River (strongly urbanized, according to Table 1); the pH (mean = 6.9 ± 0.7) had a minimum value of 5.7 in Formoso River (moderately urbanized), and a maximum value of 7.6 of the Timbó River (low urbanized). Finally, the dissolved oxygen (mean = 7.3 ± 4.9 mg L−1) presented a minimum value of 1.4 in Capibaribe and a maximum value of 7.5 for the Timbó River. These results show that despite the constancy of environmental parameters, some that change with the degree of urbanization and impacts and may change according to the condition of the environment.
| Timbó River | Santa Cruz Channel | Catuama inlet | Capibaribe River | Formoso River | Suape bay | |
|---|---|---|---|---|---|---|
| Temperature (°C) | 29.46 ± 0.2 | 25 ± 0 | 29.0 ± 0.2 | 27.9 ± 0.3 | 28.4 ± 0.2 | 28.3 ± 0.1 |
| pH | 7.5 ± 0.1 | 7.2 ± 0.8 | 7.4 ± 1.2 | 7.1 ± 0.03 | 6.4 ± 0.6 | 7.0 ± 0.8 |
| Turbidity | 3.5 ± 1.1 | – | 12.1 ± 3.6 | 59.1 ± 37.9 | 10.2 ± 2.8 | 11.3 ± 3.2 |
| Dissolved oxygen (mg L−1) | 6.0 ± 1.3 | – | 3.1 ± 0.8 | 2.2 ± 1.1 | 10.4 ± 4.8 | 6.1 ± 1.1 |
| Total dissolved solids (mg L−1) | 30.4 ± 1.2 | – | 30.9 ± 0.7 | 8.1 ± 6.5 | 26.4 ± 5.4 | 31.7 ± 0.1 |
| Salinity | 33.1 ± 1.8 | – | 33.9 ± 0.9 | 0.8 ± 0.6 | 30.2 ± 4.0 | 34.8 ± 0.1 |
| Chlorophyll-α (mg m−3) | 25.1 ± 3.3 | 26.6 ± 2.0 | 7.7 ± 1.0 | 40.14 ± 18.6 | 36.0 ± 13.0 | 3.2 ± 2.0 |
- Note: (−) The variable could not be measured.
3.2 Zooplankton community
Forty-six taxa representing the following phyla were recorded: Foraminifera, Ciliophora (Tintinnina), Rotifera, Mollusca (Gastropoda and Bivalvia), Annelida (Polychaeta), Arthropoda (Amphipoda, Copepoda, Cirripedia and Decapoda), Chaetognatha, Appendicularia and Teleostei (eggs; Table 3). Copepods were the most abundant and representative taxa in the study, with 12 species belonging to the families: Acartiidae, Cyclopidae, Corycaeidae, Ectinosomatidae, Oithonidae, Paracalanidae, Pseudodiaptomidae, Sapphirinidae, Tachidiidae and Temoridae (Table 3). The naupliar stages of Oithonidae and the adult organisms of the species Oithona oswaldocruzi were the most frequent organisms (35.7%), followed by the adults of Acartiia lillgeborgi and Euterpina acutifrons with a frequency of 33.3%. For the other groups, the highest frequency were Acartiidae and Cyclopidae nauplii (31%). Total zooplankton abundance in all periods of the ecosystems' rivers had the mean of 6900.99 (±8674.87) ind. m−3, with the minimum value of 90.23 ind. m−3 in the Capibaribe River, an environment categorized as strongly urbanized, according to Table 1, and maximum of the 31,996.66 ind. m−3 in the Santa Cruz Channel, an environment categorized as low urbanized, according to Table 1. The coefficient of variation of the abundance between areas was 125.7% (Table 3, Figure 2).
| Taxa | F.O. (%)* | Average density (ind. m−3) | Ab. relative (%) |
|---|---|---|---|
| Foraminifera | 23.8 | 222.65 | 1.71 |
| Tintinnina | 28.6 | 528.35 | 6.96 |
| Rotifera | |||
| Bdelloidea | 2.4 | 0.51 | 0.03 |
| Brachionus angularis Gosse, 1851 | 7.1 | 10.21 | 0.56 |
| Brachionus calyciflorus Pallas, 1766 | 7.1 | 12.09 | 0.64 |
| Brachionus caudatus Barrois and Daday, 1894 | 2.4 | 1.46 | 0.07 |
| Brachionus plicatilis Müller, 1786 | 7.1 | 47.04 | 2.56 |
| Epiphanes sp. | 2.4 | 2.04 | 0.14 |
| Keratella americana Carlin, 1943 | 2.4 | 0.42 | 0.02 |
| Mollusca | |||
| Bivalvia (veliger) | 9.5 | 85.82 | 1.06 |
| Gastropoda (veliger) | 28.6 | 165.41 | 1.42 |
| Polychaeta (larva) | 26.0 | 459.73 | 3.17 |
| Copepoda | |||
| Acartia (Odontacartia) lilljeborgii Giesbrecht, 1889 | 33.3 | 153.23 | 4.50 |
| Apocyclops procerus (Herbst, 1955) | 11.9 | 92.69 | 0.95 |
| Corycaeus sp. | 7.1 | 25.12 | 0.20 |
| Euterpina acutifrons (Dana, 1847) | 33.3 | 1348.66 | |
| Oithona oswaldocruzi Oliveira, 1945 | 35.7 | 1124.03 | 7.08 |
| Oithona nana Giesbrecht, 1893 | 29.6 | 2201.44 | 7.54 |
| Oithona hebes Giesbrecht, 1891 | 9.5 | 31.69 | 0.56 |
| Paracalanus quasimodo Bowman, 1971 | 21.4 | 52.98 | 0.93 |
| Pseudodiaptomus acutus (Dahl F., 1894) | 7.1 | 34.55 | 0.39 |
| Sapphirina nigromaculata Claus, 1863 | 7.1 | 10.67 | 0.33 |
| Temora turbinata (Dana, 1849) | 7.1 | 55.47 | 0.36 |
| Temora stylifera (Dana, 1849) | 9.5 | – | – |
| Microsetella sp. | 2.38 | 272.74 | 0.66 |
| Oithonidae (Copepodite) | 23.8 | 127.36 | 0.87 |
| Pseudodiaptomidae (Copepodite) | 2.4 | 0.32 | 0.01 |
| Euterpinidae (Copepodite) | 9.5 | 40.97 | 0.67 |
| Acartiidae (Nauplius) | 31.0 | 811.58 | 9.69 |
| Calanidae (Nauplius) | 19.0 | 123.09 | 1.04 |
| Cyclopidae (Nauplius) | 31.0 | 592.79 | 4.46 |
| Euterpinidae (Nauplius) | 19.0 | 904.82 | 4.44 |
| Oithonidae (Nauplius) | 35.7 | 1078.14 | 11.95 |
| Longipediidae (Nauplius) | 11.9 | 83.62 | 1.83 |
| Paracalanidae (Nauplius) | 19.0 | 153.23 | 1.13 |
| Pontellidae (Nauplius) | 16.7 | 51.29 | 1.79 |
| Pseudodiaptomidae (Nauplius) | 16.7 | 242.14 | 1.16 |
| Temoridae (Nauplius) | 26.2 | – | – |
| Cirripedia (Nauplius) | 9.5 | 1138.99 | 4.99 |
| Amphipoda | 4.8 | 7.72 | 0.07 |
| Decapoda | |||
| Brachyura (Zoea) | 4.8 | 20.75 | 0.21 |
| Porcellanidae (Zoea) | 7.1 | – | – |
| Chaetognatha | 7.1 | 108.11 | 0.77 |
| Chordata | |||
| Appendicularia | 11.9 | 240.21 | 2.01 |
- Note: (−) Rare species or that only appeared in some of the estuaries; (*) Frequency of occurrence (%).
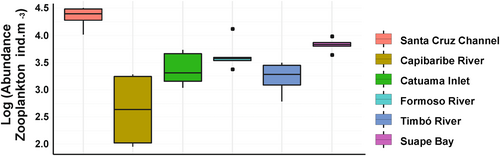
Total abundance of copepods ranged from 32.58 ind. m−3 in the Capibaribe River (strongly urbanized) to 28,070.18 ind. m−3 in the Santa Cruz Channel (low urban) (mean: 5300.60 ± 7277.13 ind. m−3), and showed a different distribution between environments (PERMANOVA—One Way, p < .05). Copepods were significantly more abundant in the Santa Cruz Channel than in other environments categorized also as low urban, like Timbó River, Formoso River, and Catuama River. The abundance of the low urbanized river ecosystems was higher than in the Capibaribe River, categorized as strongly urbanized (Dunn's post hoc, p < .05; Table 6).
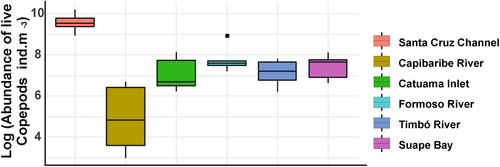
On the contrary, the total abundance of living copepods showed an average 3915.84 ind. m−3 (±5877.62 ind. m−3, CV: 66.6%), with a minimum value of 20.05 ind. m−3 in the strongly urbanized Capibaribe River, whereas the maximum value was 26,760.23 ind. m−3 in the Santa Cruz Channel, an environment categorized as low urban, which was the environment with the highest value for live copepod abundance (PERMANOVA—One Way, p < .05; Dunn's post hoc, p < .05; Figure 3; Details in Table 6).
3.3 Percentage of carcasses and non-predatory mortality rate
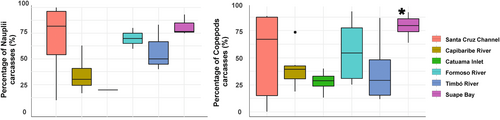
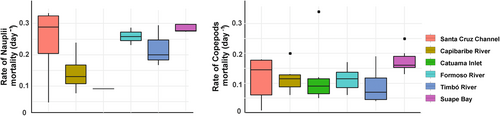
In terms of copepod carcasses, the mean percentage was 51.34% (±30.16, CV: 58.7%), with values ranging from 4% in the Timbó River (low urban) to 93.3% in Suape Bay (moderately urbanized). The percentage of carcasses of adult copepods was significantly different among the estuarine environments (PERMANOVA—One Way, p < .05; Table 4), and Suape Bay was the environment with most abundant adult carcasses and was significantly higher than other environments, like the strongly urbanized Capibaribe River and the low urbanized Timbó River and Catuama Inlet (Dunn's post hoc, p < .05, Figure 4). In terms of adult families, the dominant groups were Oithonidae (48.21 ± 28.78%; CV: 59.7%) and Tachidiidae (46.11 ± 31.22%; CV: 67.7%) present in all environments. There were no significant differences in the nauplii carcasses among environments (PERMANOVA—One Way, p > .05).
| Environments | t | p (perm) | p (MC) |
|---|---|---|---|
| Timbó River, SC Channel | 2.1085 | .016 | .028 |
| Timbó River, Formoso River | 2.6387 | .004 | .007 |
| Timbó River, Catuama | 0.93321 | .408 | .399 |
| Timbó River, Suape | 1.646 | .074 | .123 |
| Timbó River, Capibaribe River | 1.4764 | .144 | .156 |
In terms of families, Paracalanidae (0.55 ± 0.26 day−1; CV: 47%) and Oithonidae (0.119 ± 0.06 day−1; CV: 51.7%) presented the highest values around all estuarine environments. Although the Tachidiidae family did not show high mean values (0.09 ± 0.05 day−1), it varied the most during the study (56.9%). Regarding the number of days for carcass decomposition, this mean was 4.4 days (±1.14 day−1, CV: 25.9%); the families Paracalanidae, Acartiidae, and Oithonidae decomposed in 3 days, both in environments considered as low urbanized (Rio Timbó, Santa Cruz Channel and Catuama), and in moderately urbanized (Suape Bay) and highly urbanized (Capibaribe River). In spite of presenting minimum values, Suape was also the one that presented the longest decomposition time, that of 7 days for the Tachidiidae family.
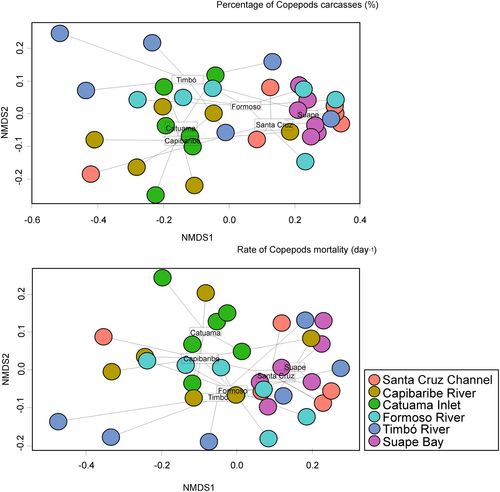
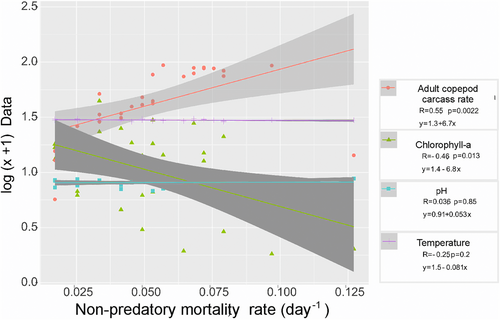
There are small groups that are separated in a not very clear and defined way concerning the percentages of carcasses and for the non-predatory mortality rate among estuarine environments (Figure 5). In general, two small groups were observed: Catuama and Capibaribe are the small group in relation to the other estuarine ecosystems (Figure 5). These were different regarding the percentage rates of carcasses between the environments and for the non-predatory mortality rate (multivariate PERMANOVA, p < .05). Almost all the environments are structured in the same way in terms of carcass percentages and non-predatory mortality rate (a posteriori tests by PERMANOVA p > .05, Tables 4 and 5). The small groups differed only between estuaries with classifications between low and moderately urbanized: the Timbó River (low urban) and the Formoso River (moderately urbanized) and between the low urban Timbó River and Santa Cruz Channel. In terms of carcasses and non-predatory mortality rate, the same structure occurred for Timbó River (low urban) and Formoso River (moderately urbanized), (p < .05, Tables 4, 5). Regarding the non-predatory mortality rate, the mean of total copepod adults was 0.15 day−1(±0.31 day−1, CV: 211.1%), with minimum values of 0.01 day−1 (Santa Cruz Channel, low urban) and maximum of 2.80 day−1 (Suape Bay, moderately urbanized). Besides that, there was no significant difference between the environments and the non-predatory mortality rate (PERMANOVA—One Way, p > .05, Figure 6), but it was negatively related to Chlorophyll-ɑ (R = −.46) and positively with the carcass rate of adult copepods (R = .55, Figure 7).
| Environments | t | p (perm) | p (MC) |
|---|---|---|---|
| Timbó River, Santa Cruz Channel | 2.1929 | .016 | .02 |
| Timbó River, Formoso River | 2.4514 | .006 | .01 |
| Timbó River, Catuama | 0.94224 | .398 | .39 |
| Timbó River, Suape | 1.3365 | .23 | .199 |
| Timbó River, Capibaribe River | 1.4613 | .154 | .168 |
| Total abundance | Abundance of live copepods | ||
|---|---|---|---|
| Environments | p | Environments | p |
| Timbó River, SC Channel | <.05 | Timbó River, SC Channel | <.05 |
| Timbó River, Suape | <.05 | SC Channel, Formoso River | <.05 |
| SC Channel, river Formoso | <.05 | Channel SC, Capibaribe | <.05 |
| SC Channel, Capibaribe | <.05 | SC Channel, Suape | <.05 |
| SC Channel, Catuama | <.05 | SC Channel, Catuama | <.05 |
| Formoso River, Capibaribe River | <.05 | River Formoso, Capibaribe River | <.05 |
| Suape, Capibaribe River | <.05 | Suape, Capibaribe River | <.05 |
- Abbreviation: SC, Santa Cruz.
4 DISCUSSION
This study was the first to characterize the contribution of carcasses and the rate of non-predatory mortality of copepods in estuarine ecosystems of northeastern Brazil, showing variations in the percentage values of carcasses from non-predatory mortality among environments with different degrees of urbanization. Among the environments studied, there was little differentiation between these percentages in most environments, not supporting the hypothesis that the higher rates of non-predatory copepod mortality were related to large urban centers. In general, the estuaries of Pernambuco state show an almost proportional contribution of carcasses and non-predatory mortality in all areas except a few environments, supporting the hypothesis that there is a spatial variation regarding carcasses in tropical estuaries.
4.1 Environmental variability
Even though the samples were collected in different environments and months, including some in periods of temporal variation, there was no meaningful variation among the abiotic data during the research, chlorophyll-ɑ, pH, and dissolved oxygen. All these variables may be closely related to the effluents of domestic and industrial origin that are discharged daily into some of these environments (Silva, 2004). The large nutrient input provides an increase in the activity of the phytoplankton primary producers, i.e. an increase in chlorophyll and consequently a decrease in oxygen rates, the latter of which is consumed by the phytoplankton, and may even cause an increase in pH, due to the removal of CO2 by the algae during periods of maximum insolation, altering the carbonate system (Esteves, 1998; Margalef, 1983; Vieira, 2019). These processes are the case of the Capibaribe River: in this study, in this extremely urbanized environment, a maximum value of Chlorophyll-ɑ (61.06 mg m−3) was recorded, as well as the minimum value of dissolved oxygen. Values of chlorophyll-ɑ were considered high and characteristic of eutrophied environments. These eutrophied environments were observed in estuaries of the same region, such as in the Botafogo River estuary (59.75 mg m−3; Otsuka et al., 2014) and Pina Basin (187.37 mg m−3; Santos et al., 2009), part of the last portion of the Capibaribe River. Nevertheless, all variables remained close to values already found for estuaries in the region (Cavalcanti et al., 2008; Figueiredo et al., 2006; Grego et al., 2004; Silva et al., 2009).
4.2 Copepod carcasses and non-predatory mortality rate dynamics
According to Day Jr et al. (1989), copepods are considered the most abundant holoplanktonic organisms. In this study, they were the main contributors, being mainly represented by the families Oithonidae, Acartiidae, and Tachidiidae, which have already been recorded in other estuarine environments of the Northeast of Brazil (e.g., Cavalcanti et al., 2008; Lopes et al., 1998; Neumann-Leitão et al., 2005; Resgalla et al., 2010). Their carcasses play an important role as microbial hotspots, strongly important for the microbial loop and an essential alternative carbon transport pathway, generating a higher flux than fecal pellets (Frangoulis et al., 2011; Glud et al., 2015; Tang et al., 2019) (Frangoulis et al., 2011; Glud et al., 2015; Tang et al., 2019). During the study, the average percentage contribution of carcasses was close to 51.34%, an expected value for marine ecosystems, which according to Tang et al. (2014) should be between 11.6% and 59.8%.
The families with the highest percentages of carcasses in estuarine environments of the Northeast of Brazil were Tachidiidae and Oithonidae. This finding was also recorded in a coastal area influenced by the estuarine plume in the same region (Tamandaré Bay and Ilhetas and Mamucabas River; Silva et al., 2020). In general, in other studies such as the work of Jyothibabu et al. (2015) in the Cochin backwater and Di Capua & Mazzocchi (2017) in the Gulf of Naples, in which they used a 200 μm net, the highest carcass values were for calanoid copepods. In the Valdivia River estuary, Giesecke et al. (2017) also used a 200 μm mesh opening and observed that the smaller organisms had the lowest percentage of dead and the opposite occurred for the larger size groups. In the present study, however, the families Paracalanidae and Acartiidae contributed a lower proportion of carcasses. For the non-predatory mortality rate, the main families were Oithonidae, Paracalanidae, and Tachidiidae, regardless of the region studied. These families are formed by small organisms with diverse feeding habits, and they are omnivorous, detritivorous, and herbivorous, according to Dubovskaya (2009). Copepods with herbivorous feeding habits seem to have poor food quality, microparasites, and other biotic factors as their main cause of non-predatory mortality, which explains the high values of this group during the study, considering that even in the absence of anthropogenic external factors, they are susceptible to mortality.
In waters with high dilution, physical stress and other factors such as the presence of contaminants can increase the rate of non-predatory mortality (Martinez et al., 2013). In estuaries, this type of mortality is mainly conditioned by tides and river discharges, increasing horizontal and vertical mixing and causing higher mortality values (Giesecke et al., 2017), in addition to the anthropic impacts typical of each environment. On the whole, we found a much higher non-predatory mortality rate (for both adult copepods and nauplii) than already observed in the work of Silva et al. (2020) in Tamandaré Bay (0.06 day−1), where the family Oithonidae (adults) showed the maximum values with the value of 0.05 ± 0.03 day−1, 2.9 times less than those recorded in this study. In the research conducted by Di Capua and Mazzocchi (2017) in the Gulf of Naples, the values were well above those, with a maximum of 0.76 day−1 for copepods of the genus Clausocalanus and taking a maximum of 7.08 days and a minimum of 3.29 days to decompose. For non-predatory mortality, there was no difference between estuarine ecosystems during this study. It was expected that environments with a wide variety of impacts and urbanization would show higher values of carcasses and non-predatory mortality. This result can be explained by the possible large concentration of bacteria present in environments with a higher degree of anthropogenic influence and urbanization, which provide intense decomposition of carcasses in these locations, with only a fraction of living organisms remaining in the water column (Litvinyuk et al., 2022; Mukhanov & Litvinyuk, 2017). This leads to the erroneous interpretation that less anthropogenically affected estuaries show higher mortality rate values.
4.3 Suape Bay: Highlights of the moderately urbanized estuarine ecosystem
Observing the environments separately, Suape (moderately urbanized) region was the one that presented Oithonidae, Paracalanidae, and Tachidiidae families in higher proportions for non-predatory mortality and percentage of carcasses. This area was the only one that differed among the environments' percentages of carcasses, which had a major contribution of carcasses to pelagic or benthic systems. This result was not expected, since it was still less urbanized than Capibaribe, a result that may be associated with the intense traffic of ships, which by generating turbulence and even marine pollution, derived from the fuel, negatively influences these values. Due to this, the Suape environment deserves special attention for future studies regarding the real contribution of copepods or zooplankton carcasses exported from these environments. Some studies in this area show the higher contribution of zooplankton and copepods in this area (Neumann-Leitão & Matsumura-Tundisi, 1998; Pessoa et al., 2009), but do not show results about the real contribution of living organisms.
Suape Bay, the only environment to differ from the others with respect to carcasses, is only one of several environments studied that suffer anthropic impacts. The Capibaribe River, for example, classified as strongly urbanized and which, according to the literature, suffers daily from domestic and industrial effluents and solid waste, was not among the environments that presented the highest percentage of carcasses and mortality rate, presenting values close to estuaries such as the Formoso River, categorized as moderately urbanized. Another possible explanation is that the organisms present there and in other estuaries in similar situations are possibly adapted to local environmental stress and do not exhibit high non-predatory mortality rates related to the degree of pollution. In the future, long-term studies involving bacterioplankton, sedimentation rate of carcasses, and environmental characterization should be done to try to understand possible relationships between impacts and non-predatory mortality rates.
5 CONCLUSION
The zooplankton community, mainly copepods, can provide the estuarine food web with a significant portion of planktonic copepod carcasses in the tropical Atlantic region (Northeast Brazil), and this contribution may vary in terms of families and environment, not showing a gradient according to the degree of urbanization of the environment. Overall, distinguishing organisms in terms of living and dead individuals may contribute to a better understanding of how planktonic copepods participate in marine trophic webs, which is almost always mistakenly related only to the route of classical trophic chains. We present new information for this understanding, showing that these same groups can significantly contribute, alternatively and in spatial terms, to the detritus chain route.
ACKNOWLEDGMENTS
We would like to thank the Federal Rural University of Pernambuco (UFRPE), the Graduate Program in Biodiversity (PPGBio), the Programa Pesquisa em Movimento, the Dean of Research and Graduate Studies, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, through the PROAP 2021 grant. We are also grateful to the Tamandaré Long-Term Ecological Research Program, the Centro de Pesquisa e Conservação da Biodiversidade Marinha do Nordeste (CEPENE), and the Federal University of Pernambuco (UFPE). M.M.O.C. and S.M.A.L. acknowledge a PhD and postdoctoral fellowships, respectively, received from Fundação de Amparo à Ciência e Tecnologia de Pernambuco (#PBPG-0713-1.08/20) and the TRIATLAS project (triatlas.w.uib.no), within the EU Horizon 2020 program (#817578).
FUNDING INFORMATION
Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE), Brazil (#APQ-0685-2.05/19) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil (PROAP 2021 grant).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




