Trophic structure of mesopelagic fishes in the Gulf of Mexico revealed by gut content and stable isotope analyses
Abstract
Mesopelagic fishes represent an important component of the marine food web due to their global distributions, high abundances and ability to transport organic material throughout a large part of the water column. This study combined stable isotope (SIAs) and gut content analyses (GCAs) to characterize the trophic structure of mesopelagic fishes in the North-Central Gulf of Mexico. Additionally, this study examined whether mesopelagic fishes utilized chemosynthetic energy from cold seeps. Specimens were collected (9–25 August 2007) over three deep (>1,000 m) cold seeps at discrete depths (surface to 1,503 m) over the diurnal cycle. GCA classified 31 species (five families) of mesopelagic fishes into five feeding guilds: piscivores, large crustacean consumers, copepod consumers, generalists and mixed zooplanktivores. However, these guilds were less clearly defined based on stable isotope mixing model (MixSIAR) results, suggesting diets may be more mixed over longer time periods (weeks–months) and across co-occurring species. Copepods were likely important for the majority of mesopelagic fishes, consistent with GCA (this study) and previous literature. MixSIAR results also identified non-crustacean prey items, including salps and pteropods, as potentially important prey items for mesopelagic fishes, including those fishes not analysed in GCA (Sternoptyx spp. and Melamphaidae). Salps and other soft-bodied species are often missed in GCAs. Mesopelagic fishes had δ13C results consistent with particulate organic matter serving as the baseline organic carbon source, fueling up to three trophic levels. Fishes that undergo diel vertical migration were depleted in 15N relative to weak migrators, consistent with depth-specific isotope trends in sources and consumers, and assimilation of 15N-depleted organic matter in surface waters. Linear correlations between fish size and δ15N values suggested ontogenetic changes in fish diets for several species. While there was no direct measure of mesopelagic fishes assimilating chemosynthetic material, detection of infrequent consumption of this food resource may be hindered by the assimilation of isotopically enriched photosynthetic organic matter. By utilizing multiple dietary metrics (e.g. GCA, δ13C, δ15N, MixSIAR), this study better defined the trophic structure of mesopelagic fishes and allowed for insights on feeding, ultimately providing useful baseline information from which to track mesopelagic trophodynamics over time and space.
1 INTRODUCTION
Mesopelagic fishes constitute an important part of the pelagic food web due to their high abundances, vertical migratory behavior and global distribution (Cornejo & Koppelmann, 2006; Gjøsaeter & Kawaguchi, 1980). In the epipelagic zone, mesopelagic fishes may consume approximately 5%–10% of the daily zooplankton production (Hopkins, Sutton, & Lancraft, 1996). Some of this production is then transferred to higher trophic levels when mesopelagic fishes are consumed by other marine fauna, such as tuna (Potier et al., 2007) and penguins (Adams, Moloney, & Navarro, 1993). Many mesopelagic fishes undergo diel vertical migrations (DVMs) where they swim up to the epipelagic zone at night to feed and return to deeper depths during the day. This movement represents a major mechanism for transporting organic material below the euphotic zone (Ashjian, Smith, Flagg, & Idrisi, 2002; Brodeur & Yamamura, 2005; Hidaka, Kawaguchi, Murakami, & Takahashi, 2001). However, mesopelagic fishes often avoid capture in nets, resulting in underestimates of abundance and biomass (Kaartvedt, Staby, & Aksnes, 2012). Therefore, the influence of mesopelagic fishes on organic matter transport may be greatly underestimated.
Mesopelagic fish aggregations have been documented near the benthos along topographic features (e.g. deep-sea coral mounds, seamounts; Gartner, Sulak, Ross, & Necaise, 2008) and in areas with enhanced productivity (e.g. upwelling areas, continental slopes; Young & Blaber, 1986; Gartner et al., 2008). Cold seeps represent one type of benthic habitat in the deep sea containing high levels of primary productivity and rugged topography. Non-seep benthic fauna observed near seeps (Carney, 1994; Levin, 2005; MacAvoy, Carney, Fisher, & Macko, 2002) can consume chemosynthetic material and serve as an important component of chemoautotrophic energy transfer (MacAvoy, Morgan, Carney, & Macko, 2008; MacAvoy et al., 2002). Whether mesopelagic fishes utilize chemosynthetic production indirectly or directly, either in the water column above seep areas or by interactions with associated benthic communities, has not been examined. Although the distribution of mesopelagic fishes does not seem to be related solely to the presence of cold seeps (Ross, Quattrini, Roa-Varόn, & McClain, 2010), it is unclear whether these productive benthic habitats attract mesopelagic fishes or their prey. Thus, a better understanding of the trophic structure of mesopelagic fishes near seeps should provide insight on whether these fishes transfer seep-derived energy through the water column to higher trophic levels.
Previous diet studies on mesopelagic fishes (Butler, Bollens, Burkhalter, Madin, & Horgan, 2001; Hopkins & Baird, 1985a,b; Hopkins et al., 1996; Lancraft, Hopkins, & Torres, 1988; Pusch, Hulley, & Kock, 2004) utilized gut content analyses (GCAs) to determine trophic relationships. Results generally classified mesopelagic fishes into three major feeding guilds: zooplanktivores, micronektonivores and generalists (Gartner, Crabtree, & Sulak, 1997). However, accuracy of guild assignments can be affected by factors that occur over long time periods (weeks, months, years), including changes in prey abundance (Kawaguchi & Mauchline, 1982), seasonality of prey resources (Kawaguchi & Mauchline, 1982) and ontogeny (Beamish et al., 1999; Butler et al., 2001; Hopkins et al., 1996; Kawaguchi & Mauchline, 1982; Williams, Koslow, Terauds, & Haskard, 2001; Young & Blaber, 1986). While GCA provides detailed taxonomic data on consumed prey that is well preserved, GCA requires time, taxonomic knowledge of potential prey (Rybczynski, Walters, Fritz, & Johnson, 2008), and only represents prey consumed over a short (<24 hr) period (Hadwen, Russell, & Arthington, 2007). Additionally, accurate guild placement is not possible for specimens with empty stomachs or with unidentifiable material, a common occurrence in midwater fishes (Gartner et al., 1997). Difficulty in sampling mesopelagic fishes also inhibits GCA, due to the challenges with collecting sufficient sample sizes to accurately document their diets.
Stable isotope analysis (SIA) is an alternative method used to examine trophic interactions among species. SIA provides time-integrated diet information of an organism (Fry, 2006), while using less processing time and taxonomic expertise than GCA. Trophic positions within a food web (Fry, 1988; Peterson & Fry, 1987) can be estimated from SIA using the isotopic ratio of nitrogen (15N/14N), which increases from 0‰ to 4‰ per trophic level, with an average of 2‰ (McCutchan, Lewis, Kendall, & McGrath, 2003) estimated for invertebrate and fish consumers. While it may be difficult to directly assess trophic levels due to the variability in fractionation of nitrogen isotopes in space and time (Mancinelli, Vizzini, Mazzola, Maci, & Basset, 2013; Post, 2003), this challenge can be addressed by selecting appropriate baseline consumers for trophic position calculations (Post, 2002). An ideal baseline consumer will incorporate the temporal variation in the isotopic composition of the primary producers, and integrate sources over a similar time scale as longer-lived consumers (Post, 2002). Similar to GCA, isotope ratios can be affected by ontogeny and seasonality, as well as diet, metabolic processes, tissue type, feeding depth and spatial location (Caut, Angulo, & Courchamp, 2009; Choy et al., 2012; Jardine, McGeachy, Paton, Savoie, & Cunjak, 2003; Jennings & Warr, 2003; Martínez del Rio, Wolf, Carleton, & Gannes, 2009; McCutchan et al., 2003; Mintenbeck, Jacob, Knust, Arntz, & Brey, 2007; Vanderklift & Ponsard, 2003). Additionally, the basal organic carbon source can be estimated through the isotopic ratio of carbon (13C/12C), which can be temporally and spatially variable. Fractionation of δ13C can range from −2.7‰ to 3.4‰, with an average of 0.4‰, between trophic levels (McCutchan et al., 2003). Carbon isotopes are also useful for distinguishing between photosynthetic and chemosynthetic carbon sources in areas such as cold seeps. Distinct δ13C ranges are known for marine phytoplankton (−22‰ to −16‰, Fry & Sherr, 1984; Post, 2002; Fry, 2006; Demopoulos, Gualtieri, & Kovacs, 2010) and chemosynthetic derived organic matter (−75‰ to −28‰, Paull, Jull, Toolin, & Linick, 1985; Brooks et al., 1987; Kennicutt et al., 1992).
Mixing models are tools used in conjunction with SIA to estimate contributions of various food sources to a mixture, i.e. the consumer (Moore & Semmens, 2008; Phillips, 2012; Phillips & Gregg, 2003). This approach is particularly useful when no dietary information is available, as in the case of empty stomachs and unidentifiable stomach content material. MixSIAR is a Bayesian mixing model that allows users to address the issue of underdetermined systems (Fry, 2013; Stock & Semmens, 2015) where there may be more potential food sources than isotopes available for analysis. Mixing model estimates involve several assumptions, including diet assimilation, trophic enrichment factors (TEFs) and isotope ratio variability (Martínez del Rio et al., 2009; Phillips et al., 2014). These estimates are also influenced by the number of isotopes (e.g. C, N and/or S), the number of sources (i.e. endmembers) and the endmembers' isotopic composition (Fry, 2013). Models that only include sources assumed to be dominant in the system can lead to overestimations of primary source contributions at the expense of other, less dominant, sources. This may result in misinterpretation of what are important primary sources in the system (Benstead, March, Fry, Ewel, & Pringle, 2006). Phillips et al. (2014) suggested guidelines for using mixing models, which included using available prior knowledge about the system and consumers (e.g. including known food sources) and assigning appropriate TEFs either based on experimental evidence or best available estimates from the literature. In addition, plotting consumer and source isotope data prior to mixing model analysis helps to ensure that the source data bound the consumer data (Phillips et al., 2014) and identifies if sources need to be included as individuals or combined into groups. Lastly, proportional contributions estimated from mixing models should be reported as range estimates rather than limited to the mean to avoid misrepresenting the uniqueness of the results (Phillips & Gregg, 2003; Phillips et al., 2014).
Given the potentially important role of mesopelagic fishes in energy use and export from productive areas, like seeps, enhanced understanding of their food webs can be gained by combining GCA and SIA, which provides both short-term (GCA) and long-term (SIA) assimilated diet data. While using isotope data alone should be interpreted with caution, when used in combination with GCA (Churchill et al., 2015), isotopes can help clarify trophic interactions (e.g. Amezcua, Muro-Torres, & Soto-Jiménez, 2015; Davis, Blanchette, Pusey, Jardine, & Pearson, 2012; Drazen et al., 2008; Fanelli & Cartes, 2010; Hadwen et al., 2007; Rybczynski et al., 2008; Vander Zanden, Cabana, & Rasmussen, 1997). To our knowledge, no combined analyses have been applied to the mesopelagic fish community, with the exception of that by Choy et al. (2012), who compared isotope data to previously published stomach content data for only two fish species. Our study utilized both GCA and SIA to characterize the trophic structure of mesopelagic fishes (31 species) over deep-sea chemosynthetic cold seeps (>1,000 m) in the North-Central Gulf of Mexico (GOM), providing the opportunity to examine whether chemosynthetic production is utilized by mesopelagic fishes in the vicinity of active cold seeps. In addition, given feeding depth can influence consumer isotopic values, we examined whether there were differences in the isotopic composition between fishes that perform DVMs and fishes that undergo weak migration patterns, to help constrain the interpretation of mesopelagic fishes isotopic compositions.
2 MATERIAL AND METHODS
2.1 Study area
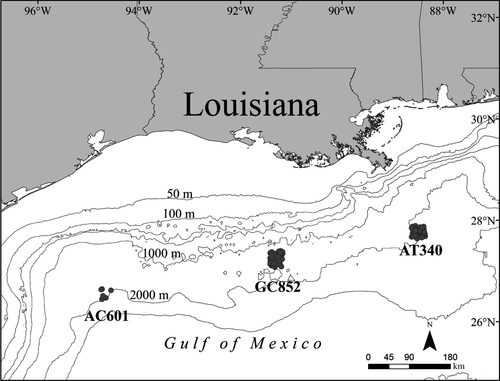
Sampling was conducted over three cold seep sites containing chemosynthetic habitats, including vestimentiferan tube worms, mussel beds and microbial mats, on the middle to lower continental slope in the North-Central GOM: Alaminos Canyon Block 601 (AC601, 2,340 m), Green Canyon Block 852 (GC852, 1,450 m) and Atwater Valley Block 340 (AT340, 2,216 m) (Figure 1). Site-specific details are available in Brooks et al. (2008) and Ross et al. (2010).
2.2 Sample collection
Sampling was conducted during 24-h operations from 9 to 25 August 2007 at 173 stations. Tucker trawls, neuston nets and plankton nets were used to sample fauna within the upper 1,500 m of the water column, with intense sampling occurring in the upper 1,000 m. Tucker trawling consisted of 123 tows (33 day and 90 night) at the three sites (AC601, GC852 and AT340); however, due to inclement weather, minimal sampling (n = 5, night only) was conducted at AC601. For information on individual stations and detailed sampling methods, refer to Ross et al. (2010).
Mesopelagic fishes and invertebrates were collected using a Tucker trawl (2 × 2 m, 1.59 mm mesh, 505 μm cod end) with a plankton net (0.5 m diameter, 335 μm mesh) attached inside the trawl mouth to simultaneously sample smaller organisms. Trawls were equipped with a Sea-Bird SBE39 temperature-depth recorder (TDR) attached to the upper frame bar to record time, depth and temperature during deployment. The Tucker trawl was deployed open, and it was assumed no significant fishing occurred during deployment due to the rapid lowering, steep wire angle and minimal forward movement of the vessel (Gartner et al., 2008; Ross et al., 2010). Trawls fished at a target depth for approximately 30 min before being triggered closed. The mean depth for each Tucker trawl tow was calculated by averaging all depths recorded by the TDR from the start to the end of each tow. Depth ranges sampled were: 45–584 m for AC601, 21–1,067 m for GC852 and 63–1,503 m for AT340.
Additional surface sampling targeted potential prey items of mesopelagic fishes for SIAs. Zooplankton samples were collected from a 1.1 × 2.4 m neuston net (6.4 mm mesh body and 3.2 mm tail bag) or plankton nets (0.5 m diameter, 335 μm and 1.0 m diameter, 505 μm mesh) deployed at the surface and towed for 15–30 min. Particulate organic material (POM) was collected by filtering surface seawater (<1 m depth) through precombusted glass fiber filters (Demopoulos et al., 2010).
Voucher specimens were preserved as follows. Fishes and gelatinous invertebrates were preserved in 10% seawater-formalin solution, while all other invertebrates were preserved in 70% ethanol. Fishes were later transferred to 50% isopropyl alcohol for storage until dietary analyses. Specimens were identified to the lowest practicable taxon and standard length (SL, fishes) or total length (TL, invertebrates) was measured to the nearest millimeter. Life-history stage (i.e. adult or juvenile) was also documented for fishes based on the presence, absence or condition of gonads; specimens were classified as juvenile when either gonads could not be discerned or they appeared to be immature. Fishes were assigned to a migration category, either vertical migrators (VM) or weak/non-migrators (WM), based on the mesopelagic fish community examined in Ross et al. (2010) (Table 1). No conclusions on the migratory patterns of Melamphaidae and Sternoptyx spp. were possible due to small sample sizes (Ross et al., 2010); however, based on literature, Sternoptyx spp. were considered weak/non-migrators (Hopkins & Baird, 1985a), while Melamphaidae were considered migrators (Willis & Pearcy, 1982).
| Species | MIG | GCA | SIA | SL range |
|---|---|---|---|---|
| Fishes | ||||
| Gonostomatidae | ||||
| Cyclothone acclinidens | WM | 1 | 46 | |
| Cyclothone alba | WM | 290 | 5 | 10–33 |
| Cyclothone braueri | WM | 319 | 11–28 | |
| Cyclothone pallida | WM | 362 | 10 | 12–51 |
| Cyclothone pseudopallida | WM | 332 | 10 | 12–45 |
| Gonostoma elongatum | VM | 88 | 12 | 21–190 |
| Sternoptychidae | ||||
| Argyropelecus aculeatus | VM | 40 | 10 | 13–56 |
| Argyropelecus hemigymnus | WM | 30 | 10 | 8–30 |
| Sternoptyx diaphana ^ | WM | 5 | 22–28 | |
| Sternoptyx pseudobscura ^ | WM | 4 | 32–49 | |
| Valenciennellus tripunctulatus | WM | 147 | 15 | 13–30 |
| Phosichthyidae | ||||
| Pollichthys mauli | VM | 63 | 10 | 14–51 |
| Vinciguerria poweriae | VM | 155 | 15 | 12–35 |
| Stomiidae | ||||
| Astronesthes macropogon * | VM | 2 | 21, 34 | |
| Astronesthes similus * | VM | 3 | 20–31 | |
| Bathophilus longipinnis * | VM | 2 | 41–65 | |
| Bathophilus pawneei * | VM | 1 | 31 | |
| Chauliodus sloani | VM | 63 | 6 | 20–132 |
| Eustomias lipochirus * | VM | 1 | 1 | 80 |
| Eustomias schmidti * | VM | 1 | 1 | 80 |
| Leptostomias bilobatus * | VM | 1 | 1 | 105 |
| Melanostomias biseriatus * | VM | 1 | 40 | |
| Melanostomias valdiviae * | VM | 1 | 31 | |
| Photonectes margarita * | VM | 1 | 260 | |
| Photostomias guernei * | VM | 8 | 5 | 49–117 |
| Stomias affinis * | VM | 14 | 2 | 23–90 |
| Stomias longibarbatus * | VM | 1 | 86 | |
| Myctophidae | ||||
| Benthosema suborbitale | VM | 234 | 6 | 10–30 |
| Ceratoscopelus warmingii | VM | 88 | 12 | 15–45 |
| Diaphus lucidus ^ | VM | 2 | 27, 67 | |
| Diaphus mollis ^ | VM | 34 | 4 | 12–55 |
| Diaphus problematicus | VM | 7 | 45–74 | |
| Hygophum benoiti | WM | 111 | 12–22 | |
| Lampanyctus alatus | VM | 72 | 15 | 14–52 |
| Lepidophanes guentheri | VM | 157 | 10 | 14–66 |
| Myctophum affine | VM | 61 | 10 | 13–18 |
| Notolychnus valdiviae | VM | 343 | 11–22 | |
| Melamphaidae | ||||
| Melamphaes simus * | VM | 7 | 14–26 | |
| Melamphaes typhlops * | VM | 2 | 22, 25 | |
| Scopelogadus mizolepis * | VM | 1 | 24 | |
| Invertebrates | ||||
| Amphipoda | ||||
| Phrosinidae | ||||
| Anchylomera blossevillei | 1 | |||
| Platyscelidae | 1 | |||
| Platyscelus sp. | 2 | |||
| Pronoidae | ||||
| Parapronoe sp. | 1 | |||
| Cephalopoda | ||||
| Bolitaenidae | ||||
| Japetella diaphana | 1 | |||
| Enoploteuthidae | ||||
| Ancistrocheirus lesuerii | 1 | |||
| Histioteuthidae | ||||
| Stigmatoteuthis arcturi | 3 | |||
| Chaetognatha | 5 | |||
| Cnidaria | ||||
| Rhopalonematidae | ||||
| Colobonema sericeum | 1 | |||
| Atollidae | 1 | |||
| Atolla vanhoeffeni | 1 | |||
| Copepoda | 6 | |||
| Megacalanidae | ||||
| Bathycalanus princeps | 4 | |||
| Pontellidae | ||||
| Labidocera sp. | 2 | |||
| Decapoda | ||||
| Benthesicymidae | ||||
| Gennadas valens | 14 | |||
| Oplophoridae | ||||
| Acanthephyra purpurea | 5 | |||
| Systellaspis debilis | 7 | |||
| Sergestidae | ||||
| Sergia sp. | 1 | |||
| Euphausiacea | 11 | |||
| Euphausiidae | ||||
| Nematoscelis megalops | 3 | |||
| Thysanopoda sp. | 2 | |||
| Thysanopoda tricuspida | 2 | |||
| Gastropoda | ||||
| Cavoliniidae | ||||
| Cavolinia tridentata | 1 | |||
| Diacavolinia sp. | 2 | |||
| Salpida | ||||
| Salpidae | ||||
| Salpa cylindrica | 4 | |||
| Salpa sp. | 6 | |||
| Zooplankton | 11 | |||
- MIG = migration pattern, where VM = vertical migrators, WM = weak/non-migrators; GCA = gut content analysis; SIA = stable isotope analysis; SL range = standard length size range (mm).
- Fish species marked with * were grouped at family level for all analyses. Fish species marked with ^ were grouped at genera for stable isotope analyses.
2.3 Dietary analyses
2.3.1 GCA
Gut content analysis was conducted on five of the most abundant mesopelagic fish families (31 species) using methods outlined in Ross and Moser (1995). Analyses focused on species with >30 individuals collected except for species in the family Stomiidae. In order to increase sample size for Stomiidae, all stomiids, with the exception of Chauliodus sloani, were grouped together and from here on referred to as Stomiidae spp. Highly abundant fish species, Cyclothone alba, Cyclothone braueri, Cyclothone pallida, Cyclothone pseudopallida, Valenciennellus tripunctulatus and Notolychnus valdiviae, were subsampled with selected specimens spanning the size range of the species in the collections, encompassing all depths sampled (see Table 1 for details on numbers of individuals per subsample).
Fishes were dissected and only stomachs were removed for analyses. Stomach fullness was estimated as 0%, 5%, 25%, 50%, 75% or 100%. Empty stomachs were documented, but excluded from all analyses. Stomach contents were placed on a Petri dish, examined underneath a dissecting microscope and identified to the lowest practicable taxon. Similar prey items were grouped together on a grid of 1 mm squares and flattened to a uniform height, which was measured. Volume (mm3) was calculated by multiplying the height by the number of squares occupied by the food item. The frequency of occurrence of a prey item equaled the number of times a prey item occurred in the fish species examined divided by the total number of stomachs analysed for that species.
Individual fish captured in the same haul are not independent of each other. Deep-sea scattering layers show aggregations of mesopelagic fishes migrating (Gartner et al., 2008) and likely feeding together in the water column. However given the variability in trawl catches and difficulty in obtaining sufficient sample sizes for mesopelagic fishes we needed to analyse all individuals. Each species was collected in multiple trawls, and stomach fullness varied in each trawl. We also recognize that net feeding is possible with trawled fishes. We used criteria similar to Hopkins et al. (1996) and assumed net feeding introduced minimal bias because (i) tow duration was short (30 min), (ii) often, the majority of fishes were damaged and therefore too stressed to eat, (iii) there were a large number of empty stomachs despite prey availability in the net, (iv) diets were similar for individuals within a species captured from different trawls, and (v) diets were variable for different species captured in the same tow.
2.3.2 SIA
Specimens were haphazardly selected from trawls for SIA, with attempts to get at least five individuals per site for SIA. Tissue was dissected from individuals and frozen at sea prior to preservation. Similar tissue was dissected from each taxon to minimize potential isotopic variability in different tissue types (Caut et al., 2009; Vanderklift & Ponsard, 2003). White muscle tissue was removed from the dorsal region of most fishes, the caudal region of shrimps, the legs of crabs and the mantle of mollusks. For small fish and invertebrate specimens, the entire body was used for SIA, and when possible the head, scales, carapace, photophores and/or internal organs were removed prior to isotope sampling. Specimens taken whole were identified prior to tissue collection, or a voucher specimen was saved for future identification.
The majority of tissue samples were dried to a constant weight in a convection oven at 50–60°C then manually crushed into a powder, with approximately 30 additional samples freeze dried in a VirTis Benchtop 3.3 Vac-Freeze. Different drying techniques do not yield significant differences in stable isotope ratios (Bosley & Wainright, 1999). Invertebrate samples were split into two vials: one untreated and the other vial treated with 1.0 N HCl to remove any inorganic carbon and then redried. To avoid potential acidification effects on 15N, δ15N values were reported from non-acidified samples and δ13C values from acidified samples (Jacob, Mintenbeck, Brey, Knust, & Beyer, 2005; Pinnegar & Polunin, 1999).
When possible, at least five specimens for each fish species and prey category from each site were haphazardly selected for analysis. For each sample, 400–600 μg was placed into a tin capsule and combusted in an Elemental Combustion System Model 4010 coupled to a Delta V Plus Isotope Ratio Mass Spectrometer (IRMS) via Conflo II interface at the University of North Carolina Wilmington (UNCW). Additional samples (49 fishes, 24 invertebrates) were analysed by IRMS at Washington State University (WSU) using a Costech (Valencia, USA) elemental analyser interfaced to a GV Instruments (Manchester, UK) Isoprime IRMS. Precision of the IRMS was verified by repeated analysis of standards U.S. Geological Survey reference material 40 (USGS 40), USGS 41 and egg albumin powder, which were incorporated into each run, with SE <0.1‰ for USGS 40 and 41 and <0.04‰ for egg albumin, for both C and N isotopes. Standard USGS 40 was analysed at both labs, with a SE of <0.1‰ at UNCW and <0.06‰ at WSU for both C and N isotopes. There was no statistical difference between the two labs for carbon isotopes (Mann–Whitney, p = .145); however, USGS 40 analysed at UNCW had significantly higher δ15N values (Mann–Whitney U = 161, n1 = 15, n2 = 77, p < .001). We corrected for instrument bias by adding the difference between the average δ15N values between the two labs (0.3‰) to the reported δ15N value from WSU. Raw delta values were corrected for linearity and normalized to these known reference materials. Reproducibility was monitored using organic reference standards bovine muscle and apple leaves (Fry, 2007). Tissue samples were analysed for stable carbon and nitrogen isotopes, with isotope ratios expressed as δ13C or δ15N in per mil (‰), referenced to Vienna PeeDee Belemnite and atmospheric nitrogen (air). Data were examined after SIA to determine whether lipids may have significantly impacted the isotope results. Samples with C:N > 4 are likely affected by the presence of lipids (Hoffman & Sutton, 2010; Post et al., 2007). With the exception of one stomiid (C:N = 4.1), our results (all C:N < 4, and 91.5% fishes have C:N < 3.5) indicated that lipids did not significantly affect the isotope ratios of fishes. Therefore, no lipid extraction methods or lipid correction factors were applied to the isotope data. POM isotope data were previously reported in Demopoulos et al. (2010).
A minimum of five samples was analysed per fish species and reported with the mean ± 1 SE for each species. For Stomiidae spp., the mean and SE calculations incorporated data from all stomiid specimens, except Chauliodus sloani, which had enough specimens to be analysed on its own. Similarly, three melamphaeid species (Melamphaes simus, Melamphaes typhlops and Scopelogadus mizolepis) were grouped together to increase sample sizes and were referred to as Melamphaidae, while Diaphus spp. included Diaphus mollis and Diaphus lucidus, and Sternoptyx spp. included Sternoptyx diaphana and Sternoptyx pseudobscura.
2.3.3 Isotope mixing model
MixSIAR, a Bayesian stable isotope mixing model (Stock & Semmens, 2015), was used to determine proportional contributions of different prey resources to a fish's diet. The MixSIAR graphical user interface and model are written in open source languages R (R Development Core Team, 2013) and Just Another Gibbs Sampler. We conducted Markov chain Monte Carlo sampling based on the following parameters: number of chains = 3; chain length = 100,000; burn in = 50,000; and thin = 50. Diagnostic tests (Gelmin–Rubin, Heidelberger–Welch and Geweke) and trace plots were examined for model convergence.
Site-specific MixSIAR analysis was not possible due to limited sample sizes and unsuccessful capture of all potential prey sources at every site. Instead the North-Central GOM was treated as a single area and MixSIAR analyses examined the overall general feeding ecology of mesopelagic fishes. We used measured isotope values for sources and their SD as reported in Table S1. POM, collected at all sites, had similar isotopic compositions and ranges reported, with no significant differences among sites AC601, GC852 and AT340 (Demopoulos et al., 2010); therefore, a single value averaged from all sites was used for the POM endmember. Other potential prey sources (endmembers) also were averaged across all sites. The endmembers used for MixSIAR calculations were as follows: Zooplankton, Cnidaria, Pteropoda, Salpida, Cephalopoda, Decapoda – Shrimp, Euphausiid, Fish, Chemo and POM (Table S1). Isotope values for the Chemo endmember were taken from samples analysed in Demopoulos et al. (2010) from sites AC601, GC852 and AT340, and represented the average Beggiatoa sp. δ13C and δ15N values across the three sites. Trophic enrichment factors used were 0.4 ± 1.3‰ and 2.0 ± 1.8‰ for δ13C and δ15N values, respectively, based on McCutchan et al. (2003). We recognize that TEFs can vary by environment, tissue type, taxon and methodology (Caut et al., 2009), as well as among individuals within a species (Amezcua et al., 2015). One TEF for δ15N [2.0 ± 1.8‰] and δ13C (0.4 ± 1.3‰) was used because (i) similar tissue was taken from all fish specimens, (ii) available literature indicated diet similarity among mesopelagic fish species, with zooplankton as the dominant prey (Butler et al., 2001; Hopkins & Baird, 1985a,b; Hopkins et al., 1996; Lancraft et al., 1988; Pusch et al., 2004), (iii) invertebrate consumers can have a lower TEF (McCutchan et al., 2003), and (iv) TEF-corrected fish isotope data were bounded by the polygon defined by the source isotope data after plotting our data, as suggested by Phillips et al. (2014).
2.4 Trophic position analysis
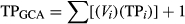 (1)
(1)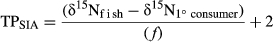 (2)
(2)2.5 Statistical analyses
The effects of fish size and location (AC601, GC852, AT340) on diet composition were examined using multivariate analyses in PRIMER-E v. 6.1 (Clarke & Gorley, 2006; Clarke & Warwick, 2001). Size was divided by 5-mm increments (10 to >115 mm), resulting in 22 size classes. Organic material and animal parts (e.g. amphipod parts, copepod parts, decapod parts) were excluded prior to analyses as these food items were ambiguous and may be pieces of previously identified prey items. Prey accumulation curves were constructed for each fish species at each site to assess whether the analysis appropriately estimated the diversity of potential prey items. For each fish species, the volumetric data were standardized by dividing the volume of each prey item by the total volume of the stomach to account for the variability in stomach fullness. Standardized volumes were square-root transformed to down-weight the contributions of more abundant prey items. Similarities among fish species of certain size classes and similar locations were calculated using a Bray–Curtis similarity co-efficient. A one-way analysis of similarities (ANOSIM) was used to test whether there were significant differences in diets from different locations or from different size classes. Resulting R values from the ANOSIM analysis can range from 0, when diets are similar, to 1, when diets are different from each other (Clarke & Warwick, 2001), with significance reported when p < .05 and R > .2 (Clarke & Gorley, 2006). When significant differences were documented, similarity percentages routine (SIMPER) was utilized to determine what prey items contributed to the dissimilarities.
To compare general feeding guilds, a similar multivariate procedure was implemented for diet comparisons among all specimens from the 31 fish species, disregarding size and location. General prey categories (Amphipoda, Annelida, Chaetognatha, Cnidaria, Copepoda, Crustacea spp., Decapoda, Euphausiacea, Fish, Mollusca, Ostracoda, Salpida and Other) were used and included identifiable animal parts (i.e. copepod parts were included in Copepoda). However, organic material and unidentifiable animal parts were excluded. With a large sample size (n = 1,327), a non-metric multidimensional scaling (MDS) plot can become cluttered with substantial ‘noise’ in individual samples. Therefore, volumetric diet data for each fish species were averaged by PRIMER prior to constructing a Bray–Curtis similarity matrix (Clarke & Gorley, 2006) and results were hierarchically clustered with group average linkage and visualized with MDS plots. A similarity profile test (SIMPROF) was conducted post cluster analysis to determine significant clusters in fishes.
Isotope data were analysed for normality and homogeneity of variance using Shapiro–Wilk and Levene Median tests in R. Fishes were tested for significant differences using one-way analysis of variance (ANOVA) and a post-hoc Tukey test was used to determine specific differences among groups; this test is useful in cases when group sizes are unequal. Isotope data that failed normality or equal variance tests were analysed with Kruskal–Wallis tests and the post-hoc Wilcoxon pairwise tests with Holm corrections. Due to sample size limitation (n < 5), statistical significance among locations was tested only for the following fish species: Gonostoma elongatum, Stomiidae spp., Argyropelecus aculeatus, Vinciguerria poweriae, Valenciennellus tripunctulatus and Lampanyctus alatus. In addition we tested for isotopic differences between fishes that undergo DVMs (VM) with those that weakly migrate or do not migrate (WM), including paired comparisons between copepod feeders and mixed zooplanktivores. These feeding guilds were chosen because they had fish species represented in both VM and WM groups. Linear regression analysis of δ15N values against fish SL were conducted to test whether ontogeny was significantly correlated with higher trophic level (inferred from δ15N values). Species with low sample sizes (n < 5) were not statistically analysed.
3 RESULTS
3.1 Catch data
A total of 8702 fishes (at least 124 species in 29 families) was collected. Collections were dominated (97.7%) by five families: Gonostomatidae (58.8%), Myctophidae (27.4%), Phosichthyidae (5.8%), Sternoptychidae (4.4%) and Stomiidae (1.3%). Size classes did not appear related to depth, with size classes for each species spread over a range of depths.
3.2 GCA
Gut content analysis was conducted on 31 species from the five most abundant mesopelagic fish families (Table 1). Of the 3025 fishes analysed, 79% were juveniles and empty stomachs occurred in 55% of the fishes. A total of 125 prey items (45 species, 37 families) was identified in the stomachs of all mesopelagic fishes, and these items were grouped into 13 general taxonomic categories. Copepods occurred more frequently in stomachs than any other prey item and were documented in 79% of the fish stomachs analysed, with the copepod Pleuromamma sp. recorded in 30 fish species. Chauliodus sloani was the only fish species that did not have copepods documented in stomachs. Location was not a significant factor affecting fish diets (Table 2, ANOSIM), providing support that GCA data could be pooled in order to examine diet similarities among species collected across the three locations. While all available specimens were analysed in the GCA for C. sloani, Stomiidae spp., Gonostoma elongatum, Diaphus mollis, Hygophum benoiti, Argyropelecus aculeatus, Argyropelecus hemigymnus, Pollichthys mauli, Vinciguerria poweriae, Benthosema suborbitale, Lampanyctus alatus, Lepidophanes guentheri and Myctophum affine, prey accumulation curves did not reach asymptotes at any site, indicating GCA may not have sufficiently captured the diet composition of these fishes (Figure S2). Similarly, prey accumulation curves for fishes subsampled for GCA (Cyclothone alba, Cyclothone braueri, Cyclothone pallida, Cyclothone pseudopallida, Valenciennellus tripunctulatus, Notolychnus valdiviae) did not reach an asymptote at any site (Figure S2).
| Species | Size | Location | Significant differences | ||
|---|---|---|---|---|---|
| Global R | p | Global R | p | ||
| Argyropelecus aculeatus | .19 | .05 | −.09 | .89 | no |
| Argyropelecus hemigymnus | .24 | .10 | −.21 | .88 | no |
| Benthosema suborbitale | .06 | .06 | −.06 | .84 | no |
| Ceratoscopelus warmingii | .01 | .44 | .07 | .12 | no |
| Cyclothone alba | −.02 | .84 | −.01 | .67 | no |
| Cyclothone braueri | −.06 | 1.00 | −.02 | .77 | no |
| Cyclothone pallida | −.14 | .90 | −.11 | 1.00 | no |
| Cyclothone pseudopallida | .06 | .13 | −.02 | .58 | no |
| Diaphus mollis | −.11 | .87 | .03 | .32 | no |
| Gonostoma elongatum | .06 | .22 | .07 | .13 | no |
| Hygophum benoiti | −.03 | .68 | no | ||
| Lampanyctus alatus | −.01 | .52 | −.07 | .85 | no |
| Lepidophanes guentheri | .17 | .03 | .02 | .33 | no |
| Myctophum affine | −.10 | .72 | .25 | .09 | no |
| Notolychnus valdiviae | .03 | .24 | .06 | .01 | no |
| Pollichthys mauli | .38 | .00 | .18 | .18 | size |
| Stomiidae spp. | .47 | .12 | −.20 | 1.00 | no |
| Valenciennellus tripunctulatus | −.07 | .94 | .03 | .15 | no |
| Vinciguerria poweriae | .10 | .01 | .03 | .09 | no |
Size did not appear to affect fish diets in the majority of species analysed (Table 2, ANOSIM), which may be because the majority of specimens analysed for GCA were juveniles (79%). Size was only significant in explaining the diets of P. mauli (ANOSIM, R = .38, p < .001). Differences in diet for P. mauli occurred between size classes 2 (15–19 mm SL) and 6 (35–39 mm SL) (R = .73, p = .006, 100% dissimilar), 3 (20–24 mm SL) and 6 (R = .591, p = .028, 100% dissimilar), 2 (15–19 mm SL) and 5 (30–34 mm SL) (R = .488, p = .014, 75.2% dissimilar), and 2 and 3 (R = .251, p = .028, 59.6% dissimilar). These differences were driven by the smaller specimens (15–24 mm SL) consuming almost exclusively copepods, whereas larger specimens consumed additional taxa, such as ostracods, amphipods, euphausiids and decapods. The overall difference between size groups 2 and 3 was nominal (R = .251). Size group 2 fed primarily on copepods alone, whereas size class 3 consumed a small amount of ostracods in addition to copepods. Size also appeared to make a slight difference in diet composition for A. aculeatus (R = .19, p = .05) and L. guentheri (R = .17, p = .03). Although not significant, size may be a factor explaining Stomiidae spp. diet composition (R = .47, p = .12), which may result from diet changes as the fish grows. However, the lack of statistical significance indicates there are other, unmeasured factors influencing the diet composition of this group. In the case of V. poweriae, prey composition was significantly different based on fish size, but the low R-value (R = .10) indicated that size was not sufficient to explain all the variance in the composition data.
Five general feeding guilds for all the fishes were identified from SIMPROF (Figure 2): piscivores, large crustacean consumers, copepod consumers, mixed zooplanktivores, and generalists. Notolychnus valdiviae collected at AC601 represented a distinct group from the other fishes. As this group was based on only one specimen, whose stomach contained only ostracods, we do not consider this single specimen as a distinctly separate feeding guild.
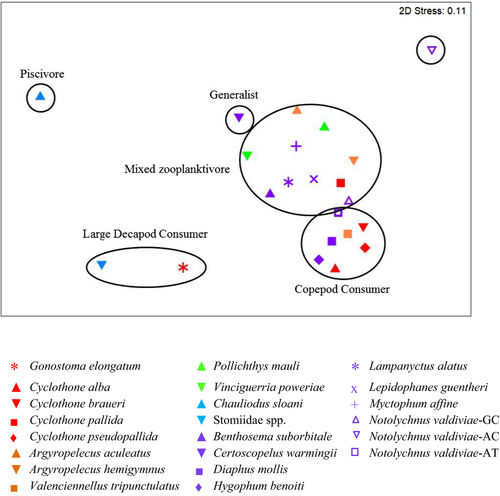
The piscivore guild was only represented by C. sloani (Figure 2, n = 63). Myctophidae and the gadiform fish, Bregmaceros spp., were the most important prey items for this fish (Figure 3). No identifiable invertebrates were documented (Table S2); however, empty stomachs occurred in 83% of all analysed C. sloani stomachs, and crustacean parts were found in a single specimen.
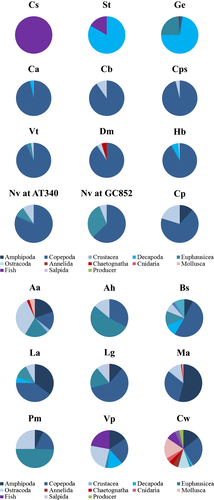
The large crustacean consumer guild consisted of G. elongatum and Stomiidae spp. (Figure 2), whose prey were volumetrically dominated (>70%) by decapods (Figure 3). Overall, the diet of G. elongatum was more variable than the combined diets of Stomiidae spp. (Figure 3). Copepods, particularly calanoids, were frequently consumed by G. elongatum (Table S3), while the diet of Stomiidae spp. was dominated by decapods and myctophids (Figure 3, Table S4). Empty stomachs occurred less frequently in G. elongatum (24%) compared with Stomiidae spp. (69%).
All remaining mesopelagic fishes were zooplanktivores classified as either copepod consumers, mixed zooplanktivores or generalists (Figure 2). The copepod consumers included C. alba, C. braueri, C. pseudopallida, V. tripunctulatus, D. mollis, H. benoiti and N. valdiviae (Tables S5–S11). While Copepoda was the major prey category consumed by species in this group (Figure 3), stomachs also contained a high richness of non-copepod prey, ranging from 13 different identified prey groups for H. benoiti to 36 prey groups for V. tripunctulatus. Copepod species consumed varied among fishes. Pleuromamma spp. were consumed more often and in higher volumes by C. alba, V. tripunctulatus, D. mollis and N. valdiviae, whereas Aegisthus mucronatus was more important in the diets of C. braueri. Valdiviella minor was the dominant prey volumetrically in the diet of C. pseudopallida, but Lubbockia spp. occurred more frequently. Calanoid copepods were volumetrically important in the diets of H. benoiti, but cyclopoid copepods occurred more frequently. A high percentage (>68%) of empty stomachs occurred in C. alba, C. braueri, C. pseudopallida and H. benoiti, while fewer empty stomachs were documented in N. valdiviae (43%), V. tripunctulatus (17%) and D. mollis (9%).
Mixed zooplanktivores were defined by a general crustacean-based zooplankton diet with taxa other than copepods consumed (Figure 3). This group included N. valdiviae collected at GC852, C. pallida, A. aculeatus, A. hemigymnus, P. mauli, V. poweriae, B. suborbitale, L. alatus, L. guentheri and M. affine (Figure 2). The overall diet richness for mixed zooplanktivores was similar to that of copepod consumers, ranging from 10 prey items for C. pallida to 42 prey items for V. poweriae (Tables S11–S20). Amphipoda was most important volumetrically in the diet of C. pallida and M. affine, while Conchoecinae (Ostracoda) was most important for A. aculeatus and A. hemigymnus (Figure 3). Ostracods, particularly Conchoecinae, also occurred frequently in the stomachs of C. pallida, A. aculeatus and P. mauli, while calanoid copepods (particularly Peuromamma spp.) occurred frequently in the stomachs of M. affine, A. hemigymnus, B. suborbitale, L. alatus, L. guentheri and N. valdiviae. Similar to copepod consumers, P. mauli, B. suborbitale, L. alatus, L. guentheri and N. valdiviae frequently consumed Pleuromamma spp. and other calanoid copepods; however, decapods, euphausiids and ostracods were important volumetrically. The diet of V. poweriae differed from other mixed zooplanktivores, with myctophids and Candaciidae (Copepoda) dominating volumetrically, but Conchoecinae and calanoid copepods occurring more frequently. The presence of empty stomachs in mixed zooplanktivores was also variable, ranging from 21% (L. alatus) to 94% (C. pallida).
The generalist group contained only Ceratoscopelus warmingii, which consumed more non-crustacean prey than any other zooplanktivore and fed on a variety of prey items (n = 13 taxon categories; Figure 3; Table S21). Crustaceans comprised roughly 60% of all identifiable stomach volume and non-crustacean prey comprised the remaining 40% for this group (Figure 3). Fishes, mollusks and copepods were important in the overall percent volume of the diet (Figure 3, Table S21). However, copepods occurred more frequently than any other prey item [>45% frequency, at all locations and time of day (Table S21)]. Empty stomachs occurred in 18% of C. warmingii.
3.3 SIAs
Stable isotope analyses were conducted on 299 samples (Tables 1 and 3), representing 30 fish species (six families) and 10 invertebrate taxa (Amphipoda, Cephalopoda, Chaetognatha, Cnidaria, Copepoda, Decapoda, Euphausiacea, Gastropoda, Salpida, Other zooplankton). Based on the TEF assumed for δ15N (2.0 ± 1.8‰), most zooplankton, including Copepoda, Euphausiacea and Amphipoda, represented primary consumers and the majority of mesopelagic fishes were secondary consumers (Figure 4). Stable carbon isotope data indicated that chemosynthetically derived resources were not assimilated by mesopelagic fishes (Table 3). All of the fishes were enriched in 13C (−23.2‰ to −16.4‰) relative to chemosynthetically derived organic matter (e.g. −75‰ to −28‰, Paull et al., 1985; Brooks et al., 1987; Kennicutt et al., 1992), with values more consistent with assimilating photosynthetically based organic material.
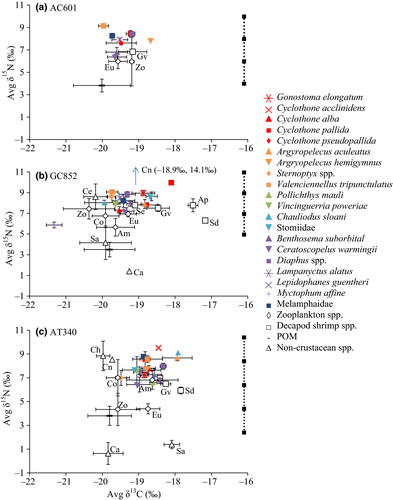
While location did not affect fish diet composition based on GCA, location did significantly affect stable isotope values for some fish species. Stable carbon isotope values were significantly lower at GC852 compared to AT340 for Vinciguerria poweriae (one-way ANOVA: df = 1, F = 20.62, p < .001), and at AC601 compared to AT340 for Valenciennellus tripunctulatus (one-way ANOVA: df = 2, F = 6.13, p = .0146, Tukey, p = .0156). Stable 15N values were significantly enriched at GC852 compared to AT340 for Gonostoma elongatum (one-way ANOVA: df = 1, F = 24.08, p < .001) and Lampanyctus alatus (one-way ANOVA: df = 2, F = 5.55, p = .0197). There were no differences in stable isotope values based on location for the following fishes: Stomiidae spp. or Argyropelecus aculeatus (both δ13C and δ15N), G. elongatum (δ13C), L. alatus (δ13C), V. poweriae (δ15N) and V. tripunctulatus (δ15N).
Mesopelagic fish isotope data were examined in an isotope biplot to determine if prey detected from GCA occupied one trophic level below the fish consumer (Figure 4). If prey isotope values were located one trophic level below fish then those prey items may be assimilated by the fishes, which complements the feeding guilds proposed by GCA. Myctophids were one trophic level below Chauliodus sloani at AT340, consistent with piscivory for C. sloani. However, this was not the case at GC852, where C. sloani occupied a similar trophic level to myctophids. Both large crustacean consumers, G. elongatum and Stomiidae spp., had similar isotope values at sites AT340 and GC852, with zooplankton taxa approximately one trophic level below these fishes. Zooplanktivores at sites AT340, GC852 and AC601, with the exception of Myctophum affine, were one trophic level above zooplankton taxa.
Isotope data were further compared among similar migrating species within GCA feeding guilds of large crustacean consumers or zooplanktivores at each location. VM were depleted in 15N relative to WM for all fishes analysed (Kruskal-Wallis test, χ2 = 11.812, df = 1, p < .001); however, there was no significant difference in δ13C values. For the mixed zooplanktivore feeding guild, VM were depleted in 15N relative to WM (ANOVA, df = 1, F = 10.558, p = .001), whereas there was no significant difference in δ13C values. Copepod consumers had no significant differences in δ13C or δ15N values for VM versus WM.
| Species | δ15N | δ13C | ||||
|---|---|---|---|---|---|---|
| AC601 | GC852 | AT340 | AC601 | GC852 | AT340 | |
| Fishes | ||||||
| Gonostomatidae | ||||||
| Cyclothone acclinidens | 9.5 (1) | −18.5 (1) | ||||
| Cyclothone alba | 7.3 ± 0.3 (5) | −19.5 ± 0.1 (5) | ||||
| Cyclothone pallida | 8.4 ± 0.3 (5) | 10.3 (1) | 8.6 ± 0.6 (4) | −19.2 ± 0.1 (5) | −18.1 (1) | −18.8 ± 0.2 (4) |
| Cyclothone pseudopallida | 7.8 ± 0.2 (4) | 8.0 ± 0.1 (3) | 7.7 ± 0.3 (3) | −19.5 ± 0.4 (4) | −18.8 ± 0.3 (3) | −18.7 ± 0.4 (3) |
| Gonostoma elongatum | 9.0 ± 0.3 (6) | 7.2 ± 0.2 (6) | −18.9 ± 0.3 (6) | −18.8 ± 0.1 (6) | ||
| Sternoptychidae | ||||||
| Argyropelecus aculeatus | 7.9 ± 0.4 (5) | 8.7 ± 0.2 (5) | −18.8 ± 0.1 (5) | −17.9 ± 0.4 (5) | ||
| Argyropelecus hemigymnus | 8.1 (1) | 7.9 ± 0.2 (9) | −18.6 (1) | −18.7 ± 0.2 (9) | ||
| Sternoptyx spp.* | 8.6 ± 0.3 (7) | 7.3 ± 0.6 (2) | −19.8 ± 0.1 (7) | −19.45 ± 0.2 (2) | ||
| Valenciennellus tripunctulatus | 9.2 ± 0.1 (5) | 9.1 ± 0.2 (5) | 8.6 ± 0.3 (5) | −20.0 ± 0.1 (5) | −19.7 ± 0.2 (5) | −18.8 ± 0.4 (5) |
| Phosichthyidae | ||||||
| Pollichthys mauli | 6.6 ± 0.2 (10) | −18.6 ± 0.1 (10) | ||||
| Vinciguerria poweriae | 7.9 ± 0.1 (10) | 7.6 ± 0.3 (5) | −19.6 ± 0.1 (10) | −19.0 ± 0.1 (5) | ||
| Stomiidae | ||||||
| Stomiidae spp.* | 8.6 ± 0.4 (11) | 8.0 ± 1.1 (6) | −19.2 ± 0.3 (11) | −18.9 ± 0.4 (6) | ||
| Chauliodus sloani | 8.0 ± 0.2 (5) | 9.4 (1) | −20.0 ± 0.5 (5) | −17.9 (1) | ||
| Myctophidae | ||||||
| Benthosema suborbitale | 7.1 ± 0.4 (3) | 7.6 ± 0.5 (3) | −19.6 ± 0.3 (3) | −18.6 ± 0.2 (3) | ||
| Ceratoscopelus warmingii | 6.4 ± 0.3 (3) | 7.2 ± 0.3 (5) | 6.4 ± 0.6 (4) | −19.6 ± 0.3 (3) | −19.3 ± 0.2 (5) | −19.0 ± 0.3 (4) |
| Diaphus spp.* | 8.7 (1) | 8.8 ± 0.7 (5) | −19.2 (1) | −19.3 ± 0.3 (5) | ||
| Diaphus problematicus | 9.2 (1) | 8.3 ± 0.3 (6) | −19.2 (1) | −18.3 ± 0.01 (6) | ||
| Lampanyctus alatus | 7.9 ± 0.2 (3) | 8.5 ± 0.2 (7) | 7.5 ± 0.3 (5) | −19.5 ± 0.2 (3) | −19.5 ± 0.1 (7) | −19.0 ± 0.12 (5) |
| Lepidophanes guentheri | 7.9 ± 0.3 (4) | 6.8 ± 0.2 (6) | −19.2 ± 0.1 (4) | −18.4 ± 0.0 (6) | ||
| Myctophum affine | 6.0 ± 0.3 (10) | −21.3 ± 0.2 (10) | ||||
| Melamphaidae* | 8.6 ± 0.2 (5) | 8.5 ± 0.7 (4) | 9.1 (1) | −19.7 ± 0.1 (5) | −19.4 ± 0.3 (4) | −18.9 (1) |
| Cnidaria | ||||||
| Atollidae | 8.8 (1) | −19.7 (1) | ||||
| Atolla vanhoeffeni | 13.1 (1) | −19.1 (1) | ||||
| Rhopalonematidae | ||||||
| Colobonema sericeum | 15.8 (1) | −18.7 (1) | ||||
| Salpida | ||||||
| Salpidae | ||||||
| Salpa cylindrica | 1.8 ± 0.7 (4) | −18.3 ± 0.2 (4) | ||||
| Salpa sp. | 4.5 ± 1.6 (3) | 1.1 ± 0.1 (3) | −19.9 ± 0.7 (3) | −17.9 ± 0.4 (3) | ||
| Cephalopoda | ||||||
| Bolitaenidae | ||||||
| Japetella diaphana | 5.7 (1) | −19.6 (1) | ||||
| Enoploteuthidae | ||||||
| Ancistrocheirus lesuerii | 6.2 (1) | −19.3 (1) | ||||
| Histioteuthidae | ||||||
| Stigmatoteuthis arcturi | 10.4 ± 1.0 (3) | −20.7 ± 0.1 (3) | ||||
| Gastropoda | ||||||
| Cavoliniidae | ||||||
| Cavolinia tridentata | 1.9 (1) | −19.4 (1) | ||||
| Diacavolinia sp. | 1.7 (1) | 0.0 (1) | −19.3 (1) | −20.3 (1) | ||
| Chaetognatha | 8.8 ± 1.2 (5) | −20.0 ± 0.2 (5) | ||||
| Amphipoda | ||||||
| Phrosinidae | ||||||
| Anchylomera blossevillei | 4.3 (1) | −20.7 (1) | ||||
| Platyscelidae | ||||||
| Platyscelidae sp. | 6.3 (1) | −17.9 (1) | ||||
| Platyscelus sp. | 6.8 ± 0.0 (2) | −19.1 ± 0.1 (2) | ||||
| Pronoidae | ||||||
| Parapronoe sp. | 7.9 (1) | −19.3 (1) | ||||
| Copepoda | 3.7 (1) | 7.0 ± 1.5 (5) | −19.1 (1) | −19.6 ± 0.3 (5) | ||
| Megacalanidae | ||||||
| Bathycalanus princeps | 9.2 ± 0.5 (4) | −20.6 ± 0.3 (4) | ||||
| Pontellidae | ||||||
| Labidocera sp. | 3.6 ± 0.3 (2) | −19.0 ± 0.12 (2) | ||||
| Euphausiacea | ||||||
| Euphausiidae | 6.0 ± 0.7 (4) | 6.6 ± 0.3 (2) | 5.0 ± 0.2 (5) | −19.6 ± 0.3 (4) | −19.2 ± 0.0 (2) | −19.14 ± 0.2 (5) |
| Nematodcelis megalops | 6.6 ± 0.3 (3) | −19.5 ± 0.5 (3) | ||||
| Thysanopoda sp. | 8.0 ± 0.6 (2) | −19.1 ± 0.54 (2) | ||||
| Thysanopoda tricuspida | 3.2 ± 0.5 (2) | −17.7 ± 0.4 (2) | ||||
| Decapoda | ||||||
| Benthesicymidae | ||||||
| Gennadas valens | 6.8 ± 0.1 (4) | 7.5 ± 0.3 (6) | 6.5 ± 0.1 (4) | −19.2 ± 0.4 (4) | −18.5 ± 0.31 (6) | −18.3 ± 0.1 (4) |
| Oplophoridae | ||||||
| Acanthephyra purpurea | 7.9 ± 0.5 (4) | 7.0 (1) | −17.5 ± 0.1 (4) | −18.4 (1) | ||
| Systellaspis debilis | 6.3 (1) | 5.9 ± 0.3 (6) | −17.2 (1) | −17.84 ± 0.1 (6) | ||
| Sergestidae | ||||||
| Sergia sp. | 7.8 (1) | −19.1 (1) | ||||
| Zooplankton | 6.0 ± 2.2 (2) | 7.4 ± 1.0 (5) | 4.3 ± 1.4 (4) | −19.2 ± 0.1 (2) | −20.4 ± 0.70 (5) | −19.6 ± 0.6 (4) |
| Autotroph | ||||||
| POMa | 3.8 ± 0.6 (5) | 3.5 ± 0.7 (15) | 3.8 ± 0.4 (9) | −20.0 ± 0.8 (5) | −19.8 ± 0.7 (15) | −19.8 ± 0.6 (9) |
- aData previously published by Demopoulos et al. (2010).
While size did not play a large role in affecting diet composition based on GCA, size may be a significant factor affecting isotope data. SL was positively correlated with δ15N values in 19 of the 20 fish species analysed (Figure S1; R2 ranged from .113 to .922, p ranged from <.001 to .646). This relationship was significant for nine taxa: Cyclothone pseudopallida (R2 = .807, p < .001), G. elongatum (R2 = .618, p = .002), V. poweriae (R2 = .614, p < .001), C. sloani (R2 = .922, p = .002), Stomiidae spp. (R2 = .390, p < .040), Argyropelecus hemigymnus (R2 = .541, p = .024), Diaphus problematicus (R2 = .678, p = .023), Diaphus spp. (R2 = .838, p = .029) and Melamphaidae (R2 = .687, p = .003). In contrast, there was a significant negative correlation between SL and δ15N for M. affine (R2 = .401, p = .049).
3.4 Mixing model results (MixSIAR)
For the piscivore Chauliodus sloani, fish contributed 14.5% [Table 4; 0.5%–48.3% credible interval (CI)], but other invertebrates contributed to their diet, including pteropods (12.3%; 0.5%–34.8% CI) and euphausiids (10.9%; 0.5%–42.7% CI). Fish also contributed to the diets of the large crustacean consumers, Stomiidae spp. (13.9%; 0.6%–45.2% CI) and Gonostoma elongatum (12.4%, 0.4%–40.5% CI), and, to a lesser degree, to the diets of the generalist Ceratoscopelus warmingii (8.0%; 0.3%–28.5% CI) and mixed zooplanktivore Vinciguerria poweriae (8.2%; 0.3%–30.9% CI). All zooplanktivores (copepod consumers, mixed zooplanktivores and the generalist) had a mixture. Of prey sources, with non-crustacean prey contributing to all mesopelagic fish diets. The copepod consumer, Valenciennellus tripunctulatus, and the mixed zooplanktivore, Lampanyctus alatus, had higher proportions of zooplankton (15.1%; 0.8%–47.6% CI and 15.4%; 0.7%–44.7% CI, respectively) compared to the other mesopelagic fishes. MixSIAR results indicated that pteropods may be an important food source for copepod consumers (Cyclothone alba), mixed zooplanktivores (Pollichthys mauli, V. poweriae, Benthosema suborbitale, L. alatus, Lepidophanes guentheri), and the generalist feeder, C. warmingii, ranging from 20.3% to 33.1% (1.6%–52.5% CI). However, the mixed zooplanktivores Argyropelecus aculeatus and Diaphus problematicus differed from other zooplanktivores with a high proportion of decapods (23.5%; 1.5%–53.1% CI and 23.8%; 2.0%–50.9% CI, respectively) contributing to their diets. The relative contribution of cephalopods to the diets of V. tripunctulatus (27.5%; 3.2%–54.5% CI), L. alatus (25.0%; 4.2%–47.8% CI) and V. poweriae (21.3%; 2.8%–44.0% CI) was higher than for most other species. Although not analysed in the GCA, Melamphaidae and Sternoptyx spp. had a mixture of food sources similar to other mixed zooplanktivores, with cephalopods contributing the highest portion to their diets (24.3%; 2.8%–50.8% CI and 27.2%; 3.0%–54.2% CI, respectively), followed by pteropods and zooplankton. For Myctophum affine, no feasible solution was generated unless chemosynthetic material was included as a source in the MixSIAR analysis. The estimated contribution of chemosynthetic material to the diet of M. affine was 18.6% (13.4%–23.8% CI).
| Guild | Species | Zooplankton | Cnidaria | Pteropoda | Salpida | Cephalopoda | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50% | Range | 50% | Range | 50% | Range | 50% | Range | 50% | Range | ||
| PIS | Chauliodus sloani | 9.9 | 0.3–37.8 | 7.2 | 0.3–28.1 | 12.3 | 0.5–34.8 | 8.4 | 0.3–32.0 | 9.7 | 0.4–36.9 |
| LGC | Stomiidae spp. | 8.6 | 0.3–32.3 | 11.5 | 0.7–33.4 | 12.3 | 0.5–34.5 | 9.4 | 0.4–30.6 | 8.4 | 0.4–30.5 |
| Gonostoma elongatum | 9.9 | 0.4–34.7 | 7.8 | 0.3–26.4 | 14.2 | 1.0–35.5 | 10.1 | 0.6–30.3 | 8.9 | 0.4–29.8 | |
| COP | Cyclothone alba | 12.2 | 0.5–40.4 | 6.1 | 0.2–27.1 | 23.7 | 2.0–48.6 | 11.2 | 0.6–40.4 | 15.1 | 0.7–41.9 |
| Cyclothone pseudopallida | 12.6 | 0.7–40.6 | 8.6 | 0.4–26.6 | 19.1 | 1.6–40.8 | 11.2 | 0.3–34.4 | 13.5 | 0.9–35.9 | |
| Valenciennellus tripunctulatus | 15.1 | 0.8–47.6 | 9.4 | 0.6–28.6 | 11.3 | 0.9–30.5 | 7.4 | 0.3–25.9 | 27.5 | 3.2–54.5 | |
| Diaphus spp. | 12.8 | 0.5–42.4 | 11.6 | 0.7–33.9 | 12.0 | 0.7–35.2 | 8.7 | 0.4–30.0 | 18.7 | 1.5–47.1 | |
| MZO | Cyclothone pallida | 13.4 | 0.6–40.3 | 13.8 | 1.0–34.0 | 12.9 | 0.5–34.0 | 8.5 | 0.5–30.2 | 15.5 | 1.1–38.9 |
| Argyropelecus aculeatus | 10.5 | 0.3–36.1 | 10.1 | 0.8–27.3 | 10.2 | 0.5–32.2 | 11.5 | 0.6–35.2 | 8.7 | 0.3–31.5 | |
| Argyropelecus hemigymnus | 11.1 | 0.5–37.0 | 9.8 | 0.5–27.1 | 16.4 | 1.1–38.6 | 12.4 | 0.7–35.3 | 10.7 | 0.7–32.0 | |
| Pollichthys mauli | 8.8 | 0.4–29.0 | 4.8 | 0.2–17.0 | 28.7 | 4.7–49.3 | 19.0 | 1.2–44.1 | 5.8 | 0.3–22.4 | |
| Vinciguerria poweriae | 11.6 | 0.7–39.1 | 4.3 | 0.2–18.8 | 23.2 | 3.9–40.3 | 8.4 | 0.3–29.8 | 21.3 | 2.8–44.0 | |
| Benthosema suborbitale | 12.0 | 0.6–40.4 | 7.0 | 0.3–24.3 | 22.6 | 1.7–46.9 | 12.8 | 0.6–39.7 | 11.1 | 0.6–35.0 | |
| Diaphus problematicus | 10.5 | 0.3–36.6 | 10.8 | 0.6–27.7 | 9.4 | 0.4–31.7 | 11.9 | 0.6–33.6 | 9.0 | 0.6–30.5 | |
| Lampanyctus alatus | 15.4 | 0.7–44.7 | 5.5 | 0.2–21.1 | 20.3 | 2.2–39.0 | 8.9 | 0.3–31.8 | 25.0 | 4.2–47.8 | |
| Lepidophanes guentheri | 13.1 | 0.9–38.4 | 5.4 | 0.2–19.1 | 20.3 | 1.6–40.9 | 15.8 | 1.2–41.5 | 9.6 | 0.6–32.5 | |
| Myctophum affine b | 7.2 | 0.3–27.2 | 6.2 | 0.3–21.0 | 11.6 | 0.4–34.4 | 8.5 | 0.4–28.2 | 7.0 | 0.3–25.3 | |
| GEN | Ceratoscopelus warmingii | 9.5 | 0.5–33.0 | 3.7 | 0.1–17.4 | 33.1 | 6.5–52.5 | 11.0 | 0.6–39.2 | 10.0 | 0.5–29.7 |
| NA | Sternoptyx sp. | 13.5 | 0.9–45.3 | 6.5 | 0.2–27.6 | 18.3 | 1.8–39.6 | 7.8 | 0.4–29.0 | 27.2 | 3.0–54.2 |
| Melamphaidae | 13.7 | 0.7–44.8 | 10.5 | 0.5–35.7 | 14.6 | 0.9–35.4 | 7.4 | 0.7–26.7 | 24.3 | 2.8–50.8 | |
| Guild | Species | Decapoda | Euphausiid | Fisha | POM | Chemo | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50% | Range | 50% | Range | 50% | Range | 50% | Range | 50% | Range | ||
| PIS | Chauliodus sloani | 8.5 | 0.4–37.2 | 10.9 | 0.5–42.7 | 14.5 | 0.5–48.3 | ||||
| LGC | Stomiidae spp. | 10.6 | 0.5–35.7 | 10.1 | 0.3–38.7 | 13.9 | 0.6–45.2 | ||||
| Gonostoma elongatum | 11.1 | 0.5–33.3 | 12.5 | 0.6–39.6 | 12.4 | 0.4–40.5 | |||||
| COP | Cyclothone alba | 6.5 | 0.3–29.3 | 11.9 | 0.7–41.6 | ||||||
| Cyclothone pseudopallida | 10.2 | 0.4–34.2 | 14.0 | 0.8–43.9 | |||||||
| Valenciennellus tripunctulatus | 6.6 | 0.3–26.6 | 11.6 | 0.5–39.5 | |||||||
| Diaphus spp. | 10.1 | 0.5–36.5 | 13.0 | 0.7–43.0 | |||||||
| MZO | Cyclothone pallida | 11.3 | 0.5–34.7 | 13.9 | 0.7–43.7 | ||||||
| Argyropelecus aculeatus | 23.5 | 1.5–53.1 | 14.0 | 0.8–45.1 | |||||||
| Argyropelecus hemigymnus | 16.0 | 1.1–40.5 | 14.0 | 0.6–42.9 | |||||||
| Pollichthys mauli | 13.5 | 0.9–33.2 | 12.2 | 0.6–38.5 | |||||||
| Vinciguerria poweriae | 3.5 | 0.1–18.1 | 8.3 | 0.3–32.4 | 8.2 | 0.3–30.9 | |||||
| Benthosema suborbitale | 9.5 | 0.3–34.9 | 12.8 | 0.5–42.8 | |||||||
| Diaphus problematicus | 23.8 | 2.0–50.9 | 14.3 | 0.8–45.2 | |||||||
| Lampanyctus alatus | 5.0 | 0.2–20.2 | 11.1 | 0.6–39.3 | |||||||
| Lepidophanes guentheri | 11.6 | 0.7–31.3 | 14.9 | 0.6–43.9 | |||||||
| Myctophum affine b | 8.8 | 0.3–31.8 | 9.4 | 0.5–33.3 | 9.2 | 0.5–32.0 | 18.6 | 13.4–23.8 | |||
| GEN | Ceratoscopelus warmingii | 4.7 | 0.2–20.7 | 8.9 | 0.4–32.7 | 8.0 | 0.3–28.5 | ||||
| NA | Sternoptyx sp. | 4.7 | 0.2–25.0 | 9.9 | 0.4–39.1 | ||||||
| Melamphaidae | 5.9 | 0.3–25.8 | 11.9 | 0.6–41.2 | |||||||
- aFish were an average of all myctophid species.
- bResults for M. affine are based on the inclusion of chemosynthetic material calculated from Beggiatoa sp. material collected at the three study sites (Demopoulos et al., 2010), as this species was not bound by the photosynthetic based prey sources analysed in this study.
- Endmember values were averaged across sites and were as follows zooplankton (δ15N = 6.8 ± 2.6, δ13C = −19.6 ± 0.9), Cnidaria (δ15N = 12.6 ± 3.5, δ13C = −19.2 ± 0.5), Pteropoda (δ15N = 1.8 ± 0.1, δ13C = −19.4 ± 0.1), Salpida (δ15N = 2.4 ± 2.1, δ13C = −18.7 ± 1.2), Cephalopoda (δ15N = 8.6 ± 2.8, δ13C = −20.2 ± 0.7), Decapoda – Shrimp (δ15N = 6.9 ± 1.0, δ13C = −18.2 ± 0.7), euphausiid (δ15N = 5.8 ± 1.5, δ13C = −19.1 ± 0.7), Fisha (δ15N = 7.4 ± 1.1, δ13C = −19.4 ± 1.0), Chemo (δ15N = −2.2 ± 2.7, δ13C = −32.3 ± 2.9) and particulate organic matter (POM; δ15N = 3.7 ± 2.4, δ13C = −19.8 ± 2.2)
3.5 TP calculation comparisons
The mean TPGCA ranged from 3.0 (multiple species) to 4.0 (Chauliodus sloani, Table 5), whereas the mean TPSIA had a larger range [1.7 (Myctophum affine) to 3.6 (Diaphus problematicus)]. Qualitative comparisons between TP estimates suggest that the overall mean TPSIA was higher than mean estimates calculated from GCA for Valenciennellus tripunctulatus, Cyclothone pallida, Argyropelecus aculeatus and Argyropelecus hemigymnus (Table 5). All other mesopelagic fishes (n = 13) had mean TPSIA lower than mean TPGCA (Table 5). There was overlap in the reported ranges for TPGCA and TPSIA for all fish species except Cyclothone alba and M. affine, which had lower TPSIA values compared to TPGCA.
| Guild | Species | GCA | SIA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | SD | Range | n | SL | TP | Err | Range | n | SL | ||
| PIS | Chauliodus sloani | 4.0 | 0.0 | 4.0–4.0 | 5 | 20–132 | 2.9 | 0.9 | 2.4–4.2 | 6 | 25–105 |
| LGC | Stomiidae spp. | 3.9 | 0.2 | 3.5–4.0 | 8 | 20–104 | 3.2 | 1.6 | 2.3–5.0 | 11 | 59–117 |
| Gonostoma elongatum | 3.2 | 0.3 | 3.0–3.9 | 29 | 21–180 | 3.1 | 0.5 | 2.8–3.6 | 12 | 41–188 | |
| COP | Cyclothone alba | 3.0 | 0.1 | 3.0–3.5 | 57 | 10–28 | 2.3 | 0.5 | 2.0–2.7 | 5 | 19–33 |
| Cyclothone braueri | 3.0 | 0.0 | 3.0–3.0 | 60 | 11–28 | ||||||
| Cyclothone pseudopallida | 3.0 | 0.1 | 3.0–3.5 | 51 | 12–45 | 3.0 | 0.7 | 2.6–3.6 | 10 | 15–41 | |
| Valenciennellus tripunctulatus | 3.0 | 0.1 | 3.0–3.5 | 87 | 13–30 | 3.5 | 0.7 | 2.9–4.0 | 15 | 18–26 | |
| Diaphus spp. | 3.2 | 0.3 | 3.0–4.0 | 27 | 12–55 | 3.1 | 0.5 | 2.5–3.4 | 6 | 27–65 | |
| Hygophum benoiti | 3.0 | 0.1 | 3.0–3.3 | 13 | 12–18 | ||||||
| Notolychnus valdiviae | 3.0 | 0.1 | 3.0–4.0 | 151 | 11–22 | ||||||
| MZO | Cyclothone pallida | 3.0 | 0.0 | 3.0–3.0 | 9 | 13–51 | 3.5 | 0.9 | 2.8–4.3 | 10 | 27–48 |
| Argyropelecus aculeatus | 3.1 | 0.2 | 3.0–3.5 | 25 | 14–46 | 3.2 | 1.3 | 1.8–4.1 | 10 | 18–56 | |
| Argyropelecus hemigymnus | 3.0 | 0.1 | 3.0–3.4 | 11 | 13–30 | 3.4 | 0.5 | 3.0–3.9 | 10 | 8–11 | |
| Pollichthys mauli | 3.1 | 0.2 | 3.0–3.5 | 34 | 33–36 | 2.8 | 0.4 | 2.4–3.3 | 10 | 25–37 | |
| Vinciguerria poweriae | 3.1 | 0.2 | 3.0–4.0 | 69 | 12–35 | 2.9 | 0.7 | 2.4–3.7 | 15 | 20–31 | |
| Benthosema suborbitale | 3.1 | 0.2 | 3.0–4.0 | 83 | 10–29 | 2.9 | 0.9 | 2.2–3.6 | 6 | * | |
| Diaphus problematicus | 3.6 | 0.5 | 3.3–4.2 | 7 | * | ||||||
| Lampanyctus alatus | 3.0 | 0.1 | 3.0–3.5 | 46 | 14–45 | 3.0 | 0.6 | 2.4–3.8 | 15 | 30–52 | |
| Lepidophanes guentheri | 3.1 | 0.1 | 2.9–3.5 | 91 | 14–57 | 2.8 | 0.5 | 2.3–3.3 | 10 | 37–66 | |
| Myctophum affine | 3.1 | 0.2 | 3.0–4.0 | 24 | 13–18 | 1.7 | 0.7 | 1.0–2.3 | 10 | 16–17 | |
| GEN | Ceratoscopelus warmingii | 3.1 | 0.3 | 2.9–4.0 | 56 | 2.4 | 0.9 | 1.7–3.5 | 12 | 20–44 | |
| NA | Melamphaidae | 3.2 | 0.9 | 2.4–4.0 | 10 | NA | |||||
| Sternoptyx spp. | 3.0 | 0.4 | 2.8–3.5 | 9 | 22–49 | ||||||
- Guilds were based on GCA and defined as PIS = piscivore, LGC = large crustacean consumer, COP = copepod consumer, MZO = mixed zooplanktivore, GEN = generalist. NA under guild means not available as these species were not analysed using GCA in this study.
- *Indicates specimens were damaged and SL not recorded for all specimens.
- Err is the propagated error associated with all parameters in the TP calculations from SIA data.
4 DISCUSSION
4.1 General trophic structure
The trophic structure of mesopelagic fishes was similar among the five dominant families examined in this study. Location did not appear to be a major factor influencing the mesopelagic fish trophic structure, supporting previous studies in the Eastern GOM (Hopkins et al., 1996). However, δ15N value differences between the VM and WM migration categories indicated that feeding depth influences the isotopic composition of these fishes and, consequently, our calculations of trophic position. Overall, both GCA and SIA indicated mesopelagic fishes were secondary consumers, occupying the third trophic level. Zooplankton taxa, particularly copepods, were important in the diets of mesopelagic fishes and, based on SIA and MixSIAR, may be assimilated by multiple fish species (Table 4), assuming the sources sampled are isotopically similar over time (i.e. zooplankton sampled in this study are isotopically similar to zooplankton consumed at an earlier time point and assimilated into the tissues). While no studies exist on the tissue turnover rate of mesopelagic fishes, fish δ15N values can be affected by growth, with juveniles having faster (weeks) tissue turnover compared to adult fishes (Vander Zanden, Clayton, Moody, Solomon, & Weidel, 2015). The prevalence of smaller juvenile mesopelagic fishes examined in this study may also explain the dominance of zooplankton in their diets, as juvenile fishes may not have switched over to larger prey items. For larger taxa (e.g. Stomiidae), zooplankton may serve as an intermediate resource between larger meals (Sutton, 2005). Thus, mesopelagic fishes likely constitute an important control on zooplankton communities as a result of the high consumption of zooplankton prey (Bernal, Oliver, Maynou, & Fernández de Puelles, 2015), with predation highest in the epipelagic zone at night (Hopkins et al., 1996).
The five feeding guilds (piscivore, large decapod consumer, copepod consumer, mixed zooplanktivore, generalist) documented using GCA were less well defined using SIA, possibly due to consistent consumption of zooplankton (Gartner et al., 1997) inferred from SIA (this study). Only three feeding guilds were previously reported for mesopelagic fishes [Gartner et al., 1997 (using GCA) and Valls et al., 2014 (using SIA)]. It is possible that juvenile mesopelagic fishes have more specialized diets than adults. However, given the diet composition was not well sampled based on prey accumulation curves, the analysis of additional stomachs, particularly adult specimens, could increase diet variability and result in fewer distinct feeding groups. The variations in prey preferences within feeding groups (GCA results) were also apparent in the isotope data among mixed zooplanktivore and among copepod consumer species. Diet variation over time, including both short and long time scales, suggests juvenile mesopelagic fishes are able to partition resources.
Photosynthetically derived POM serves as the primary carbon source fueling the zooplankton consumed by mesopelagic communities. Although surface POM in this study was similar across sites (Demopoulos et al., 2010), the isotopic composition of POM can be influenced by multiple factors, such as depth, freshwater and terrestrial input of nutrients and/or diazotrophy, resulting in δ15N values from −2‰ to 8‰ in the GOM (Dorado, Rooker, Wissel, & Quigg, 2012). While POM isotope values can increase with depth due to microbial activity, coagulation, and particle size (Mintenbeck et al., 2007), many mesopelagic fishes undergo VMs and feed in the epipelagic zone at night, potentially decoupling the relationship between isotopic enrichment and depth.
Fish isotopic composition can be influenced by various facets including depth of feeding, metabolic activity, locomotion, trophic level and fish size. Higher δ15N values would be expected for fishes feeding at depth due to the enrichment in 15N of the baseline POM as depth increases. This may explain the higher δ15N values of Cyclothone pallida, a WM (Ross et al., 2010), compared to other migrating mixed zooplanktivores, as it primarily feeds at depth on isotopically enriched zooplankton. Similar isotopic patterns were reported for mesopelagic fishes in the Mediterranean, where higher δ15N values were associated with bottom dwelling or non-migrating mesopelagic fishes (Valls et al., 2014). In addition to utilizing prey enriched in 15N, fishes occupying cooler, deep water may have reduced metabolic activity, allowing mesopelagic fishes (both VM and WM) to convert more energy to growth (McLaren, 1963; Enright, 1977; Herring, 2002) or be more efficient in N use (Trueman, McGill, & Guyard, 2005). Both of these factors can minimize their fractionation of assimilated nitrogen. Fish size can also affect trophic level (Trueman et al., 2005), whereby larger fishes with bigger gapes can feed on larger, trophically higher prey resources. This would explain the significant positive correlation between δ15N values and SL reported for nine species in this study. There was no apparent relationship between fish size and depth (i.e. larger specimens were not captured deeper than smaller specimens). However because most of the fishes examined in this study were juveniles (>79%), including more adult size classes and collecting at discrete depths will help address how ontogeny affects fish δ15N composition.
Zooplanktivory was common for most mesopelagic fishes (19 out of 24 species with prey documented in the stomachs) examined here. Although GCA alone was not sufficient to assess trophic guild for Sternoptyx spp. and Melamphaidae given few specimens were collected, SIA classified them as zooplanktivores, consistent with previously published diet analyses (Gartner & Musick, 1989; Hopkins & Baird, 1985a; Hopkins et al., 1996). Species-specific prey preferences provided by GCA enabled the separation of zooplanktivorous midwater fishes into copepod consumers, mixed zooplanktivores and generalists. While copepods comprised over 90% of the diets of copepod consumers, competitive pressure on individual copepod species may not be high because different mesopelagic fish species consumed different copepod species, consistent with previous studies (Hopkins & Baird, 1981; Hopkins et al., 1996). Unlike copepod consumers, mixed zooplanktivores consumed ostracods, euphausiids and amphipods (Hopkins & Baird, 1981, 1985a; Hopkins & Gartner, 1992; Hopkins et al., 1996; Sutton, Hopkins, & Lancraft, 1998; present study). The importance of euphausiids in the diets of Lampanyctus alatus and Lepidophanes guentheri also increased with size (Hopkins & Baird, 1985b; Hopkins & Gartner, 1992; present study).
Feeding selectivity by co-occurring zooplanktivorous mesopelagic fishes could be driven by competitive pressures among species inhabiting similar depths (Sutton et al., 2008), as suggested for assemblages in the Mid-Atlantic Ridge (Carmo, Sutton, Menezes, Falkenhaug, & Bergstad, 2015), leading to resource partitioning in the midwater community (Hopkins & Sutton, 1998). Depth is an important factor in the mesopelagic fish assemblages in the GOM, with the assemblage divided into VM and WM (Ross et al., 2010). VM may reduce competitive pressures for zooplankton prey among mesopelagic fishes, with the majority of these species undergoing migrations (Ross et al., 2010). Certain mesopelagic fishes, such as Diaphus mollis, are able to feed on dense prey populations in the epipelagic zone by following the migrational patterns of crustacean prey, e.g. decapods or the copepod Pleuromamma spp. (Hopkins & Sutton, 1998; Pusch et al., 2004). In contrast, Cyclothone pseudopallida, a WM (Ross et al., 2010), consumed deepwater prey, e.g. the copepod Aegisthus mucronatus, known to occupy depths from 500 to 1,500 m (Deevey & Brooks, 1977), suggesting this fish fed at depth. Stomiids can have asynchronous migrations (only part of the population migrates; Sutton & Hopkins, 1996), which may reduce direct competition for resources in the epipelagic zone. Stomiidae spp. may play a key role in transporting phytoplankton-derived surface production utilized in the mesopelagic zone further down the water column to the bathypelagic zone and benthos (Sutton & Hopkins, 1996), which may also be true for other mesopelagic fish species that feed at deeper depths. Future work incorporating depth as a factor in trophic structure would provide a more direct measure of competition and insights on niche overlap in this study area.
The relative importance of non-crustacean prey, particularly gelatinous organisms, to mesopelagic fish diets and their role in energy transport may be underestimated. In part this is due to difficulty in identifying these easily digested prey (Gartner et al., 1997), which are considered ‘trophic dead ends’ (Mianzan, Pájaro, Colombo, & Madirolas, 2001) and consequently missed in diet studies. MixSIAR revealed potential contributions of non-crustacean prey to the diets of all mesopelagic fishes, with estimates of >20% contribution for certain copepod consumers, mixed zooplanktivores and generalists. The importance of non-crustacean prey for Melamphaidae documented in MixSIAR is also supported by previous literature (Gartner & Musick, 1989). MixSIAR also revealed the potential contributions of gelatinous organisms and cephalopods to the copepod consumers Cyclothone alba and Valenciennellus tripunctulatus. Salps in particular were estimated to be an important food source (this study), and were previously reported in the diets for several mesopelagic fish species examined in the GOM, Gulf of California, Central Pacific and Northeast Atlantic (Kashkina, 1986). As these gelatinous taxa had lower δ15N values compared to crustacean prey, their consumption and assimilation by midwater fishes may explain the lower estimates of TPSIA compared to TPGCA for many of zooplanktivorous fishes examined in this study. Therefore, gelatinous prey may play a more crucial role than previously attributed in the trophic structure of mesopelagic fishes.
As previously done in freshwater systems (Rybczynski et al., 2008; Vander Zanden et al., 1997; Woodward & Hildrew, 2002), the TP calculations conducted here provided a characterization of the trophic structure of midwater fishes and enabled a qualitative comparison between GCA and SIA (Amezcua et al., 2015). In most cases, SIA placed fish species at a trophically lower position than GCA. GCA is less sensitive to detecting mixed diets over long time scales, and, as a result, GCA may overestimate TP by only using identifiable prey items. Mesopelagic fishes may also be trophically plastic, whereby species within the same feeding guild and occupying similar TPs may consume different prey resources, as was the case with C. alba, Cyclothone braueri and Cyclothone pseudopallida. Differences in prey selection may account for TP (both GCA and SIA) variability among individuals within a species. Hopkins and Gartner (1992) reported higher than expected trophic diversity in myctophid fishes using GCA. The large ranges in TP estimates, including TPSIA, from the current study suggest that high trophic diversity (e.g. Layman, Arrington, Montaña, & Post, 2007) may be associated with the entire GOM mesopelagic community, not just limited to myctophids. It should be noted that TPSIA is a function of the TEF from the literature, and assumed to be the same across all fishes analysed; however, piscivorous fishes might have a higher TEF than invertebrate feeders (e.g. McCutchan et al., 2003; Post, 2002). Other studies analysing mesopelagic fishes with SIA (Choy et al., 2012; Valls et al., 2014) used a higher TEF (3‰–3.15‰), also derived from published values on other, non-mesopelagic, fish species. If we assume a higher TEF for Chauliodus sloani, the calculated TP would be lower than the current estimate, which is inconsistent with its diet of higher trophic level prey (i.e. fishes). Based on the data analysed in our study, the assumed TEF of 2‰ used for all fishes seemed appropriate. The utilization of a different primary consumer could also affect our TP calculations. Some euphausiid species can be omnivores or carnivores (Kinsey & Hopkins, 1994) and will have higher δ15N values than herbivores. However, our measured euphausiid δ15N values were approximately one trophic level above POM, consistent with euphausiids serving as primary consumers and providing the best available representative of a primary consumer present at all sites.
Model results informed by GCA provided a baseline for determining prey contributions. Surprisingly, decapods were identified as an important source for the zooplanktivores, Diaphus spp., Argyropelecus aculeatus and Argyropelecus hemigymnus. It is unlikely that these fishes are consuming large decapods; however, some decapods captured in this study were of similar size to euphausiids and therefore could be consumed by these fishes. Contributions of fish to the diets of Vinciguerria poweriae (8.2%; 0.3%–30.9% CI) were lower than expected based on GCA. Given the GCA results classified this species as a mixed zooplanktivore, it is probable that V. poweriae only occasionally consumes fishes, which is reflected in the isotope mixing model results (MixSIAR) and lower estimated TP (2.9). Large ranges in potential contributions make it difficult to estimate dominant prey sources for individual fishes. In addition, the lack of species-specific information on TEF values also influences the model results. The assumed TEF was suitable for our purposes because it allowed fishes to be contained by the mixing polygon created from endmember isotope ratios. Additional work utilizing compound-specific SIAs could also enable better resolution in determining source contributions.
4.2 Role of chemosynthetic production in mesopelagic fish diets
Chemosynthetically derived production did not appear to be a primary food source for mesopelagic fishes, except for possibly Myctophum affine, based on SIA and MixSIAR analyses. Mesopelagic fishes may be limited by the bottom water depth and unable to directly utilize chemosynthetic production at sites >1,000 m. Reports of mesopelagic fish aggregations occurring on the sea floor and exploiting benthic productivity (Gartner et al., 2008) were previously documented from shallower bottom depths (184–900 m) compared to this study (1,450–2,340 m). However, our sampling did not occur all the way to the benthos, so it is possible that certain, as yet unmeasured, mesopelagic fishes aggregate at deeper depths near the benthos, potentially utilizing chemosynthetic production.
While MixSIAR analysis reported a contribution of chemosynthetic derived organic matter to M. affine, it is unclear if this was truly the case or a result of mixing model assumptions. The POM values used in the model were collected at the same time and location as the fishes analysed, representing the best available approximation of this endmember resource. However, isotope values of POM are variable in the GOM and using a lower δ13C value for POM in the model would reduce the contribution of chemosynthetic derived material. Implementation of compound-specific SIA of M. affine tissues would help resolve the contribution of chemosynthetic material to the diet of this species (MacAvoy, Macko, & Carney, 2003).
4.3 Species-specific trophic ecology
While Chauliodus sloani was the only strictly piscivorous mesopelagic fish species in our study based on GCA, MixSIAR results for C. sloani suggest a diet comprised of fishes, zooplankton, and decapod and euphausiid crustaceans. Chauliodus sloani is well suited for piscivory given its specific physical adaptations like large, curved teeth and an expansive oral cavity (Borodulina, 1972). Isotope data from AT340 supported piscivory in C. sloani, with myctophids identified as a potential food source, occurring one trophic level below C. sloani. However, piscivory was less evident at GC852 despite the capture of larger juvenile specimens at that site, with lower δ15N values for C. sloani compared to a few zooplanktivorous species (Cyclothone pallida, Cyclothone pseudopallida and Valenciennellus tripunctulatus). Chauliodus sloani may feed on isotopically depleted food resources, e.g. Myctophum affine or zooplankton (Butler et al., 2001; Clarke, 1982; Hopkins et al., 1996; Sutton & Hopkins, 1996), which would result in lower than expected δ15N values for this piscivore. In a previous study, crustaceans, particularly euphausiids, were consumed more frequently by smaller (<120 mm) specimens of C. sloani (Roe & Badcock, 1984), which may be less efficient at capturing fishes. The large number of empty stomachs recorded in this study and in previous work (Sutton & Hopkins, 1996) suggests foraging may not always be successful, and C. sloani may rely on smaller zooplankton to sustain energetic needs between successful foraging events on larger taxa, including fishes (recorded in this study and in previous work Sutton, 2005). Consumption and assimilation of zooplankton between larger fish meals would result in a lower mean TPSIA than TPGCA for C. sloani, as was found here. Given the significant positive correlation between fish size (SL) and δ15N, these fish may switch from zooplankton to more fish-dominated prey as they grow, consistent with published GCA results (Butler et al., 2001; Clarke, 1982; Roe & Badcock, 1984; Sutton, 2005) for this species.
In contrast to C. sloani, other stomiids examined in this study were large crustacean consumers. Decapods, as well as euphausiids and copepods, were documented in the stomachs of stomiid genera, including Astronesthes, Photostomias and Malacosteus, whereas other genera, such as Idiacanthus and Stomias, are known piscivores (Clarke, 1982; Hopkins et al., 1996; Sutton & Hopkins, 1996; Sutton, 2005; present study). These dietary differences among stomiid species indicate that one feeding guild was insufficient to characterize the diet of Stomiidae. Although Stomiidae spp. were classified in a different guild than C. sloani, TP estimates suggest stomiids occupy the same trophic position as a tertiary consumer. However, there was a large range in TPSIA among individuals, suggesting high trophic diversity (e.g. Layman et al., 2007). Stomiidae spp. also had a high number of empty stomachs (69%), similar to C. sloani, indicating they may utilize a similar feeding strategy and consume lower trophic level taxa (e.g. zooplankton) between meals of higher order consumers, e.g. myctophids or the decapod Gennadas valens (Hopkins, Flock, Gartner, & Torres, 1994; present study). As a result, Stomiidae spp. have a high predation impact on both primary and secondary consumers (Hopkins et al., 1996; Sutton & Hopkins, 1996).
The gonostomatid Gonostoma elongatum was identified as a large crustacean consumer in this study, occupying the third trophic level. While decapods and euphausiids had the highest maximum contribution to its diet based on GCA, inclusion of smaller zooplankton was documented, consistent with previous data from the Eastern GOM (Hopkins et al., 1996; Lancraft et al., 1988). MixSIAR did not identify a dominant food source for this species, potentially a result of a highly variable diet composed of small and large crustaceans. As a migrator (Ross et al., 2010), G. elongatum may consume 15N depleted prey in the epipelagic zone, resulting in a lower estimated TP than other large crustacean consumers. Clarke (1982) suggested that G. elongatum consumes increasingly larger crustaceans as it grows, which is supported by the significant increase in δ15N values with increasing fish size (this study). While G. elongatum's diet may shift over its life cycle, both SIA and GCA identified zooplankton as a food resource, suggesting diet similarity over the time scales recorded in fish muscle (weeks) and stomach contents (days).
The high diversity of prey consumed by the generalist zooplanktivore Ceratoscopelus warmingii documented in this study was consistent with previous diet studies (Hopkins & Baird, 1975; Hopkins et al., 1996). This generalist feeding strategy may explain why C. warmingii had among the lowest TP for zooplanktivores. In addition to herbivory (Robison, 1984; present study), MixSIAR reported 15N depleted pteropods as important prey items. Thus, C. warmingii occupies a unique trophic niche among zooplanktivores by utilizing a generalist feeding strategy (Robison, 1984).
Myctophum affine utilize a more generalist feeding strategy like that of C. warmingii, with M. affine occupying the lowest trophic level. As a mixed zooplanktivore, M. affine feed selectively on amphipods (Hopkins & Gartner, 1992; present study) and copepods (present study). However, MixSIAR results indicated all sources had similar contributions. Small specimens of M. affine may have been selecting prey items not sampled in this study, while larger specimens may have switched to a generalist feeding strategy, with POM and non-crustacean prey documented in the GCA. Although not reported for M. affine, larger myctophids can occupy shallower layers (Benoit-Bird & Au, 2006), consuming 15N-depleted food resources (i.e. POM and non-crustacean prey). This would account for not only the low TP but also the negative correlation between size and δ15N values documented in this study.
5 CONCLUSIONS
This study highlights the utility of incorporating multiple techniques to discern trophic relationships (Amezcua et al., 2015; Churchill et al., 2015; Rybczynski et al., 2008; Vander Zanden et al., 1997; Woodward & Hildrew, 2002) among midwater fishes. This combined approach is particularly useful when limitations (i.e. empty stomachs, lack of species-specific diet information, overlapping carbon isotope values for potential food resources) are present for one method. When we combined the species-specific data provided by GCA with the long-term diet perspective provided by SIA, we determined that multiple mesopelagic fishes, not just myctophids, were utilizing zooplankton prey sources and not opportunistically feeding. This is consistent with previous diet analyses of myctophids using only GCA (Hopkins & Gartner, 1992). Feeding guild classification became more robust when different methods produced similar results, as in the case of Gonostoma elongatum. While GCA revealed five distinct guilds, SIA did not consistently support those guilds. Considering SIA integrates diets over longer time scales than GCA (including ontogenetic changes), the boundaries of these guilds may be more fluid, with mesopelagic fishes assimilating a variety of prey resources over time. Through the use of SIA, we noted that mesopelagic fishes may be assimilating gelatinous prey, potentially serving as a significant, yet previously underestimated, food resource. SIA did not reveal a reliance upon chemosynthetically derived carbon in most of the mesopelagic fish diets. However, it is possible that mesopelagic fishes utilize seep-derived organic matter intermittently, but detecting the seep isotopic signal is difficult due to longer-term assimilation of isotopically enriched, non-seep food resources, including POM. To our knowledge, this is the first time that both GCA and SIA have been applied concomitantly to understand multiple midwater fish diets and estimate the relative contribution of dominant food resources. Ultimately, the combined approach provides a more complete understanding of the trophic structure of these communities residing in the GOM.
ACKNOWLEDGEMENTS
This project was largely funded by the Department of the Interior U.S. Geological Survey under Cooperative Agreement No. 05HQAG0009, sub agreement 05099HS004 to S.W.R. and from the USGS Environments Program to A.W.J.D. We appreciate the support and assistance of Dr G. Brewer (formerly USGS) during this project. Special thanks to the crew of the R/V Cape Hatteras and all scientific personnel for assisting with fishing operations and sample processing. S. Artabane, A. Roa-Varón and Dr A. Quattrini assisted with fish identifications and C. Ames assisted with invertebrate identifications. We also thank S. Artabane, T. McIver, A. Roa-Varón and Dr. J. Bourque for their assistance with analyses. Thanks to our anonymous reviewers for their insights that improved this paper. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.




