Effects of the ridge-and-runnel system on macrofaunal spatial distribution on a macrotidal sandy beach in the Brazilian Amazon coast
Abstract
Ridge-and-runnel systems are common morphological features across the intertidal zone of dissipative macrotidal beaches. However, these systems have often been overlooked by macrofaunal zonation studies. The present study investigated the effects of this system on the spatial distribution of the macrofaunal community on an Amazonian macrotidal sandy beach. Samples (macrobenthos and sediments) were collected at seven equidistant levels (50 m apart) from the high tide water mark to the swash zone, in two different areas: (1) with and (2) without ridge-and-runnel systems. The results showed a significant increase in macrofaunal abundance and richness in area with runnels, associated with muddier sediment and higher organic content. The macrofauna in the runnels, both in upper-intertidal and lower-intertidal zones, had higher density and richness than that in the sandbars. There was no difference between tidal levels in area 2, although we observed an increasing tendency of abundance and richness towards the low tide line. Taxonomic composition and trophic groups also differed between areas, with predominance of species that are typical of sandy-mud substrate and higher participation of nonselective deposit feeders in area 1. Predatory species typical of sandy substrates prevailed in area 2. These findings add knowledge to sandy beach ecology and indicate the importance of including aspects of habitat complexity in both across- and alongshore macrofaunal distribution studies.
1 INTRODUCTION
In recent decades, numerous studies carried out on beaches of subtropical and temperate regions have identified the factors that regulate macrofaunal community and population patterns, particularly spatial distribution (McLachlan & Defeo, 2018). Overall, differences in zoobenthic populations and communities between beaches and latitudes have been explained by the autecological hypothesis and by morphodynamic states (Brazeiro, 2001; Defeo & McLachlan, 2011; McLachlan et al., 1993; McLachlan & Defeo, 2018). On the other hand, organism distribution within a beach (alongshore and across-shore) is mostly considered a response to environmental gradients, such as exposure to air, temperature, moisture, grain size, and food availability (Defeo & McLachlan, 2013; McLachlan & Defeo, 2018; Takada et al., 2016). However, aspects of habitat heterogeneity in spatial patterns are an underexplored field in beach ecology and are often overlooked, despite important findings on intertidal benthic community zonation (Gingold et al., 2010, 2011; Maria et al., 2013; Schlacher et al., 2014).
Macrotidal sandy beaches frequently have increased complexity of morphological features, which add topographical heterogeneity and new habitats for benthic organisms to the beach landscape (Gingold et al., 2011). A good example of these morphological features is the ridge-and-runnel systems, a series of asymmetrical ridges (sandbars) running parallel to the coast and separated by shallow troughs (runnels) (Mulrennan, 1992; Wright & Short, 1984). The development of this topography is favoured by moderate wave-energy conditions acting on a flat beach with abundant sediment supply (Barbosa et al., 2007; King et al., 1949; Mulrennan, 1992; Scott et al., 2011). Ridge-and-runnel systems may comprise a varying number of sandbars and runnels, which accumulate water during ebb tide (Kroon & Masselink, 2002; Wright & Short, 1984). Runnels remain partially submerged during low tide and partly protected against across-shore currents and are therefore less exposed than sandbars (Gingold et al., 2010).
Studies on the horizontal distribution of benthic communities developed on meso- and macrotidal beaches usually overlook the fauna in the runnels (Gingold et al., 2011). Studies on macrofaunal distribution along ridge-and-runnel systems have been rare so far, and the few studies conducted only refer to specific taxonomic groups (Nonomura et al., 2007; Patel & Desai, 2009). For example, Nonomura et al. (2007), who studied mysids on a beach (low-tide terrace) in Japan, found two typical infralittoral species with high density in the runnels of the mid-littoral. In India, Patel and Desai (2009) found higher bioturbation rates in runnels than in sandbars, associating these findings with more heterogeneous sediment, higher food availability, and bidirectional water flow found in runnels. Indeed, extensive research about this topic is nearly limited to meiofauna (Gingold et al., 2011; Maria et al., 2013, 2018). Meiofaunal studies conducted on ultra-dissipative beaches of the coast of Mexico (Gingold et al., 2011) and Belgium (Maria et al., 2013, 2018) found higher taxonomic and functional diversity within runnels, associated with smaller grain size and higher concentrations of chlorophyll-a in these environments.
In the Amazonian coast, dominated by macrotidal regimes, ridge-and-runnel systems are commonly found in sandy beaches, and may be associated with tidal runnels and ebb-tidal deltas, which drain floodplains containing mangroves and transport fine sediments and nutrients to beaches (Barbosa et al., 2007; El-Robrini et al., 2006; Ranieri & El-Robrini, 2016). Therefore, Amazonian beaches provide an opportunity to assess the potential effects of this type of morphology on macrofaunal structure. Thus, the present study aimed to assess whether the ridge-and-runnel systems affect macrofauna structure (composition, richness, and abundance) and spatial distribution on a Brazilian Amazonian Beach.
2 MATERIALS AND METHODS
2.1 Study area
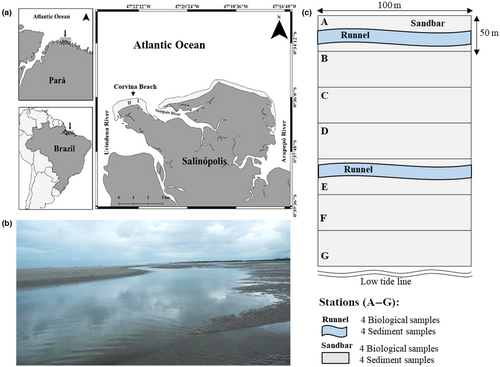
Corvina Beach (0°36′17.2′′S; 47°23′04.7′′W to 0°36′14.6′′S; 47°22′04.4′′W) is located on the Brazilian Amazonian coast (Salinópolis County) and faces the Atlantic Ocean (Figure 1a). This beach is 2 km long and morphodynamically characterized as ultra-dissipative, with low slope (≤1.5°), large intertidal zone (300–600 m), and occurrence of ridge-and-runnel systems, particularly in its eastern portion (El-Robrini et al., 2006; Ranieri & El-Robrini, 2016). The region is dominated by a macrotidal regime, ranging from 4 to 5.3 m (Ranieri & El-Robrini, 2015; Souza Filho, 2005). The supralittoral zone is comprised of dunes, which reach a mangrove in the upper zone. Climate is humid tropical with a mean annual temperature of 27.7 ± 1.1°C (Martorano et al., 1993) and annual rainfall (30-year series) ranging from 2200 to 2800 mm (Moraes et al., 2005).
2.2 Sampling and laboratory procedures
Sampling was carried out during dry season (September 2017), as salinity is higher during this season, and consequently, macrofauna species richness is higher (Rosa Filho et al., 2018); this allows a better assessment of the effect of the ridge-and-runnel system on macrofauna assemblage. Samples were collected in two different intertidal areas (50 m × 100 m; equidistant ∼700 m) of the beach: area 1, with a ridge-and-runnel system, formed by two runnels parallel to the water line; and area 2, without runnels. In each area, seven (A–G) equidistant (50 m apart) stations (ST) were established from the high tide watermark to the swash zone. Sampling occurred during spring low tide period, simultaneously in the two areas. Four biological samples were collected in the ST without runnels (area 2). In area 1 (in A and E STs), four samples were taken from the central portion of the runnel and four were taken from the sandbar (approximately 2 m from the runnel). The upper channel (A ST) was permanently covered by a water slide during low tide both in September and in the months following the sampling of this study (November and December 2017; March and April 2018) (Cardoso E.M.P., personal observation). Along with biological sampling, a sediment sample was collected from each ST (sandbar and runnel) for granulometric and organic matter analyses. For the biological and abiotic sampling, a diameter cylindrical sampler (0.0314 m2) was inserted into the substrate down to a depth of 20 cm. Biological samples were sieved through a 0.3-mm mesh screen and were fixed with 5% saline formalin. Abiotic samples were cooled during transportation to the laboratory, where they were stored in a freezer at −20°C until the analysis.
In the laboratory, biological samples were analysed under a stereoscopic microscope, and all organisms were counted and identified to the lowest possible taxonomic level. The granulometric analysis was performed by sieving out the coarse sediments and pipetting the fine sediments. Mean particle diameter, sorting, % sand, and % gravel were calculated using Sysgran (Camargo, 2006). Dried samples were combusted at 550°C for 5 h to determine organic content (Walkley & Black, 1934).
2.3 Statistical analysis
Richness (total number of taxa) and density (ind m−2) were calculated in each biological sample. These descriptors were compared between areas (1 and 2) and stations (A-G), using a two-way analysis of variance (ANOVA). In area A, independent stations (STs: A and A rn; E and E rn) were considered in-runnel and out-runnel sites. Thus, the station was nested within the area factor. Before the analysis, data normality and homogeneity of variances were tested by applying Kolmogorov–Smirnov and Levene's tests, respectively. Where required, data were fourth-root transformed. When ANOVA results were significant, Tukey's a posteriori test was applied for pairwise comparisons. Applying the same design, ANOVA was also used to assess sediment data.
To assess multivariate patterns of macrobenthic communities, principal coordinate analysis (PCoA) and a permutational multivariate analysis of variance (PERMANOVA) were run on a Bray–Curtis similarity matrix of the fourth root-transformed species-abundance data. To identify the species that characterized each area and tidal station, those species that had a correlation (Spearman's coefficient) of more than 60% with one of the first two axes were plotted in PCO plots.
A distance-based linear model (DistLM; McArdle & Anderson, 2001) was performed to link overall macrofauna structure, richness, and density with environmental variables. This technique analyses and models the relationship among a multivariate data cloud, as described by a resemblance matrix and by predictor variables. The best models in DistLM were chosen using sequential stepwise tests using adjusted R2 and based on AIC selection criterion (Anderson et al., 2008). Prior to the DistLM, the variables that were strongly correlated (Spearman R = .8) were excluded, to avoid high collinearity among environmental predictors. A 5% significance level was considered in all analyses.
3 RESULTS
3.1 Characteristics of sediment
Sediment in both areas was classified as fine sand, well sorted (area 1), and very well sorted (area 2). In general, sediments in area 1 had finer grains, although coarse sandy fraction did occur (STs: B–C) only in this area (Supplementary Material 1). Most sediment parameters varied significantly among areas (Table 1). In area 1, aside from the smaller grain size, there were higher sorting values, per cent of fines (silt and clay) and fine sand, and organic matter. Sediment characteristics of runnels (area 1) did not differ from each other, but were significantly different from most sandbars, which had smaller grain size and higher sorting degree. Furthermore, STs B and C had the largest grain size in area 1. In area 2, there was no difference between tidal stations regarding sediment parameters (Table 1; Supplementary Material).
| ANOVA (F) | Mean grain (Φ) | Sorting | % fines | % VFS | % FS | %MS | %CS | OM % |
|---|---|---|---|---|---|---|---|---|
| Area | 33.3* | 1126.9* | 48.4* | 0.3 | 4.9* | 0.1 | 5.4* | 20.6* |
| Area 2 > Area 1 | Area 1 > Area 2 | Area 1 > Area 2 | ≈ | Area 2 > Area 1 | ≈ | Area 1 > Area 2 | Area 1 > Area 2 | |
| Station | 4.0* | 3.0* | 5.1* | 3.9* | 2.6* | 1.2 | 0.8 | 3.1* |
| rn = runnel |
Area 1: A rn ≈ E rn < B, C Area 2: ≈ |
Area 1: A run ≈ E rn < C Area 2: ≈ |
Area 1: A rn > B–F A ≈ E rn > C Area 2: ≈ |
Area 1: E > C Area 2: ≈ |
Area 1: C > A, D, E rn Area 2: ≈ |
Area 1: ≈ Area 2: ≈ |
Area 1: ≈ Area 2: ≈ |
Area 1: A rn > A–F, E rn Area 2: ≈ |
- Abbreviations: CS, coarse sand; FS, fine sand; MS, medium sand; VFS, very-fine sand.
- * Significant values F (p < .05).
3.2 Macrofauna
Forty-two macrobenthic species (Supplementary Material 2) were reported in the present study; 36 were found in area 1 and 16 were found in area 2. Twenty-four species were associated with runnels; among those, eight were found exclusively in this type of habitat. Annelida was the phylum with the largest number of taxa (14), and was the most abundant group in both areas, despite its different distribution along tidal stations (Figure 2a). In area 1, polychaetes were dominant in the runnels and sandbars of the upper (A–B) and lower SLs (G). Molluscs and crustaceans had higher densities in the intermediate (C–D) and lower SLs (E–F). Insects occurred nearly throughout the tidal zone of area 1, while they were restricted to the upper SLs in area 2 (Figure 2a). In area 2, crustaceans were dominant only in the upper SL (A), while polychaetes dominated all other stations.
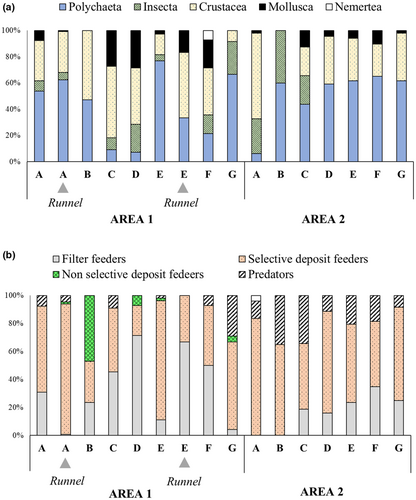
Regarding the contribution of feeding guilds to total abundance, selective deposit feeders dominated in both areas, although nonselective deposit feeders and filter feeders were more abundant in area 1. Filter feeders were present in all STs of area 1 (Figure 2b). In area 2, filter feeders did not occur in the upper STs (A–B) and predators were relatively more abundant (Figure 2b).
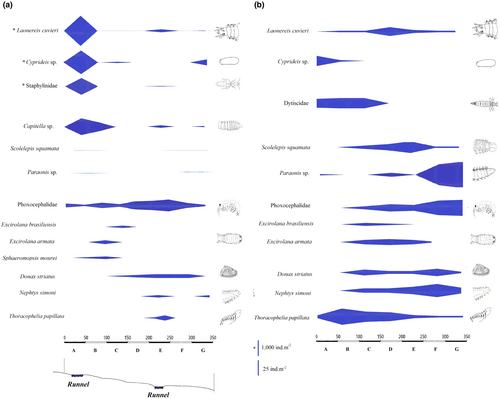
There were clear spatial distribution patterns (areas and stations) for the most abundant macrobenthic taxa. In area 1, the upper runnel (ST: A) was dominated by the polychaetes Laeonereis culveri (Webster, 1879) and Capitella sp., the insect Staphylinidae, and the ostracod Cyprideis sp. (Figure 3a). Phoxocephalidae amphipods were the most abundant taxon in the lower runnel (ST: E), as well as in sandbars (upper and lower STs). The polychaete Thoracophelia papillata (Santos et al., 2004) was restricted to the intermediate and lower SLs (C–F) of this area. Conversely, the upper STs (A–B) in area 2 were dominated by T. papillata and Dytiscidae (insects), while Paraonis sp., Nephtys simoni (Perkins, 1980), and Phoxocephalidae sp. dominated the lower SLs (F-G), and Excirolana armata (Dana, 1853), Scolelepis squamata (Müller, 1806), and L. culveri dominated the middle STs (Figure 3b).
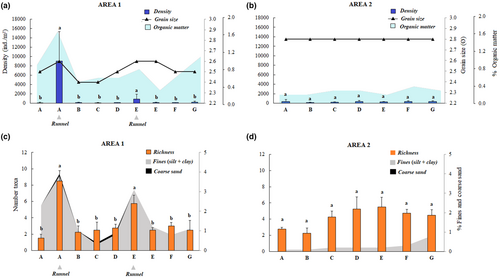
Mean macroinvertebrate densities varied significantly among areas (F1–48= 4.9; p = .03) and stations (F14–48 = 7.9; p < .01). In area 1, the higher mean values were recorded in stations with runnels (mean = 4960 ± 2114 ind m−2) compared to sandbars (126 ± 21 ind m−2) (Figure 4a). Significant differences in richness also were found among areas (F1–48= 4.7; p = .04) and stations (F14–48 = 4.8; p < .01). Richness was higher in area 1 and within the stations with runnels, which differed from sandbars (Figure 4). Therefore, these descriptors did not vary among stations in area 2 (Figure 4b).
PERMANOVA indicated differences in macrofaunal structure among areas and stations (Table 2). In area 1, pairwise tests indicated no differences between the upper (ST: A) and lower (ST: E) runnels, but there were differences between runnels and most sandbars. The macrofauna in runnel E was similar to that of the sandbars of the upper stations (STs: A–B). A significant dissimilarity in sandbars only occurred between A and E STs. In area 2, differences occurred between the upper STs (A–C) and the lower STs (D–G) (Table 2).
| Source | Df | MS | Pseudo—F | P (perm) |
|---|---|---|---|---|
| Area | 1 | 15,510 | 4.230 | 0.001* |
| Station (ST) | 12 | 3768.9 | 2.379 | 0.001* |
| Residual | 50 | 1584.4 | ||
| Total | 63 |
| Pairwise test (stations) | |||||||
|---|---|---|---|---|---|---|---|
| Area 1 | A rn | A | B | C | D | E rn | E |
| A | * | ||||||
| B | * | ns | |||||
| C | * | ns | ns | ||||
| D | * | ns | ns | ns | |||
| E rn | ns | ns | ns | * | * | ||
| E | * | * | ns | ns | ns | ns | |
| F | * | ns | ns | ns | ns | ns | ns |
| G | * | ns | ns | ns | ns | ns | ns |
- Abbreviations: df, degrees of freedom; MS, mean squares; Rn, runnel.
- * Significant differences (p < .05); ns = not significant (p > .05).
The PCoA plots confirmed a distinct spatial configuration of macrofaunal samples from different areas (Figure 5). In area 1 (Figure 5a), axis 1 explained 37.7% of the variation in data and distinguished the macrofaunal samples between habitats (runnels and sandbars). The taxa that were most correlated with the negative side of this axis were Dolichopodidae, Staphilynidae, L. culveri, and Capitella sp., which were more abundant in runnels. On the other hand, on the positive side of axis 1, N. simoni, D. striatus, and Phoxocephalidae were most closely associated with the sandbars. There was no clear separation of sandbars samples from upper and lower STs (Figure 5a).
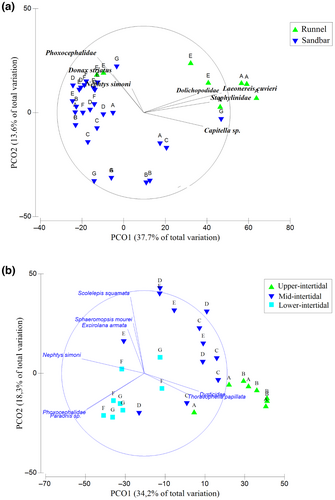
In area 2, two principal groups of samples were identified: group 1 (positive side of axis 1) gathered samples from the upper-intermediate STs (A–D), mostly correlated to T. papilata and Dytiscidae, and group 2 (negative side of axis 1), formed by samples from the lower STs (E–G), was highly correlated to the polychaetes N. simoni, S. squamata, Paraonis sp., and peracarids Phoxocephalidae, Sphaeromopsis morei, and E. armata.
The best DistLM explained approximately 30% of variation in macrofauna structure (Table 3). Organic matter, per cent of fines (silt and clay), and grain size were the environmental variables that were best correlated with overall macrofaunal structure. Regarding richness, per cent of fine and coarse sand was the best correlated. Aside from coarse sand, organic matter and grain size had a higher effect on macrofauna density (Table 3). These variables are shown on the secondary Y-axes of the graphs in Figure 4 for a better appreciation of the relationship between environmental and biological features.
| AIC | r 2 | Variable included | p | |
|---|---|---|---|---|
| Structure | 486.71 | .289 | % OM | .001 |
| % Fine | .001 | |||
| Grain size | .002 |
| Descriptors | Relation | ||||
|---|---|---|---|---|---|
| Richness | 423.05 | .319 | % CS | .028 | Positive |
| % Fines | .048 | Positive | |||
| Density | 457.02 | .326 | % OM | .065 | Positive |
| % CS | .019 | Positive | |||
| Grain size | .050 | Negative | |||
4 DISCUSSION
The effects of the ridge-and-runnel system in alongshore and across-shore macrofaunal distribution show the importance of this system as driver of macrofaunal structure. The increased macrofaunal diversity and density, in area 1 and within runnels, are similar to the findings of previous studies on meiofauna (Gingold et al., 2010, 2011; Maria et al., 2013). The factors that might explain this increase in macrofaunal descriptors, as well as changes in composition, might be summarized by the following aspects: (1) mitigation of the typical environmental harshness of sandy beaches, particularly in the permanently submerged upper runnel (Gingold et al., 2011); (2) changes in sediment, including granulometry and increased food resources; (3) and increased environmental heterogeneity with the presence of microhabitats (sandbar and runnels) across the beach (Gingold et al., 2010, 2011). Although the first points have been considered key factors in the regulation of macrobenthic communities of sandy beaches (Barboza & Defeo, 2015; Barreira et al., 2001; Defeo & McLachlan, 2005; Degraer et al., 2003; James & Fairweather, 1996; Jaramillo & McLachlan, 1993; Neves et al., 2007), habitat heterogeneity approaches have been poorly explored (McLachlan & Defeo, 2018).
Most empirical studies show a positive relationship between habitat heterogeneity and biological diversity (Gallucci et al., 2020), due to the increase in range of resources and decrease in niche overlap, which in turn leads to species coexistence (Chesson, 1991; Downes et al., 1998; Gallucci et al., 2020). ‘Heterogeneity’ or ‘complexity’ implies the existence of different ‘kinds’ of elements that comprise an environment and it occurs at several scales, either spatial or temporal (Hewitt et al., 2007; Tokeshi & Arakaki, 2012). Regarding marine benthic communities, environmental heterogeneity at small spatial scales (cm to few meters) is mostly due to biologically produced habitats, while environmental factors may prevail over biotic features at larger spatial scales (10's m to km) (Gallucci et al., 2020). Macrobenthic distribution on sandy beaches is mostly affected by changes in morphodynamics and beach morphology, resulting in patchiness on different scales, which tends to be much higher (difference of up to one hundred meters) on dissipative beaches than on reflective beaches (McLachlan & Defeo, 2018). The formation of the ridge-and-runnel system in beach facies generates local scale complexity that affects the structure of benthic communities (Gingold et al., 2010, 2011).
Contrasting energy and substrate conditions can be found in ridge-and-runnel macrotidal systems due to their heterogeneous topography (Gingold et al., 2010). Sandbars are environments with high mobility and with higher wave rework, while channels are more protected from the action of waves (Aagaard, 1991; Kroon & Masselink, 2002; Mulrennan, 1992). In addition, channels tend to accumulate water, debris, microalgae, and particulate organic matter and are more subject to cohesion and stabilization of sediment, which might be comprised of an array of types, from mud to gravel (Christie et al., 2000; Degraer et al., 2003; Gingold et al., 2010, 2011; Maria et al., 2013; Patel & Desai, 2009). In area 1 of Corvina Beach, grain heterogeneity (fine, fine sands, medium and coarse sands), which means less sorted sediments, might be an indicator of higher sedimentation and lower action of waves and currents in grain transport, especially within the channels.
In area 1, the presence of species that are typical of both sandy and muddier sediments results in an increase in density and in both taxonomic and functional richness of the benthic assembly. For instance, the polychaetes Heteromastus sp., Notomastus sp. and Isolda pulchella, which occurred exclusively inside the channels, are deposit feeders typical of muddy environments with high organic content, such as mangroves (Fernandes, 2003; Rosa Filho et al., 2006). The species Laeonereis culveri, Capitella sp. and Cyprideis sp., which had high abundance in area 1, are also deposit feeders with higher affinity to muddy sediments (Angonesi, 2005; Dean, 2001; Lana, 1986; Santos et al., 2020). In addition, the polychaetes T. papillata, N. simoni and S. squamata, typical of Amazonian sandy beaches, also occurred in this area (Rosa Filho et al., 2009, 2011; Santos et al., 2021, 2022; Santos & Aviz, 2018, 2020).
Although deposit feeder is the feeding habit of most macrofaunal taxa on Corvina Beach, as is common on dissipative beaches with fine sediment (Brown & McLachlan, 1990; Dewarumez, 1983), some significant changes in trophic group distribution were observed between areas. In area 1 (with channels), the higher participation of nonselective deposit feeders (e.g., Capitella sp. and Heteromastus sp.), might be due to the organically richer sediment (Fauchald & Jumars, 1979). In the same area, filter feeders occurred at all mid-littoral stations, while in the area without channels they were present from the station C (approximately 100 m from the swash line) onwards, thus avoiding desiccation of the upper stations (STs: A–B). The fact that filter feeders are limited to the intermediate and upper mid-littorals and thus spend a longer period of time submerged by the tide is an expected trend for sandy beaches (Defeo & McLachlan, 2011). Therefore, the presence of runnels in the upper intertidal allowed the occurrence of filter feeders in this part of the beach, for example, the bivalve Austromacoma constricta and the shrimp Ogyrides occidentalis.
The differences in across-shore macrofaunal distribution were remarkable between sampling areas. The runnels, even in the upper tidal station, were richer and much denser than sandbars, even the runnels located at lower tidal stations. Increase in food resources (organic matter) favours the colonization by organisms, mainly detritivores, which often exhibit opportunistic behaviour (Angonesi, 2005). On the other hand, in the area without channels, richness and density increased towards the low tide line, reflecting the physical gradients on the beach (Armonies & Reise, 2000; McLachlan, 1980; McLachlan & Jaramillo, 1995; Santos et al., 2021).
Macrofaunal composition was similar between channels, even though they were located at a station far from the beach. In addition, macrofaunal similarity between the ST channel E and sandbars in upper beach stations (STs: A–B), as well as between upper and lower sandbars, indicates that some species are largely distributed throughout the beach. These results suggest that although the habitat heterogeneity increased in the area 1, physical gradients (e.g., moisture) that usually define the macrofaunal zonation patterns could have been mitigated. In fact, the high moisture levels in the runnels can sometimes occur on the sandbars (Vanhée et al., 2002). The macrobenthos vertical zonation has been reported in Amazonian macrotidal sandy beaches, with two to three faunal zones being identified (Rosa Filho et al., 2009, 2011; Santos et al., 2021).
The channel allowed the colonization by some species at the upper stations of the beach, as previously discussed on distribution of filter feeders. Another example is the amphipod Phoxocephalidae, which was reported at all tidal stations in area 1, but did not occur in the upper ST (A) in area 2. One more peculiar finding in area 1 was the high density of insects and their distribution throughout the mid-littoral. The high density of insect larvae in the upper channel (ST: A) might be due to the accumulation of water and organic matter, which thus created environments favourable to egg laying by adults. The presence of these larvae throughout the mid-littoral might result from their transport during tidal movements. Insects were expected to be limited to parts of the upper mid-littoral, as occurred in the area of Corvina Beach without channels and as observed on other Amazonian beaches (Rosa Filho et al., 2009, 2011; Santos et al., 2021, 2022).
Macrofaunal composition was dissimilar in the area without channels, especially comparing the upper stations (A–C) to the lower stations (E–G). The organisms that predominated in the upper stations (A–B) are those with higher tolerance to desiccation and stress caused by the tidal cycle, such as insects and T. papillata, a polychaete with high ability for vertical migration (down to 20 m of depth in the substrate) and tolerance to anoxia (Fauchald & Jumars, 1979; Neves et al., 2007; Rouse & Pleijel, 2001; Ruby & Fox, 1976). In the intermediate and lower parts, which are more moist, other polychaete species were more abundant (e.g., N. simoni, Paraonis sp. and S. quamata), as was the amphipod Phoxocephalidae.
Interestingly, the polychaete T. papillata, which was an abundant species present at all stations in area 2, occurred only in ST E in area 1. The low occurrence of T. papillata in the area with ridge-and-runnel system might be due to the increase in fine sediments, since this species is associated with sandy sediments (Dales, 1952; Neves et al., 2007), or even due to the higher competition with other infaunal deposit feeders, such as L. culveri and Capitella sp. Changes in sediment and/or competition might also explain why N. simoni, Paraonis sp. and S. squamata, which were abundant in area 2, had a very low abundance in area 1. There is massive evidence that biological interactions play a significant role in structuring sandy beach macrofaunal communities, which tend to have an increasing importance under milder environmental conditions (e.g., dissipative beaches or undisturbed systems) (Defeo & McLachlan, 2005).
The distribution of the bivalve D. striatus seems also to have been affected by the presence of channels. The highest densities of this species occurred in the area without channels, and D. striatus was most closely associated with sandbars in the area with channels. The absence of D. striatus in the upper intertidal of area 1 (ST: B) contrasts with the presence of this bivalve in the same station of area 2. In addition, the presence of D. striatus at the upper intertidal stations (ST: A and B) on two Amazonian beaches without runnels (Santos et al., 2022) reinforces that these microhabitats are not favourable to this bivalve.
Areas with ridge-and-runnel system may be less favourable to D. striatus because channels provide increased protection against wave action and are microhabitats less exposed to cross-shore currents than sandbars (Gingold et al., 2010). Donacids are tidal migrant filter feeders typically found in habitats characterized by strong wave action or high current speed (Allen, 1983), such as the swash zone of sandy beaches (Brazeiro & Defeo, 1996; Brown & McLachlan, 1990; Gianuca, 1983; Neves & Bemvenuti, 2009). Clams of the Donax species migrate seaward during falling tides and shoreward during rising tides and tend to ride the largest waves that have the largest excursion (Ellers, 1995). Therefore, donacids prefer more exposed shores and high hydrodynamics, which keep organic detritus in suspension and promote tidal migration.
5 CONCLUSIONS
The results shown here add knowledge to sandy beach ecology, considering that the patterns established for macrofauna nowadays are driven by data obtained in temperate and subtropical beaches, with microtidal regime. The present study is pioneering as it describes changes in macrobenthic community distribution in response to the ridge-and-runnel systems, which is a common morphology in dissipative beaches. The results showed composition changes and a significant increase in macrofaunal abundance and richness in a beach area with this system, as well as within runnels.
ACKNOWLEDGMENTS
The first author was granted scholarship by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brazil (CAPES), Number 1732800. We also thank our LOB-UFPA colleagues who helped in the field and particularly Afonso Quaresma de Lima, Technician from the Institute of Geosciences of UFPA. Thanks are also due to the anonymous reviewers for their comments, which helped us to improve the manuscript.
CONFLICT OF INTEREST STATEMENT
We have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




