Lack of population genetic structure in the marine nematodes Ptycholaimellus pandispiculatus and Terschellingia longicaudata in beaches of the Persian Gulf, Iran
Abstract
We investigated genetic diversity and population genetic structure of two common benthic nematode species, Ptycholaimellus pandispiculatus and Terschellingia longicaudata, from sandy beaches in the area of Bandar Abbas (Iran), Persian Gulf. Based upon partial mitochondrial cytochrome oxidase c subunit 1 (COI) gene data, 17 and two haplotypes were found for P. pandispiculatus and Te. longicaudata, respectively. Analysis of molecular variance did not reveal a significant population genetic structure for either species. The absence of genetic structuring indicates substantial dispersal and gene flow in our study area. To assess the species structure of Te. longicaudata at a larger geographic scale, we compared 18S rDNA and COI sequences from Iran and the Scheldt Estuary in The Netherlands to ascertain whether they truly belong to the same species. Our data confirmed previous studies that Te. longicaudata likely constitutes a complex of multiple cryptic species, with one of these species having a (near) cosmopolitan distribution.
1 INTRODUCTION
Connectivity among marine populations is determined by the dispersal capacities of adults and/or larvae, as well as by the “permeability” of the environment. Dispersal is important for persistence of species because it allows organisms to escape from unsuitable environmental conditions, avoid competition and increase their distribution range. Dispersal distances and directions have a profound effect on gene flow and genetic differentiation within species (Froukh & Kochzius, 2007). Species with low dispersal capacities are expected to have a stronger population genetic structure than species with high dispersal abilities (Avise, 2004). Both physical (e.g. ocean currents and habitat characteristics) and biological (e.g. spawning season, predation, larval and adult behavior) factors affect dispersal, and consequently also population genetic structure, of species in marine environments (Derycke, Backeljau, & Moens, 2013; Hohenlohe, 2004). Sandy beaches are dynamic and physically stressful environments, principally driven by the forces of waves, tides and sediment movements (McLachlan, 1983; Rodil & Lastra, 2004; Short, 1999). These factors are important in shaping population structure, dynamics and connectivity of their inhabitants.
Free-living marine nematodes are the most abundant and species-rich metazoan fauna in sandy beaches and most other marine soft sediments, and are characterized by a wide variety of morphologies, life histories and feeding strategies (Giere, 2009; Heip, Vincx, & Vranken, 1985). Because most marine nematodes have endobenthic life styles, and because they lack planktonic or pelagic dispersal stages, marine nematodes are generally assumed to have limited dispersal capacity. However, they may be able to disperse passively through movement of sediments, currents, and ballast water of ships (Boeckner, Sharma, & Proctor, 2009; Palmer, 1988; Radziejewska, Gruszka, & Rokicka-Praxmajer, 2006). In addition, some nematode species may actively emerge into the water column and swim over short distances (Jensen, 1981; Schratzberger, Whomersley, Warr, Bolam, & Rees, 2004).
Many genera of marine nematodes have cosmopolitan or nearly cosmopolitan distributions. To some extent, this was also believed to hold at the species level (Bhadury et al., 2008), at least based on morphological criteria for species identification. More recently, however, considerable cryptic diversity has been uncovered in a number of coastal nematode species (Armenteros et al., 2014; Derycke et al., 2005, 2007; Derycke, Tandingan et al., 2010), raising the question as to whether alleged cosmopolitan morphospecies in fact represent multiple cryptic species with more restricted geographic ranges. For example, a population genetic study based on the mitochondrial cytochrome oxidase c subunit 1 (COI) locus of the nematode species complex Litoditis marina revealed that only one out of 10 cryptic species had a transatlantic distribution, whilst the remaining species had narrower geographic ranges (Derycke, Remerie et al., 2008). As L. marina usually lives in association with macroalgae and has been shown to raft on drifting algae, its chances of long-distance transport are expected to exceed those of most benthic marine nematodes (Derycke, Remerie et al., 2008).
Cryptic species diversity was also uncovered in the endobenthic nematode Terschellingia longicaudata based on nuclear 18S rDNA sequence data (Bhadury et al., 2008), with one species clade showing a very broad geographic distribution (including samples from Europe, the Atlantic coast of Mexico, and Malaysia). These results were not anticipated because Te. longicaudata typically frequents hypoxic or anoxic (and hence deeper) layers of sediment and would, therefore, not be expected to rapidly emerge from sediments and passively disperse over larger distances. As 18S rDNA sequences do not always show sufficient differentiation between closely related nematode species, the conclusion of Bhadury et al. (2008) should be confirmed using more variable marker genes. Nevertheless, the alleged cosmopolitan distribution of an endobenthic species with limited dispersal capacity is thought-provoking. Indeed, several recent studies on population genetic structure in coastal and estuarine nematodes have invariably highlighted a significant population genetic structure at local scales of 100 km and less in species that are considered more prone to passive dispersal (L. marina, Halomonhystera disjuncta and Thoracostoma trachygaster) because of their association with macroalgae (Derycke, Tandingan et al., 2010; Derycke et al., 2005, 2007, 2013). The vast majority of marine nematode species are, however, (endo)benthic and may, depending on their habitat and position in the sediment, be more or less prone to erosion and thus passive dispersal.
In this study, we investigated the population genetic structure of two abundant benthic nematode species from sandy beaches in the area of Bandar Abbas (Iran), Persian Gulf: Ptycholaimellus pandispiculatus Hopper, 1961, and Te. longicaudata de Man, 1907. Terschellingia longicaudata (Linhomoeidae) is common in inter-tidal and shallow subtidal sediments that are rich in organic matter and have sharp chemoclines (Heip et al., 1990; Schratzberger, Bolam, Whomersley, & Warr, 2006). Its peak abundances are typically reached below the top 2 cm of sediment, and the nematode can withstand hypoxia/anoxia (Steyaert et al., 2007). Carbon stable isotope ratios of Te. longicaudata from seagrass, mangrove and estuarine tidal flat sediments have demonstrated a clear trophic link with chemoautotrophic bacteria (Vafeiadou, Materatski, Adão, De Troch, & Moens, 2014). In contrast, species in the genus Ptycholaimellus (Chromadoridae) are epigrowth feeders that probably derive most of their nutrition from microalgae such as diatoms (Moens & Vincx, 1997). Members of this genus are particularly common in the surface layer (upper 1 cm) of inter-tidal sediments (Commito & Tita, 2002; Steyaert et al., 2003; Van Colen et al., 2009), where microphytobenthos tends to concentrate. Because of their nearly epibenthic life style, they can be expected to be more susceptible to resuspension and passive transport compared to the endobenthic Terschellingia (Commito & Tita, 2002).
Here, we used the mitochondrial COI gene to test the following hypotheses: (i) population genetic structure of both species will be limited on a scale of tens of kilometers because of the “homogeneity” of the study area, lack of clear dispersal barriers, and strong hydrodynamics of the beaches, which may lead to regular erosion and passive transport, thus facilitating gene flow; (ii) Ptycholaimellus, living at the surface of the sediment, will show less population genetic structure compared to the deeper-living Terschellingia. In addition, (iii) we compared 18S rDNA (18S) and COI gene sequences from Te. longicaudata populations from the Persian Gulf and from an estuarine tidal flat of the Scheldt Estuary, The Netherlands, to ascertain whether they truly belong to the same species or, alternatively, their morphology is hiding cryptic diversity. Phylogenetic affinities of Te. longicaudata from the Persian Gulf were assessed based on 18S phylogenetic analysis of published sequences sampled from a broad geographic range.
2 MATERIAL AND METHODS
2.1 Study area
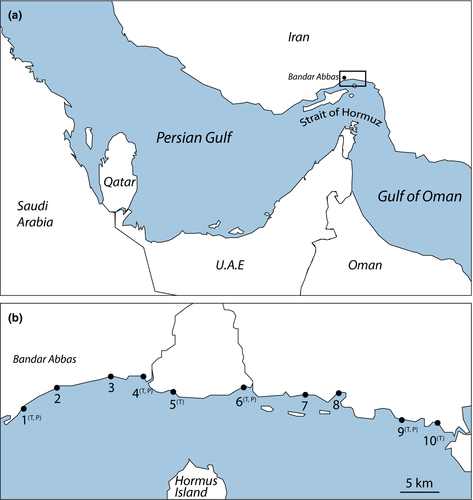
Samples were collected at low tide in September 2012 from the mid-tidal level at 10 beach locations along the Strait of Hormuz, Persian Gulf, spanning 52 km of Iranian coastline (Figure 1, Table 1). Stations 1–4 are located immediately in front of the city of Bandar Abbas and are subject to variable types and degrees of anthropogenic disturbance (sewage inputs, tourism etc..); they correspond to the beaches of Suro, Haghani, Dolat Park and Terminal, respectively (N. Sahraean, personal observations, December 2008). The other beaches are located eastward of Bandar Abbas and – at least at first glance – are less subject to local anthropogenic impacts.
| COI | haplotype | Loc 1 | Loc 4 | Loc 5 | Loc 6 | Loc 9 | Loc 10 | n |
|---|---|---|---|---|---|---|---|---|
| Terschellingia longicaudata | haplotype 1 | 1 | 0 | 1 | 0 | 1 | 2 | 5 |
| haplotype 2 | 16 | 15 | 18 | 14 | 16 | 17 | 96 | |
| haplotype 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Ptycholaimellus pandispiculatus | haplotype 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| haplotype 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| haplotype 4 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| haplotype 5 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| haplotype 6 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| haplotype 7 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| haplotype 8 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | |
| haplotype 9 | 2 | 7 | 0 | 7 | 3 | 0 | 19 | |
| haplotype 10 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| haplotype 11 | 8 | 6 | 0 | 10 | 7 | 0 | 31 | |
| haplotype 12 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| haplotype 13 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| haplotype 14 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| haplotype 15 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| haplotype 16 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| haplotype 17 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
- Numbers of the different haplotypes at six beach locations (Loc) in the Persian Gulf. Sufficient individuals for population genetic analysis (at least 15) of Te. longicaudata and P. pandispiculatus were obtained from six and four beaches, respectively. COI, mitochondrial cytochrome oxidase c subunit 1; n, number of individuals.
For Te. longicaudata, we also sampled the Paulina inter-tidal mudflat in the polyhaline reach of the Scheldt Estuary, The Netherlands, in July 2014. Details on the Paulina tidal flat are given in Gallucci, Steyaert, and Moens (2005); the sampling station corresponded to station H4 in Cnudde et al. (2015).
2.2 Sampling
From each sampling location, five samples were collected using 3.5-cm diameter PVC cores pushed into the sediment down to a depth of 5 cm. These samples were pooled into a single sample per location, preserved in DESS (Yoder et al., 2006). Nematodes were extracted from the sediments by vigorous washing of the samples with a jet of tap water, followed by decantation over a sieve with a mesh size of 38 μm. This procedure was repeated at least eight times, and the fraction collected on the sieve was subsequently centrifuged (ca 2000 g, 12 min) in the colloidal silicagel Ludox™ at a specific gravity of 1.18. Supernatant was decanted over a 38 μm sieve and collected in a small amount of DESS. This procedure was repeated three times and the three supernatants of a sample were pooled. Sufficient individuals of Te. longicaudata and P. pandispiculatus (at least 12 per species and sampling location) were obtained from six and four beaches, respectively (Figure 1). Fifty Te. longicaudata were collected from the Paulina mudflat.
2.3 Morphological identification and vouchering of specimens
Ptycholaimellus pandispiculatus and Te. longicaudata were handpicked from each sample using a fine needle under a stereomicroscope. They were rinsed three times with sterile distilled water to remove DESS. Each specimen was mounted individually on a temporary slide and identified using diagnostic morphological characters (Armenteros, Ruiz-Abierno, Vincx, & Decraemer, 2009) under a LEICA DM R research microscope at high magnification. Diagnostic features of P. pandispiculatus and Te. longicaudata specimens were photographed using LEICA Application software (Leica DL MB; objective 100×). Vouchered specimens were carefully removed from microscopic slides and then transferred individually into an Eppendorf tube containing 20 μl worm lysis buffer (WLB; 50 mM KCl, 10 mM Tris–HCl pH 8.3, 2.5 mM MgCl2, 0.45% Tergitol NP40, 0.45% Tween 20) and frozen (−20°C) until further processing.
2.4 Choice of marker sequences
The mitochondrial COI gene is one of the most widely used markers for species diversity assessment and population genetic analysis in animals, and for nematodes, it has proven useful for discriminating closely related species (Derycke, Vanaverbeke, Rigaux, Backeljau, & Moens, 2010). It has also been well documented that species delimitation based on single gene data often falls short due to gene tree–species tree incongruence, and that multiple markers increase the accuracy of species delimitation (Dupuis, Roe, & Sperling, 2012; Leliaert et al., 2014), hence the inclusion of a second marker, the 18S rRNA gene, in our analyses of the phylogenetic relationships of Te. longicaudata from the Persian Gulf. Although 18S is known to have lower resolution than COI when it comes to distinguishing recently diverged species, 18S has been found to be useful for assessing species diversity in the genus Terschellingia (Bhadury et al., 2008).
2.5 DNA extraction, PCR amplification and sequencing
DNA extraction followed the protocol by Williams, Schrank, Huynh, Shownkeen, and Waterston (1992). In short, proteinase K (1 μl, 10 g/ml) was added to each tube containing a single nematode in WLB, followed by incubation at 65°C for 1 hr, and denaturation of the proteinase K at 95°C for 10 min. Afterwards, tubes were centrifuged at 13,200 rpm for 1 min at 20°C and stored at 4°C.
The mitochondrial COI gene was amplified with primers LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HC02198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′; 621 bp) (Folmer, Black, Hoeh, Lutz, & Vrijenhoek, 1994) for Te. longicaudata, and primers JB3 (5′-TTTTTTGGGCCTGAGGTTTAT-3′) and JB5 (5′-AGCACCTAAACTTAAAACATAATGAAAATG-3′; 380 bp) (Bowles, Blair, & McManus, 1992) for P. pandispiculatus. PCR-mix was prepared for each primer set separately in total volumes of 25 μl for Te. longicaudata, containing 16.125 μl PCR-grade water, 2.5 μl buffer, 2.5 μl dye, 2 μl MgCl2, 0.5 μl deoxynucleotide triphosphates dNTP, (10 mM each), 0.125 μl of each primer (25 μM), 0.125 μl TopTaq polymerase (Qiagen, 5 U/μl) and 1 μl DNA. For P. pandispiculatus, essentially the same mix was prepared, but with 15.875 µl PCR-grade water, 0.250 µl of each primer (25 µM) and 2 µl DNA. The PCR conditions for Te. longicaudata were: initial denaturation of 1 min at 94°C, five cycles of (94°C for 40 s; 45°C for 40 s; 72°C for 45 s), 35 cycles of (94°C for 40 s; 51°C for 40 s; 72°C for 45 s) and a final extension of 5 min at 72°C. The PCR conditions for P. pandispiculatus were: initial denaturation of 5 min at 94°C, 35 cycles of (94°C for 30 s; 50°C for 30 s; 72°C for 45 s) and a final extension of 10 min at 72°C. Four μl of each PCR product was loaded onto 1% agarose gels (1% agarose gel in 0.5 × tris(hydroxymethyl)aminomethane acetate ethylene diamine tetraacetic acid buffer) with a negative control to check the quality and reliability of the PCR and the size of the amplified product. PCR products were sequenced at Macrogen Europe (Amsterdam, The Netherlands) with the forward primer using the fluorescent dye terminator Sanger sequencing method.
For Te. longicaudata, the 18S rRNA gene was amplified using two primers, MN18F (5′-CGCGAATRGCTCATTACAACAGC-3′) and Nem_18S_R (5′-GGGCGGTATCTGATCGCC-3′) (Bhadury et al., 2008). PCR-mix was prepared in total volumes of 14.875 μl PCR-grade water, 2.5 μl buffer, 2.5 μl dye, 2 μl MgCl2, 0.5 μl dNTP (10 mM each), 0.250 μl of each primer (25 μM; forward and reverse primer), 0.125 μl TopTaq polymerase (Qiagen, 5 U/μl) and 2 μl DNA. The final volume of the PCR-mix was 25 μl. The conditions for Te. longicaudata from the Persian Gulf were: initial denaturation of 5 min at 95°C, 40 cycles of (95°C for 1 min; 54°C for 1 min; 72°C for 2 min) and a final extension of 10 min at 72°C. PCR products were sequenced with forward and reverse primers.
2.6 Population genetic and phylogenetic analysis
Sequences were assembled using SEQMAN PROTM (Lasergene®, DNASTAR), and aligned using ClustalW in MEGA 6 (Thompson, Higgins, & Gibson, 1994). Analysis of molecular variance (AMOVA) (Excoffier, Laval, & Schneider, 2005) was performed in ARLEQUIN (version 3.1, 2005; Schneider, Kueffer, Roessli, & Excoffier, 1996) to test the distribution of genetic variability among and within populations. The significance of the variance components was tested by permuting haplotypes among populations (Excoffier, Smouse, & Quattro, 1992). A minimum spanning network was created using HAPSTAR 0.7, and was adapted to incorporate the frequencies of haplotypes using EXCEL and POWERPOINT. Uncorrected pairwise distances (p-distances) between haplotypes were calculated using MEGA 6.
Analysis of Te. longicaudata population structure and cryptic diversity on a broader geographic scale was based on COI and 18S rDNA sequence data sets. To explore COI sequence conservation in Te. longicaudata, amino acid sequences of the two Te. longicaudata haplotypes were aligned and compared with COI sequences of other species of monhysterids, and representative species of the major clades of nematodes. Alignments were analysed with GENEIOUS v. 7 (Biomatters, www.geneious.com).
The COI data set consisted of 101 sequences from the Persian Gulf and 50 sequences from the Scheldt Estuary, aligned using translation alignment in BIOEDIT (Hall, 1999). The 18S data set consisted of 128 sequences of Te. longicaudata, including 24 newly generated sequences from Iran, The Netherlands and Vietnam, and 104 sequences from GenBank from various locations (UK, France, The Netherlands, Bahrain, Mexico, Malaysia and Taiwan; Cook et al., 2005; Bhadury et al., 2006, 2008; Table S1). Eighteen sequences of Cyartonema elegans, Daptonema procerus, Daptonema setosum, Daptonema normandicum, Daptonema sp., Diplolaimelloides meyli, H. disjuncta, Metadesmolaimus sp., Monhystera sp., Sabatieria celtica, Sabatieria punctata, Sphaerolaimus hirsutus, Theristus acer, Theristus agilis and Theristus sp. were selected as the outgroup based on Meldal et al. (2007). The 18S sequences were aligned using MUSCLE (Edgar, 2004). Phylogenetic trees were estimated using maximum likelihood (ML) and rapid bootstrap analysis with RAxML under the GTRCAT model via the RAxML BlackBox web-server (http://embnet.vital-it.ch/raxml-bb/) with default settings (Stamatakis, Hoover, & Rougemont, 2008). The newly generated sequences of Terschellingia and Ptycholaimellus have been deposited in the European Nucleotide Archive (EMBL-EBI/ENA) with accession numbers LT795763-LT795772 and LT795773-LT795788, respectively.
3 RESULTS AND DISCUSSION
Recent molecular studies have indicated rampant cryptic species diversity in free-living marine nematodes, as well as different levels of genetic differentiation of populations at regional scales (reviewed in Derycke et al., 2013), comparable to the sampling scale of the present study (~52 km of coastline). Here, we investigated genetic diversity and population genetic structure of two common benthic nematode species from sandy beaches in the Persian Gulf: P. pandispiculatus and Te. longicaudata.
3.1 Population genetic structure of Ptycholaimellus pandispiculatus
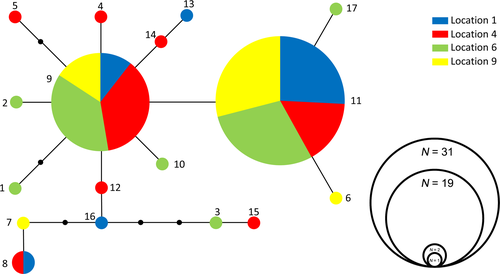
The DNA alignment of the mitochondrial COI gene fragment of the 66 individuals of P. pandispiculatus was 381 bp long and did not contain any insertions/deletions. Twenty-one sites (5.5%) were variable, six of which represented non-synonymous substitutions (i.e. resulting in amino acid changes). In total, 17 haplotypes were found (Figure 2). Uncorrected p-distances between haplotypes ranged between .003 and .018. Haplotypes 9 and 11 were considerably more frequent than the others, together comprising 75% of all sequences. They were also the only ones present at all four locations. Most other haplotypes occurred as singletons or doubletons (Figure 2). Location 4 had the highest amount of unique haplotypes (eight) and location 9 the least (four haplotypes). AMOVA did not reveal a significant population genetic structure for P. pandispiculatus (Fst = .013, p > .2; Table 2). The genetic diversity found for P. pandispiculatus is comparable to the genetic diversity found in other marine nematodes on similar geographic scales. For example, along the Belgian North Sea coast and Scheldt Estuary (~100 km), the number of COI haplotypes within single cryptic species of the H. disjuncta complex ranged from 4 to 17, with intra-specific pairwise distances of 0.003–0.026 (with one exception due to a single highly divergent haplotype; Derycke et al., 2007). A similar number of haplotypes (15) was found within the L. marina cryptic species “PmI” along the Belgian coast, Western Scheldt and Eastern Scheldt (Derycke et al., 2005), while a higher haplotype diversity (33) was found in Th. trachygaster clade II, but this study covered a larger geographic scale (>500 km; Derycke, Tandingan et al., 2010).
| Species | % variance | F st | p-value |
|---|---|---|---|
| Ptycholaimellus pandispiculatus | |||
| Among populations | 1.30 | .013 | n.s. |
| Within populations | 98.70 | ||
| Terschellingia longicaudata | |||
| Among populations | 2.76 | .027 | n.s. |
| Within populations | 97.24 | ||
- n.s., not significant, p > .1.
We did not find indications of population genetic structuring among the four sampled locations in the Strait of Hormuz, which had inter-distances of 14–52 km (Figure 1). This indicates substantial dispersal and gene flow in our study area. Our findings contrast with some recent studies on free-living marine nematodes, where subtle to strong genetic differentiation of nematode populations was observed on similar geographic scales (Derycke et al., 2013). Similarly, low (but still significant) population genetic differentiation was observed in the Scheldt Estuary for Bathylaimus assimilis, which is characterized by an endobenthic life style (Derycke et al., 2013). Passive dispersal in endobenthic species is expected to be lower and population genetic structuring higher than in species associated with exposed and transient habitats such as seaweed wrack (L. marina, H. disjuncta and Th. trachygaster; Derycke, Tandingan et al., 2010; Derycke et al., 2005, 2007). Rather than by its endobenthic life style, the absence of genetic structuring in P. pandispiculatus may be explained by passive dispersal of individuals through sediment resuspension and currents in the dynamic beach habitat (Boeckner et al., 2009; Derycke et al., 2013). Our study area represents a relatively non-interrupted environment of sandy beaches along a continuous stretch of coastline that lacks obvious dispersal barriers, and is characterized by a dominant eastward sea surface current (Kämpf & Sadrinasab, 2006; Reynolds, 1993). This contrasts with the study areas of some previous studies that included apparent differences in habitats such as coastal and estuarine environments (Derycke et al., 2005, 2007). Only one prior study on nematode population genetic structuring has entirely focused on a beach habitat: Th. trachygaster, a species associated with macroalgae, exhibited strong population genetic differentiation along the Southern Californian coast, but this coincided with well-known biogeographic barriers, such as Point Conception and the Los Angeles Region. In between these barriers, population genetic structuring was absent along large stretches of coastline (~180 km; Derycke et al., 2013; Derycke, Tandingan et al., 2010). Hydrodynamic forces of beaches result in erosion and resuspension of sediments and enhance passive dispersal of endobenthic organisms (Derycke et al., 2013; Galindo et al., 2010; Gingold, Ibarra-Obando, & Rocha-Olivares, 2011; Palmer, 1988; White et al., 2010). This is even more plausible for Ptycholaimellus species which, are known as epigrowth feeders that live near the sediment surface, and are also frequently found in resuspended sediment directly above the sediment surface (Commito & Tita, 2002; Eskin & Palmer, 1985).
Finally, active movement related to body morphology, swimming behavior and feeding strategy may be an important dispersal mechanism for some free-living nematodes (Thomas & Lana, 2011), for instance because nematodes that actively enter the water column have a higher probability of becoming passively transported over larger distances. Ptycholaimellus belongs to a nematode family (Chromadoridae) and feeding guild for which such active emergence into the water column has been observed (Jensen, 1981).
3.2 Population genetic structure and cryptic diversity of Terschellingia longicaudata
The COI alignment of the 101 individuals of Te. longicaudata from Iran was 621 bp long and did not contain any gaps. These 101 individuals belonged to only two haplotypes, with 96 specimens (95.4%) belonging to haplotype 1, and five specimens (4.95%) to haplotype 2 (Figure 3a). The two haplotypes differed from each other in 36 nucleotides, 10 of which represented non-synonymous substitutions. The uncorrected p-distance between the two haplotypes was .057. Haplotype 1 was found at all six locations, while haplotype 2 was found in four locations: 1, 5, 9 and 10. The sequences from the 50 individuals from The Netherlands were all identical to haplotype 1. Clearly, AMOVA did not reveal any genetic differentiation among the Persian Gulf populations (Fst = .027, p > .9; Table 2). The presence of the common COI haplotype in all sampled locations of the Persian Gulf suggests high gene flow, which is somewhat surprising given the fact that Te. longicaudata lives endobenthically, with peak abundances typically below the top 2 cm of sediment. This would suggest limited resuspension in the water column and thus a low passive dispersal potential. However, beach hydrodynamics may regularly erode and resuspend sediments down to considerable depths (Bell & Sherman, 1980).
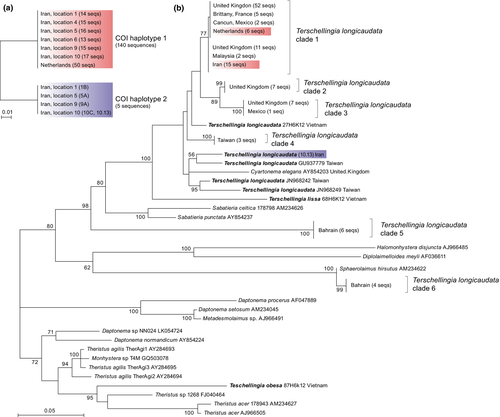
The 18S rDNA alignment of Te. longicaudata (including 12 outgroup taxa) was 951 bp long, including 465 (48.9%) variable sites. The ML phylogenetic analysis was congruent with the published 18S phylogeny in Bhadury et al. (2008), and showed that the genus Terschellingia, as well as the species Te. longicaudata (as traditionally circumscribed based on morphology) were non-monophyletic. At least six distinct Te. longicaudata clades were recovered, in addition to five singleton sequences (Figure 3a). One of these clades (clade 1 in Figure 3a) included sequences from distant localities (Europe, Iran, Mexico and Malaysia). All individuals with the dominant COI haplotype from The Netherlands and the Persian Gulf also fall within that clade, which is consistent with the COI results, which showed that the individuals from The Netherlands and the Persian Gulf belong to the same COI haplotype (haplotype 1). We were only able to obtain one partial 18S sequence from a Persian Gulf individual with the rare COI haplotype 2 (specimen 10.13). This sequence of 342 bp long differed from the common 18S sequences in 11 positions, corresponding with a p-distance of .033, and formed a different branch in the 18S tree that was more closely related to a Te. longicaudata sequence from Taiwan (although strong bootstrap support was lacking; Figure 3b). The presence of two distinct clades in the Persian Gulf that were concordantly recovered by the COI and 18S data suggests the presence of two different species in the Persian Gulf. Although there are no universal threshold values of genetic distances for distinguishing species, the large genetic distances between the two COI haplotypes (p-distance .057) and between the two 18S ribotypes (p-distance .033) hint at the presence of two species in the Persian Gulf. The intra-specific COI variation reported for some nematode species by Derycke et al. (2005), Derycke, Vanaverbeke, et al., 2010, Derycke, Tandingan et al., 2010, and the threshold of 5% COI sequence divergence (Armenteros et al., 2014) supports our claim of two cryptic species of Te. longicaudata. The genealogical concordance of the two unlinked loci provides further evidence for the existence of two distinct species (Derycke et al., 2007; Leliaert et al., 2014).
Our data confirm the presence of one widespread cryptic species of Te. longicaudata (18S clade 1), which has been collected from distant locations, including Europe, Iran, Mexico and Malaysia. The remaining cryptic species of Te. longicaudata seem to have a narrower geographic range, based on the data available. Several other cryptic species of free-living marine nematodes have been shown to have wide geographic ranges based on molecular data (Bik, Lambshead, Thomas, & Lunt, 2010; Derycke, Remerie, et al., 2008).
Admittedly, in the absence of a thorough morphological analysis of specimens of the different putative species of Te. longicaudata, we cannot prove that they are cryptic species in the true sense of the word, meaning that they cannot be differentiated morphologically and/or morphometrically. In other marine morphospecies complexes, the discovery of substantial genetic divergence went ahead of the discovery of morphological differentiation. Using a reverse taxonomic approach, “cryptic” species of the L. marina and H. disjuncta complexes could be differentiated based on a combination of morphometric measurements (Derycke, Fonseca et al., 2008; Fonseca, Derycke, & Moens, 2008), whereas unique characteristics differentiated species within the Th. trachygaster complex (De Oliveira et al., 2012). Given the paucity of diagnostic characters and the high morphological plasticity of most species of Terschellingia (Armenteros et al., 2009), an integrative approach to the taxonomy of this genus, combining multilocus molecular and dedicated morphological tools, is warranted.
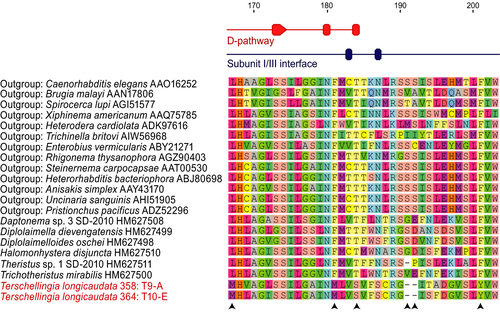
In addition to the wide geographic range of a single COI haplotype, the lack of intra-specific genetic diversity in the COI gene could be indicative of a selective sweep, in which variation of the COI gene has been eliminated due to strong selective pressure on the COI gene itself, or due to strong natural selection on another genomic region, which eventually resulted in low genetic variation of the COI gene by genetic hitchhiking (Barton, 2000). Selective pressure of the COI gene in Te. longicaudata, a nematode that shows strong preferences for hypoxic environments, is a plausible scenario. This gene encodes the subunit 1 of the cytochrome c oxidase complex (a.k.a. respiratory complex IV), and is a key enzyme in aerobic metabolism. It is also the largest and most conserved subunit of cytochrome c oxidase (Michel, Behr, Harrenga, & Kannt, 1998). Subunit I contains two haem centers (haem a, which acts as an electron input device to the haem a3, and haem a3, which is part of a binuclear center and is the site of oxygen reduction), in addition to two proton-conducting pathways (D- and K-pathways), and an electron transfer pathway (Dürr et al., 2008). The amino acid composition around these active sites may determine the affinity with oxygen, which may be more crucial for an organism living in hypoxic environments.
In order to find clues for possible positive selection of the COI gene in Te. longicaudata, we constructed a COI amino acid alignment of the two Te. longicaudata haplotypes, 11 other species of monhysterid nematodes, and 13 outgroup species representing some of the main nematode lineages (Figure 4, Table S1). A region surrounding the end of the D-pathway (position 167–203) includes four amino acid changes and two deletions that seem to be unique in Te. longicaudata. We did not find evidence for divergent amino acid composition of Te. longicaudata in the two haem centers (Table S1). It should be noted that taxon sampling in our alignment is low and that more extensive sampling, especially in the monhysterids, is needed to confirm these observations in order to find further indications for possible selection of COI linked to hypoxic environments.
4 CONCLUSIONS
Our data indicate the absence of genetic structure of both endobenthic nematode species (Te. longicaudata and P. pandispiculatus), which probably reflects substantial passive dispersal and gene flow in our study area in the Persian Gulf. As a result, both populations appear to be genetically homogenous. As such, our first hypothesis (limited population genetic structure for both species) was confirmed, whereas our second hypothesis (less population genetic structure in P. pandispiculatus, living at the surface of the sediment, compared to the deeper-living species Te. longicaudata) was rejected. Genetic diversity in Te. longicaudata was very low with only two COI haplotypes recovered (one dominant and one rare). The COI data, combined with 18S rDNA sequences, also confirmed previous studies that Te. longicaudata likely constitutes a complex of multiple cryptic species, with one of these species having a wide geographic distribution.
ACKNOWLEDGEMENTS
We acknowledge the research council of Ghent University for funding of this work through GOA project 01G01911. Bart Braeckman and Sofie Derycke provided useful suggestions for the discussion of this paper, while Tania Nara Bezerra and Jan Vanaverbeke introduced the first author to the identification of marine nematodes.




