Disentangling exploitation of the intertidal grazer Phorcus sauciatus (Gastropoda: Trochidae) in an oceanic archipelago: Implications for conservation
Abstract
Harvesting of intertidal grazers such as topshells is known to affect negatively the exploited populations by altering population structure and decreasing abundance. Phorcus sauciatus has a wide geographic distribution in the North-eastern Atlantic Ocean and is subject to increasing levels of harvesting pressure due to the expansion of human population on coastal areas. The effect of proximity to human settlements and coastal accessibility on the size structure and abundance of P. sauciatus populations was examined in Madeira archipelago. Mean size, proportion of reproductive individuals, and abundance of this species were generally smaller in areas closer to human settlements and in more accessible coastal areas. Marine protected areas returned the highest mean sizes evidencing their effectiveness in preserving the size structure of this species. The results highlight the necessity to regulate the harvest of P. sauciatus in Madeira archipelago, as well as the implementation of management measures aiming at the sustainable exploitation and conservation of this species, exploited in this region since the early 15th century.
1 INTRODUCTION
Intertidal reefs are highly productive ecosystems supporting an extremely diverse range of assemblages of algae and animals (Gamfeldt & Bracken, 2009). Their accessibility has made them susceptible to a variety of human-induced impacts such as harvesting (Thompson, Crowe, & Hawkins, 2002). Highly exploited populations are characterized by low abundances and a decrease of mean size of the targeted species (Castilla & Durán, 1985; Riera et al., 2016). However, the effects of harvesting are not limited to changes in targeted species, but they extend through cascading trophic effects to the whole habitat (Scheffer, Carpenter, & Young, 2005). Mollusks are one of the most exploited intertidal organisms all over the globe (Roy, Collins, Becker, Begovic, & Engle, 2003; Sagarin et al., 2007) due to the easy accessibility to their habitat, having been extensively harvested in several geographic regions since prehistoric times (Martins, Jenkins, Hawkins, Neto, & Thompson, 2008; Siegfried, 1994; Turrero, Múñoz-Colmenero, Pola, Arbizu, & García-Vázquez, 2012).
The harvesting of intertidal organisms is steadily increasing throughout the last decades due to the expansion of human population to coastal areas (Neumann, Vafeidis, Zimmermann, & Nicholls, 2015). Several species are considered highly threatened such as the limpet Patella ferruginea Gmelin, 1791, a Mediterranean species that is currently concentrated in a limited number of coastal locations (Espinosa et al., 2014), and the Hawaiian endemic limpet species Cellana sandwicensis (Pease, 1861), Cellana exarata (Reeve, 1854), and Cellana talcosa (Gould, 1846), that have been disappearing in extensive intertidal areas of islands with high population density (Valledor, 2000). However, the situation concerning other intertidal mollusk species, for example sea snails, still remains overlooked for most of their geographic regions though having recently received some attention in Atlantic oceanic islands (Navarro et al., 2005; Ramírez et al., 2005; Sousa et al., 2018).
The lack of management measures for the conservation of sea snails is alarming, especially in coastal isolated places where no adjacent populations are present to supply larvae for settlement and recruitment, though it has been observed that a high proportion of larvae settle <100 km from their source population (Cowen, Lwiza, Sponaugle, Paris, & Olson, 2000), and large genetic connectivity among populations has been shown in Atlantic and Mediterranean populations of Osilinus and Phorcus (Donald et al., 2012).
The Macaronesian region, comprising the oceanic archipelagos of Azores, Madeira, Selvagens, and Canaries, is characterized by high harvesting pressure on intertidal ecosystems (Martins, Thompson, Neto, Hawkins, & Jenkins, 2010; Núñez, Brito, Riera, Docoito, & Monterroso, 2003). This situation has been demonstrated through a sharp decrease of exploited mollusks populations in Azores (Martins, 2009), Madeira (Sousa et al., 2018) and the Canaries (Ramírez, Tuya, & Haroun, 2009); however, food culture greatly varies among these archipelagos. For example, Madeira has an old tradition of harvesting sea snails, namely the species P. sauciatus (Koch, 1845; Sousa et al., 2018), while the consumption of this species is not extensive in the Canaries and varies among islands, being more intense in western islands (Moro & Herrera, 2000; Ramírez et al., 2009; Tuya et al., 2006). Thus, the harvesting pressure of P. sauciatus show variations throughout the Macaronesian region and adjacent areas, and conservation measures need to be context-dependent and focused on local harvesting pressures to ensure the viability of this sea snail species in the region.
The topshells of the genus Phorcus are ecologically important algae grazers that play a key role in regulating the ecological balance of their habitat (Ramírez et al., 2005). These species occupy the rocky shores from the supratidal to the subtidal and are subject to an array of environmental pressures due to their extended vertical distribution, resulting in structural adaptations since their position relative to the shore influences their exposure to wave action, temperature variation, desiccation, and tidal width (Ramírez et al., 2005; Sousa et al., 2018). They are essentially preyed by prawns, crabs, and fishes (Crothers, 2001).
Phorcus sauciatus has a wide geographic distribution in the North-eastern Atlantic, including the Macaronesian archipelagos of Azores, Madeira, and Canaries with its northern boundary in the Iberian Peninsula and its southern limit in the African mainland, with small genetic differentiation between them, suggesting either recent or continuing dispersal among these areas (Ávila et al., 2015; Donald et al., 2012; Rubal, Veiga, Moreira, & Sousa-Pinto, 2014).
Herein, the effects of harvesting pressure on the size structure and abundance of populations of P. sauciatus in Madeira (NE Atlantic Ocean), where this species has been historically highly exploited, were investigated considering: (a) that proximity to human settlements would result in a decrease in topshell mean size and lower abundance in “near” stations (<1 km from human settlements) compared to “far” stations (>3 km from human settlements), (b) and that accessibility to topshell populations affects negatively the size structure and abundance of the more accessible populations (South sectors) compared to the least accessible populations (North sectors).
2 METHODS
The study was conducted on specimens randomly collected from the upper to the lower intertidal zones of the rocky shores of the Madeira archipelago (Figure 1), during low tide. Each harvesting set was performed by the same experienced harvester during a standardized period of 15 min.
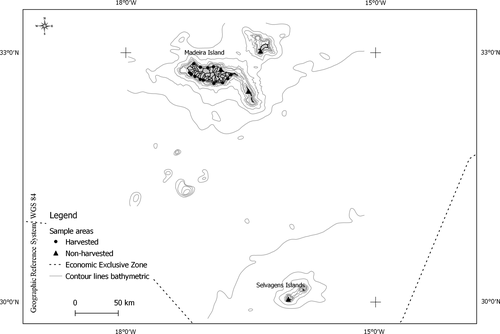
A total of 17 coastal settlements (locations) throughout the rocky shores were sampled between January 2017 and May 2018 (13 in harvested and four in not harvested zones). The locations were as similar as possible to each other and were selected considering the coastal settlements with analogous conditions (e.g., type of substrate, slope of the coast, rugosity).
All specimens were measured (total shell length, TL, mm) using a Vernier calliper to the nearest 0.01 mm.
Data were analyzed for deviations to the parametric assumptions of analysis of variance (ANOVA). Three factors were considered: (a) Locations (random factor), (b) Proximity (fixed factor, with three levels “Near” and “Far” from coastal settlements and MPAs), and (c) Accessibility (fixed factor, with three levels “North”, “South,” and MPAs). Normality of the distribution of the data was verified through the Kolmogorov-Smirnov 2-sample test, and homogeneity of variance was determined using Levene's statistics. Analysis of variance was performed considering the Brown-Forsythe F test, when the variance of the data was not homogeneous. All statistical analyses were performed using SPSS v.24.0 (IBM Corp, ). For all tests, statistical significance was accepted when p < 0.001 to reduce the chance of false positives.
2.1 Effect of proximity and accessibility on size structure and CPUEn
The effect of proximity was analyzed for each coastal settlement of Madeira archipelago, by selecting a minimum of two sites according to proximity to human settlement as described by Riera et al., (2016). Sites at <1 km from the nearest human settlement were classified as “near,” and sites >3 km from the nearest human settlement were considered as “far.” Sites were also grouped according to accessibility to the coast, samples from the northern coast, the least accessible due to rough seas were classified as “North,” and samples from the more accessible southern coast, due to milder conditions, were classified as “South.” Four Marine Protected Areas (MPAs), where topshell harvest is not allowed, were also sampled as areas with no harvesting pressure throughout the year, because of their inaccessibility and protection measures, namely Porto Santo Island (NE Madeira); Rocha do Navio (N Madeira); Desertas Islands (SE Madeira); and Selvagens Islands (between Madeira and the Canaries).
A univariate comparison of the size of P. sauciatus was performed using an ANOVA. For the comparison of the proportion of reproductive individuals, considering the size at first maturity as 12.95 mm TL (Sousa et al., 2018), a statistical Pearson chi-square test was applied. Proximity to human settlements and accessibility were considered within the data to determine the influence of harvesting on P. sauciatus size structure throughout Madeira archipelago.
Relative abundance was estimated using the catch per unit of effort in number (CPUEn), corresponding to the ratio between the total number of captured specimens at each harvesting set and time. The effect of proximity and of accessibility on the abundance of P. sauciatus was evaluated using a Pearson chi-square and a Mann–Whitney U test, respectively.
3 RESULTS
A total of 6,329 specimens of P. sauciatus from 13 selected locations of rocky shores and four MPAs of Madeira archipelago were analyzed. The mean size of total topshells sampled in Madeira was 14.39 ± 3.44 mm TL. The size-frequency distribution showed that the sampled data had a normal distribution (Z = 1.883, p < 0.001). Size did not exhibit homogeneous variance for proximity (W = 55.125, p < 0.001); however, it was homogenous for accessibility (W = 1.613, p = 0.204).
3.1 Effect of proximity and accessibility on size structure and CPUEn
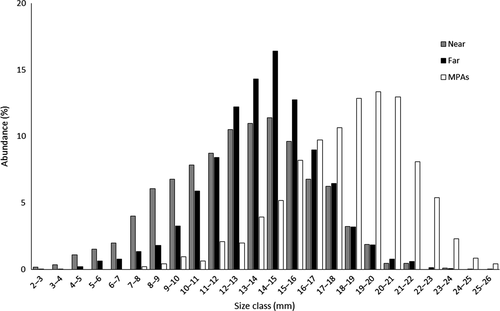
The study populations were characterized by a wide range of sizes, from 2.40 to 23.49 mm in near areas, from 2.91 to 25.07 mm in far areas, and from 7.35 to 25.79 mm in MPAs. The size distribution showed that reproductive specimens (>12.95 mm TL) were dominant, representing 51% of the total specimens in “near” areas, 65% in “far” areas, and 94% in MPAs (Figure 2). This suggests that most specimens are reproductive individuals, having reached the size at first maturity. The differences in the proportion of reproductive individuals according to proximity were statistically significant (χ2 = 682.946, p < 0.001). Pairwise comparisons showed significant differences in the proportion of reproductive specimens between near and far sites (χ2 = 67.203, p < 0.001), near sites and MPAs (χ2 = 223.514, p < 0.001) and far sites and MPAs (χ2 = 607.858, p < 0.001). Statistical differences were also observed between the proportion of reproductive specimens according to the level of proximity on each of the 13 studied localities (Pearson Qui-square statistical test, p < 0.001).
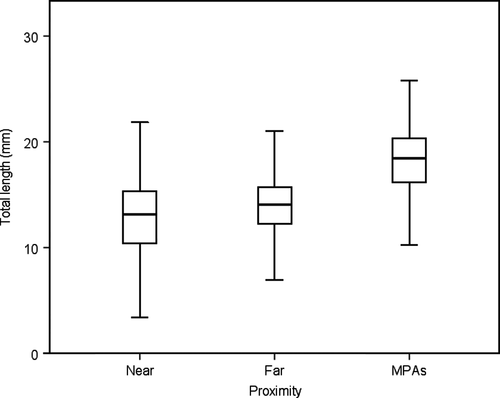
Mean size of the specimens caught in “near” areas was 12.87 ± 3.52 mm TL, 13.94 ± 2.86 mm TL in “far” areas, and 18.13 ± 3.10 mm TL in MPAs (Figure 3). Statistical differences in mean size among levels of proximity were significant (F = 806.524, p < 0.001; Table 1). Post hoc analysis confirms significant differences between near and far sites (ANOVA Tamhane's T2 statistical test, p < 0.001), near sites and MPAs (ANOVA Tamhane's T2 statistical test, p < 0.001) and far sites and MPAs (ANOVA Tamhane's T2 statistical test, p < 0.001). Statistical differences were also observed between levels of proximity on each of the 13 studied localities (ANOVA, p < 0.001).
| Factor | Sum of squares | df | Mean square | F | p-Value |
|---|---|---|---|---|---|
| Locality | 1,833.904 | 12 | 15.825 | 17.763 | 0.000 |
| Proximity | 16,959.324 | 2 | 8,479.662 | 806.524 | 0.000 |
| Accessibility | 4,285.856 | 2 | 4,285.856 | 422.210 | 0.000 |
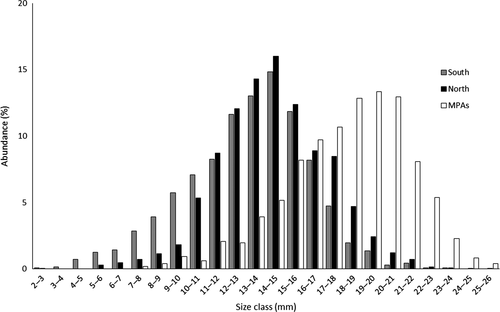
Regarding accessibility, specimens ranged from 2.40 to 23.39 mm in the more accessible areas and from 2.97 to 25.07 mm in the least accessible areas. The size distribution showed that reproductive individuals were more dominant in the least accessible areas (70%) than in the more accessible areas (57%). The differences in the proportion of reproductive individuals among levels of accessibility were statistically significant (χ2 = 1,052.511, p < 0.001; Figure 4). Pairwise comparisons confirmed significant differences in the proportion of reproductive specimens between north and south (χ2 = 56.057, p < 0.001), north and MPAs (χ2 = 585.149, p < 0.001) and south and MPAs (χ2 = 791.710, p < 0.001).
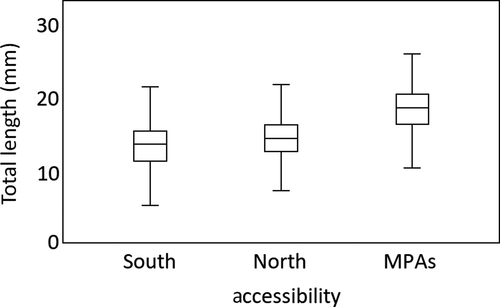
The analysis of specimens mean size showed larger individuals on less accessible areas (north coast), with a mean size of 14.92 ± 3.24 mm TL, than on more accessible areas (south coast) with a mean size of 13.18 ± 3.14 mm TL (Figure 5). The differences observed in mean size of topshell according to accessibility were statistically significant (F = 422.210, p < 0.001; Table 1). Post hoc analysis also showed significant differences within the north and south coasts (ANOVA Tamhane's T2 statistical test, p < 0.001), north and MPAs (ANOVA Tamhane's T2 statistical test, p < 0.001) and south and MPAs (ANOVA Tamhane's T2 statistical test, p < 0.001).
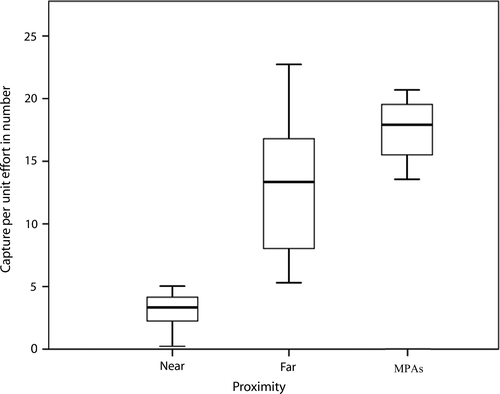
Concerning the CPUEn, abundances ranged from 0.23 to 22.73 specimens per minute with a mean abundance of 8.30 ± 6.20 specimens/min. The highest mean abundances were recorded for MPAs (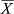 = 17.30 ± 2.93 specimens/min) and “far” areas (
= 17.30 ± 2.93 specimens/min) and “far” areas (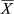 = 13.38 ± 5.89 specimens/min), and the lowest for “near” areas (
= 13.38 ± 5.89 specimens/min), and the lowest for “near” areas (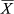 = 3.40 ± 2.13 specimens/min; Figure 6). Differences found in mean abundances between areas were statistically significant (KW = 8.160, p < 0.001). Pairwise comparisons showed significant differences within the near and far sites (U = 3.000, p < 0.001), near sites and MPAs (U = 0.000, p < 0.001). Concerning far sites and MPAs, the observed differences were not statistically significant (U = 11.000, p = 0.240).
= 3.40 ± 2.13 specimens/min; Figure 6). Differences found in mean abundances between areas were statistically significant (KW = 8.160, p < 0.001). Pairwise comparisons showed significant differences within the near and far sites (U = 3.000, p < 0.001), near sites and MPAs (U = 0.000, p < 0.001). Concerning far sites and MPAs, the observed differences were not statistically significant (U = 11.000, p = 0.240).
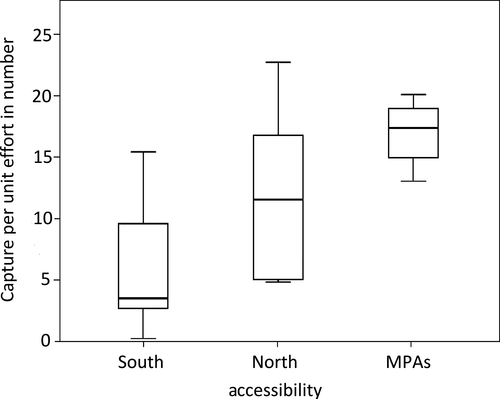
Regarding accessibility, the highest abundance occurred at the least accessible coast ( = 12.08 ± 7.26 specimens/min) and the lowest at the more accessible coast (
= 12.08 ± 7.26 specimens/min) and the lowest at the more accessible coast ( = 6.58 ± 5.89 specimens/min; Figure 7). Statistical differences found in mean abundance according to accessibility were statistically significant (KW = 8.160, p = 0.017). The pairwise comparisons showed significant differences between the south and MPAs (U = 5.000, p < 0.001) contrarily to the observed differences between south and north coasts (U = 22.000, p = 0.080) and between north and MPAs (U = 6.000, p = 0.201) which were not statistically significant.
= 6.58 ± 5.89 specimens/min; Figure 7). Statistical differences found in mean abundance according to accessibility were statistically significant (KW = 8.160, p = 0.017). The pairwise comparisons showed significant differences between the south and MPAs (U = 5.000, p < 0.001) contrarily to the observed differences between south and north coasts (U = 22.000, p = 0.080) and between north and MPAs (U = 6.000, p = 0.201) which were not statistically significant.
4 DISCUSSION
The limited habitat and its easy accessibility to human activity make intertidal and shallow-water grazers extremely vulnerable to anthropogenic pressures (Nakin & McQuaid, 2014). Harvesting of intertidal grazers such as topshells and limpets is known to affect negatively the exploited populations by altering their population structure, resulting in a decrease of abundance, and altered size structure (Riera et al., 2016; Tuya et al., 2006). Additionally, the removal of grazers can often lead to an imbalance on the population dynamics of the species inhabiting rocky shores due to both direct and indirect effects on the trophic chains, with potential cascading effects (Branch & Moreno, 1994; Crowder & Norse, 2008).
The overall present results indicated that the populations of P. sauciatus in Madeira archipelago are dominated by reproductive specimens (>12.95 mm TL). However, in areas closer (<1 km) to human settlements the mean size and the proportion of these specimens are smaller than in distant areas (>3 km) as a result of size-selective harvesting. The effect of proximity is more pronounced on the south coast due to a greater density of human population allied to an easier accessibility to this coast. This effect is even more pronounced when comparing with MPAs where harvesting is not allowed, and >90% of individuals are mature. Even though, reproductive individuals are overly more abundant, it is known that in exploited populations of broadcast spawners, such as topshells and limpets the decrease of larger individuals, results in the reduction of their reproductive success and can lead to their decline over time (Guerra-García, Corzo, Espinosa, & García-Gómez, 2004; Núñez et al., 2003). In fact, present results suggest that, for P. sauciatus in Madeira, harvesting has led to a decrease in reproductive individuals according to a gradient of proximity to human settlements, varying from 90% in no take zones to 51% in areas closest to human settlements. This trend currently exists although there are many MPAs in and around Madeira Island from where larval dispersal and settlement may occur and provide recruits to nearby harvested populations.
The effect of human influence has also been demonstrated for Phorcus atratus (Wood, 1828) and P. sauciatus in the Canaries (Ramírez et al., 2009), where significant differences in size structure of these species among islands have been observed. Ramírez et al., (2009) also verified that proximity to human settlements resulted in the disappearance of larger individuals and a decrease in number for the majority of size ranges of these species in the Canaries. The reduction in size and the increase in mortality rates are the primary effects of size-selective harvest; however, harvest also prompts shifts on the reproductive potential of these species through changes in reproductive investment and relative fecundity. Additionally, it promotes ecological effects like changes in community structure and interspecific competition (Fenberg et al., 2012; Fenberg & Roy, 2008).
The size structure of P. sauciatus populations is also influenced by accessibility, with larger individuals being found in the least accessible coast (North) and it is clear that on the south coast there are more significant differences in average sizes between localities. This pattern may be due to the fact that populations on the north coast are more protected, having less harvesting pressure and therefore being more balanced in terms of population structure. When considering MPAs, where harvesting is not allowed, it is evident that these populations have a more balanced size distribution, with higher mean shell length and greater abundance of larger size class specimens.
The effect of accessibility on the abundance of P. sauciatus, however, did not return significant differences between northern and southern populations. Regardless, least accessible populations still showed higher abundance than more accessible populations due to the existence of rougher environmental conditions and lower human density in the North coast. These impacts on the spatial distribution of P. sauciatus abundance in Madeira archipelago, as a result of proximity and accessibility to human settlements, have also been reported for Phorcus articulatus (Lamarck, 1822) along the coast of Tunisia by Cheour, Cherif, Messaoud, Aloui-Bejaoui, and Ali (2014) and in the Canaries by Ramírez et al., (2009).
As expected, harvested populations always showed smaller mean size and lower proportion of reproductive individuals than non-harvested populations, suggesting that harvesting is impacting the population dynamics of P. sauciatus. However, differences in environmental conditions, such as sea surface temperature, tidal width, degree of exposure to wave action, and productivity, between locations may also impact the population dynamics of these populations. These factors were not considered in the current analysis; however, most of these factors are not expected to vary greatly between the studied locations, for instance sea surface temperature and productivity. On the other hand, the degree of exposure to wave action is likey to affect the population dynamics of P. sauciatus as shown by our data, locations subject to harsher wave action have larger sized and more reproductively active individuals, as an indirect consequence to lower levels of exploitation due to these populations being less accessible to humans.
The present results highlight the necessity to regulate the harvest of P. sauciatus in Madeira archipelago, as well as the implementation of management measures aiming at the sustainable exploitation of this species. The effect of regulating topshell harvest is evidenced by the smaller size in harvested compared to non-harvested areas in Madeira. These management measures should be considered together with no take zones, as it has been shown that they contribute to the preservation of the size structure of P. sauciatus and complemented with continuous monitoring effort of the exploited local (Madeira) and regional (Macaronesia) populations.
Further studies with a wide range of regions and considering the harvesting pressure together with the biotic (interspecific and intraspecific completion) and abiotic factors (temperature, productivity, salinity) of the regions are warranted to evaluate the role of these factors in the size structure and abundance of P. sauciatus in the north-eastern Atlantic.
ACKNOWLEDGEMENTS
The authors are grateful to the Fisheries Research Service (DSI) of the Regional Directorate of Fisheries of the Autonomous Region of Madeira. We acknowledge Dr.ª Antonieta Amorim for providing the map with de stations used in this work and are also grateful to André Pinto, Filipe Andrade and Jorge Lucas for their help during this work, namely in biological sampling and harvesting surveys and to Drª Carolina Santos and the IFCN (Instituto das Florestas e Conservação da Natureza da RAM) for allowing and collaborating in the collection of topshells in the natural reserve zones. The first author (RS) was supported by a grant from ARDITI OOM/2016/010 (M1420-01-0145-FEDER-000001-Observatório Oceânico da Madeira-OOM) and the second author (JV) by a grant from FCT (SFRH/BSAB/143056/2018). This study was also supported by the UE FEDER in the framework of the Projects MARISCOMAC-MAC/2.3d/097 and MACAROFOOD - MAC/2.3d/015, the Regional Government of Madeira and FCT, through the strategic project UID/MAR/04292/2019 granted to MARE.
AUTHORS′ CONTRIBUTIONS
RS: Study design, data acquisition, statistical analysis, data interpretation, writing the paper; JV: Data interpretation, writing the paper; RR: Study design, critical analysis, revision of the paper; JD: Critical analysis, revision of the paper; JAG: Critical analysis, revision of the paper; MF: Data acquisition; PH: Study design, data acquisition, statistical analysis, data interpretation, writing the paper. All authors read and approved the final manuscript.




