A study of crude oil-degrading bacteria from mangrove forests in the Persian Gulf
Abstract
Mangroves are coastal ecosystems, found in tropical and subtropical regions around the world. They are found in the transitional zones between land, sea, and rivers. Petroleum hydrocarbons are the most common environmental pollutants, and oil spills pose a great hazard to mangroves forests. This research was focused on the isolation and characterization of crude oil-degrading bacteria from mangrove ecosystems at the Persian Gulf. Sixty-one crude oil-degrading bacteria were isolated from mangrove samples (plant, sediment, and seawater) that enriched in ONR7a medium with crude oil as only carbon source. Some screening tests such as growth at high concentration of crude oil, bioemulsifier production, and surface hydrophobicity were done to select the most efficient strains for crude oil degradation. Molecular identification of strains was carried out by amplification of the 16S rRNA gene by PCR. The results of this study were indicated that the quantity of crude oil-degrading bacteria was higher in the root of mangrove plants compare to other mangrove samples (sediment and seawater). Also, identification results confirmed that these isolated strains belong to Vibrio sp. strain NW4, Idiomarina sp. strain BW32, Kangiella sp. strain DP40, Marinobacter sp. strain DW44, Halomonas sp. strain BS53, and Vibrio sp. strain DS35. The application of bioremediation strategies with these bacteria can reduce crude oil pollution in this important marine environment.
1 INTRODUCTION
Mangroves are coastal ecosystems found in tropical and subtropical regions around the world. They are found in the transitional zones between land, sea, and rivers; regarding their geographical distribution, these ecosystems are found in the Americas, Africa, Asia, and Oceania (Brito et al., 2006). Mangroves can tolerate a wide range of sediment types, temperature changes, nutrient, salinity, and oxygen levels. Mangrove plant species vary in their tolerance to these factors, forming characteristic templates or zones of vegetation (Lovelock, 1993). This ecosystem provides various natural functions of great ecological and economic importance. Some of these functions include being an important nursery for fish, crustaceans, mollusks, reptiles, mammals, bird nesting, a site of carbon and nutrient accumulation, a location of marine biomass reconstruction and offering protection against coastal erosion (Alongi, 2002).
Mangrove forests were first recorded in the Persian Gulf and Oman Sea by Eratosthenes (194–276 BC), a geographer from Alexandria (Safiari, 2002). Nowadays, mangroves are found along the Iranian beaches of the Persian Gulf and Oman Sea, as well as around Bahrain, Qatar, Saudi Arabia, and the United Arab Emirates (Bayoumi & El-Nagar, 2009). Mangrove forests on the southern coast of Iran are given the common title “The Hara forests,” and they cover several locations between the 25°11′ and 27°52′ parallels longitude (Safiari, 2002). Mangrove forests inIran are composed of two species of trees, namely Avicennia marina and Rhizophora mucronata, with Avicennia marina scrub being the most prolific, covering over 90% of the Oman Sea and Persian Gulf mangrove habitats. R. mucronata growth is limited and is usually restricted to creeks of the Syriac region (included Gas and Hara River Delta). Other sites, however, are dominated by A. marina, known locally as the “Hara.”
Petroleum hydrocarbons are the most common environmental pollutants, and oil spills pose a great hazard to terrestrial and marine ecosystems. Oil pollution may arise either accidentally or operationally whenever oil is produced, transported, stored, processed, or used at sea or on land. Oil spills are a major threat to the environment as they severely damage the surrounding ecosystems (Hassanshahian, 2014a). Many oil products are highly viscous in special crude oils, and heavy fuel oils can be deposited on shorelines and shoreline resources in thick, sticky layers that may either disrupt or completely prevent normal biological processes of exchange with the environment (Bayat, Hassanshahian, & Askeri, 2015, 2016). Crude oil physically covers plants and animals, and they die from suffocation, starvation, or other physical interference with normal physiological function (Hassanshahian, 2014bb; Hoff et al., 2010). A number of researchers consider mangroves to be the most sensitive of all coastal ecosystem types to oil spills (Brito et al., 2006; Cappello, Russo, Santisi, & Calogero, 2012).
Biodegradation by natural populations of microorganisms is the basic and the most reliable mechanism by which thousands of xenobiotic pollutants, including crude oil, are removed from the environment (Hassanshahian, 2014a). Bioremediation is a strategy to utilize biological activities as much as possible for quick remediation of environmental pollutants (Hassanshahian, Yakimov, Denaro, Genovese, & Cappello, 2014). Growth stimulation of indigenous microorganisms, biostimulation, along with inoculation of foreign oil-degrading bacteria is a promising means of accelerating detoxifying and degrading activities at a polluted site with minimum impact on the ecological systems (Cappello et al., 2012).
Many crude oil-degrading bacteria have been isolated from oil contaminated sites of mangrove ecosystem forests (Brito et al., 2006; Cappello et al., 2012; Yu, Wong, Yau, Wong, & Tam, 2005). For example, Brito et al. (2006) isolated some hydrocarbonoclastic bacterial inhabiting mangrove sediments at Brazil by combining molecular and culture-dependent approaches. Crude oil-degrading bacteria that characterized by these researchers belong to Pseudomonas, Marinobacter, Alcanivorax, Microbulbifer, Sphingomonas, Micrococcus, Cellulomonas, Dietzia, and Gordonia genera. Bayoumi and El-Nagar (2009) enriched and describe some crude oil-degrading bacteria from Red Sea mangrove forests at India. These bacteria belong to Bacillus and Pseudomonas genera. Lin and Cai (2008) describe PAH-degrading bacteria from mangrove forests at China.
Since the petroleum hydrocarbons do not dissolve in water, then the bacteria must have a specific mechanism to growth on hydrocarbons. Many bacteria are capable of emulsifying hydrocarbons in solution by producing surface active agents such as biosurfactants that enhance the adhesion of cells to the substrate. Biosurfactants reduce surface tension by accumulating at the interface of immiscible fluids, increasing the surface area of insoluble compounds which leads to increased bioavailability and subsequent biodegradation of the hydrocarbons (Batista, Mounteer, Amorim, & Totola, 2006). Although the mangrove ecosystems have been extensively studied, a few studies have focused on the capacity for crude oil biodegradation. Most of these studies report growth stimulation of hydrocarbon degrading microorganisms (Brito et al., 2006).
This research aims to study the diversity and quantity of crude oil-degrading bacteria in mangrove forests at the Persian Gulf (Bushehr province) regarding their capability to producing biosurfactant and biodegradation of crude oil.
2 MATERIALS AND METHODS
2.1 Sampling
Mangrove plants (root sample), seawater, and mangrove sediment samples were collected from three oil contaminated sites at the Persian Gulf. The situation of these sites was located near the Bushehr city (BW [Bushehr sea Water], BP [Bushehr Plant], BS [Bushehr Sediment] 46°15, N; 84°15, E), Deyr port (Dw [Deyr sea Water], DP [Deyr Plant], DS [Deyr Sediment] 51°30, N; 27°15, E), and Nayband zone (NW [Nayband sea Water], NP [Nayband Plant], NS [Nayband Sediment], 52°69, N; 27°49, E). Figure 1 shows the map of sampling sites. Collected samples were transported on ice to the laboratory. The plant samples of mangrove were washed with sterile seawater. Also, the root samples were homogenized in the buffer.
2.2 Isolation and selection of crude oil-degrading bacteria
Crude oil-degrading bacteria were isolated in ONR7a medium supplemented with 1% (v/v) of crude oil (Iranian light crude oil) as sole carbon and energy source. ONR7a contained (per liter of distilled water) 40 g of NaCl, 11.18 g of MgCl2·6H2O, 3.98 g of Na2SO4, 1.46 g of CaC12·2H2O, 1.3 g of TAPSO (3-N Tris hydroxyl methyl Amino 2-hydroxy Propane sulfonic acid), 0.72 g of KCl, 0.27 g of NH4Cl, 89 mg of Na2HPO4·7H2O, 83 mg of NaBr, 31 mg of NaHCO3, 27 mg of H3BO3, 24 mg of SrCl2·6H2O, 2.6 mg of NaF, and 2 mg of FeCl2·4H2O. For solid media, bacterial agar (15 g/L) was added to the solution (Hasanshahian & Emtiazi, 2008).
Solution of plant samples (5 ml), condensed seawater (5 ml), and portion of sediments (10 g) were added to Erlenmeyer flasks containing 100 ml of medium, and the flasks were incubated for 7 days at 30°C on rotary shaker (180 rpm, INFORS AG). Then, 5 ml of samples were transported to fresh medium. After a series of four further subcultures, inoculums from the flask were streaked out, and phenotypically different colonies on ONR7a agar were purified. Phenotypically different colonies obtained from the plates were transferred to fresh medium with and without crude oil to eliminate autotrophic and agar utilizing bacteria. The procedure was repeated, and only isolates exhibiting pronounced growth on crude oil were stored in stock media with glycerol at −20°C for further characterization (Hasanshahian & Emtiazi, 2008).
2.3 Enumeration of bacteria
2.3.1 Quantification of cultivable heterotrophic and crude oil-degrading bacteria
Measurements of bacterial abundance within the mangrove plant materials, seawater, and mangrove sediment samples were carried out by colony-forming units (CFU) and most probable number (MPN) procedures (Cappello et al., 2012; Fuchsluger, Preims, & Fritz, 2010). Heterotrophic bacteria were estimated by spreading 100 μl of 10-fold diluents tubes on plates of marine agar medium (MA) and incubating at 30°C for 3 days. Also, crude oil-degrading bacteria were estimated by spreading 100 μl of 10-fold diluents flask on plates of ONR7a agar medium with crude oil and incubating at 30°C for 7 days. The bacteria quantification results were expressed as CFU/ml (Cappello et al., 2012).
2.3.2 Most Probable Number of heterotrophic and crude oil-degrading bacteria
The Most Probable Number (MPN) method for crude oil-degrading bacteria was done in sterile 24-well microplates using sample aliquots with corresponding dilutions and cultivation media. 1,700 µl ONR7a medium was provided in each well of the microplates, and then, dilution series of samples (usually 10-fold dilution until 10−1–10−3) were prepared in ONR7a medium. Wells were inoculated with 100 μl of sample diluents. Following sample inoculation, 100 μl of sterile Iranian light crude oil was applied at the center of each well. Plates were incubated at 30°C for 21 days. The positive index for MPN was turbidity that visually checked. The positive test tube was counted, and MPN was statistically evaluated (Fuchsluger et al., 2010). The MPN method for heterotrophic bacteria was done in sterile 24-well microplates. Each well of the microplates was provided with 1,700 µl marine broth (MB) and 100 μl dilution 10−3–10−4 of the sample, and then, plates were incubated at 30°C for 14 days. All samples from each station were used for MPN count. MPN counts were analyzed with the computer program MPN calculator version 4.2 (Fuchsluger et al., 2010).
2.4 Identification of the isolates
2.4.1 Biochemical characterization
The following characteristics were determined according to the Bergey's Manual of Determinative Bacteriology: The Gram stain, motility, starch hydrolysis, indole, H2S production, catalase and oxidase, oxidation/fermentation, reduction in nitrate, growth and acidification of carbohydrates tests were carried out (Holt, Kriey, Sneath, Staley, & Williams, 1998).
2.4.2 Molecular identification
The 16S rRNA gene was amplified for taxonomically characterize the isolated strains. Total DNA of bacterial strains was extracted with the CTAB method. PCR amplification of 16S rRNA genes was performed using the general bacteria primer 27F (5-AGAGTTTGATCCTGGCTCAG-3) and universal reverse primer 1492R (5-TACGYTACCTTGTTACGACTT-3; Cappello et al., 2012). The amplification reaction was carried out in a total volume of 25 μl consisting 2 mM MgCl2 (1 μl), 10× PCR buffer (200 mM Tris; 500 mM KCl) (2.5 μl), 2 mM each dNTP (2 μl), 0.15 mM each primer (1 µl), 1U (0.5 µl) Taq DNA polymerase (Qiagen, Hilden, Germany), and 2 µl of template DNA (50 pmol). Total volume was brought up to 15 μl using sterile milliQ water. PCR program consisted of 35 cycles was performed in a thermal cycler Gene Amp 5700 (PE Applied Biosystem, Foster City, CA, USA). The temperature profile for PCR was kept, 94°C for 5 min, 94°C for 1 min, 54°C for 1 min, 72°C for 1.5 min, 35 cycles and then 72°C for 10 min and finally storage at 4°C. The 16S rRNA gene sequences amplified were sequenced with BigDye Terminator V3.1 cycle sequencing kit on an automated capillary sequencer (model 3100 Avanti Genetic Analyzer, Applied Biosystems). Similarity rank from the Ribosomal Database Project (RDP) and FASTA Nucleotide Database Query was used to determine partial 16S rRNA sequences to estimate the degree of similarity to other 16S rRNA gene sequences. Analysis and phylogenetic affiliates of sequences were performed as following the protocol described by Yakimov, Timmis, & Golyshin, 2007. Phylogenetic trees were drawn by MEGA5 software with neighbor-joining method.
2.5 Measurement of bacterial growth and crude oil degradation
Bacterial isolates were grown at 30°C for 15 days on rotator shaker (180 rpm). The growth of the isolates was routinely assessed indirectly by measuring the turbidity (OD600 nm) using UV-visible spectrophotometer (Shimadzu UV-160, Japan). The crude oil removal assay was carried out by dissolving the residual crude oil in the medium in dichloromethane (DCM; 50 ml) and reading the optical density of the oil extract against blank (distilled water) at wavelength of 420 nm (Rahman, Thahira-Rahman, Lakshmanaperumalsamy, & Banat, 2004). The standard curve was used to calculate the rate of degradation (Hassanshahian, Bayat, Cappello, Smedile, & Yakimov, 2016).
2.6 Gas chromatography (GC) of residual crude oil
In order to better and precise detection of crude oil degradation, the GC-FID protocol was used. In this method first, the residual crude oil in all samples was extracted. The extraction protocol was as follows: The same volume of DCM (ratio 1:1 DCM:sample) was added to each flask, and residual crude oil was extracted and then treated with anhydrous sodium sulfate (Na2SO4) to remove residual water. Extracts were concentrated by separating funnel (Yu et al., 2005). Analyses were done by GC-FID (Varian 3800 model, USA) equipped with a SE-54 capillary column (25 m × 0.32 mm × 0.1 μm) and flame ionization detector (FID). Helium was used as the carrier gas (30 ml/min). The oven was programmed as follows: 100°C (1 min) then increased to 300°C (2 min) at a rate of 30°C/min. The samples were quantified according to previously described protocols (Hassanshahian, Zeynalipour, & Hosseinzadeh, 2014; Tebyanian, Hassanshahian, & Kariminik, 2013).
2.7 Measurement of emulsification activity and bacterial adherence to hydrocarbons (BATH)
The emulsification activity (E24) was determined by the addition of hexadecane to the same volume of cell-free culture broth. After mixing with vortex for 2 min and leaving to stand for 24 hr, the E24 index is given as the percentage of the height of emulsified layer (in millimeters) divided by total height of the liquid column (in millimeters). Measurement of the bacterial adhesion to hydrocarbon was performed as described by Pruthi and Cameotra (1997).
2.8 Effect of different concentrations of crude oil on degradation by isolated strains
The effect of different concentrations of crude oil (1%, 2.5%, 4%, and 5.5%) on the growth and degradation of crude oil by the selected bacterial strains was studied using ONR7a medium at 30°C and 180 rpm for 15 days (Karimi & Hassanshahian, 2016). Bacterial growth and crude oil degradation were calculated by read absorbance at 600 and 420 nm, respectively. The experiments were conducted in triplicates, and the average values were considered (Karimi & Hassanshahian, 2016; Sathishkumar, Binupriya, Baik, & Yun, 2008).
3 RESULTS
3.1 The quantity of heterotrophic and crude oil-degrading bacteria in mangrove samples (CFU and MPN)
The number (quantity) of heterotrophic and crude oil-degrading bacteria was calculated in all collected mangrove samples by two enumeration methods: CFU and MPN. The results are shown in Table 1. As shown in this table, the numbers of heterotrophic bacteria in BP and NW were maximum (respectively, 3.73 × 106, 2.71 × 106 CFU/ml) compared with other samples. BP and NS samples have maximum abundance of crude oil-degrading bacteria (respectively, 2.4 × 104, 4.6 × 105 CFU/ml). Also, the MPN values for sample NS and DP (respectively, 5.8 × 106, 5.1 × 105 cell/g) have maximum number of crude oil-degrading bacteria compared with other samples and the maximum quantity (MPN) of heterotrophic bacteria related to BP and NW samples (respectively, 9.3 × 108, 8.3 × 108 cell/g). Also, Table 1 illustrates that the MPN values in all samples were higher than CFU values.
| Sample | Quantity of heterotrophic bacteria (cfu/ml) | Quantity of crude oil-degrading bacteria (cfu/ml) | MPN of heterotrophic bacteria (cell/ml) | MPN of crude oil-degrading bacteria (cell/ml) |
|---|---|---|---|---|
| BW | 2.11 × 106 | 7.4 × 101 | 1.5 × 108 | 2.9 × 105 |
| BP | 3.73 × 106 | 2.4 × 104 | 9.3 × 108 | 2.3 × 105 |
| BS | 9.8 × 105 | 9.3 × 103 | 3.9 × 108 | 1.7 × 105 |
| DW | 3.15 × 105 | 9.2 × 10 | 6.4 × 108 | 3 × 104 |
| DP | 2.1 × 105 | 2.8 × 104 | 6.3 × 107 | 5.1 × 105 |
| DS | 3.8 × 105 | 4.3 × 103 | 5.3 × 108 | 7 × 105 |
| NW | 2.71 × 106 | 2.1 × 103 | 8.3 × 108 | 2 × 105 |
| NP | 3 × 104 | 2 × 104 | 3.4 × 108 | 1.9 × 105 |
| NS | 7.3 × 105 | 4.6 × 105 | 4.3 × 107 | 5.8 × 106 |
Note
- All data present in this figure are the average of three replicate of enumeration in the same dilution.
3.2 Isolation and identification of bacteria
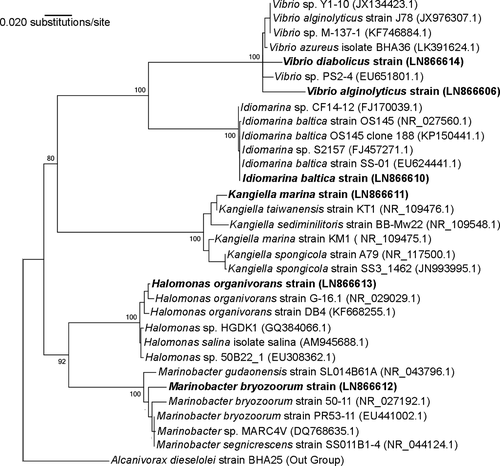
Sixty-one crude oil-degrading bacteria were isolated from collected mangrove samples. Sixteen isolated strains that show maximum growth on crude oil were selected for further study. These strains were first identified by biochemical tests. The results are presented in Table 2. As shown in this table, most of the isolated strains were Gram-negative. Six strains were identified molecularly by amplification and sequencing the 16S rRNA gene sequencing and comparing them to the database of known 16S rRNA sequences. The results of the identification procedure were showed that six isolated bacteria belong to Vibrio sp. strain NW4, Idiomarina sp. strain BW32, Kangiella sp. strain DP40, Marinobacter sp. strain DW44, Halomonas sp. strain BS53, and Vibrio sp. strain DS35. All sequences of six bacteria were submitted to the Genetic Sequence Database at the National Center for Biotechnology Information (NCBI). The GeneBank IDs of these strains in NCBI were as follows: LN866606 (for NW4), LN866610 (for BW32), LN866611 (for DP40), LN866612 (for DW44), LN866613 (for BS53), and LN866614 (for DS35). The phylogenic tree of these six isolated strains is illustrated in Figure 2. This figure shows that strains DP40 and DW44 have high similarity with other sequences that present in GeneBank database, although the similarity of other strains is less in compare to these two strains.
| Bacterial strain | Gram staining | Oxidase | Catalase | Motility | H2S | Indole | Nitrate | Citrate | TSI | O/F |
|---|---|---|---|---|---|---|---|---|---|---|
| NW1 | Positive | + | + | + | + | − | + | + | K/A | ± |
| NW2 | Negative | + | + | + | + | − | − | + | K/A | +/+ |
| NW4 | Negative | + | + | + | + | − | + | + | K/A | −/+ |
| BW30 | Positive | − | + | − | − | − | + | + | K/K | ± |
| BW31 | Negative | − | − | − | − | − | + | + | K/K | +/+ |
| BW32 | Negative | + | + | + | − | − | + | + | K/K | −/+ |
| DS34 | Negative | − | − | − | − | − | + | + | K/K | ± |
| DS35 | Negative | − | + | + | + | − | + | + | K/A | −/+ |
| DS36 | Negative | + | + | − | − | + | + | + | K/K | −/− |
| DP38 | Negative | − | + | − | + | + | + | + | A/A | ± |
| DP39 | Negative | − | − | − | − | − | + | + | K/A | −/− |
| DP40 | Negative | + | + | − | − | − | + | − | K/K | +/+ |
| DW44 | Negative | + | + | − | − | − | − | − | K/K | ± |
| BS53 | Negative | + | + | + | − | − | + | + | K/K | −/− |
| BS55 | Negative | + | + | − | − | + | + | + | A/K | −/− |
| BS56 | Negative | − | − | − | − | − | + | + | K/K | −/− |
3.3 Growth rate, crude oil removal, cell surface hydrophobicity, and emulsification activity of most efficient isolated strains
All bacterial strains (six strains) were grown in 1% crude oil for 15 days in a shaker incubator. After 15 days, the levels of microbial growth and crude oil degradation measured using spectrometry and GC-FID method. According to the Table 3, the strain BW32 exhibited highest level (88.18%) and the strain DP40 exhibited the lowest levels (53.73%) of crude oil degradation between all isolated strains. Also, for confirmation of the results, the GC chromatographs of these two strains are shown and compared to blank in Figure 3. Emulsification activity and bacterial adhesion to hydrocarbon (BATH) were analyzed for each strain separately. The data obtained from these tests are presented in Table 3. Strain BW32 has the highest value of emulsification activity (E24: 54%), and the high value of cell surface hydrophobicity relates to strain NW4 (BATH: 42.4%).
| Bacterial strain | Growth (OD600 nm) | Percentage of oil removal (spectrometry) | Percentage of oil removal (GC method) | Emulsification activity (E24%) | Cell surface hydrophobicity (BATH %) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |
| NW4 | 1.54 | 0.69 | 66.12 | 0.82 | 71.53 | 0.37 | 24 | 0.81 | 42.4 | 0.67 |
| BW32 | 1.92 | 0.18 | 88.13 | 0.58 | 88.18 | 0.55 | 54 | 1.82 | 27 | 0.51 |
| DS35 | 1.67 | 0.25 | 70.30 | 0.80 | 74.21 | 0.81 | 8 | 0.79 | 16 | 0.78 |
| DP40 | 1.12 | 0.56 | 53.73 | 1.2 | 61.22 | 1.02 | 7 | 0.64 | 20 | 0.98 |
| DW44 | 1.43 | 0.11 | 60.82 | 1.16 | 67.8 | 0.85 | 24 | 0.45 | 17 | 1.08 |
| BS53 | 1.8 | 0.35 | 75.72 | 0.51 | 83.15 | 1.24 | 34 | 1.09 | 21 | 1.06 |
Note
- SD: standard deviation.

3.4 Effect of different concentrations of crude oil on degradation of crude oil by selected isolated strains
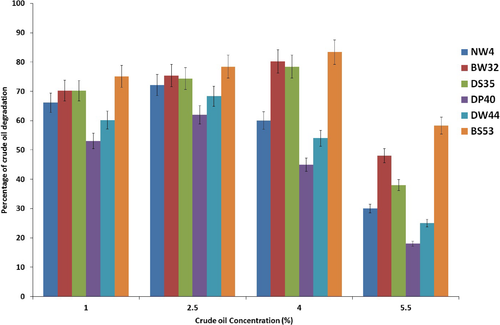
Effect of different concentrations of crude oil on degradation was determined. The results are presented in Figure 4. As shown in this figure, the highest degradation of crude oil related to 2.5% concentration for strains NW4, DP40, and DW44 but the optimum concentration of crude oil for strains BW32, DS53, and BS53 was 4% concentration. However, crude oil degradation was decreased when concentrations of crude oil were increased.
4 DISCUSSION
The mangrove forests which are near human activities are susceptible to a variety of pollutions such as crude oil pollution of anthropogenic sources. The present microorganisms in the polluted sediments of the mangroves are able to degrade petroleum compositions because of their genetic potential (Hassanshahian & Mohamadian, 2011; Yu et al., 2005).
In current research, some crude oil-degrading bacteria were isolated from mangrove ecosystem that located at the Persian Gulf. These mangrove forests routinely damaged by oil pollution that happen at the Persian Gulf. Since over the 60% of crude oil transition occurred in this Gulf, thus Persian Gulf is chronically polluted by oil and petroleum products. Sixty-one crude oil-degrading bacteria were isolated in primary screening that means crude oil-degrading bacteria have sufficient diversity in this ecosystem. Some screening tests such as growth in high concentration of crude oil, bioemulsifier production, and surface hydrophobicity were done to selecting the best and prevalent strains for crude oil degradation that decrease degrading bacteria to 16 strains. These strains were first characterized by biochemical tests, and then, similar bacteria were deleted and the remaining strains identified by molecular method. Five different genera were reported in this research as crude oil-degrading bacteria from mangrove forests at the Persian Gulf: Marinobacter, Kangiella, Vibrio, Idiomarina, and Halomonas.
These genera previously described as crude oil-degrading bacteria by other researchers. But this research is the first report on the isolation of these genera from mangrove forests at the Persian Gulf.
The bacterial quantification results of this research confirmed that the quantity of crude oil-degrading bacteria in the mangrove sediments and root of mangrove plants were higher than other zones of this ecosystem. Some reasons can be explained for this finding. For example, the organic materials near the root of the plant were higher than seawater or sediments, also the growth of bacteria was better supported and the quantity was increased. Also, plants produce some growth promotion factors such as vitamins and hormones that increased the quantity of rhizosphere bacteria.
Crude oil contains many different organic molecules that are difficult for bacteria to utilize. Thus, these organic molecules in the crude oil are usually classified as recalcitrant organic matter (Dang & Jiao, 2014; Ghanavati, Emtiazi, & Hassanshahian, 2008). The exudates of plant roots mainly contain labile organic matter that is easy for bacteria to utilize. The stimulation effect of labile organic matter to recalcitrant organic matter utilization is a hot topic in environmental microbiology and carbon biogeochemical cycling, and it is formally termed as the “priming effect” (Dang & Lovell., 2016). The gain (in term of carbon sources and energy) from utilization of recalcitrant organic matter is usually marginal to the bacteria, as the degradation of recalcitrant organic matter costs quite an amount of cellular energy. Therefore, the gene expression for utilization of recalcitrant organic matter is likely to be highly regulated. The physiological status and especially the energetic environment of the bacteria are important factors that may control the initiation of gene expression in bacterial degradation of recalcitrant organic matter. Thus, the “priming effect” should also be viewed from the bioenergetics perspectives, as the consumption and respiration of labile organic matter secreted by the plant roots may provide the root-colonizing bacteria with energy that is needed to express the genes and produce the enzymes to break down the recalcitrant organic molecules such as the constituents in crude oil. The composition, abundance, and dynamics of metabolizable organic compounds secreted by the plant roots may play a predominant role in controlling the composition, community succession, and ecophysiology of the root-associated microbiota, and the plant physiological status and environmental conditions may influence the composition, abundance, and dynamics of metabolizable organic compounds secreted by the plant roots (Dang & Lovell, 2016). It is highly likely that the varying organic compounds secreted by the mangrove plant roots in response to the varying environments and plant physiology lead to the increase in diversity and abundance of bacteria that can degrade crude oil. These results are coordinated with the results that previously reported by other researcher that works with different mangrove forests at worldwide (Batista et al., 2006; Brito et al., 2006; Fuchsluger et al., 2010).
The results of this research suggest that optimum concentration for crude oil degradation was: 2.5% and 4% concentration of crude oil. Fusey and Oudot (1984) reported that when the concentrations of crude oil were increased hydrocarbon biodegradation decreased. This pattern can be related to oxygen or nutrients limitation in higher concentrations of crude oil. Hasanshahian and Emtiazi (2008) demonstrated that maximum crude oil removal levels have been carried out at 1% concentration. Zahed, Hamidi, Isa, and Mohajeri (2010) reported that most of the crude oil removal occurred at 100 mg/L and lower concentrations of crude oil are more efficient for crude oil removal.
5 CONCLUSION
The mangrove forests ecosystems are the most susceptible marine ecosystem against oil contamination, and thus, protection of these ecosystems is important for reserve the marine animal and plant niches. The results obtained from this research confirmed that crude oil-degrading bacteria have sufficient diversity and density in this ecosystem at the Persian Gulf. By using bioremediation strategies (biostimulation and bioaugmentation) with these bacteria, crude oil pollution can be reduced in this important marine environment.
ACKNOWLEDGEMENT
This work was supported financially by Shahid Bahonar University of Kerman.
CONFLICT OF INTEREST
There are not any conflicted of interest between authors.




