Spatial and seasonal variations of dinoflagellates and ciliates in the Kongsfjorden, Svalbard
Abstract
Abundance and assemblages of dinoflagellates and ciliates were studied in water samples collected from three different depths at five locations in the Kongsfjorden, during summer (June 14–21, 2011) and fall (September 15–27, 2012). Generally, athecate dinoflagellates were ubiquitously dominant during both seasons. Surface dinoflagellates abundance ranged from 1.87 × 103 cells/L (KF1) to 11.62 × 103 cells/L (KF4) and column integrated abundance ranged from 20.3 × 106 cells/m2 (KF1) to 126 × 106 cells/m2 (KF2) during summer. Dinoflagellate abundance was relatively lower during fall ranging from 0.02 × 103 cells/L (KF5) to 0.66 × 103 cells/L (KF3) at surface, and correspondingly, a low column integrated abundance ranging from 2.34 × 106 cells/m2 (KF5) to 19.1 × 106 cells/m2 (KF1) was observed. Amphidinium sp., Gyrodinium fusiforme, Gyrodinium estuarile dominated during summer, while Gymnodinium sp. was dominant during fall. Among ciliates, aloricate ciliates were more dominant than loricates. Ciliates at surface ranged from as low as 0.069 × 103 cells/L (KF1) to 3.69 × 103 cells/L (KF4) during summer. Ciliate abundance increased with depth (up to 20 m). Strombidium spp. (55.28%) and Mesodinium rubrum (36.66%) were dominant during summer. Among the loricates and the aloricates, Strombidium spp. (85.72%) and Tintinnid spp. (92.15%) dominated in fall. The presence of dominant aloricates with characteristic cleptochloroplasts reflected high grazing activity in these waters during both seasons. Diversity study indicates that the dinoflagellates and ciliates are well represented during both seasons. Statistical analyses of the dinoflagellates and ciliates with hydrographic data do not show dominant role of any hydrographical parameters on their diversity, and the same is discussed vis-à-vis Atlantification of the fjord.
1 INTRODUCTION
Ciliates and dinoflagellates fall in the size range of 20–200 μm and constitute a major portion of the zooplankton biomass in most of marine and estuarine systems (Porter, Sherr, Sherr, Pace, & Sanders, 1985). They are the primary consumers and graze mostly on bacteria, flagellates, and diatoms in the food web of any aquatic ecosystem (Johansson, Gorokhova, & Larsson, 2004; Sherr & Sherr, 2007) and act as a significant food source for higher vertebrate and invertebrate predators (Fukami, Watanabe, Fujita, Yamaoka, & Nishijima, 1999; Stoecker & Capuzzo, 1990). They display unique feeding habits that allow them to graze upon cells more than their own volume (Hansen & Calado, 1999). They are known to effectively graze on bacteria and influence the removal of bacterial biomass out of the microbial loop. Further, they have high growth rates which equal or exceed prey growth and therefore can serve as a viable food source to higher organisms (Gifford & Dagg, 1991; Stoecker & Capuzzo, 1990). Thus, it forms a crucial component for transferring organic carbon from heterotrophic bacteria to higher trophic levels (Azam et al., 1983). Moreover, studies have shown that dinoflagellates and ciliates can be dominant consumers of phytoplankton in both oligotrophic and eutrophic regions (Capriulo, Sherr, & Sherr, 1991; Lessard & Murrell, 1998) consuming >100% of the primary production at times (Lessard & Murrell, 1998).
The protozoan community abundance is known to be controlled by strong biotic factors such as phytoplankton abundance (Sherr, Sherr, Wheeler, & Thompson, 2003) and copepods (Levinsen & Nielsen, 2002; Rysgaard, Nielsen, & Hansen, 1999), thus showcasing both strong top-down and bottom-up control in different regions. Some dinoflagellates and few ciliates are hosts to photosynthetic symbionts and as a result are kleptoplastidic (algal chloroplasts are sequestered by host organisms) in nature (Esteban, Fenchel, & Finlay, 2010). They are also known to control the primary production. In the Arctic waters and nearby cold water regions, dinoflagellates and ciliates are responsible for the consumption of 22%–75% of the daily production (Landry & Calbet, 2004; Paranjape, 1990; Sherr & Sherr, 2009; Sherr, Sherr, & Ross, 2013). Due to their varied range of trophic nature, their species composition could be useful in identifying the differences in the microbial food webs in the aquatic system (Onda et al., 2017).
A study between different geographical regimes-a sub polar (Disko Bay) and a temperate (Kattegat) regime reported ciliates and dinoflagellates to be having similar patterns in abundance, composition, and grazing impact in both the systems (Levinsen & Nielsen, 2002). A number of studies have explored their role in the Arctic marine systems (Hansen, Christiansen, & Pedersen, 1996; Sherr, Sherr, & Hartz, 2009; Sherr et al., 2003). However, apart from Seuthe, Iversen, and Narcy (2011) who discussed the temporal variation of the dinoflagellates and heterotrophic ciliates from a single station study, Kubiszyn, Piwosz, Wiktor, and Wiktor (2014) studied the interannual variability of the protists during summer along the West Spitsbergen waters.
The Kongsfjorden system in Svalbard archipelago is an extremely vulnerable ecosystem which is experiencing effects of climate change. “Atlantification” of Kongsfjorden marine system has been reported due to increased ice melt, warmer temperatures in summer (Węsławski, Bucholz, Głuchowska, & Weydmann, 2017), intrusion of warm north Atlantic waters (AW) into the fjord and resultant altered diversity in microplankton (Smoła et al., 2017) and changes in food web (Vihtakari et al., 2018). Earlier studies have found influence of AW on the composition, age and biomass of planktonic communities especially for protists (Kubiszyn et al., 2014) and mesozooplankton (Gluchowska et al., 2016; Kwasniewski, Walkusz, Cottier, & Leu, 2013). However, not much is known about the probable changes that might occur within the dinoflagellate and ciliate community as a result of increased “Atlantification” during two different seasons.
In order to understand the functional diversity of the dinoflagellates and ciliates, it is essential to carry out studies on the species composition, abundance, seasonal distribution and succession patterns and the biotic and abiotic factors affecting them. Therefore, in this study we have discussed the distribution and composition of the dinoflagellates and ciliates at five stations along the central transect of the Kongsfjorden during summer (2011) and fall (2012) season and investigated the factors (both hydrography and chemistry) that might influence their distribution in this regime.
2 MATERIAL AND METHODS
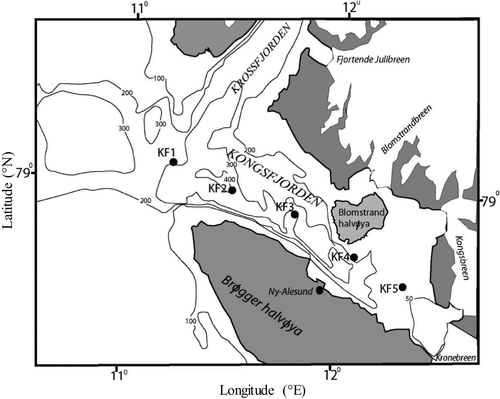
The Kongsfjorden is a part of the Kongsfjord-Krossfjord system that is situated in the west coast of Svalbard Archipelago (78°55′N, 11°56′E). It is a glacial fjord that is oriented southeast to northwest direction. It is ~20 km long and its width ranges from 4 to 10 km (Svendsen et al., 2002) and represents a unique site for seasonal studies of the Arctic region.
Five stations along the center of the fjord (Figure 1) were sampled during summer (June 14–21, 2011) and fall (September 15–27, 2012). The stations were located so as to cover the inner fjord (KF4 and KF5) that are highly influenced by freshwater runoffs from glaciers, mid fjord (stations KF3), and fjord mouth (stations KF2 and KF1) situated at the outer region of the basin that is comparatively more influenced by the AW. The overall depths along the sampling transect ranges from <50 m at KF5 to >300 m at KF1. Water samples were collected from 5 stations (from near surface, 10 and 20 m) by hydrocast using Niskin sampler for nutrients (nitrate and phosphate), chlorophyll a (chl a), phytoplankton, dinoflagellates and ciliates abundance and composition. Prior to each sampling, hydrography of the water column including temperature, salinity, and photosynthetically active radiation (PAR) was obtained using portable conductivity-temperature-depth (CTD) sensors (SBE-19 plus, Sea-Bird Electronics, USA) attached to the winch cable onboard RV-Teisten. Based on PAR% data (Figure S1) samples for nutrients, chl a and microscopy were restricted to top 20 m. Nutrients from surface, 10 m and 20 m, were estimated spectrophotometrically (Seal Autoanalyser Model AA3) following the method of Grasshoff, Ehrhardt, and Kremling (1983). Chl a was measured by HPLC (summer 2011) and spectrophotometrically (fall 2012) for surface, 10 m and 20 m depth. Bhaskar, Tripathy, Sabu, Laluraj, and Rajan (2016) and Krishnan, Sinha, and Atri (2012) describe the detailed methodology used for chl a analysis. The depth-integrated values were calculated using the trapezoidal method.
Water samples (500–1,000 ml) was collected from all stations and fixed with acid Lugol's iodine (1% w/v) and stored in dark at 4°C until analyses. For microscopic analyses, a settling and siphoning procedure was followed (Utermöhl, 1958) to concentrate samples from 500 ml to <50 ml from which 1 ml sample was then counted under inverted microscope (Olympus IX 100) using 200 and 400x magnification. The dinoflagellates and ciliates were then extrapolated to per liter and were usually identified up to class or genus level and wherever possible identification up to species level (generally for dinoflagellates) was carried out using standard reference material (Meunier, 1910; Tomas, 1997). Further, the dinoflagellates were divided into thecates and athecates. Since fixation with acidic Lugol's solution prohibits the examination of the presence of chloroplasts, heterotrophic dinoflagellates were identified based on literature (Tomas, 1997). Ciliates were distinguished between loricate and aloricate forms. Biovolume was calculated using closest geometric shapes of dominant genera and species from which biomass was calculated for dinoflagellates, loricates, and aloricates as described in Seuthe et al. (2011).
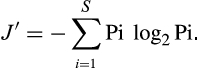
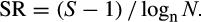
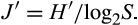
Evenness is the ratio of observed diversity to maximum diversity. The latter is achieved when most species in a collection are equally abundant (Pielou 1966).
Detailed explanation about the statistical analyses is given in Paul (2007) and Paul, Ramaiah, Gauns, and Fernandes (2007 and references therein).
3 RESULTS
3.1 Hydrography
Hydrography was carried out for the entire water column (up to 10 m above bottom) at all stations. However, PAR <1% depth ranged from 20 m (KF1) to <10 m (KF5) during summer 2011 and 36 m (KF1) to 20 m (KF5) during fall 2012 (Figure S1). In order for better comparison between the two seasons, sampling for microscopy samples was restricted to top 20 m in this study. Detailed description about the variations in hydrography has been discussed previously (Bhaskar et al., 2016; David & Krishnan, 2017); however, a brief description of the hydrography is given here.
The sea surface temperature (SST) varied from 3.02 (KF3) to 9°C (KF4) and decreased with depth in summer. In fall, the SST varied from 3.58°C (KF4) to 4.14°C (KF2) and increased with depth unlike the summer season. Salinity at near surface ranged from 32.72 (KF4) to 34.22 (KF5) and increased with depth. All stations except KF4 and KF5 had lower salinity. Stations KF4 and KF5 had minimal vertical salinity variation (Table 1), and vertical profiles showed well-mixed waters below 10 m depth as described by David and Krishnan (2017). There was not much variation in the salinity during fall throughout the transect, and the salinity ranged from 33.39 to 33.66 at KF1 and KF5, respectively. Variable stratification was observed in the top 16 m water column throughout the transect. Strongly stratified surface waters (SW) were restricted to KF4 and KF5 due to glacial meltwater in both seasons (Bhaskar et al., 2016; David & Krishnan, 2017).
| Stations | Depth (m) | Seasons | |||
|---|---|---|---|---|---|
| Summer | Fall | ||||
| Temp (°C) | Salinity | Temp (°C) | Salinity | ||
| KF1 | 0 | 6.38 | 33.45 | 4.07 | 33.39 |
| 10 | 3.61 | 34.43 | 4.08 | 33.40 | |
| 20 | 2.05 | 34.44 | 4.15 | 33.47 | |
| KF2 | 0 | 6.22 | 33.46 | 4.14 | 33.39 |
| 10 | 3.35 | 34.46 | 4.13 | 33.40 | |
| 20 | 2.35 | 34.45 | 4.16 | 33.63 | |
| KF3 | 0 | 3.02 | 34.22 | 3.88 | 33.53 |
| 10 | 2.79 | 34.33 | 4.01 | 33.54 | |
| 20 | 1.96 | 34.49 | 4.27 | 33.85 | |
| KF4 | 0 | 7.32 | 32.80 | 3.58 | 33.46 |
| 10 | 5.50 | 34.00 | 3.56 | 33.50 | |
| 20 | 3.13 | 34.47 | 3.90 | 33.86 | |
| KF5 | 0 | 3.27 | 34.16 | 3.81 | 33.66 |
| 10 | 3.20 | 34.37 | 3.88 | 33.69 | |
| 20 | 2.36 | 34.46 | 4.14 | 34.01 | |
Depth averaged nitrate (NO3) concentrations increased from 1.85 µM near mouth (KF2) to a high of 4.31 µM at the glacial end (KF5) during summer; similarly, the depth average NO3 concentrations ranged from 0.54 µM (KF1) to a high of 1.93 µM (KF5) during fall. In summer, phosphate (PO4) concentrations were detected only at stations close to the glacier and the depth average concentrations were in the range of 0.20 μM (KF4) to 18.3 μM (KF5). In contrast to NO3, higher concentrations of PO4 were observed in fall and its concentration varied from 0.32 µM (KF1) to 0.76 µM (KF4). Silicate (SiO4) could not be estimated during summer and in fall the depth average concentration of SiO4 was >1 μM in all stations (Table 2). Also, during fall, mean nitrate to phosphate ratio (N:P) ranged from 1.69 at KF2 to 4.35 at KF5, whereas mean NO3 to SiO4 ratio (N:Si) was >1 only at KF5 (Bhaskar et al., 2016).
| Seasons | |||||
|---|---|---|---|---|---|
| Stations | Summer | Fall | |||
| Nitrate | Phosphate | Nitrate | Phosphate | Silicate | |
| KF1 | 2.43 (1.98–3.20) | 0.01 (0.00–0.04) | 0.54 (0.31–0.77) | 0.32 (0.25–0.38) | 1.87 (1.78–1.97) |
| KF2 | 1.85 (1.67–1.98) | 0.10 (0.00–0.18) | 1.14 (0.99–1.29) | 0.68 (0.42–0.93) | 1.51 (1.38–1.64) |
| KF3 | 3.67 (3.24–4.21) | 0.07 (0.00–0.12) | 1.11 (1.11–1.12) | 0.42 (0.08–0.72) | 1.82 (0.90–3.00) |
| KF4 | 3.69 (3.30–3.90) | 0.20 (0.18–0.22) | 1.53 (1.36–1.70) | 0.76 (0.63–0.89) | 1.55 (0.99–2.10) |
| KF5 | 4.31 (4.28–4.34) | 18.33 (5.0–35.0) | 1.93 (1.80–2.06) | 0.44 (0.38–0.51) | 1.51 (1.32–1.71) |
Note
- Concentration ranges are given in parenthesis.
3.2 Dinoflagellates and ciliates abundance and composition
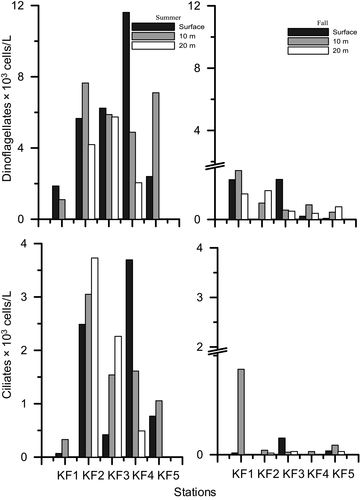
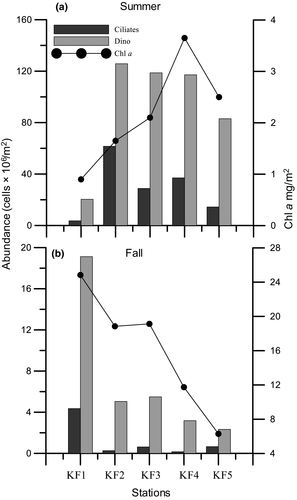
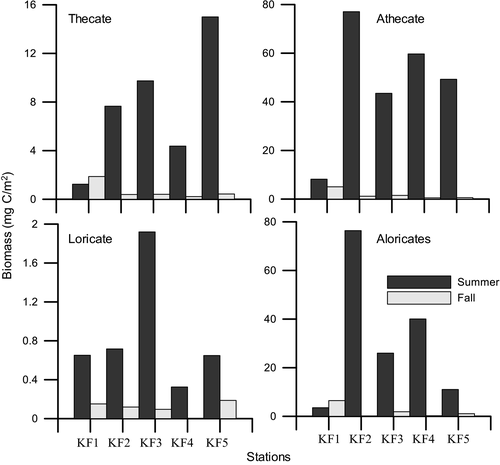
Overall, the dinoflagellates abundance during summer was greater by more than an order of magnitude than fall in all stations and depths (Figure 2). During summer, the surface abundance ranged from 1.87 × 103 cells/L (KF1) to a high of 11.62 × 103 cells/L (KF4). The dinoflagellates abundance decreased with depth with an exception of KF5 and KF3 wherein a subsurface maximum was observed at 10 m. The column integrated abundance of dinoflagellates ranged from a low of 20.3 × 106 cells/m2 (KF1) to a high of 126 × 106 cells/m2 (KF3, Figure 3a). During fall, the dinoflagellates abundance at surface ranged from 0.02 × 103 cells/L (KF5) to 0.66 × 103 cells/L (KF3). A subsurface maximum of abundance was observed at 10 m in stations KF4 and KF1. Column integrated abundance of dinoflagellates during fall ranged from 2.34 × 106 cells/m2 (KF5) to 19.1 × 106 cells/m2 (KF1). Overall, the athecates dominated during two seasons in terms of both biomass and numbers. The total abundance of athecates was greater, and they contributed up to 55,031 cells/L during summer and contributed up to 2,817 cells/L during fall, while thecates formed only 11,355 cells/L and 2,058 cells/L during summer and fall, respectively (Table 3). Column integrated athecate biomass varied from 8.18 (KF1) to 77.07 mg C/m2 at KF2 and from 0.50 (KF4) to 5.04 mg C/m2 (KF1) during summer and fall, respectively (Figure 4).
| No | Dinoflagellates | Summer | Fall | No | Ciliates | Summer | Fall |
|---|---|---|---|---|---|---|---|
| Thecate Dinoflagellates | Loricate ciliates | ||||||
| 1 | Alexandrium sp | 4.80 | 28 | Acanthostomella cf. norwegica | 37.85 | 7.85 | |
| 2 | Ceratium horridum | 1.13 | 29 | Tintinnopsis cf. tubulosoides | 14.53 | ||
| 3 | Dinophysis sp | 1.17 | 5.48 | 30 | Cyttarrocylis spp | 29.31 | |
| 4 | Dinophysis roundata a | 1.85 | 31 | Tintinnids | 13.52 | 82.72 | |
| 5 | Diplosalis sp | 2.92 | 32 | Tintinnids sp1 | 1.20 | 6.81 | |
| 6 | Fragilidium sp | 1.69 | 33 | Tintinnids sp2 | 3.59 | 2.62 | |
| 7 | Peridinium sp | 10.55 | 0.43 | Total loricates | 1,405.00 | 152.80 | |
| 8 | Protoperidinium bipes a | 7.21 | 14.36 | Aloricate ciliates | |||
| 9 | Protoperidinium spa | 14.60 | 5.62 | 34 | Didinium sp | 1.53 | |
| 10 | Protoperidinium sp1a | 4.52 | 0.82 | 35 | Didinium cf. gargantua | 2.32 | |
| 11 | Protoperidinium sp2a | 1.69 | 36 | Laboea strobila b | 2.07 | 10.20 | |
| 12 | Protoperidinium sp3a | 1.32 | 37 | Mesodinium rubrum b | 36.66 | ||
| 13 | Prorocentrum sp | 23.45 | 41.99 | 38 | Strombidium sp1 | 25.91 | 83.61 |
| 14 | Scripsiella trochoidea | 17.03 | 6.41 | 39 | Strombidium sp2 | 1.26 | 2.11 |
| 15 | Scripsiella sp | 6.32 | 21.05 | 40 | Strombidium spb | 27.81 | |
| Total Thecates dinos | 11,355 | 2,058.4 | 41 | Ciliate 1 | 1.06 | ||
| Athecate dinoflagellates | 42 | Ciliate 2 | 2.30 | 3.02 | |||
| 16 | Amphidinium operculatum a | 2.27 | Total aloricates | 11,960.00 | 529.60 | ||
| 17 | Amphidinium spa | 18.15 | 3.42 | ||||
| 18 | Amphidinium sphaenoids a | 3.27 | 3.62 | ||||
| 19 | Gyrodinium fusiformi a | 17.69 | 14.24 | ||||
| 20 | Gyrodinium esturaile a | 18.08 | |||||
| 21 | Gymnodinium sp | 0.38 | 32.72 | ||||
| 22 | Gyrodinium sp | 9.58 | 7.19 | ||||
| 23 | Torodinium spc | 0.39 | 2.34 | ||||
| 24 | Athecate Dino | 7.97 | 21.71 | ||||
| 25 | Athecate Dino1 | 12.38 | 13.97 | ||||
| 26 | Unidentified dino cyst | 3.10 | |||||
| 27 | Unidentified dino1 | 5.54 | 1.06 | ||||
| Total athecates | 55,031.00 | 2,817.00 | |||||
- a Heterotrophic nature of the species.
- b Mixotrophic nature of the species.
- c Species that are reported to contain chloroplasts of cleptochloroplasts.
Moreover, heterotrophic dinoflagellates abundance was greater contributing up to 88.80% in summer and 42.08% in fall (Table 3). The heterotrophic dinoflagellates identified during the study were Dinophysis roundata, Protoperidinium bipes, Protoperidinium spp, Amphidinium operculatum, Amphidinium sp, Amphidinium sphaenoides, Gyrodinium fusiformis, and Gyrodinium estuariale. As genus Protoperidinium is a heterotrophic thecate dinoflagellate and is a raptorial feeder (Kjæret, Naustvoll, & Paasche, 2000), it was considered to be heterotrophic although the species was not identified. Generally, the species diversity was greater in summer than fall for both thecates and athecates (Table 4). The SR for thecates was 1.93 in summer and 1.31 in fall. Athecates had a SR of 1.92 during summer and 1.13 in fall. The species evenness (J′) was >0.6 for all the groups during summer but was >0.6 only for the thecates and athecates during fall (Table 4).
| Season | SR | H′ | J′ | |
|---|---|---|---|---|
| Summer | Thecates | 1.93 | 3.48 | 0.82 |
| Athecates | 1.92 | 3.26 | 0.73 | |
| Loricates | 0.69 | 2.09 | 0.81 | |
| Aloricates | 0.91 | 2.10 | 0.63 | |
| Fall | Thecates | 1.31 | 2.45 | 0.71 |
| Athecates | 1.13 | 2.60 | 0.78 | |
| Loricates | 0.60 | 0.92 | 0.46 | |
| Aloricates | 0.64 | 0.89 | 0.38 |
The total surface ciliates abundance during summer ranged from 0.07 × 103 cells/L at KF1 to a high of 3.69 × 103 cells/L at KF4. There was an increase in the abundance of ciliates in KF2 and KF3 at 20 m depth. However, in other stations the numbers declined after 10 m. The ciliate column integrated abundance was observed to be highest at KF2 (61.6 × 106 cells/m2) while the lowest abundance was observed at the mouth station, KF1 (3.65×106 cells/m2, Figure 3a). During fall, the surface abundance of ciliates ranged from 0.01 × 103 cells/L (KF1) to a 0.08 × 103 cells/L (KF3). The ciliate column integrated abundance was highest at KF1 (4.36 × 106 cells/m2) and lowest in KF4 (0.16 × 106 cells/m2, Figure 3b). Overall abundance of ciliates was higher in summer than fall. During both seasons (Table 2), the aloricates dominated than the loricates contributing to as high as 11,960 cells/L (summer) and 530 cells/L (fall). The column integrated biomass of the aloricates was higher during both seasons as compared to the loricates (Figure 4). The ciliate assemblages were dominated by the plastidic aloricates Strombidium sp. (27.81%), Strombidium sp. 1 (25.91%), and Mesodinium rubrum (36.66%) in summer. While in fall, Strombidium sp. 1 and Laboea strobila were the dominant aloricates and contributed to 83.61% and 10.20%, respectively.
In summer, the temperature and nutrients did not have any significant correlation with the dinoflagellates and ciliates (Table 5). Overall chlorophyll a (chl a) showed a positive significant correlation with dinoflagellates (r = .881, p < .001) and the dominant dinoflagellates, Amphidinium sp. (r = .772, p < .005) and G. fusiformis (r = .717, p < .013). At surface, Gyrodinium spp. had a significant correlation with ciliates (r = .9, p < .037), with chl a (r = .9, p < .037) and G. fusiformis had a similar significant correlation with ciliates (r = .9, p < .037) and with chl a (r = .9, p < .037). However at 10 m, chl a did not show any significant correlation either with dinoflagellates or the ciliates, while at 20 m, Gyrodinium spp. had a negative significant correlation with ciliates (r = −.999, p < .014). In fall, only nitrate showed a significant negative correlation with dinoflagellates, thecates and aloricates (Table 5). At surface, dinoflagellates had a significant positive correlation with Strombidium spp. (r = .894, p < .040) and G. fusiformis (r = .894, p < .028). While at 10 m depth, chl a had a significant correlation with dinoflagellates (r = .9, p < .037), Tintinnid spp. (r = .9, p < .037), and P. bipes (r = .97, p < .0048). Tintinnids spp. had a positive relation with P. bipes (r = .974, p < .004) too at the same depth, while at 20 m Gymnodinium spp. had a significant correlation with Tintinnid spp. (r = .968, p < .0067).
| Seasons | Variables | Dino | Athecates | Thecates | Loricates | Aloricates | |
|---|---|---|---|---|---|---|---|
| Summer | Temperature | R | .108 | .240 | −.376 | −.295 | .020 |
| p | .714 | .409 | .185 | .328 | .946 | ||
| Salinity | R | −.134 | −.235 | .147 | .028 | .010 | |
| p | .647 | .418 | .615 | .929 | .973 | ||
| Nitrate | R | −.198 | −.262 | −.271 | .052 | −.378 | |
| p | .497 | .366 | .349 | .865 | .183 | ||
| Phosphate | R | −.078 | −.129 | −.311 | −.260 | .008 | |
| p | .791 | .660 | .278 | .391 | .979 | ||
| Fall | Temperature | R | .225 | .181 | .242 | −.115 | .188 |
| p | .419 | .519 | .385 | .683 | .503 | ||
| Salinity | R | −.274 | −.138 | −.313 | .109 | −.277 | |
| p | .324 | .624 | .255 | .698 | .317 | ||
| Nitrate | R | −.709 | −.370 | −.888 | .006 | −.661 | |
| p | .022 | .293 | .001 | .986 | .038 | ||
| Phosphate | R | −.079 | −.103 | −.058 | −.060 | −.049 | |
| p | .828 | .776 | .874 | .870 | .894 | ||
| Silicate | R | −.073 | −.456 | .445 | .119 | −.018 | |
| p | .841 | .185 | .197 | .743 | .960 | ||
Note
- The significant values are in bold.
4 DISCUSSION
This study focuses on the distribution of dinoflagellates and ciliates along the fjord in two different seasons. In spite of the importance of dinoflagellates and ciliates in the pelagic food web system, little information is available about their diversity and composition from the fjord (Aberle et al., 2013; Seuthe et al., 2011) and the Svalbard region (Monti & Minocci, 2013). During both seasons, dinoflagellates dominated the Kongsfjorden at all stations and all depths. Spatially, the dominance of dinoflagellates and ciliates was higher in the mid fjord than the fjord mouth (KF1) and fjord head (KF5). Previous studies have shown that zooplankton biomass to be higher in the outer fjord during summer (Hop et al., 2006) while Walkusz et al. (2009) reported higher abundance of zooplankton at the glacial end of the fjord during both summer and fall. The glacial end of the fjord (KF5) had a lesser dominance of both dinoflagellates and ciliates during this study and could be attributed to higher grazing impact of the mesozooplankton (Walkusz et al., 2009). The abundance of athecate dinoflagellates was higher than the thecate dinoflagellates which were also reported earlier during spring season (Hodal, Falk-Petersen, Hop, Kristiansen, & Reigstad, 2012). Usually, dinoflagellates are less diverse in the Svalbard region than the diatoms (Hasle & von Quillfeldt, 1996). However, they are reported to be more dominant and diverse in summer and early fall (Hasle & von Quillfeldt, 1996; Hodal et al., 2012; Okolodkov, Hapter, & Semovski, 2000; Seuthe et al., 2011). This corroborated with our study where the dinoflagellates diversity was higher during both seasons as compared to ciliates (Table 3). Moreover, the athecates dominated irrespective of the seasons.
Kongsfjord is a warmer fjord in the Svalbard region. Long-term hydrographic studies have indicated an increase in the SST and the volume of intrusion of AW in the Kongsfjord (Walczowski, 2014) leading to the Atlantification of the fjord. Due to the presence of a deep canyon the Kongsfjordrenna, the warm AW is directed from the outer shelf to the innermost part of the Kongsfjord (Węsławski et al., 2017). This creates a natural variability from freshwater to marine and vice versa within the Kongsfjord, thereby creating a natural environment for more eurythermal species to thrive within the fjord. Nevertheless, in the present study, hydrography of the sampling stations clearly showed the presence of SW and intermediate waters in the top 40 m while AW were found below 75 m in both seasons (Bhaskar et al., 2016; David & Krishnan, 2017). Since the sampling depths were restricted to top 20 m, the role of subsurface AW in the distribution of the dinoflagellates and ciliates is minimal. Previous studies show that SW are influenced by remote forcings like tides, upper layer circulation, and glacial meltwater induced changes (Svendsen et al., 2002). Other factors including physical (stratification, mixed layer depth, euphotic depth, salinity, temperature, eddies, and upwelling), chemical (nutrient types and availability), and biological (grazers and their predators) may also play an important role in controlling/ regulating the plankton distribution. Such ecological underpinnings bring about changes in composition, metabolism, reproduction, and growth of the planktonic organisms (Eppley, Koeller, & Wallace, 1978), which is reflected in their diversity.
Experimental studies in the Barents Sea have shown that the ciliates and athecates attain maximum growth rate when the temperatures are below 5°C and the thecates usually grow faster when the temperatures are >5°C (Franzè & Lavrentyev, 2014). During our study period, the overall temperature averaged around 3.77°C in summer and 3.98°C in fall (Table 1). The higher presence of athecates and ciliates in the fjord could be attributed to lower temperatures during the study period. Predator prey relationships are expected to be negative which is indicative of significant top-down grazer impacts (Schmoker, Hernández-León, & Calbet, 2013). However, some studies have also found positive correlations between presumptive predator and prey (Yang, Choi, & Hyun, 2008) which may be indicative of a strong bottom-up forcing and rapid response of the protistan consumers to prey dynamics.
Ciliates were previously reported to be affected by various environmental factors such as light availability, temperature, nutrient level, and predator prey interactions (Sanders, 1995). However, in this study no significant relation was observed between the ciliates and the environmental factors. Recent studies have shown that ciliates are less sensitive to changes in temperature and salinity (Lawrence & Menden-Deuer, 2012) and its distribution is regulated by prey community and its availability (Anderson & Harvey, 2019). Since food source controls ciliate populations, the influence of the environmental parameters is more likely to be indirect (Faure-Fremiet, 1967). Moreover, during the study period the temperature and salinity did not show any large scale variations to affect the ciliate distribution within the fjord which could be attributed to the lack of relation between the ciliates and the environmental parameters. Existence of the eurythermal and euryhaline species such as M. rubrum, L. strobila and Strombidium spp could be one of the reasons there were no significant correlations between the ciliate and the environmental parameters (Węsławski et al., 2017). The photosynthetic ciliate, M. rubrum (an obligate phototroph), was observed only in the summer season and was conspicuous by its absence in fall. High abundance of this ciliate is reportedly observed in the month of May and June in the Baltic Sea just after the spring bloom (Lindholm, 1985; Rychert, 2004). This species is known to proliferate in warmer waters, decreased salinity, and a stable water column along with nitrate depleted waters (Johnson, Stoecker, & Marshall, 2013; Lindholm & Mörk, 1990; Montagnes et al., 2008). These conditions were prevalent during summer in the Kongsfjord and could be attributed to high abundance of M. rubrum during the study period.
Chlorophyll a showed a positive significant correlation with dinoflagellates (r = .881, p < .001) including the dominant mixotrophic dinoflagellates, Amphidinium sp. (r = .772, p < .005) and G. fusiformis (r = .717, p < .013) suggesting a possible herbivory by these dinoflagellates on the autotrophs. Field and laboratory experiments have shown that phosphate limitation induces and stimulates increased feeding in Gyrodinium spp. (Li & Stoecker, 2000), which could be the one of the possible reasons for higher abundance of Gyrodinium spp. during both the seasons. This is reflected by the significant negative correlation the dinoflagellates Gyrodinium estuarile had with nitrate at 10 m during summer (r = −0.97, p < .004) and phosphate (r = −.999, p < .014), while Gyrodinium fusiforme with nitrate (r = −.75, p < .01) and phosphate (r = −.66, p < .004) during fall.
Dinoflagellates are not selective grazers and are known to graze upon ciliates (Bockstahler & Coats, 1993) and phytoplankton; hence, they play an important role in regulating the phytoplankton biomass in the water column. In the present study, integrated chl a was fivefolds higher during fall while the abundance of grazers was sixfolds higher during summer (Figure 3). A tight coupling between dinoflagellates like Gyrodinium sp. with chl a and ciliates during summer in SW indicates prey-predator relationship. At 20 m depth, an inverse correlation with ciliates may be due to active grazing by Gyrodinium sp. upon ciliates in the absence of phytoplankton cells. The intensity of grazing may have reduced in fall as column integrated ciliate, and dinoflagellates abundance was extremely low at all stations (Figure 3b). Aloricate ciliates like Strombidium sp and naked dinoflagellates graze upon same prey (diatoms and phototrophic nanoflagellates). Moreover, grazing rates of Strombidium sp. are an order of magnitude higher than dinoflagellates like Gymnodinium sp. (Vargas & Martinez, 2009). Hence, higher abundance of aloricate ciliates such as Strombidium spp., M. rubrum, and L. strobila and athecate dinoflagellates further corroborates that grazing pressure on the phytoplankton biomass influenced the column integrated chl a during the seasons.
Species composition is an important aspect of community or ecosystem ecology. A stable system is one that remains at or returns to some sort of an equilibrium when a disturbing force or environmental stress is applied (Connell & Souza, 1983). During this study, too very little variation in the composition of dinoflagellates and ciliates was observed. In this study, during summer J′ averaged >0.7 for the whole dinoflagellates and cilliates community, while during fall only the dinoflagellates averaged >0.7 suggesting that most of the dinoflagellates and ciliates were well represented with an exception of ciliates in fall. Although the relative abundance of the species differed seasonally, the composition of the community remained unchanged to a large extent. Most of the dinoflagellates and ciliates species listed in this study have been previously reported from the Kongsfjord (Kubiszyn et al., 2014; Seuthe et al., 2011) and the Svalbard (Monti & Minocci, 2013) region.
5 CONCLUSION
The present study reflects the importance of the dinoflagellates and ciliates in the Kongsfjorden region. A large seasonal variation in their biomass was observed. However, the dinoflagellates and ciliates community composition is similar to previous studies and there are no visible changes in the community composition in the different seasons in the fjord. In the advent of reported Atlantification of the fjord, an increased contribution of marine species is expected in the fjord. Nevertheless, our study shows that such changes in the dinoflagellates and ciliates community is not yet observed and is similar to the recent reports on the robustness of the fjord plankton community (Weslawski et al., 2017). The Arctic system is subjected to strong seasonality in terms of light availability, temperature, ice cover, and nutrients. Although the environmental factors play a role in controlling their distribution and abundance, in this study it was observed that different parameters played the controlling factor in different seasons. Moreover, these parameters indirectly affect the dinoflagellates and ciliates abundance and distribution which is primarily controlled by the availability of food. Considering their importance in the microbial food web and sustenance of the higher trophic levels in the fjord with limited studies on their distribution in the Kongsfjorden (Nelson et al., 2014; Seuthe et al., 2011), it is the need of the hour for long-term diversity and speciation study of the protists to better understand the changes happening in Kongsfjorden due to Atlantification.
ACKNOWLEDGEMENTS
The authors thank Ministry of Earth Sciences for financial aid to conduct this study and Dr. K. P. Krishnan for providing logistics support. The authors would like to thank Dr. M. Ravichandran, Director NCPOR for support to carry out this work. This work was carried out as part of the Long Term Environmental Monitoring Program of the Kongsfjorden and Department of Science and Technology (DST) Fast Track Young Scientist funded project titled “Impact of Glacial meltwater on the distribution and composition of phytoplankton in the Kongsfjorden” awarded to JTB. JTB acknowledges funding given by DST Fast Track Young Scientist and DST-Women Scientist scheme-A (DST-WOS-A) and support by NCPOR. This is NCPOR contribution no: J-71/2019-20.
CONFLICT OF INTEREST
On behalf of all the authors, the corresponding author states that there is no conflict of interest.




