Contrasting host use and feeding behavior by sympatric mesograzers
Abstract
Marine mesograzers use macroalgae as food and habitat and may have strong top-down effects on plants and macroalgae. Sympatric mesograzer species often differ regarding host use and feeding behavior, which may lead to distinct impacts by these consumers on primary producers. The amphipods Hyale niger and Ampithoe marcuzzii are mesograzers abundant that co-occur in Brazilian waters and, although they use several macroalgae as habitat, it is unknown how much the food value of these hosts explains the host use pattern by the two mesograzers. Herein, we investigated the abundance and feeding behavior of the sympatric amphipods H. niger and A. marcuzzii. For that, their abundance on the macroalgal hosts Sargassum filipendula, Padina gymnospora, and Dichotomaria marginata was evaluated seasonally in a rocky shore at Fortaleza Beach, state of São Paulo, Brazil. Also, multiple-choice and no-choice feeding experiments were carried out at laboratory to evaluate the feeding behavior of these mesograzers. The abundance of H. niger was similar among the three macroalgal hosts (except during winter), while A. marcuzzii was more abundant on Dichotomaria and Padina than Sargassum in all seasons. Moreover, H. niger consumed more Padina than Dichotomaria and Sargassum in both feeding experiments. In turn, A. marcuzzii preferred to feed on Padina when it had a choice and consumed the three macroalgal hosts at similar levels in the no-choice feeding experiment. Overall, H. niger and A. marcuzzii differ from each other regarding their feeding behavior and host use pattern. In particular, the food value of macroalgae seems to partially explain the host use by A. marcuzzi, but not by H. niger. The differences between H. niger and A. marcuzzii may result in varying impacts on primary producers.
1 INTRODUCTION
Herbivory has a central role in aquatic and terrestrial ecosystems via top-down control of primary producers (Hillebrand, 2009; Hillebrand et al., 2007; Poore et al., 2012). Although the importance attributed to primary consumers as a trophic group, it has been long acknowledged that sympatric herbivore species often differ in traits affecting the consumption of plants and seaweeds, such as nutritional requirements, feeding behavior, and tolerance to chemical defenses (Behmer & Joern, 2008; Best & Stachowicz, 2012; Cruz-Rivera & Hay, 2000; Hay et al., 1987), which in turn may result in different impacts on primary producers (Bakker et al., 2006; Duffy & Harvilicz, 2001; Erwin et al., 2013; Jaschinski & Sommer, 2008). Also, the variation in such traits may have implications for ecosystems, with some ecosystem processes changing with the increase of herbivore diversity (Blake & Duffy, 2010; Duffy et al., 2003). In this context, understanding the factors driving the interaction between primary producers and sympatric herbivores is an important step to predict the effects of these consumers on communities and ecosystems.
Macroalgae have an important role on the primary productivity of coastal ecosystems and contribute to marine carbon sequestration (Duarte et al., 2022; Krause-Jensen & Duarte, 2016). Also, besides their trophic role on marine food webs (Koenigs et al., 2015), these primary producers can be considered as habitat-forming organisms, buffering adverse biotic (e.g., predation) (Coull & Wells, 1983) and abiotic factors (e.g., desiccation) (Bertness et al., 1999) and, thus, facilitating the occurrence of a diverse fauna (Christie et al., 2009). The abundant fauna associated with macroalgae encompass majorly invertebrate species (Lippert et al., 2001; Tanaka & Leite, 2003; Taylor & Cole, 1994) and represent an important food source for fishes and large decapods (e.g., crabs, shrimps) (Dubiaski-Silva & Masunari, 2008; Zamzow et al., 2011). Among this associated fauna, mesograzers, such as amphipods, isopods, and gastropods, not only use macroalgae as a habitat, but also feed on macroalgal hosts and/or their epiphytes (Duffy, 1990; Pavia et al., 1999; Karez et al., 2000; Råberg & Kautsky, 2007; Jacobucci & Leite, 2014) and, thus, may impact the abundance and diversity of marine primary producers (Berthelsen & Taylor, 2014; Duffy & Hay, 2000; Enge et al., 2013; Råberg & Kautsky, 2007).
The host use by mesograzers is often driven by the food and refuge values of macroalgae, which in turn are related to some macroalgal traits (Duffy & Hay, 1991; Lasley-Rasher et al., 2011; Poore & Steinberg, 1999). Overall, tissue toughness, chemical defenses, and nutrient content of macroalgae determine the role of these hosts as food by affecting the feeding behavior and fitness of herbivores (Cruz-Rivera & Hay, 2001; Duarte et al., 2010; Duffy & Hay, 1991, 1994; Nicotri, 1980), while the morphology and chemical compounds of macroalgae can be related to their importance as refuge since these traits mediate the vulnerability of mesograzers to predation and dislodgement by currents (Hay et al., 1990; Sotka, 2007; Zamzow et al., 2010). However, a macroalgal host that represents a high-quality food not necessarily is a suitable refuge for mesograzers (or vice-versa) and, thus, these consumers must solve a trade-off between their demands for food and refuge (Duffy & Hay, 1991; Lasley-Rasher et al., 2011; Machado, Ferreira, & Leite, 2019). In this regard, investigating the feeding preference and rate of mesograzers is a way of evaluating the food value of macroalgae and, with data on the field abundance of these consumers, has been a useful and widely applied approach to infer how much the food value of macroalgae can explain the host use by these herbivores (Duffy & Hay, 1991; McDonald & Bingham, 2010; Poore & Steinberg, 1999; Sotka, 2007; Taylor & Brown, 2006).
Mesograzer species often differ regarding their host use and feeding behavior (Cruz-Rivera & Hay, 2000; Duffy & Hay, 1994; McDonald & Bingham, 2010; Nicotri, 1980). For example, at local scale, some species of mesograzers are found using different hosts (Duffy & Hay, 1994; McDonald & Bingham, 2010; Poore et al., 2000). Even when sharing the same host, mesograzers may differ regarding their food source, either feeding on host's tissues, epiphytes, or both (Duffy, 1990; Jacobucci & Leite, 2014; Pavia et al., 1999). Also, sympatric mesograzers often show distinct patterns of feeding preference and rate (Best & Stachowicz, 2012; Duffy & Hay, 1994; Machado et al., 2017). All these differences may lead to varying impacts by these consumers on primary producers (Duffy & Harvilicz, 2001; Jaschinski & Sommer, 2008; Råberg & Kautsky, 2007).
The amphipods Hyale niger (Haswell, 1879) (family Hyalidae) and Ampithoe marcuzzii Ruffo, 1954 (family Ampithoidae) are mesograzers abundant on marine macroalgae (Bueno et al., 2017; Ferreira et al., 2019; Jacobucci et al., 2009; Machado, Ferreira, Bueno, et al., 2019). Hyale niger has been reported in Australia and Madagascar waters, while A. marcuzzii has been found in Florida and Caribbean Sea. Although these mesograzers have a quite distinct geographic distribution, they co-occur in Brazilian waters (Serejo & Siqueira, 2018). For instance, in the southeastern coast of Brazil, both amphipods can be found in association with Sargassum species and other macroalgae (Bueno et al., 2017, 2019; Machado, Ferreira, Bueno, et al., 2019; Tanaka & Leite, 2003). Hyale niger has a free-living lifestyle and, when associated with the brown macroalgae Sargassum, it prefers to feed on epiphytes rather than on its macroalgal host (Jacobucci & Leite, 2014). In contrast, A. marcuzzii is a tube-dwelling amphipod that prefers to consume Sargassum than its epiphytes (Duffy, 1990). Although H. niger and A. marcuzzii use several macroalgae as habitat, it is unknown how much the food value of these hosts explains the host use pattern by the two mesograzers. Herein, we investigated the abundance and feeding behavior of the sympatric amphipods H. niger and A. marcuzzii. Specifically, we asked (1) Does the abundance of these mesograzers vary temporally and among macroalgal hosts? (2) Does the host use pattern by H. niger and A. marcuzzii related to their consumption in multiple-choice (i.e., feeding preference) and no-choice feeding experiments? (3) Do these two mesograzers differ from each other regarding their feeding behavior and association with macroalgal hosts?
2 MATERIALS AND METHODS
2.1 Study area
Amphipods and macroalgae were collected in a rocky shore at Fortaleza beach (23°32′S, 45°10′W), Ubatuba, on the north coast of the state of São Paulo, Brazil. Sargassum filipendula is the main macroalgae in the subtidal zone at this site, while Padina gymnospora and Dichotomaria marginata can be found in small patches among Sargassum fronds. All these macroalgae harbor epiphytes and a diverse amphipod assemblage (Machado, Ferreira, Bueno, et al., 2019).
2.2 Host use pattern in the field
To investigate the effect of macroalgal host species on the abundance of H. niger and A. marcuzzii, sampling was carried out twice in each season throughout 2014: summer (January and February), autumn (April and May), winter (July and August), and spring (October and November). The sampling was carried out as described by Machado, Ferreira, Bueno, et al. (2019). Briefly, at each sampling month, five fronds of S. filipendula, P. gymnospora and D. marginata were sampled underwater (for each season, N = 10 for each algal host), stored in bags (0.2 mm of mesh size), and frozen. In the laboratory, all fauna and epiphytic algae were removed from macroalgal samples under freshwater. Mobile fauna was preserved in 70% ethanol and then the number of H. niger and A. marcuzzii amphipods was recorded. As a measure of the availability of macroalgal substrate, the wet weight of epiphytes (epiphytic load) and hosts was obtained after removing the excess of water using a salad spinner.
2.3 Feeding experiments
To test if the pattern of host use by H. niger and A. marcuzzii could be explained by its feeding preference, a multiple-choice feeding experiment was performed using their macroalgal hosts (i.e., D. marginata, P. gymnospora, and S. filipendula). Also, to test if these mesograzers were able to feed on Dichotomaria, Sargassum, and Padina without the interference of other foods, and to estimate their consumption on each of those hosts, a no-choice feeding experiment was performed. Macroalgae were collected at the study area using the same procedure described above. After sampling, macroalgae were inspected on plastic trays and the associated fauna was carefully removed from macroalgal fronds using small brushes and plastic pipettes. Then, H. niger and A. marcuzzii amphipods were kept in plastic containers with seawater and transported to the laboratory in thermal boxes along with macroalgal hosts. Macroalgae and amphipods were kept in the laboratory in tanks (20 L) with seawater (one species per tank) and air pump and aerator, under 12:12 photoperiod and at a temperature of 23°C (see Machado et al., 2017). Amphipods were fed on a mix of seaweeds commonly found in the study area, such as Sargassum, Dichotomaria, Padina, and epiphytes. All experiments were carried out inside a germination chamber (12:12 photoperiod and at a temperature of 23°C).
The following feeding experiments were carried out based on Machado et al. (2017); Machado, Ferreira, and Leite (2019). In short, adult amphipods previously fed on macroalgal hosts were individually kept in cups with seawater (200 mL) and approximately 60 mg of each macroalgal species for 70 h (Hyale) or 48 h (Ampithoe) (N = 20 cups per mesograzer species). Based on previous studies (Machado et al., 2017; Machado, Ferreira, & Leite, 2019), we selected an amount of food and period that allowed amphipods to feed on macroalgae without depleting all the food available during the experiment. Cups were kept with a plastic cover to avoid the loss of water. Also, replicates with macroalgae, but no amphipods (i.e., control) (N = 20), were used to account for autogenic changes in macroalgal weight (Peterson & Renaud, 1989). Macroalgal pieces were patted dry in a sheet paper and weighed at the beginning and the end of the experiment. The consumption by amphipods was estimated as the difference between the initial macroalgal weight (corrected by the change in macroalgal mass of control cups) and the final macroalgal weight (see Cronin & Hay, 1996). Negative values of mass change were assumed as zero (i.e., no consumption). Replicates with dead amphipods at end of the experiment were not considered for analysis (one replicate for Hyale). Also, some replicates were excluded from analysis because they had newborn juveniles released by ovigerous females during the experiment (three replicates for Ampithoe), which could have affected the amount of food consumed in that replicates. For both amphipod species, the multiple choice-experiment was carried out in February 2020. For the no-choice feeding experiment, we followed the procedures described above, except that adult amphipods were individually kept in cups with seawater (60 mL) and a single macroalgal species (~ 60 mg) was offered for 70 h (Hyale) or 65 h (Ampithoe) (N = 15 cups per macroalgal species for Hyale, except for Padina treatment, which had N = 14; and N = 12 cups per macroalgal species for Ampithoe). Also, replicates with dead amphipods (Hyale: one replicate with Sargassum; Ampithoe: two replicates with Padina) or with newborn juveniles (Hyale: one replicate with Sargassum, four replicates with Dichotomaria, five replicates with Padina; Ampithoe: four replicates with Dichotomaria, four replicates with Padina) at end of the experiment were not considered for analysis. For Hyale, this experiment was carried out in December 2020, while it was conducted in May 2021 for Ampithoe.
2.4 Data analysis
The abundance of H. niger and A. marcuzzii was analyzed using Generalized Linear Models (GLM) with a negative binomial distribution and logarithmic link function. Macroalgal host species and season were used as factors and epiphytic load as co-variable, while the mass of macroalgal hosts (log transformed) was used as an offset variable. From a model with all predictor variables, generalized variance inflation factors (GVIF) were estimated to test for the presence of collinearity among predictor variables (Fox, 2002). Using the “vif” function from “car” package, GVIF[1/(2*df)] (a correction of GVIF values) was used to account for predictor variables with different degrees of freedom (e.g., categorical and continuous variables). No evidence for collinearity was found for any variable (GVIF[1/(2*df)] < 2) (Table S1). A model selection was performed using Akaike Information Criterion (AIC) (Table S2). We considered as equivalent the models with delta AIC <2 (Burnham & Anderson, 2002). Also, for model validation, we used functions from DHARMa package (Hartig, 2022) to check for model dispersion and homogeneity of variances (Figures S2–S5). Possible temporal autocorrelation was verified using the Durbin-Watson test and auto-correlation function plots (Table S3 and Figure S1). A posteriori tests were used to explore the effects of factors, co-variable and/or their interaction (see Results for details). Furthermore, two-way ANOVA was used to evaluate if epiphytic load and host biomass varied among seasons and macroalgal host species, while Pearson's correlation coefficient was used to evaluate the relationship between epiphyte and host biomasses for each macroalgal species.
For the multiple-choice experiment, the proportional consumption of each food regarding the total mass of food consumption in a cup was used as response variable and compared among foods using Hotelling T2 test, a multivariate approach that is suitable when there is dependence among the measured response variables (Lockwood, 1998). Pairwise comparisons were carried out to check for differences in consumption among macroalgal hosts (see Lockwood, 1998). For the no-choice experiment, consumption was compared among macroalgal hosts using one-way ANOVA. The assumptions of normality and homogeneity of variances for ANOVAs were checked graphically and, when necessary, data were transformed by log (X + 1). When a factor (or interaction between factors) was significant, a Tukey's test was used to verify differences between groups. Analyses were performed using R 3.6.3 (R Core Team, 2020).
3 RESULTS
3.1 Host use pattern in the field
The abundance of H. niger was affected by macroalgal host species and season, but not epiphytic load (Table 1). In this case, a significant interaction between the factors host species and season was detected. The abundance of this mesograzer was higher on Dichotomaria than Padina and Sargassum during winter, while it did not vary among hosts in the other seasons (Figure 1a). Also, a temporal variation in the abundance of H. niger was detected on Sargassum and Dichotomaria, but not Padina. Hyale niger was more abundant on Sargassum during autumn than winter, with other seasons supporting intermediate values (Figure 1a). On Dichotomaria, H. niger was more abundant in autumn and winter than spring, occurring in intermediate abundance during summer (Figure 1a).
| Source of variation | Df | Deviance | Residual df | Residual deviance | p (>|χ2|) |
|---|---|---|---|---|---|
| Hyale niger | |||||
| NULL | – | – | 119 | 216.75 | – |
| Macroalgae (Al) | 2 | 36.02 | 117 | 180.73 | <.001 |
| Season (Se) | 3 | 35.51 | 114 | 145.22 | <.001 |
| Epiphytic load (Ep) | 1 | 2.26 | 113 | 142.97 | .133 |
| Al X Se | 6 | 13.76 | 107 | 129.20 | .032 |
| Al X Ep | 2 | 4.74 | 105 | 124.46 | .093 |
| Ampithoe marcuzzii | |||||
| NULL | – | – | 119 | 272.89 | – |
| Macroalgae (Al) | 2 | 115.84 | 117 | 157.06 | <.001 |
| Season (Se) | 3 | 7.00 | 114 | 150.06 | .072 |
| Epiphytic load (Ep) | 1 | 0.18 | 113 | 149.88 | .669 |
| Al X Se | 6 | 14.33 | 107 | 135.54 | .026 |
| Se X Ep | 3 | 10.00 | 104 | 125.55 | .019 |
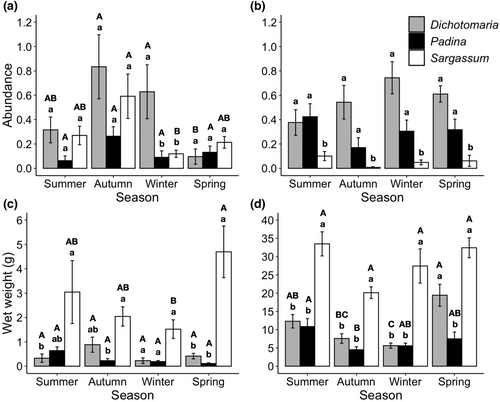
In turn, the abundance of A. marcuzzii was affected by macroalgal host species and epiphytic load (Table 1). In all seasons, the abundance of this mesograzer was higher on Dichotomaria and Padina than Sargassum (Figure 1b). No temporal variation in the abundance of A. marcuzzii was detected within each host (Figure 1b). Furthermore, the abundance of A. marcuzzii was negatively correlated with the epiphytic load during summer (GLM, Intercept = −0.86, Slope = −0.20, Z = −2.18, p = .030) and spring (GLM, Intercept = −0.60, Slope = −0.25, Z = −2.67, p = .008), but not during autumn (GLM, Intercept = −0.84, Slope = −0.44, Z = −1.46, p = .145) and winter (GLM, Intercept = −0.46, Slope = −0.54, Z = −1.89, p = .058).
Moreover, the epiphytic load was affected by macroalgal host species, but it depended on season (Table 2). Overall, Sargassum had greater epiphytic load than Dichotomaria and Padina, except during winter, when no difference in epiphyte's wet weight was observed among macroalgal hosts (Figure 1c). The epiphytic load varied among seasons only in Sargassum, with higher wet weight in spring than in winter (Figure 1c). However, these results should be interpreted with caution since the ANOVA's assumption of homogeneity was not met even after data transformation.
| Source of variation | Df | MS | F | p |
|---|---|---|---|---|
| Epiphytes | ||||
| Macroalgae (Al) | 2 | 9.00 | 44.57 | <.001 |
| Season (Se) | 3 | 0.35 | 1.74 | .163 |
| Al X Se | 6 | 0.50 | 2.46 | .029 |
| Residuals | 108 | 0.20 | ||
| Hosts | ||||
| Macroalgae (Al) | 2 | 20.15 | 104.09 | <.001 |
| Season (Se) | 3 | 2.63 | 13.60 | <.001 |
| Al X Se | 6 | 0.57 | 2.92 | .011 |
| Residuals | 108 | 0.19 | ||
The host biomass also varied among macroalgal host species and seasons, with a significant interaction between both factors (Table 2). The biomass of Sargassum was higher than Dichotomaria and Padina in all seasons, except during spring, when it was similar to the biomass of Dichomaria (Figure 1d). Also, temporal variation in macroalgal biomass was detected for Dichotomaria and Padina, with the highest levels during spring and summer, respectively. No temporal variation in the biomass of Sargassum was found (Figure 1d). Moreover, a positive correlation between macroalgal host and epiphyte biomasses was observed for Padina (Pearson's correlation, t = 3.16, df = 38, p = .003, r = 0.46), but not for Dichotomaria (Pearson's correlation, t = 0.113, df = 38, p = .910, r = 0.02) and Sargassum (Pearson's correlation, t = 1.31, df = 38, p = .197, r = 0.21).
3.2 Feeding experiments
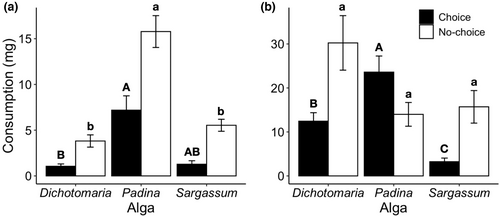
Overall, Padina was more consumed than Dichotomaria and Sargassum by H. niger (Hotelling T2 test, T2 = 25.69, F2,17 = 12.13, p < .05) and A. marcuzzii (Hotelling T2 test, T2 = 63.39, F2,15 = 29.71, p < .05) in the multiple-choice feeding experiment. Hyale niger consumed about seven and six times more Padina than Dichotomaria (p < .05) and Sargassum (not statistically significant), respectively (Figure 2a). In turn, A. marcuzzii consumed about two and seven times more Padina than Dichotomaria (p < .05) and Sargassum (p < .05), respectively, and consumed four times more Dichotomaria than Sargassum (p < .05) (Figure 2b). In the no-choice feeding experiment, the feeding behavior of H. niger was similar to that observed in the multiple-choice experiment, being Padina more consumed than the other macroalgae (ANOVA, F2,30 = 36.47, p < .001) (Figure 2a). Also, although there was a significant effect of macroalgal species on the consumption of A. marcuzzii (ANOVA, F2,23 = 3.51, p = .047), no significant difference among algae was detected in the a posteriori Tukey test (Figure 2b).
4 DISCUSSION
The mesograzers H. niger and A. marcuzzii presented different patterns of host use (i.e., field abundance) and feeding behavior (i.e., consumption in multiple-choice and no-choice feeding experiments). Hyale niger was more abundant on Dichotomaria than other macroalgae only during winter but consumed more Padina than Dichotomaria and Sargassum in both feeding experiments. In turn, A. marcuzzii was more abundant on Dichotomaria and Padina than Sargassum in all seasons. Also, this mesograzer preferred to feed on Padina when it had a choice and consumed the three macroalgal hosts at similar level in the no-choice feeding experiment. Thus, the food value of macroalgae seems to be an important factor on the host use by A. marcuzzi, but not for H. niger.
The abundance of H. niger on the macroalgal hosts Sargassum and Dichotomaria varied among seasons, with high values during autumn. Temporal differences in amphipod assemblage associated with macroalgal beds have been related to the temporal variation of epiphytic load and the biomass of macroalgal hosts (Bueno et al., 2019; Jacobucci et al., 2009; Mukai, 1971; Russo, 1989). The epiphytic load differed among seasons only in Sargassum, with higher levels in summer than winter. Although H. niger can use epiphytes as a food source (Jacobucci & Leite, 2014), an influence of epiphytic load on the abundance of this mesograzer was not found in the present study. Also, temporal variation in macroalgal biomass was detected for Dichotomaria, with the highest levels during spring, while no temporal variation in the biomass of Sargassum was found. These results suggest epiphytic load and biomass of macroalgal hosts are not sufficient to explain temporal variation in the abundance of H. niger. Other factors not investigated in the present study, such as predation pressure (e.g., Holmlund et al., 1990) and canopy structure (Hacker & Steneck, 1990), may help to explain the seasonal differences in H. niger's abundance and, thus, require further investigation.
Moreover, although the abundance of A. marcuzzii was similar among seasons, it was negatively correlated with epiphytic load during summer and spring, but not during other seasons. This could be a result of the indirect effect of epiphytic load on the abundance of A. marcuzzii via reduction of the availability of macroalgal hosts as consequence of high epiphytism levels (e.g., Buschmann & Gómez, 1993; D'Antonio, 1985). Since A. marcuzzii seems to prefer to feed on macroalgal hosts rather than epiphytes (Duffy, 1990), significant reductions on the availability of these hosts may affect its abundance. However, our results do not support such suggestion since the relationship between epiphyte and host biomasses was not negative for any macroalgal host species. Alternatively, this result could reflect differences among macroalgal hosts in levels of epiphytism and, thus, be related to spurious relationship between variables (i.e., epiphytic load and A. marcuzzii's abundance). In fact, we found that Sargassum, the host that harbored the lowest abundance values of A. marcuzzi, support a higher epiphytic load than Padina and Dichotomaria in all seasons, except during winter.
Hyale niger and A. marcuzzii presented different patterns of host use in the field. The abundance of H. niger was similar among macroalgal hosts (except during winter, when Dichotomaria harbored more H. niger than other macroalgae), while A. marcuzzii was more abundant on Dichotomaria and Padina than Sargassum in all seasons. These macroalgal hosts differ from each other in structural traits and morphology (Bueno et al., 2017; Machado, Ferreira, Bueno, et al., 2019). Moreover, in the present study, Sargassum supported higher epiphytic load and had greater biomass than other macroalgal hosts. Although those differences, Sargassum harbored an abundance of amphipods similar (for Hyale) or lower (for Ampithoe) than Dichotomaria and Padina. In this case, differences in the food value among these macroalgae could help to explain the patterns of host use by H. niger and A. marcuzzii.
Both mesograzer species preferred to feed on Padina than Dichotomaria and Sargassum. Also, when these amphipods were restricted to a single food source, H. niger presented the same pattern of consumption observed in the multiple-choice feeding experiment, while A. marcuzzii consumed all three macroalgae at similar level. In similar multiple-choice feeding experiments, it has been found that other sympatric mesograzers also rejected Dichotomaria when it was offered with Padina and Sargassum simultaneously (G.B.O Machado, unpublished data; Machado, Ferreira, & Leite, 2019). Interestingly, although Sargassum is preferentially consumed by other co-occurring small herbivores (Jacobucci & Leite, 2014; Machado et al., 2017; Machado, Ferreira, & Leite, 2019), it was rejected by both H. niger and A. marcuzzii in the multiple-choice feeding experiments, corroborating the notion that sympatric mesograzers may differ regarding their feeding behavior (Cruz-Rivera & Hay, 2000; Duffy & Hay, 1994; McDonald & Bingham, 2010; Nicotri, 1980).
The host use by A. marcuzzii may be partially related to the food value of macroalgae. Ampithoe marcuzzii occurred in low abundance on Sargassum, which was the macroalgae less consumed by this mesograzer in the multiple-choice feeding experiment, suggesting that the rejection of Sargassum as food by A. marcuzzi may explain its infrequent association with this host. However, although A. marcuzzii preferentially consumed Padina over Dichotomaria, it was found on both hosts in similar abundances. Also, A. marcuzzii was able to feed on Sargassum and Dichotomaria when these were the only foods available, suggesting this mesograzer may use a compensatory feeding strategy. Consumers can meet their nutritional requirements by using different behavioral strategies, such as feeding on higher quality foods (i.e., selective diet), ingesting complementary foods varying in nutrient content (i.e., mixed diet), and increasing the consumption when only lower quality foods are available (i.e., compensatory feeding behavior) (Aquilino et al., 2012; Cruz-Rivera & Hay, 2000, 2001; Pennings et al., 1993; Stachowicz & Hay, 1999). For low mobility herbivores, such as tube-dwelling amphipods, a compensatory feeding may be advantageous, since it allows these consumers to feed on macroalgae available nearby, including those with poor-nutrient content, without foraging for long distances and, thus, reducing their risk of predation (Cruz-Rivera & Hay, 2000, 2001; Stachowicz & Hay, 1999). A compensatory feeding behavior, along with the fact that Dichotomaria was the second most consumed food on the multiple-choice feeding experiment, may explain the occurrence of this mesograzer on Dichotomaria.
In contrast, the host use by H. niger does not seem to be explained by the food value of hosts, since this mesograzer consumed more Padina than other macroalgae in both multiple-choice and no-choice feeding experiments but had similar (or lower) abundance on Padina than on Dichotomaria and Sargassum. In this case, it is possible that H. niger's diet relies on other food items besides their macroalgal hosts. In fact, this mesograzer prefers to feed on epiphytes over macroalgal hosts, such as Sargassum (Jacobucci & Leite, 2014). Also, the ingestion of detritus and small invertebrates are common among amphipods from the family Hyalidae (Guerra-García et al., 2014). The consumption of alternative food items may make H. niger less dependent on specific macroalgal hosts and, thus, explain the mismatch between the food value of macroalgae and the host use by this mesograzer found in the present study. Also, it is possible that there is a temporal separation of food and shelter use by H. niger. It has been suggested that high mobility mesograzers, such as those with a free-living lifestyle, may meet their requirements for food and habitat by living on some macroalgal species during day while feeding on others at night (Buschmann, 1990).
Mesograzers have strong effects on macroalgae (Duffy & Hay, 2000; Poore et al., 2012) and, thus, understanding the factors mediating the host use by sympatric mesograzer species is crucial to predict the impacts of this abundant and diverse group on marine ecosystems. By carrying out field sampling and laboratory experiments, we found the mesograzers H. niger and A. marcuzzii differ from each other regarding their feeding behavior and host use pattern. In particular, the food value of macroalgae seems to partially explain the host use by A. marcuzzi, but not by H. niger. The differences between H. niger and A. marcuzzii may result in varying impacts on primary producers.
ACKNOWLEDGMENTS
We thank Aline Neufeld and Silvana Siqueira for assistance with field and laboratory work. We thank Marília Bueno and Pedro Bergamo for their valuable comments. This study was financially supported by the São Paulo Research Foundation (FAPESP) Grant to GBO Machado (No. 2013/17629-9 and No. 2018/11803-0) and TM Costa (No. 2020/03171-4) and the Brazilian National Council for Scientific and Technological Development (CNPq) Grant to CA Paula and DJ Borges (No. 135271/2020-7).
FUNDING INFORMATION
This study was financially supported by the São Paulo Research Foundation (FAPESP) Grant to GBO Machado (No. 2013/17629–9 and No. 2018/11803–0) and TM Costa (No. 2020/03171–4) and the Brazilian National Council for Scientific and Technological Development (CNPq) Grant to CA Paula and DJ Borges (No. 135271/2020–7).
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




