Effects of acidified seawater on calcification, photosynthetic efficiencies and the recovery processes from strong light exposure in the coral Stylophora pistillata
Abstract
The aim of this study was to investigate whether coral photosynthetic efficiencies and recovery processes are affected by CO2-driven ocean acidification in symbiont photosynthesis and coral calcification. We investigated the effects of five CO2 partial pressure (pCO2) levels in adjusted seawater ranging from 300 μatm (pre-industrial) to 800 μatm (near-future) and strong and weak light intensity on maximum photosynthetic efficiency and calcification of a branching coral, Stylophora pistillata, as this species has often been used in rearing experiments to investigate the effects of acidified seawater on calcification and photosynthetic algae of corals. We found that, the photosynthetic efficiencies and recovery patterns under different light conditions did not differ among pCO2 treatments. Furthermore, calcification of S. pistillata was not affected by acidified seawater under weak or strong light conditions. Our results indicate that the photosynthetic efficiency and calcification of S. pistillata are insensitive to changes in ocean acidity.
1 INTRODUCTION
Reef-building corals are the main calcifying organisms forming coral reefs, which are biologically diverse ecosystems and provide many different ecological goods and services such as fisheries, tourisms etc. (Moberg & Folke, 1999). Thus, the decrease of corals and consequent deterioration of the coral reef ecosystem is a serious problem not only for many reef organisms but also in terms of human activity. Corals are threatened by various environmental changes at local (e.g. inputs of sediment and nutrients; Bellwood, Hughes, Folke, & Nystrom, 2004) and global scales (e.g. global warming is considered the key stressor leading to coral bleaching; Hoegh-Guldberg, 1999). Understanding how these environmental changes lead to the decrease of corals is one of the most important topics in coral reef conservation biology. Laboratory experiments are one of the approaches that have contributed to the understanding of the stress responses of corals (reviewed in Fabricius, 2005), for example, investigating the effects of changes in temperature (Inoue et al., 2012), light (Bhagooli & Hidaka, 2004) and nutrients (Tanaka, Miyajima, Koike, Hayashibara, & Ogawa, 2007; Tanaka, Ogawa, & Miyajima, 2010).
In addition to the environmental changes above, ocean acidification has been recognized as a severe threat to corals because their calcification rates are generally reduced by decreases in carbonate ion concentrations (reviewed in Hoegh-Guldberg et al., 2007; Kroeker et al., 2013). Laboratory rearing experiments with acidified seawater have contributed greatly to the understanding of the responses of corals to ocean acidification (reviewed in Kroeker et al., 2013), demonstrating that significant decreases in coral calcification occur in acidified seawater (e.g. Anthony, Kline, Diaz-Pulido, Dove, & Hoegh-Guldberg, 2008; Gattuso, Frankignoulle, Bourgeb, Romaine, & Buddemeier, 1998; Iguchi et al., 2012, 2014; Marubini, Christine, Ferrier-Pages, Furla, & Allemand, 2008). Several recent studies suggest that the responses of corals to acidified seawater are complex, and that physiological conditions alter the calcification responses under environmental changes (Comeau, Carpenter, & Edmunds, 2013b; Comeau, Edmunds, Spindel, & Carpenter, 2013a; Edmunds, 2011; Ohki et al., 2013; Ries, Cohen, & McCorkle, 2009; Takahashi & Kurihara, 2013).
One important aspect to consider when investigating stress responses of corals is the existence of photosynthetic symbiotic dinoflagellates, zooxanthellae (genus Symbiodinium). It has been reported that acidified seawater could affect both coral calcification and photosynthesis of symbiotic algae through the decrease in CO32− and the increase in HCO3− (Comeau, Carpenter, & Edmunds, 2013c; Jury, Whitehead, & Szmant, 2010; Marubini et al., 2008). The decrease in calcification by calcifying organisms, including corals, is generally attributed to the decrease in CO32− (Hoegh-Guldberg et al., 2007). It has also been reported that the changes in the concentration of HCO3− have greater influence on coral calcification than changes in the concentration of CO32−, Ωarag, and pH (Jury et al., 2010). Considering this information, increased CO2 partial pressure (pCO2) might play a role in the fertilization of photosynthesis (Comeau et al., 2013c; Marubini et al., 2008). Therefore, the effects of acidified seawater on corals are complex.
It is also reported that ocean acidification facilitates coral bleaching in two coral species (Acropora intermedia and Porites lobata; Anthony et al., 2008), although the mechanisms are still unknown and information on the effects of acidified seawater on coral symbiotic algae is limited (Crawley, Kline, Dunn, Anthony, & Dove, 2010). It is suggested that corals can recover from bleaching if the period of thermal stress is not fatal to them (Grottoli, Rodrigues, & Palardy, 2006; Suzuki et al., 2003). However, it is poorly understood whether the recovery processes of corals from bleaching are affected by ocean acidification.
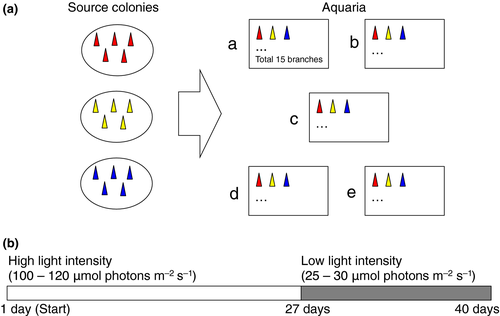
In the present study, we determined whether coral calcification, photosynthetic efficiencies and the recovery processes are affected by ocean acidification. We examined the effects of five levels of pCO2 adjusted seawater, ranging from 300 μatm (pre-industrial) to 800 μatm (near-future), enabling us to examine non-linear responses to ocean acidification (Ries, Cohen, & McCorkle, 2010). Furthermore, we examined two different light intensity (100–120 and 25–30 μmol photons m−2 s−1) on photosynthetic efficiency and calcification of a branching coral, Stylophora pistillata, a species commonly used in laboratory rearing experiments to investigate the effects of ocean acidification on calcification and photosynthetic algae of corals (Gattuso et al., 1998; Godinot, Houlbrèque, Grover, & Ferrier-Pagès, 2011; Houlbrèque et al., 2012; Marubini et al., 2008; Reynaud et al., 2003).
2 MATERIAL AND METHODS
2.1 Preparation of acidified seawater
Five pCO2 levels (approximately 300–800 μatm) were prepared in our experiment (Figure 1). These pCO2 values represent pre-industrial (low pCO2), present-day (control) and three near-future (higher pCO2) pCO2 levels, respectively (Table 1). Precise and stable pCO2 conditions (each pCO2 value was maintained within 10% during the experimental period) were achieved by using a high-precision pCO2 control system (Fujita et al., 2011), in which the pCO2 values were monitored by using a non-dispersive infra-red absorption system with a LI-COR 840 detector (LI-COR Biosciences Co., Lincoln, NE, USA). Seawater pH was also manually checked in each aquarium to confirm whether each pCO2 condition was properly maintained. Seawater was filtered using an inline filter system (1 μm). The temperature was recorded every 30 min by using data loggers (Water Temp Pro, Onset, MA, USA) and was maintained at approximately 27°C (Table 1), which is the ambient water temperature during the coral spawning season in Okinawa. Each aquarium (12 L) was filled with seawater adjusted to each pCO2 value, and each aquarium was maintained as a flow-through system. The seawater within each aquarium was stirred by submersible pumps (Kotobuki mini box, Kotobuki, Japan). The chemical and physical conditions of each treatment are summarized in Table 1. The pH, HCO3−, CO32− and Ωarg were estimated from pCO2, temperature, mean total alkalinity of 2228 ± 60 μmol/kg (mean ± SD, n = 4), which was measured three times in source water in aliquots of 50 ml by the potentiometric acid titration method with a Radiometer automated burette (Model ABU91) at 25°C (Kawahata, Suzuki, Ayukai, & Goto, 2000), and salinity of 34.5 by using the computer program CO2SYS (Lewis & Wallace 1998). The dissolved inorganic nitrogen and phosphorus concentrations in the seawater used in this study were less than 1 and 0.1 μm, respectively; thus, the concentrations were usual levels found in coral reef seawaters (Haas, Al-Zibdah, & Wild, 2009; Tanaka et al., 2010).
| Treatment | Temperature | pHT | pCO2 (μatm) | HCO3 (μmol/kg) | CO32− (μmol/kg) | Ωarg |
|---|---|---|---|---|---|---|
| a | 26.8 ± 0.5 | 8.13 ± 0.03 | 304 ± 25 | 1,616 ± 30 | 248 ± 12 | 3.98 ± 0.19 |
| b | 27.0 ± 0.3 | 8.02 ± 0.02 | 413 ± 19 | 1,717 ± 14 | 207 ± 5 | 3.33 ± 0.09 |
| c | 27.2 ± 0.1 | 7.94 ± 0.02 | 521 ± 25 | 1,786 ± 14 | 179 ± 6 | 2.89 ± 0.09 |
| d | 27.2 ± 0.2 | 7.85 ± 0.02 | 668 ± 38 | 1,856 ± 14 | 151 ± 6 | 2.43 ± 0.09 |
| e | 27.0 ± 0.2 | 7.78 ± 0.02 | 800 ± 50 | 1,904 ± 14 | 132 ± 6 | 2.11 ± 0.09 |
2.2 Preparation of coral nubbins
Three colonies of Stylophora pistillata were collected from a population of the reef slope of Oku reef, Northern Okinawa Island, Japan. All samples were collected with permission from Okinawa Prefecture. The colonies were kept in a flow-through outdoor tank in Sesoko Tropical Biosphere Research Station (University of the Ryukyus, Japan) for several years. Similar-sized branches, hereinafter called nubbins (around 1–2 cm long), were cut from the parental colonies with nippers and attached to plastic bolts with superglue. The nubbins were maintained in a tank with running seawater for around 1–2 weeks under natural light conditions until coral tissues started to spread on the surface of the plastic bolts.
2.3 Experimental set-up
Five nubbins from each colony were maintained in each aquarium as flow-through system (the amount of seawater/aquarium: 150 ml/min). Thus, we placed 15 nubbins from three colonies in each pCO2 treatment (Figure 1). During the experiment, the seawater temperature was maintained at around 27°C (Table 1). Light measurements were recorded by HOBO temperature and light loggers (Onset Computer Corp., Bourne, MA, USA) and a manual photon meter (Biospherical Instruments Inc., USA). In the first stage of the experiment (until 27 days after starting the experiment), high light intensity (about 100–120 μmol photons m−2 s−1, which was confirmed as strong in our preliminary experiment) was provided under a 12 hr:12 hr light:dark photoperiod under metal-halide lamps although accidentally two aquaria (treatments a and c) were exposed to higher light intensity for a few days (see Results). In the second stage (27–40 days after starting the experiment), low light intensity (about 25–30 μmol photons m−2 s−1) was provided under the same photoperiod as the first stage.
2.4 Measurements of maximum photosynthetic efficiency and calcification
To evaluate the photosynthetic fitness of zooxanthellae in nubbins, we measured the maximum fluorescence yield with a Diving-PAM Underwater Fluorometer (Walz, Germany) and calculated the maximum photosynthetic efficiencies [variable fluorescence/maximum fluorescence (Fv/Fm)] because this parameter can be easily traced at some time points under different environmental conditions (e.g. Berkelmans & van Oppen, 2006). Minimum fluorescence (Fo) was determined using 3-μs pulses of a light-emitting diode (blue LED, peak emission at 470 nm), and the Fm of each dark-adapted sample was measured by a 0.8-s saturation light pulse (8000 μmol photons m−2 s−1) (Schreiber, Schliwa, & Bilger, 1986). To determine the Fv/Fm values, we monitored the ratio of Fv (where Fv = Fm − Fo) to Fm for all of the samples, which were dark-adapted for 15 min prior to each measurement. Fv/Fm values were measured at 1, 2, 4, 8, 9, 10, 16, 17, 20, 24, 27, 31, 33, 38, 39 and 40 days after starting the experiment. Coral skeletal weight was measured as buoyant weight as described by Spencer-Davies (1989). The measurements were performed at 1, 27 and 40 days after starting the experiment to evaluate the calcification rates in two experimental stages. The calcification rates were calculated as percentage change in skeletal weight relative to the initial weight per day during the experiment (first stage: 1–27 days; second stage: 27–40 days).
2.5 Statistical analysis
Two-way analysis of variance (ANOVA) was applied to calcification rates by setting colony and pCO2 as fixed effects. Two-way repeated measures ANOVA was applied to Fv/Fm values by setting colony and pCO2 as fixed effects and days as a random effect. These statistical analyses were performed using R (R Development Core Team, 2015).
3 RESULTS
Fv/Fm values gradually decreased in all pCO2 treatments after the start of the experiment (Figure 2), suggesting that the light intensities were strong for our target species during the first stage under our experimental conditions. The source colonies used for this experiment were healthy during our experiment in other aquaria (data not shown). Temperature and carbon chemistry were kept almost uniform during each experimental stage (Table 1). Unfortunately, the light intensity of two aquaria (treatments a and c) became higher than planned (180–220 μmol photons m−2 s−1) for 2 days at approximately 16 days after starting the experiment. The high light was due to the detachment of a light cover from the metal-halide lamps, leading to significantly reduced Fv/Fm values in these two conditions from approximately 16 days after starting the experiment (Figure 2). During the first stage, Fv/Fm values did not differ among pCO2 treatments (two-way, mixed model ANOVA; F4,40 = 0.882, p = .48), but significant differences were detected among colonies (F2,20 = 4.697, p = .02), as well as an interaction between pCO2 treatments and colonies (F8,80 = 16.74, p < .01).
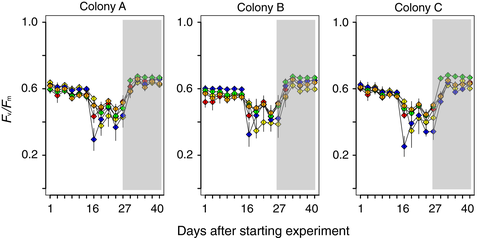
After reducing light intensity in the second stage, Fv/Fm values gradually increased and reached the values observed at the beginning of the experiment. However, there was an unclear difference in recovery speeds of photosynthetic efficiencies between low and high pCO2 treatments (Figure 2), although significant differences were detected among pCO2 treatments (F4,20 = 8.272, p < .01), colonies (F2,10 = 20.77, p < .01) and an interaction between pCO2 treatments and colonies (F8,40 = 10.87, p < .01) during the second stage.
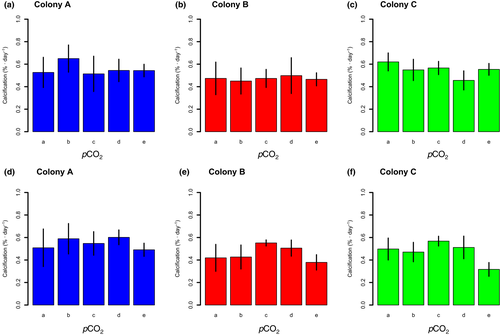
All coral nubbins showed positive calcification rates in all treatments during the experiment (Figure 3). During the first stage, calcification rates significantly differed among colonies (F2,60 = 4.772, p = .01) but not among pCO2 treatments (F4,60 = 0.518, p = .723), and the interaction between pCO2 treatments and colonies was not significant (F8,60 = 1.238, p = .293). During the second stage, calcification rates significantly differed among colonies (F2,60 = 6.559, p < .01) and pCO2 treatments (F4,60 = 6.676, p < .01), but the interaction between pCO2 treatments and colonies was not significant (F8,60 = 1.181, p = .326).
4 DISCUSSION
In the present study, we examined the effects of acidified seawater and light intensity on the photosynthetic efficiency (Fv/Fm) and calcification of the coral Stylophora pistillata. Although we found some physiological differences in S. pistillata among our experimental treatments, these patterns were complex. In our experiment, the Fv/Fm of S. pistillata was clearly affected by light intensity, but not by acidified seawater. Whereas light is essential for photosynthesis of coral–algal holobionts, excessive light exposure leads to photoinhibition, which is a light-dependent reduction in photosynthetic capacity (e.g. Bhagooli & Hidaka, 2004). In fact, high light intensity in our experiment led to decreased Fv/Fm in all three colonies of S. pistillata, but the patterns were not clearly different among pCO2 treatments. Although high light intensity in our experiment reduced the Fv/Fm of S. pistillata, low light intensity allowed recovery of the Fv/Fm (Figure 2). However, the recovery speeds of Fv/Fm did not clearly differ among pCO2 treatments.
Anthony et al. (2008) reported that coral bleaching of Acropora intermedia and Porites lobata was easily induced by acidified seawater. Therefore, we hypothesized that the photosynthetic efficiencies and recovery speeds of S. pistillata would differ among pCO2 treatments, but our results did not support this hypothesis. We speculate that this result could be attributed to low pCO2 values in the experiment, or to variation in dependences on symbiotic algae among species. Godinot et al. (2011) reported that acidified seawater (pH 7.46–8.09) did not affect Fv/Fm of S. pistillata, indicating that photosynthetic efficiencies of S. pistillata are not sensitive to acidified seawater. Similarly, Ohki et al. (2013) reported that photosynthetic efficiencies of Acropora digitifera were not affected by acidified seawater (pH 7.7–8.1), and the same pattern was also reported in Acropora cervicornis in the Caribbean (Enochs et al., 2014). Conversely, a decrease in Fv/Fm was observed in acidified seawater (pH 7.4–8.0) in the massive coral Porites australiensis in acidified seawater (Iguchi et al., 2012). As several studies show that calcification responses of marine calcifying organisms to acidified seawater are more complex than previously expected (Hikami et al., 2011; Iglesias-Rodrigues et al., 2008; Price, Hamilton, Tootell, & Smith, 2011; Ries et al., 2009), we suggest that the Fv/Fm of coral–algal holobionts in acidified seawater is also variable among species. The change in algal composition within nubbins might account for the change in Fv/Fm observed in this study as Sampayo, Ridgway, Bongaerts, and Hoegh-Guldberg (2008) reported that bleaching susceptibility is affected by different symbiont subtypes within a colony of S. pistillata. It was reported that S. pistillata harbored the C1 symbiont (LaJeunesse et al., 2004) in Zamami Island, Okinawa, Japan, which is in close proximity to our sample collection site. However, it is unclear whether the S. pistillata samples used in our experiment harbored these diverse symbionts, which might have led to the change in photosynthetic efficiencies.
Previous studies using S. pistillata have reported that calcification rates decreased in high pCO2 treatments (Gattuso et al., 1998; Marubini et al., 2008; Reynaud et al., 2003), although the effects of acidified seawater on calcification of S. pistillata changed according to the experimental temperature (Reynaud et al., 2003). In our experiment, there was no evidence of a decrease in calcification of S. pistillata in acidified seawater (Figure 3). The highest pCO2 in our experiment was approximately 800 μatm, lower than pCO2 values that clearly decreased calcification of S. pistillata in previous studies at 1000 μatm (Gattuso et al., 1998) and 1000–2000 μatm (Marubini et al., 2008). Thus, the range of pCO2 values used in our experiment may provide only weak acidifying conditions, whereby S. pistillata could maintain calcification at almost the same rates as the pre-industrial period and the present. As a next step, it would be necessary to evaluate the combined effect of acidified seawater and high seawater temperature on physiological parameters of S. pistillata in our experimental system.
ACKNOWLEDGEMENTS
This study was supported by the AICAL (Acidification Impact on CALcifiers) project funded by the Global Environment Research Fund A-0804 of the Ministry of the Environment of Japan and KAKENHI (nos 23241017, 26220102, 15H02813). We thank H. Kinjo for their help in experimental set-up.




