Do intertidal Zostera noltei meadows represent a favourable habitat for amphipods? The case of the Kneiss Islands (Gulf of Gabès: Central Mediterranean Sea)
Abstract
The structure, diversity, spatial and seasonal distributions of amphipod assemblages associated with intertidal Zostera (Zosterella) noltei meadows were studied around the Kneiss Islands (central Mediterranean Sea). This site represents a site of international interest in terms of its ornithological diversity (Important Bird Area, Ramsar Site and SPAMI). The amphipod fauna was sampled at 32 stations in spring 2014. A total of 6,482 individuals belonging to 78 species and 22 families were identified; among these taxa, Lysianassa ceratina is new for the Tunisian amphipod inventory, whereas 25 species are identified for the first time in the Gulf of Gabès. The dominant species are Cymadusa filosa, Microdeutopus gryllotalpa, Gammarus insensibilis, Dexamine spiniventris, Monocorophium insidiosum, Elasmopus rapax, Melita palmata and Leucothoe incisa. Four amphipod assemblages are identified using MDS analysis. The distribution of amphipod assemblages is linked to several environmental factors, such as sediment type, organic matter content and distance from the shore, which is itself associated with a clear inshore–offshore gradient. Seasonal variations of the amphipod assemblage patterns at eight stations, sampled between April 2014 and January 2015, show a maximum abundance and diversity during summer and autumn, with a minimum in winter. These seasonal fluctuations may be related to many factors such as variations of climatic factors (e.g. temperature, salinity), the growth and production rates of Zostera noltei meadows and seasonal patterns in the life cycle of dominant species.
1 INTRODUCTION
Among the groups that dominate benthic communities, amphipods are considered as one of the fundamental components of marine bottom communities (Balian, Segers, Lévèque, & Martens, 2008; Marques & Belllan-Santini, 1993). They are a diverse, abundant and species-rich group of peracarid crustaceans. Most are free-living marine species, although some are terrestrial, and are present in both freshwater and marine environments. In marine ecosystems, they have been recorded from the intertidal zone to hadal depths (Väinölä et al., 2008). Amphipods are mostly eurythermal and euryhaline, inhabiting a wide variety of habitats with a few species showing a cosmopolitan distribution. They influence the structure of estuarine and coastal benthic communities by virtue of their high abundance (Saunders, Attrill, Shaw, & Rowden, 2003; Tanner, 2006), and varied reproductive strategies and behaviour (Johnson, Stevens, & Watling, 2000). Moreover, amphipods play an important role in benthic communities (Zakhama-Sraieb et al., 2011; Guerra-Garcia et al., 2014) and are involved in food webs for aquatic birds and economically important demersal fish (Caine, 1991; Dauvin & Desroy, 2005). Furthermore, crustacean amphipods are used as bioindicators of marine pollution and environmental stress due to their habitat at the sediment–water interface, their relatively long lifespan and sedentary behaviour (Bellan-Santini, 1981; Dauvin & Ruellet, 2007; de-La-Ossa-Carretero, Del-Pilar-Ruso, Giménez-Casalduero, Sánchez-Lizaso, & Dauvin, 2012). They are considered as a key element of marine and estuarine monitoring programmes (Ysebaert & Herman, 2002) and may be used as a proxy in the determination of ecosystem dynamics and ecological quality status (Dauvin, Andrade, & de-La-Ossa-Carretero J.A., Del Pilar Ruso Y., Riera R., 2016). In that context, amphipod species have been included in several biotic indicators for assessing the ecological quality status (EcoQ) of marine ecosystems, as sensitive species such as opportunistic polychaetes are resistant and proliferate with increasing organic matter content in sediment (Dauvin et al., 2016; Valério-Berardo, Flynn, & Wakabara, 2000).
The Mediterranean Sea is one of the most important hot spots of amphipod diversity in the world, with more than 500 species recorded (Christodoulou, Paraskevopoulou, Syranidou, & Koukouras, 2013; Coll et al., 2010; Dauvin, Grimes, & Bakalem, 2013; Mosbahi, Dauvin, & Neifar, 2015a). Following the pioneering work on Tunisian amphipods in the early twentieth century (Chevreux, 1910, 1911), there was a long interval until the studies of Zakhama-Sraieb, Sghaier, and Charfi-Cheikhrouha (2008), Zakhama-Sraieb, Sghaier, and Charfi-Cheikhrouha (2009, 2010, 2011, 2017), mainly on the north coast of Tunisia, which led to the compilation of an inventory of the amphipod fauna in April 2017 listing 138 species belonging to 79 genera and 37 families.
Several studies have been conducted to identify the amphipod fauna of meadow habitats in the Mediterranean Sea, mainly along the northern shore (Camisa et al., 2017; Gambi, Lorenti, Russo, Scipione, & Zupo, 1992; Reynolds, Carr, & Boyer, 2012; Sanchez Jerez, Barbera, & Ramos Espla, 1999; Scipione, Gambi, Lorenti, Russo, & Zupo, 1996; Scipione & Zupo, 2010). However, data on amphipods associated with Posidonia oceanica meadows are scarce and fragmented along the southern coasts of the Mediterranean. For example, along the Tunisian coast, Zakhama-Sraieb et al. (2011) have studied the structure, diversity and spatial distribution of the amphipod fauna, recording 44 species belonging to 12 families associated with the Posidonia meadow habitat. Based on a seasonal monitoring survey in 2016–2017, Fersi, Dauvin, Pezy, and Neifar (2018) have recorded 45 amphipod species and 21 families in four subtidal channels of the Gulf of Gabès. The amphipod fauna is dominated by a small number of species characteristic of areas with detritus accumulation and seagrass meadow, mainly composed of Posidonia oceanica, Cymodocea nodosa and Halophila stipulacea.
Likewise, in the Mediterranean, dwarf eelgrass meadows associated with amphipod fauna have been seldom considered compared with other areas worldwide, likely because these seagrasses are quite rare and mostly limited to shallow coastal lagoons (Rueda & Salas, 2008; Sfriso, Birkemeyer, & Ghetti, 2001). By contrast, other seagrasses such as P. oceanica are widely represented in the shallow waters of the Mediterranean Sea. To our knowledge, there has been no scientific research on amphipod patterns associated with Zostera (Zosterella) noltei meadows. Nevertheless, these dwarf eelgrass beds are considered as the climax intertidal community in the Mediterranean (Tagliapietra, Pessa, Cornello, Zitelli, & Magni, 2016).
With a view to increasing our knowledge of the amphipods associated with Z. noltei meadows, we conducted the present study: (a) to analyse the composition and structure of the amphipod fauna associated with Z. noltei mudflats around the Kneiss Islands in the Gulf of Gabès; (b) to analyse the spatial and temporal patterns of amphipod assemblages; (c) to identify the role of the main environmental factors controlling the distribution of amphipod assemblages, and (d) to compare our results with studies carried out on other meadow habitats along the Mediterranean coasts, concerning not only amphipods but also polychaetes that form the dominant macrofauna from the intertidal zone of the Gulf of Gabès.
2 MATERIALS AND METHODS
2.1 Study area
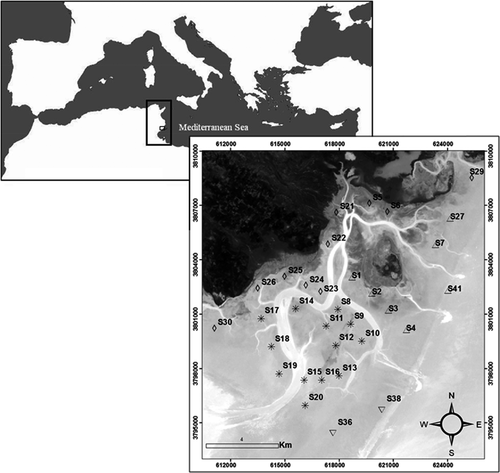
Located in the north-western part of the Gulf of Gabès, between latitudes 34˚10'N-34˚30'N and longitudes 10˚E-10˚30'E, the intertidal zone around the Kneiss Islands extends over an area of 220 km2 (Figure 1). This area displays the highest tidal range in the Mediterranean Sea; the tide is semi-diurnal, with a distinct spatial pattern in amplitude showing a maximum of 2.3 m at the margins and decreasing towards the middle of the gulf during spring tides (Sammari, Koutitonsky, & Moussa, 2006). At low tide, the Kneiss Islands are surrounded by vast mud and sand flats (Abdennadher et al., 2010), covered by eelgrass beds (Zostera (Zosterella) noltei Hornemann) (Mosbahi, Boudaya, Dauvin, & Neifar, 2015b). The Kneiss Islands represent a site of international interest in terms of their bird diversity, which was declared a national nature reserve in 1993, and then established as a “Specially Protected Area of Mediterranean Importance” (SPAMI) in 2001, an “Important Bird Area” (IBA) in 2003 and designated as a “RAMSAR site” since 2007.
2.2 Sampling and laboratory procedures
Samples of the amphipod fauna were collected at low tide using a corer with a sampling area of 0.0225 m2. Five replicates were carried out at each station: four samples for biological analysis covering a total surface area of 0.09 m2, and one for sediment analysis. Sampling consisted of collecting the topmost 20 cm of the sediment, being carried out at 32 stations in April 2014, in areas corresponding to fully extended Z. noltei seagrass beds (Figure 1). The sediment analyses (proportions of mud, fine sand, medium sand and coarse sand) and organic matter contents measured in the sediment are the same as those reported in a previous paper on polychaete structure and seasonal patterns (Mosbahi, Dauvin, & Neifar, 2017).
Moreover, to identify the seasonal changes in amphipods associated with Zostera meadow diversity, eight stations (two stations for each sediment type) were each sampled four times (in April, July and October 2014, and in January 2015). Each sample was sieved through a 1-mm mesh; the remaining fraction was fixed in 4% buffered formalin and stained with Rose Bengal. Concurrently, Z. noltei shoots were collected. In the laboratory, the leaves of each shoot were removed and their length and width measured. The Leaf Area Index, corresponding to the leaf surface area of Z. noltei per 1 m2 of surface covered (m2 per m2), was also calculated. In addition, some parameters of the water column were measured in situ during each expedition, including temperature, salinity and pH using a thermometer (WTW LF 196), a salinometer (WTW LF 196) and a pH meter (WTW 3110).
In the laboratory, the amphipod fauna was sorted, identified to species level under the stereomicroscope and binocular microscope and then counted. Species number corresponds to the total number of species recorded in 0.09 m2, whereas abundance is converted to the number of individuals per 1 m2. The nomenclature of amphipod species was updated according to the World Register of Marine Species (WoRMS, last accessed on 20 April 2020).
2.3 Data analysis
2.3.1 Univariate analysis
The specific richness (S) and abundance (A) are used to describe the type of spatial–temporal spreading involved in amphipod settlement. The degree of the presence of each species (number of stations at which the species id found) is determined to assess their qualitative and quantitative impact on the settlement. Shannon's (H') diversity index (Shannon & Weaver, 1963) and Pielou's (J') evenness index (Pielou, 1966) are the two diversity indices used in this study, giving additional information about the structure of amphipod assemblages and how individuals are distributed within the different species.
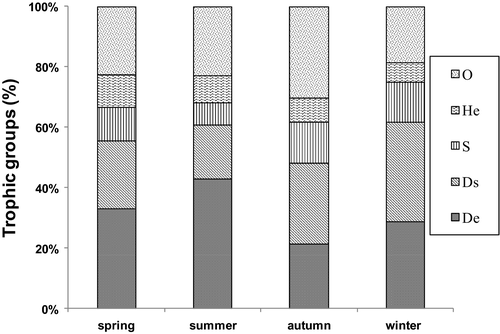
Functional biodiversity is analysed taking into account the nature, origin and food feeding mode of each species: S, suspension feeders; Ds, deposit-suspension feeders; He, herbivores; De, deposit feeders; and O, omnivores. These trophic groups are considered here according to the recent literature (Guerra-Garcia et al., 2014; Zaabar, Zakhama-Sraieb, Charfi-Cheikhrouha, & Sghaier, 2015; Zakhama-Sraieb et al., 2011).
2.3.2 Statistical analysis
ANOVA (analyses of variance) is applied with a one-factor between-subjects design for statistical testing of the differences in the species richness, abundance (for single species or total abundance), diversity index and evenness between all stations and seasonal samples. A post hoc Tukey test (p < .05) is used for a posteriori multiple comparisons. A Shapiro–Wilk normality test and a Bartlett's test for homogeneity of variances are performed prior to each ANOVA. Then, ANOVA is performed to assess the influence of environmental features (sediment type, shoot density, leaf length and width, number of leaves per shoot) and seasonal variations (spring, summer, autumn and winter) on patterns of amphipod diversity in the intertidal zone of the Kneiss Islands. These statistical procedures are carried out using the R software (ade4).
2.3.3 Multivariate analysis
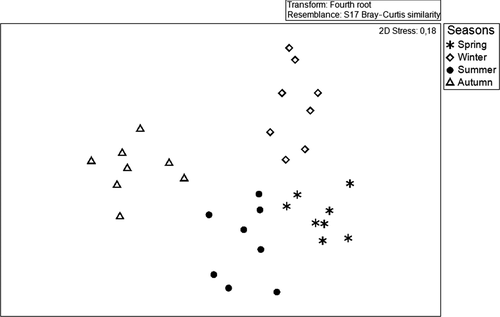
The original data consist of a “stations × species” matrix, which is obtained after removing rare species. Species are considered as rare when they occur at fewer than three stations (10 species are considered as rare). Amphipod fauna abundances are firstly square-root-transformed, to maintain the contribution of rarer species without over-weighting. A non-metric multidimensional scaling method (n-MDS) based on the Bray–Curtis similarity allows us to assess differences in amphipod assemblages between stations sampled in spring 2014. The seasonal patterns of the amphipod assemblages is also determined by non-metric multidimensional scaling (n-MDS) analyses based on the Bray–Curtis similarity using fourth-root-transformed abundances for each season. The structure of amphipod assemblages between seasons and sites is then compared by a two-way permutational multivariate analysis of variance (PERMANOVA, Anderson, 2001), with Station (St, 32 levels) and Season (Se, 4 levels). The significance of the differences between the obtained groups of samples is assessed by analysis of similarity (ANOSIM) according to the considered factors (e.g. spring samples versus. summer samples), in order to identify the groups of species inhabiting the Zostera noltei meadows throughout the year. A SIMilarity PERcentages (SIMPER) test is performed using PRIMER®-v6 (Plymouth Routines in Multivariate Ecological Research) software package (Clarke & Gorley 2006) to determine which species contribute most to within-group similarity.
3 RESULTS
3.1 Environmental parameters
The granulometric analyses revealed that there were four groups of sediment types in the intertidal zone of the Kneiss Islands (previously described in Mosbahi et al., 2017). Zostera noltei eelgrass beds colonized all the sampled stations. Physical and chemical parameters appeared similar across the 32 stations sampled in April 2014. On the contrary, these parameters showed a seasonal variability between the eight stations sampled during the four seasons from 2014 to 2015.
3.2 Seasonal variation of Zostera noltei traits
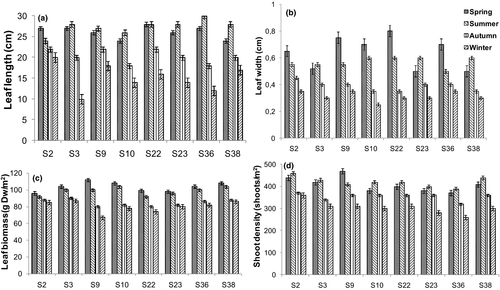
Shoot density was maximal in spring and summer (more than 370 shoots/m2) and minimum in autumn and winter (less than 370 shoots/m2), whereas leaf length and leaf width were maximal in spring and summer (more than 22 cm and 0.5 cm, respectively). Also, leaf biomass was maximal in spring and summer (above 90 g DWm−2) and minimal in winter (Figure 2). Rhizome biomass ranged from 80 to 100 g DWm−2 and remained very similar throughout the year, whereas the Leaf Area Index (LAI) was maximal in spring and summer (between 1 and 2 m2 of Z. noltei per m−2 of sediment surface). The traits of Z. noltei around the Kneiss Islands showed significant spatial and temporal variations (for the both cases, p < .05).
3.3 Faunal parameters
A total of 6,482 individuals were sampled, belonging to 78 species and 22 families (Appendix). In fact, the most diverse families were Aoridae (8 species), Lysianassidae (8 sp), Corophiidae (7 sp), Ampeliscidae (6 sp), Caprellidae and Caprellidae (5 sp). However, the most abundant families were the Gammaridae, Dexaminidae, Lysianassidae and Leucothoidae. The most dominant species were Gammarus insensibilis Stock, 1966, Dexamine spiniventris (Costa, 1853), Microdeutopus gryllotalpa Costa, 1853, Cymadusa filosa Savigny, 1816, Melita palmata (Montagu, 1804) and Leucothoe incisa Robertson, 1892. For the 32 stations sampled together in spring 2014, the faunistic parameters showed a marked variability, with abundance ranging from 1,220 to 6, 106 ind.m−2 (with a mean abundance of 2,280 ± 210 ind. m−2), specific richness from 8 to 22 taxa, Pielou's evenness from 0.66 to 0.90 and the Shannon index from 2.94 to 4.02 bits.ind. Diversity indices showed a significant difference between the four amphipod assemblages. Among the recorded species, Lysianassa ceratina was new for Tunisian waters, and 25 species were identified for the first time in the Gulf of Gabès (Appendix). According to trophic level and feeding mode of the sampled species, there were 23 deposit-suspension feeders (29.5%), 19 omnivores (24.5%), 17 plant deposit feeders (22%), 11 herbivores (14%) and 8 suspension feeders (10%; Appendix). Overall, deposit-suspension feeders and omnivores were the dominant trophic group represented in the meadow habitat.
3.4 Characteristics of the different amphipod assemblages
At 65% similarity level, the non-metric multidimensional scaling (n-MDS) plot allowed us to discriminate four groups of stations corresponding to four amphipod assemblages (Figure 3).
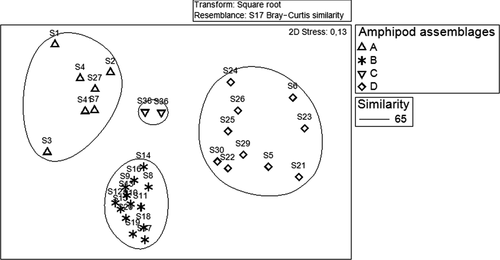
Assemblage A was clustered together at seven stations located closest to the northern coast of the Kneiss Islands, mainly made up of fine sand showing the greatest enrichment in organic matter, and was characterized by numerical dominance and a major contribution to the sampled amphipod population (62.5%). This assemblage was mostly represented by the Caprellidae species Caprella acanthifera Leach, 1814, Caprella liparotensis Haller, 1879, and Pseudoprotella phasma (Montagu, 1804) and species of Corophiidae such as Monocorophium insidiosum (Crawford, 1937) and Leptocheirus bispinosus Norman, 1908.
Assemblage B (42%) was clustered at thirteen stations composed solely of medium sand sediment. This assemblage was dominated by Microdeutopus gryllotalpa Costa, 1853, Melita palmata (Montagu, 1804), Elasmopus rapax Costa, 1853, and Quadrimaera inaequipes (A. Costa, 1857).
Assemblage C (27.5%) made up a group characterized by only two stations sampled in the deeper zone of the Kneiss Islands characterized by coarse sand, relatively poor in organic matter. This assemblage was represented by Ampelisca spp., Aora spp., Lysianassa pilicornis (Heller, 1866) and Ericthonius brasiliensis (Dana, 1853).
Finally, assemblage D (48%) comprised ten stations composed of mud sand, more strongly represented by Leucothoe incisa Robertson, 1892, Dexamine spiniventris (Costa, 1853) and Gammarus insensibilis Stock, 1966. The two-way PERMANOVA showed a significant influence of the factor St (F = 2.214, p < .005) on the structure of amphipod assemblages (Table 1).
| Group | Mean similarity (%) | Main species (% contribution to the similarity) | Mean abundance (ind.m−2) |
|---|---|---|---|
| A | 62.5 | Caprella acanthifera−47.70 | 512 |
| Caprella liparotensis−44.62 | 443 | ||
| Pseudoprotella phasma−36.42 | 438 | ||
| Monocorophium insidiosum−33.20 | 420 | ||
| Leptocheirus bispinosus−30.5 | 308 | ||
| Cymadusa filosa−19.6 | 187 | ||
| Ampithoe helleri−11.02 | 204 | ||
| B | 42 | Microdeutopus gryllotalpa−62.8 | 387 |
| Melita palmata−58.6 | 602 | ||
| Elasmopus rapax−52.5 | 460 | ||
| Quadrimaera inaequipes−34.6 | 280 | ||
| Hyale grimaldii−27.6 | 108 | ||
| Leptocheirus bispinosus−26.3 | 206 | ||
| Microdeutopus anomalus−19.2 | 246 | ||
| C | 27.5 | Ampelisca serraticaudata−56.8 | 409 |
| Ampelisca brevicornis−51.02 | 162 | ||
| Aora gracilis−43.7 | 292 | ||
| Aora spinicornis−38.9 | 255 | ||
| Lysianassa pilicornis−27.9 | 412 | ||
| Ericthonius brasiliensis−12.8 | 187 | ||
| Gammaropsis maculata−8.44 | 202 | ||
| D | 48 | Leucothoe incisa−54.2 | 422 |
| Dexamine spiniventris- 50.3 | 320 | ||
| Gammarus insensibilis−48.38 | 380 | ||
| Leucothoe spinicarpa−43. 12 | 166 | ||
| Orchestia mediterranea−36.5 | 241 | ||
| Maera hirondellei−29.12 | 168 | ||
| Dexamine spinosa−17.4 | 302 |
It was noteworthy that the four amphipod assemblages identified by n-MDS appear similar for all four groups of sediment separated by cluster analysis, indicating that the distribution of amphipod fauna in the intertidal soft bottom of the Kneiss Islands was predominantly related to edaphic factors, particularly the sediment characteristics and organic matter content. Hence, in the following section, we considered the seasonal variations of these four amphipod assemblages by analysing their ecological and diversity structure over a period of one year.
3.5 Seasonal variation of ecological and diversity indices of amphipod communities
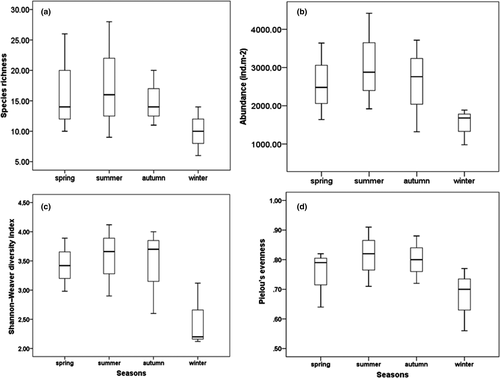
The boxplots (Figure 4) showed the seasonal variations in the diversity indices of amphipod assemblages associated with Zostera noltei meadows of the Kneiss Islands. The abundance of amphipods showed a significant seasonal variation (ANOVA; F = 13.4, p < .05), with maximum values in summer (average abundance = 3,692 ± SD 597 ind.m−2) and spring (3,097 ± 610 ind.m−2), whereas the lowest abundance was recorded in autumn (2,640 ± 212 ind.m−2) and winter (1,452 ± 371 ind.m−2). The species richness reached a value of 39 species in summer (mean: 20; max: 28; min: 10), 48 species in spring (mean: 17; max: 26; min: 12), 32 species in autumn (mean: 15; max: 20; min: 11) and 22 species in winter (mean 10; max: 14; min: 7). The species richness varied significantly between seasons (ANOVA; F = 8.06, p < .05). Shannon's and Pielou's indices showed a significant temporal variation (ANOVA; F = 2.21, p < .05; and F = 4.11, p < .01 respectively), with higher values being obtained in summer and lower values in winter. Both ecological and diversity indices displayed a significant spatial variation over the year (Table 2). Likewise, the trophic groups showed considerable temporal (ANOVA; F = 16.01, p < .01) and spatial fluctuations (ANOVA; F = 4.26, p < .05). All year round, it was noteworthy that deposit-suspension feeders, deposit feeders and omnivores were the most abundant trophic groups (Figure 5). Based on the quantitative data, the structure and composition of the amphipod assemblages showed a significantly different seasonal grouping (RANOSIM = 0.62, p < .001) (Figure 6; Table 3). Differences between spring and summer (average dissimilarity 42.5%) could be related to the higher abundance of (1) Microdeutopus gryllotalpa, Leucothoe spinicarpa and Leucothoe incisa in spring and (2) Gammarus insensibilis, Monocorophium insidiosum and Cymadusa filosa in summer. Differences between summer and autumn (39.8%) were related to the higher abundance of (1) Dexamine spiniventris, Apocorophium acutum Chevreux, 1908 () and Lysianassa pilicornis (Heller, 1866) in summer and (2) Liljeborgia dellavallei, Orchestia gammarellus (Pallas, 1766) and Elasmopus brasiliensis (Dana, 1855) in autumn. Between autumn and winter (51.3%), the differences were related to the higher abundance of (1) Pseudoprotella phasma, Leucothoe spinicarpa and Lysianassa costae (H. Milne Edwards, 1,830) in autumn and (2) Melita palmata (Montagu, 1804) and Gammarus insensibilis (Stock, 1966) in winter. Between winter and spring, the differences (62.5%) were related to the lower abundance of (1) Ericthonius brasiliensis (Dana, 1853), Lysianassa longicornis (Lucas, 1846) and Orchestia mediterranea (Costa, 1853) in winter and (2) the lower abundance of Leucothoe spinicarpa, Elasmopus rapax and Hyale grimaldii in spring. The community structure of the four amphipod assemblages showed a seasonal variability. Throughout the year, assemblage A was dominated by Monocorophium insidiosum, Cymadusa filosa and Pseudoprotella phasma. Assemblage B was strongly represented by Melita palmata Microdeutopus gryllotalpa and Microdeutopus anomalus. Assemblage C was characteristic of deeper stations away from the coast dominated by the suspension feeder Ampelisca serraticaudata and the omnivorous Lysianassa pilicornis. Finally, assemblage D was characterized by the dominance of deposit feeder species Dexamine spiniventris and Gammarus insensibilis. The two-way PERMANOVA also revealed a significant influence of factor Se on the structure of amphipod assemblages Se (F = 2.842, p < .001).
| Factors | ddl | F | p | Tukey test | |
|---|---|---|---|---|---|
| Organic matter | Season | 3 | 11.2 | <.001 | Spr ≠ Win, Sum ≠ Aut |
| Stations | 11 | 3.04 | <.001 | ||
| Temperature | Season | 3 | 19.04 | <.05 | Win ≠ Spr, Sum, Aut |
| Stations | 11 | 0.1 | .65 | ||
| Salinity | Season | 3 | 3.2 | <.001 | Win ≠ Spr, Sum, Aut |
| Stations | 11 | 0.1 | .52 | ||
| PH | Season | 3 | 1.04 | .64 | |
| Stations | 11 | 0.13 | .7 | ||
| Richness species | Season | 3 | 8.06 | <.05 | Win ≠ Spr, Sum, Aut |
| Stations | 11 | 11.32 | <.05 | ||
| Abundance | Season | 3 | 13.42 | <.05 | Win ≠ Spr, Sum, Aut |
| Stations | 11 | 24.2 | <.05 | ||
| Shannon–Weaver diversity | Season | 3 | 2.21 | <.001 | Win ≠ Spr, Sum, Aut |
| Stations | 11 | 4.2 | <.05 | ||
| Pielou's evenness | Season | 3 | 4.11 | <.001 | Win ≠ Spr, Sum, Aut |
| Stations | 11 | 2.5 | <.05 | ||
| Trophic groups | Season | 3 | 16.01 | <.01 | Win ≠ Spr≠Sum, Aut |
| Stations | 11 | 4.26 | <.05 |
| Group | SIMPER average (%) | Main species | Abundance (ind.m−2) |
|---|---|---|---|
| spring versus summer | 42.5 | Gammarus insensibilis- 58.9 | 760 |
| Microdeutopus gryllotalpa- 44.5 | 802 | ||
| Leucothoe spinicarpa- 40.2 | 380 | ||
| Leucothoe incisa- 32.8 | 512 | ||
| Monocorophium insidiosum- 29.8 | 452 | ||
| Cymadusa filosa- 25.5 | 305 | ||
| Dexamine spiniventris- 20.8 | 394 | ||
| Ampelisca serraticaudata- 12.4 | 128 | ||
| Caprella acanthifera- 6.2 | 224 | ||
| summer versus autumn | 39.8 | Dexamine spiniventris- 46 | 584 |
| Orchestia gammarellus- 42.1 | 272 | ||
| Apocorophium acutum- 36.4 | 198 | ||
| Elasmopus brasiliensis- 30.4 | 226 | ||
| Lysianassa pilicornis- 26.7 | 246 | ||
| Liljeborgia dellavallei- 18.5 | 303 | ||
| Dexamine spinosa- 16.2 | 416 | ||
| Pseudoprotella phasma−12.7 | 266 | ||
| Corophium orientale−10.3 | 382 | ||
| autumn versus winter | 51.3 | Pseudoprotella phasma- 53.4 | 408 |
| Melita palmata−48.7 | 662 | ||
| Leucothoe spinicarpa- 46.4 | 364 | ||
| Lysianassa costae−37.6 | 248 | ||
| Gammarus insensibilis−28.1 | 468 | ||
| Microdeutopus anomalus- 20.6 | 390 | ||
| Apocorophium acutum−19.5 | 104 | ||
| Elasmopus brasiliensis- 16.4 | 226 | ||
| Ampelisca serraticaudata- 12.2 | 218 | ||
| winter versus spring | 62.5 | Ericthonius brasiliensis- 48.5 | 326 |
| Lysianassa pilicornis- 42.6 | 438 | ||
| Orchestia mediterranea- 37.4 | 319 | ||
| Leucothoe spinicarpa−30.4 | 256 | ||
| Elasmopus rapax- 29.7 | 322 | ||
| Hyale grimaldii−25.6 | 182 | ||
| Maera hirondellei−22.3 | 214 | ||
| Ampithoe helleri−10.3 | 146 | ||
| Melita palmata−8.5 | 118 |
4 DISCUSSION
The amphipod fauna is a common component of the total invertebrate faunal assemblage associated with seagrass beds (Nelson, 1980; Sturaro, Lepoint, Vermeulen, & Gobert, 2015). Seagrass meadows represent a nursery habitat for amphipod communities and are therefore crucial for the conservation and restoration of marine environments (Heck, Hays, & Orth, 2003). Data on Zostera noltei beds are very rare in the Mediterranean Sea, and very few studies concern the amphipods. Such studies are of particular interest because this seagrass is scarce and at the limit of its distribution (Quintas, Moreira, & Troncoso, 2013; Rueda & Salsas, 2008).
The present study describes for the first time the spatial and temporal distribution of amphipod communities associated with Z. noltei meadows on the coasts of the central Mediterranean Sea. We also record 78 taxa belonging to 22 families associated with eelgrass beds in the intertidal zone of the Kneiss Islands. Conversely, 26 species reported by Fersi et al. (2018) are found in the subtidal tidal channels of the Kneiss, of which only 12 species are in common between the intertidal and subtidal ecosystems. During this study, a relatively high number of amphipod species associated with Z. noltei meadows were recorded on the south-eastern coast of Tunisia (78 species) when compared to other sites in the Mediterranean (44 species). As regards other meadow habitats (i.e. Zostera marina, Cymodocea nodosa and Posidonia oceanica), 24, 51 and 62 species, respectively, have been identified on the northern coast of Tunisia by Zakhama-Sraieb et al. (2011); in the northern and southern Adriatic Sea by Scipione & Zupo (2010); in the Marine Protected Area of Tavolara-Punta Coda Cavallo by Sturaro et al. (2015); in the central Tyrrhenian Sea (Italy) by Camisa et al. (2017); on the Alicante coast (Spain) by de-la-Ossa-Carretero, Del-Pilar-Ruso, Giménez-Casalduero, and Sanchez-Lizaso (2015); along Atlantic coasts (Esquete, Moreira, & Troncoso, 2011); and in the Black Sea (Karaçuha, Sezgin, & Dagli, 2009) (Table 4). Accordingly, the high species richness recorded in the Kneiss Islands compared with various beds worldwide can be attributed to the sampling methods used (e.g. quadrat, corer or airlift), the sampling effort (number of site/replicates) and sampling period (month, season or year). In the latter case, the extended sampling period would favour the collection of occasional (e.g. those using the beds only for feeding or breeding) and/or seasonal species (Urra et al., 2013). The amphipod population of the intertidal zone of the Kneiss Islands is dominated by seven species: Cymadusa filosa, Microdeutopus gryllotalpa, Gammarus insensibilis, Dexamine spiniventris, Monocorophium insidiosum, Elasmopus rapax and Leucothoe incisa. These species are characteristic of areas with detritus accumulation, associated with the occurrence of Zostera, Halophila and Cymodocea meadows. Likewise, the amphipod fauna associated with Zostera noltei meadows of the Kneiss Islands is typical of that found in other beds such as those composed of Zostera spp. (Esquete et al., 2011; Karaçuha et al., 2009), Cymodocea nodosa (Scipione & Zupo 2010) or Posidonia oceanica (Cimisa et al., 2017; Zakhama-Sraieb et al., 2011) on Mediterranean and Atlantic coasts. This shows that the amphipod group comprises many cosmopolitan species that coexist in several habitats (Dauvin & Ruellet, 2007; Gomez-Gesteira & Dauvin, 2000; Väinölä et al., 2008). The present study lists 25 new species belonging to 14 families, representing new additions to the amphipod fauna of the Gulf of Gabès, which were unreported in previous studies (Mosbahi, Boudaya, et al., 2015, 2016; Zakhama-Sraieb, Sghaier, et al., 2009; Zakhama-Sraieb et al., 2011). In addition, the species Lysianassa ceratina is recorded here for the first time in Tunisian waters. This species is reported as living among algae in shallow water, being distributed in the Mediterranean, on the Atlantic coast of Europe and North Africa from the Shetlands to Senegal, as well as in the Canary Islands and Indian Ocean (Griffiths 1974).
| Study area | Habitats | Sampling | SN | RN | RS | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Technique | Se N | S | ||||||
|
Kneiss Islands, Tunisia (Mediterranean Sea) |
Z.n | Corer (0.0225m2) | 4 | 1.08 | 12 | 4 | 78 | This study |
|
Galician Rias (NW of Spain) Atlantic Sea |
Z. spp. | Van Veen Grab (0.056m2) | 4 | 2.8 | 10 | 5 | 83 | Esquete et al., (2011) |
|
Sinop peninsula coast, Turkey Black Sea |
Z.spp. | Metal frame (0.06 m2) | 4 | 0.36 | 6 | 1 | 62 | Karaçuha et al., (2009) |
|
Northern and southern Adriatic Sea (Mediterranean Sea) |
Z.m P.o C.n |
Hand-towed net (0.2*0.4m) Airlift sampler (1m square) |
2 | - | 10 | - |
24 for Z.m 29 for C.n 31 for P.o |
Scipione and Zupo (2010) |
|
Tunisian coasts (Mediterranean Sea) |
P.o | Quadrat (0.16 m2) | 1 | 4.3 | 9 | 3 | 44 | Zakhama-Sraieb et al., (2011) |
|
Central Tyrrhenian sea, Italy (Mediterranean Sea) |
P.o | Quadrat (0.16 m2) | 4 | 2.8 | 6 | 3 | 62 | Camisa et al., (2017) |
|
Alicante, Spain (Mediterranean Sea) |
P.o | Box square | 2 | - | 11 | 3 | 62 | de-la-Ossa-Carretero et al., (2015) |
| Revellata Bay, France | P.o |
Hand-towed net, Airlift, and Light traps |
1 | - | - | - | 32 | Michel, Lepoint, Dauby, & Sturaro, 2015 |
|
Tavolara-Punta Coda Cavallo Marine Protected Area (Mediterranean Sea) |
P.o | Airlift (0.185 m2) | 1 | 5.94 | 8 | 4 | 51 | Sturaro et al., (2015) |
4.1 Characteristics of the different amphipod assemblages
In a first step, four distinct amphipod assemblages can be recognized in the apparently homogeneous Zostera noltei meadows in the intertidal zone of the Kneiss Islands. These assemblages are characterized by spatial and temporal changes in the population, and their distribution pattern seems to be entirely governed by the sediment characteristics. Sediment grain-size and organic matter content play a significant role in controlling the distribution of the amphipod communities in the Kneiss Islands. Contrariwise, the distribution of amphipods in the Kneiss subtidal channels appears mainly linked to the presence of algae and phanerogams and the availability of detritus (Fersi et al., 2018), which are the same factors responsible for the amphipod distribution in the Bizerte Lagoon (Zaabar et al., 2015). In other localities, several authors (Guerra-Garcia et al., 2014) have obtained the same results as observed in the Kneiss Islands concerning the amphipod distribution. In fact, to elucidate the processes that determine amphipod distribution, various ecological studies have been conducted in different habitats and areas: soft bottoms of shallow coastal areas (Carvalho et al., 2012), rocky shores (Jimeno & Turon, 1995), estuarine ecosystems (Cunha, Moreira, & Sorbe, 2000) and P. oceanica meadows (Zakhama-Sraieb et al., 2011; Sturaro et al., 2014). All this research has established that amphipod distribution is influenced by several natural parameters and anthropogenic factors. For example, water depth is considered as one of the major physical factors that affects amphipod assemblage distribution (Carvalho et al., 2012). However, other factors can vary progressively along the depth profile, such as the type of substrate or food availability. Evidently, some species can settle on several different substrates. Other species show active selection: a certain particle-size class or the presence of other species providing a structural habitat that may offer protection or potential food (Vázquez-Luis, Sanchez-Jerez, & Bayle-Sempere, 2009). The trophic resource availability is another important natural factor controlling amphipod species distribution (Zakhama-Sraieb et al., 2011; Carvalho et al., 2012; Sturaro et al. 2014). In addition, the amphipod distribution can be correlated with anthropic factors. Several studies have concluded that some species are more tolerant than others, resulting in changes in amphipod composition related to the degree of pollution (Bellan-Santini, 1980; de-la-Ossa-Carretero et al., 2012), and that anthropic factors can therefore be just as important as natural parameters in determining amphipod assemblage distribution (de-la-Ossa-Carretero et al., 2015). The four amphipod assemblages show a pattern of distribution very similar to that observed for the polychaete assemblages previously described in the intertidal zone of the Kneiss Islands (Mosbahi et al., 2017). This result points to the existence of four distinct macrofaunal assemblages in the apparently homogeneous Z. noltei meadow habitat. These benthic assemblages are characterized by spatial variations, and their distribution pattern is strongly correlated with edaphic characteristics of the environment such as the degree of exposure (inshore/offshore gradient), sediment type and organic matter content.
4.2 Seasonal variability of amphipod communities
In a second step, the amphipod composition is considered subject to predictable seasonal changes. There is a decline in the total abundance and numbers of species from autumn through winter for the amphipod communities, whereas diversity and evenness indices show an increase. The maximum values of diversity parameters are recorded in spring and summer. However, analysis based on year-round data show a strong seasonal variation in the species composition of amphipod assemblages, along with their abundance and diversity. This pattern corresponds to four main seasonal groups, represented by autumn, winter, spring and summer. During the warm season (spring and summer), amphipod assemblages are characterized by a high number of species and number of individuals for some dominant species such as Gammarus insensibilis, Microdeutopus gryllotalpa, Dexamine spiniventris, Ampelisca serraticaudata, Monocorophium insidiosum, Cymadusa filosa, Caprella acanthifera and Leucothoe spp. During autumn, the structure of amphipod assemblages changes and becomes dominated by Liljeborgia dellavallei, Orchestia gammarellus and Elasmopus brasiliensis. Winter yields the lowest values in terms of species diversity and abundance, characterized by the dominance of two species: Melita palmata and Gammarus insensibilis. This seasonality in amphipod assemblages is similar to that found in other parts of the world, particularly in the Mediterranean (Scipione & Zupo 2010; Camisa et al., 2017) and in temperate latitudes (Cunha, Sorbe, & Moreira, 1999; Moreira, Gestoso, & Troncoso, 2008; Karaçuha et al., 2009). Seasonal changes can occur for several reasons. The variation of seasonal climatic parameters may alter the temperature and salinity conditions in estuarine habitats, thus leading to changes in the seasonal structure of benthic assemblages (Cunha et al., 1999). In addition, these changes can be explained by the seasonality in growth and production rate of eelgrass beds (Esquete et al., 2011; Karaçuha et al., 2009; Zaabar et al., 2015). In general, the traits of eelgrass meadows are least marked in winter, when young leaves start developing, associated with the lowest production of epiphytic biomass. Contrariwise, during the warm season, eelgrasses reach their highest productivity and show a peak in epiphyte assemblages, as well as in their spatial coverage (Bedini, Pertusati, Batistini, & Piazzi, 2011; Ott, 1980), suggesting that amphipod assemblages and species coexistence may be promoted by different mechanisms, mostly related to direct and indirect effects of the changing host plant structure. A possible explanation for the high number and density of species observed during the warm season may involve a more efficient partitioning of the abundant food resources available at that time, implying a reduction in trophic niche overlap (Camisa et al., 2017; Michel et al., 2015). Moreover, life histories with seasonal patterns in reproductive activity and dispersal may explain variations of the crustacean assemblages over the annual cycle (Cunha et al., 1999; Esquete et al., 2011; Moreira et al., 2008). In addition, seasonal fluctuations can be related to the recruitment period of the dominant species in spring and summer (Reiss & Kroncke, 2005). The recruitment of benthic macrofauna affects community abundance (Colling, Bemvenuti, & Gandra, 2007; Das Neves, Silva, & Bemvenuti, 2008), although the patterns are highly variable in space and time and may be influenced by numerous biological and physical factors during the seasonal cycle (Pineda, Reyns, & Starczak., 2009). The structure of amphipod assemblages associated with eelgrass beds is also influenced by food availability, adult migration and survival, as well as intra- and interspecific competition and predation pressure (Michel et al., 2015; Nakaoka, 2002; Sanchez Jerez et al., 1999; Sturaro et al., 2016). The trophic structure of amphipods communities on the mudflats of the Kneiss Islands is dominated all the year round by deposit-suspension feeders, deposit feeders and omnivores. This contrasts with the amphipod populations of the tidal channels, which are largely dominated by herbivores, grazers and detritus feeders, living mainly on algae and phanerogams. This difference can be explained by food availability in these two particular habitats of the Gulf of Gabès.
In conclusion, amphipod assemblages are rich and diversified in the Zostera noltei meadows of the Kneiss Islands. The intertidal benthic system of these Islands yields results, which again demonstrate the important ecological role of the seagrasses. The conservation of these peculiar habitats will be crucial for preservation of the local biodiversity.
ACKNOWLEDGEMENTS
The authors thank the fishers of the Kneiss Islands for their help during the sampling, the laboratory staff of GeoResources Materials, Environment and Global Changes (FSS) for their assistance during sediment analysis, and Michael Carpenter for the English revision.
APPENDIX A
Checklist of amphipods collected in the Kneiss Islands: TG: trophic group; TIN: total individual number; S: suspension feeders; DS: deposit-suspension feeders; He: herbivores; De: plant deposit feeders; and O: omnivores. (**), new species for Tunisia waters; and (*), new species reported for the first time in the Gulf of Gabès.
| Family | Amphipod species | TG | TIN |
|---|---|---|---|
| Ampeliscidae | Ampelisca diadema (Costa, 1853) | S | 86 |
| Ampelisca brevicornis (Costa, 1853) | S | 14 | |
| Ampelisca rubella A. Costa, 1864 | S | 8 | |
| Ampelisca serraticaudata Chevreux, 1888 | S | 42 | |
| Ampelisca spinipes Boeck, 1861 | S | 7 | |
| Ampelisca unidentata Schellenberg, 1936 | S | 6 | |
| Amphilochidae | Apolochus neapolitanus (Della Valle, 1893)* | De | 19 |
| Ampithoidae | Ampithoe helleri Karaman, 1975 | He | 6 |
| Ampithoe ramondi Audouin, 1826 | He | 60 | |
| Ampithoe riedli Krapp-Schickel, 1968 | He | 14 | |
| Cymadusa filosa Savigny, 1816 | He | 464 | |
| Cymadusa crassicornis (Costa, 1853)* | He | 14 | |
| Aoridae | Aora gracilis (Spence Bate, 1857) | Ds | 22 |
| Aora spinicornis Afonso, 1976* | Ds | 8 | |
| Lembos websteri Spence Bate, 1857* | Ds | 4 | |
| Tethylembos viguieri (Chevreux, 1911) | Ds | 7 | |
| Microdeutopus anomalus (Rathke, 1843) | Ds | 316 | |
| Microdeutopus gryllotalpa Costa, 1853 | He | 509 | |
| Microdeutopus algicola Della Valle, 1893* | Ds | 64 | |
| Microdeutopus chelifer (Spence Bate, 1862)* | De | 18 | |
| Caprellidae | Caprella acanthifera Leach, 1814 | O | 123 |
| Caprella grandimana (Mayer, 1882)* | O | 14 | |
| Caprella liparotensis Haller, 1879* | O | 6 | |
| Deutella shieckei Cavedini, 1982* | O | 8 | |
| Pseudoprotella phasma (Montagu, 1804) | O | 44 | |
| Corophiidae | Apocorophium acutum (Chevreux, 1908)* | Ds | 3 |
| Corophium orientale Schellenberg, 1928 | Ds | 321 | |
| Leptocheirus guttatus (Grube, 1864)* | Ds | 4 | |
| Leptocheirus bispinosus Norman, 1908 | Ds | 8 | |
| Leptocheirus pectinatus (Norman, 1869) | Ds | 12 | |
| Monocorophium insidiosum (Crawford, 1937) | Ds | 562 | |
| Monocorophium acherusicum (Costa, 1853) | Ds | 11 | |
| Dexaminidae | Dexamine spiniventris (Costa, 1853) | De | 304 |
| Dexamine spinosa (Montagu, 1813) | De | 168 | |
| Tritaeta gibbosa (Spence Bate, 1862) | De | 4 | |
| Gammaridae | Gammarus insensibilis Stock, 1966 | De | 640 |
| Gammarus aequicauda (Martynov, 1931) | De | 5 | |
| Echinogammarus olivii (H. Milne Edwards, 1,830)* | De | 2 | |
| Hyalidae | Hyale grimaldii Chevreux, 1891 | He | 8 |
| Hyale schmidti (Heller, 1866) | He | 6 | |
| Hyale camptonyx (Heller, 1866) | He | 3 | |
| Parhyale aquilina (Costa, 1857)* | He | 3 | |
| Ischyroceridae | Ericthonius brasiliensis (Dana, 1853) | Ds | 7 |
| Ericthonius difformis H. Milne Edwards, 1,830 | Ds | 11 | |
| Ericthonius punctatus (Spence Bate, 1857) | Ds | 7 | |
| Ischyrocerus inexpectatus Ruffo, 1959* | Ds | 2 | |
| Leucothoidae | Leucothoe incisa Robertson, 1892 | O | 282 |
| Leucothoe denticulata A. Costa, 1851 | O | 187 | |
| Leucothoe venetiarum Giordani- Soika, 1950* | O | 19 | |
| Liljeborgiidae | Liljeborgia dellavallei Stebbing, 1906 | O | 228 |
| Lysianassidae | Lepidepecreum longicornis (Spence Bate, 1862)* | O | 6 |
| Lysianassa ceratina (Walker, 1889)** | O | 38 | |
| Lysianassina longicornis (Lucas, 1846) | O | 83 | |
| Lysianassa pilicornis (Heller, 1866) | O | 20 | |
| Lysianassa plumosa Boeck, 1871* | O | 8 | |
| Lysianassa costae (H. Milne Edwards, 1,830) | O | 16 | |
| Orchomene humilis (Costa, 1853) | Ds | 4 | |
| Socarnes filicornis (Heller, 1866)* | Ds | 10 | |
| Maeridae | Elasmopus brasiliensis (Dana, 1855) | S | 164 |
| Elasmopus pectenicrus (Spence Bate, 1862) | Ds | 102 | |
| Elasmopus rapax Costa, 1853 | S | 329 | |
| Maera hirondellei Chevreux, 1900 | Ds | 184 | |
| Quadrimaera inaequipes (A. Costa, 1857) | Ds | 162 | |
| Melitidae | Melita palmata (Montagu, 1804) | Ds | 332 |
| Oedicerotidae | Perioculodes aequimanus (Korssman, 1,880)* | O | 33 |
| Westwoodilla rectirostris (Della Valle, 1893 | O | 14 | |
| Phliantidae | Pereionotus testudo (Montagu, 1808)* | He | 18 |
| Photidae | Gammaropsis ostroumowi Sowinski, 1898* | De | 26 |
| Gammaropsis maculata (Johnston, 1828)* | De | 36 | |
| Gammaropsis dentata Chevreux, 1900* | De | 8 | |
| Photis longipes (Della Valle, 1893)* | De | 12 | |
| Phoxocephalidae | Metaphoxus simplex (Spence Bate, 1857)* | De | 6 |
| Podoceridae | Podocerus chelonophilus (Chevreux & Guerne, 1888) | De | 10 |
| Stenothoidae | Stenothoe gallensis Walker, 1904 | O | 17 |
| Stenothoe monoculoides (Montagu, 1815) | O | 24 | |
| Talitridae | Orchestia gammarellus (Pallas, 1766) | De | 28 |
| Orchestia mediterranea Costa, 1853 | De | 78 | |
| Talitrus saltator (Montagu, 1808) | De | 24 |




