Interactions of bacteria with diatoms: influence on natural marine biofilms
Abstract
Interactions between microfouling components influence the biofilm community and the cascading events, thus playing an important role in the biofouling process. Bacteria and diatoms are among the dominant forms reported in biofilms. Experiments were carried out with natural marine biofilms from a tropical monsoon-influenced environment to evaluate the interactions between bacteria and diatoms through application of antibiotics (streptomycin and chloramphenicol). Overall, chloramphenicol inhibited diatom communities, whereas streptomycin did not. These antibiotic-mediated changes in the fouling diatom community were consistent across the seasons. However, the rates at which the fouling communities changed depended on the initial species composition. It was also observed that elevated nutrient concentrations overrode the inhibitory effect of chloramphenicol. Maximum Vibrio enhancement was observed in the enriched conditions during the pre-monsoon and unenriched conditions during the monsoon (with naturally elevated background nutrient concentrations), highlighting the relevance of nutrient concentrations for Vibrio spp. This has interesting implications for antibiotic-mediated interactions between fouling diatom and bacterial communities under differing nutrient regimes. Although this study indicates the relevance of ‘cross-talk’ at the microfouling level, understanding the effects of additional microbial products (e.g. bacteriocins and peptidoglycan) on these community-level interactions will represent a step forward.
Introduction
Biofilm development on an immersed substrate starts with the formation of an organic ‘conditioning layer’ (Loeb & Neihof 1975), which modulates attachment and colonization of the substrate by bacteria and fouling diatoms. The sequence of establishment may vary. Although bacteria are considered to be the first colonizers on a virgin surface (Molino & Wetherbee 2008), followed by diatoms, this is not always the case. Diatoms can also attach to surfaces that are free of bacteria (Cooksey 1981).
Irrespective of the sequence, colonization of the substrate by diatoms represents an important step in the biofouling process. Fouling diatoms follow the ‘infection’ model of biofilm formation (Molino et al. 2009) which is independent of the initial diatom concentration; under favourable conditions, a single diatom cell is sufficient to initiate a successful biofilm (Molino et al. 2009). This process, termed ‘microfouling’, is usually associated with a marked increase in the biofilm biomass (Mitbavkar & Anil 2007). The ‘microfoulers’ (diatoms and bacteria) play a very important role in influencing the progression of the biofilm and are closely associated (Cole 1982). They provide signals for the settlement and metamorphosis of various macrofouling organisms ranging from algae, ascidians, barnacles, bryozoans, hydroids, oysters and polychaetes (Kirchman & Mitchell 1983; Weiner et al. 1989; Szewzyk et al. 1991; Leitz & Wagner 1993; Khandeparker et al. 2002, 2005, 2006; Lam et al. 2003; Qian et al. 2007; Huggett et al. 2009). Given their relevance, it is intriguing that very few reports (Murray et al. 1986; Harder et al. 2002; Wigglesworth-Cooksey & Cooksey 2005; Dobretsov & Thomason 2011) have addressed the relationship between diatoms and bacteria in biofilms. Molino & Wetherbee (2008) pointed out the lack of sufficient research on these aspects. Recently, Landoulsi et al. (2011) also opined that future studies need to focus on the relevance of bacteria for diatom adhesion in biofilms.
The present study analyses the interactions between diatoms and bacteria in biofilms at the community level, across three seasons. Fouling diatom biofilms were developed under natural conditions and their subsequent progression followed under controlled settings, in the presence/absence of antibiotics. Antibiotics are one class among the many metabolites produced by bacteria that helps in competition and signalling processes. The changes in fouling diatom communities when treated with antibiotics indicate the relevance of bacteria in influencing the biofilm. Streptomycin and chloramphenicol (produced naturally by actinomycetales and Streptomyces venezuelae, respectively) were used in these experiments. Both antibiotics inhibit protein synthesis in bacteria, by binding to the 30S ribosomal subunit (streptomycin) and 50S ribosomal subunit (chloramphenicol) (Josephson et al. 2000). Streptomycin may affect diatoms directly by inhibiting protein synthesis in diatom organelles (70S) (Stanier et al. 1971). However, the chances of this are slim because the majority of organelle proteins are encoded by nuclear genes due to transfer of most organelle genes to the nucleus during evolution (Keeling & Palmer 2008). Chloramphenicol, the other antibiotic used, inhibits photosystem II in diatoms (Ohad et al. 1984).
Changes in Vibrio dynamics were also noted, as some of them are important pathogens for fish (Vibrio alginolyticus, Vasconcelos et al. 1975) and humans (Vibrio cholerae and Vibrio parahaemolyticus, Rehnstam-Holm et al. 2010) and are closely linked with diatoms (Rehnstam-Holm et al. 2010; Asplund et al. 2011; and references therein). In fact, biofilms are considered a reservoir and source of dissemination for V. cholerae (Shikuma & Hadfield 2010). Nutrient concentrations in the surrounding waters also affect the progression of the biofilm community (Qian et al. 2007). Taking this into consideration, the questions addressed were:
- How do streptomycin and chloramphenicol affect biofilm diatom communities?
- Do these effects vary temporally?
- What are the accompanying changes in bacteria (mainly Vibrio spp.)?
- What is the influence of nutrient enrichment on these interactions?
Material and Methods
Development of natural biofilms
Natural diatom biofilms were developed by immersing polystyrene slides in Dona Paula Bay (15°27.5′ N, 73°48′ E), Goa, on the west coast of India. Earlier studies on fouling diatom assemblages in the study area (Patil & Anil 2005a; Mitbavkar & Anil 2007) indicated considerable seasonal variation across monsoon. Therefore, this experiment was conducted prior to the monsoon (June 2009; pre-monsoon), September 2009 (during monsoon) and October 2009 (during post-monsoon).
Fouling films in the study area tend to be dominated by microorganisms up to day 4, after which macroorganisms such as barnacles and polychaetes start proliferating. Based on this, the natural biofilms used in these experiments were developed in situ for only 2 days.
A total of 100 polystyrene slides (7.6 × 2.5 cm), hydrophobic in nature, were fitted into wooden slideholders, each having a carrying capacity of 20 slides, and suspended in seawater, approximately 1 m below the low tide level. The slideholders were suspended randomly along a pier, taking care to prevent entanglement with one another. After 2 days, the slideholders were retrieved, transferred carefully to a bucket containing seawater from the study area, and transported to the laboratory.
Laboratory incubation of biofilms
In the laboratory, the slides were carefully removed from the slideholders, taking care not to dislodge the developed biofilms. The slides were suspended in autoclaved aged seawater (unenriched and enriched) with and without antibiotics. Streptomycin and chloramphenicol were used at 0.1 mg·ml−1 and 0.02 mg·ml−6, respectively, concentrations in the range used to render diatom monocultures near axenic (Patil & Anil 2005c). The following diluents were used: aged seawater (ASW; unenriched control), ASW + streptomycin (ASW + S), ASW + chloramphenicol (ASW + C), f/2 medium (Guillard & Ryther 1962) prepared in ASW [f/2(ASW); enriched control], f/2(ASW) + streptomycin [f/2(ASW) + S] and f/2(ASW) + chloramphenicol [f/2(ASW) + C]. The ASW was filtered through GF/C (1.2 μm) filters and autoclaved before addition of nutrients and antibiotics. Aliquots of 500 ml of each diluent were poured into transparent plastic bags, and a single slide (with attached biofilm) was suspended in each bag. Nine slides were used for each diluent.
The experimental units (plastic bags) were incubated at room temperature (RT, approximately 27 ± 1 °C) under a 12 h :12 h light/dark cycle for 15 days. Every 5 days (days 5, 10 and 15), three experimental units of each diluent were sampled.
Diatom analysis
One slide from each treatment was analysed for diatoms. The slides were placed in petri dishes containing the respective diluents. The glass slides were rested horizontally on two glass rods placed at the ends. This arrangement ensured that the lower surface of the slide was not in contact with the petri dish. Diatoms in 10 randomly selected fields (area = 0.38 dm2) on each side of the slide were analysed through microscopy (100 × magnification). Diatoms were identified based on standard identification keys (Subrahmanyan 1946; Desikachary & Prema 1987; Desikachary et al. 1987; Tomas 1997; Horner 2002). Diatom abundance on both sides of the slide was calculated separately; the average values are presented as diatoms dm−2.
Bacterial analysis
Two slides from each treatment were used for bacterial analysis, one slide for total bacterial count (TBC) and the other for Vibrio analysis. The biofilm material was scraped off the slides using a nylon brush (Patil & Anil 2005b). For TBC, samples were fixed with formaldehyde (final concentration 1–2% v/v). Bacteria were quantified using acridine orange and epifluorescence microscopy (Daley & Hobbie 1975). These values are expressed as cells dm−2.
Vibrio species were quantified by spread plating diluted samples on thiosulphate-citrate-bile salts (TCBS) agar (Hi-Media, HiMedia Laboratories Pvt. Ltd., Mumbai, India), which differentiated V. cholerae, Vibrio alginolyticus and V. parahaemolyticus. The plates were incubated at 37 °C for 24 h before colonies were enumerated. As V. cholerae are known to persist in the VBNC state (Roszak & Colwell 1987; viable but non-culturable), we assumed that the bacteria grown on TCBS agar were a representative subset of the biofilm bacteria on the polystyrene slides. To minimize the uncertainties regarding the analysis of Vibrio species using TCBS agar, Vibrio colonies were randomly picked from the selective TCBS agar and purified. Subsequently, their identity was confirmed using a series of appropriate biochemical tests (Gram-staining, KOH string test, oxidase, catalase, motility, indole, gas from glucose, Methyl Red, Voges Proskaeur and citrate utilization). The Vibrio abundance thus quantified was expressed as CFU dm−2.
Chlorophyll a and nutrient analysis
Chlorophyll a content in biofilms and the medium in which the slides were suspended was analysed. The biofilm slide used for diatom analysis was used for chlorophyll analysis (single slide per treatment). The biofilm material was scraped off the slides, dispensed into autoclaved, 1.2-μm-filtered seawater, mixed well and filtered through GF/F filters (0.75 μm). Appropriate aliquots of the medium were filtered likewise. Chlorophyll was extracted by crushing the filters in 90% acetone followed by fluorometric estimation using standard procedures (Parsons et al. 1984). Concentrations of nutrients-nitrate (NO3-N), nitrite (NO2-N), ammonia (NH4-N), phosphate (PO4-P) and silicate (Si) in the medium were analysed using standard spectrophotometric procedures (Parsons et al. 1984).
Data analyses
Margalef's species richness (d), Pielou's evenness (J’) and Shannon–Wiener's diversity (H’) of the fouling diatom community were calculated, using PRIMER software version 5 (PRIMER-E Limited, Plymouth, UK). These values along with diatom abundance, TBC, V. alginolyticus, V. cholerae and V. parahaemolyticus were analysed statistically for variation across seasons and treatments. For seasonal variation, the ‘initial’ 2-day, naturally developed biofilm data was considered. For variation across treatments, day 5 values were considered separately for unenriched and enriched treatments. In the enriched treatments, >450 diatoms per viewing field (under 100× magnification) progressing to growth in multiple layers was observed. This type of growth was recorded qualitatively as matt diatom growth. These enriched treatments were not analysed statistically. Statistical analyses, wherever used, was preceded by checking for normality and homogeneity of variances using Shapiro's and Levene's tests, respectively. The datasets that fulfilled the assumptions for parametric analysis were analysed through one-way ANOVA; the non-normal datasets were analysed using Kruskal–Wallis tests. All these analyses were carried out using the STATISTICA 6 program (Stat Soft Inc., Tulsa, OK, USA).
The fouling diatom abundance in all the treatments was fourth root-transformed and subjected to clustering and ordination analysis using Bray–Curtis similarity coefficients (PRIMER software version 5). Prior to this analysis, matt diatom growth of observed species was assigned values 10 times higher than the maximum observed for the respective species. These ordinations were plotted separately for each season.
To assess the rate at which diatom communities changed in response to streptomycin and chloramphenicol, the species/percentage turnover (T) or change in species composition was calculated as T = (G + L)/(S1 + S2) × 100, where G and L are the number of taxa gained and lost between sampling events, and S1 and S2 are the number of taxa present in successive sampling events. In the formula by Diamond & May (1977) ‘sampling events’ refers to months, whereas in the present study, sampling events refer to a 15-day period.
Pearson's correlation was carried out between diatom abundance, species richness, evenness and diversity (y-axis) and total bacteria (TBC), V. alginolyticus, V. cholerae and V. parahaemolyticus (x-axis) only for the unenriched conditions (day 5 values, all sampling periods). The non-parametric datasets were transformed to achieve normality. Diatom abundance data was fifth root-transformed, whereas the TBC and Vibrio datasets were log-transformed. The r- and P-values are depicted. This analysis was done using STATISTICA 6 program.
Results
Seasonal variations in the 2-day-old naturally developed biofilms
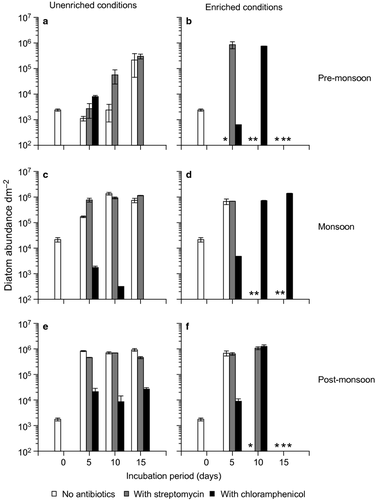
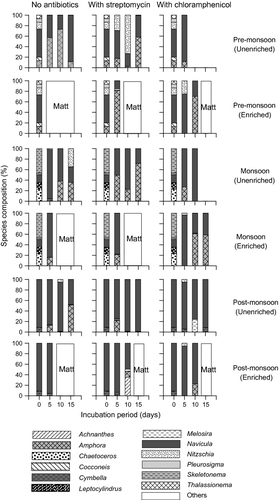
The ‘initial’ fouling diatom abundance was highest during monsoon followed by pre- and post-monsoon (Fig. 1). These variations were not statistically significant (KW test, n.s.). The monsoon period had the lowest species evenness (Fig. S1) and was dominated by Skeletonema and Chaetoceros (centric diatoms) (Fig. 2). The pre-monsoon period had the highest species richness and diversity whereas the opposite trend was observed during post-monsoon (Fig. S1). Pre-monsoon and post-monsoon periods were dominated by Navicula (pennate diatom) (Fig. 2).
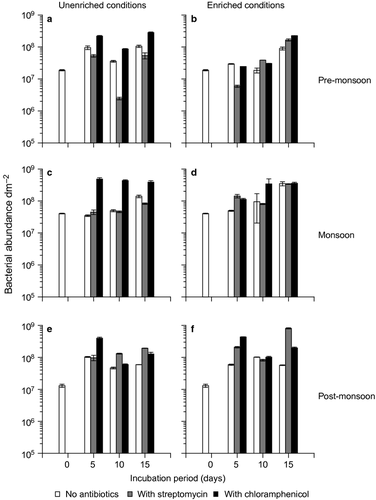
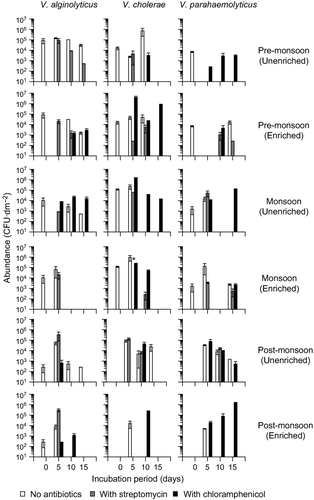
Bacterial abundance showed a similar trend as diatoms, i.e. it was the highest in monsoon followed by pre- and post-monsoon (Fig. 3). However, unlike diatoms, temporal variation in the bacterial abundance was statistically significant (one-way ANOVA, P = 0.0003). Among the Vibrio species, only V. cholerae showed a similar temporal trend as diatoms and bacteria (Fig. 4). Vibrio alginolyticus and V. parahaemolyticus were maximum in pre-monsoon followed by monsoon and post-monsoon (Fig. 4). These temporal variations in Vibrio spp., however, were not statistically significant (KW test, n.s.).
The chl a concentration in the 2-day-old naturally developed biofilms was highest in monsoon (probably a reflection of the high diatom abundance) followed by post- and pre-monsoon (Fig. S2).
Laboratory incubation of the retrieved slides under unenriched and nutrient-enriched conditions
The progression of the 2-day naturally developed biofilm community differed depending on incubation in unenriched and nutrient-enriched conditions (Tables 1-3). Incubation under unenriched conditions led to diatom abundance up to 106 dm−2 (Fig. 1). In the enriched treatments, matt diatom growth was observed on day 5 (pre-monsoon) and day 10 (monsoon and post-monsoon) (Fig. 1). The effect of nutrient enrichment was also apparent in the species turnover, especially for chloramphenicol; 33–43% (unenriched conditions) and 56–100% (enriched conditions) (Fig. 5). Such differences between unenriched and enriched treatments were not observed in species richness, evenness or diversity of the fouling diatom communities (Fig. S1). Matt diatom growth was not associated with marked changes in the total bacterial abundance (Fig. 3) or the abundance of V. alginolyticus, V. cholerae and V. parahaemolyticus (Fig. 4). However, the effect of enrichment was observed in the chlorophyll a concentration of the biofilms and the media (Fig. S2). Chlorophyll a concentration of the biofilms in enriched treatments was higher than in the unenriched treatments, especially on days 10 and 15. This trend was also observed in the media profiles on day 15 in most instances (Fig. S2). Matt diatom growth was associated with a sharp decrease in silicate and nitrate concentrations in most cases (Fig. S3), reflecting utilization by the growing fouling diatom community.
| Diatoms | Day 0 | Days 5–15 | |||||
|---|---|---|---|---|---|---|---|
| ASW | ASW + S | ASW + C | f/2(ASW) | f/2(ASW) + S | f/2(ASW) + C | ||
| Achnanthes subsessilis Kutzing | + | + | |||||
| Amphora coffeaeformis (Agardh) Kutzing | + | + | + | + | + | + | + |
| Cocconeis scutellum Ehrenberg | + | + | + | ||||
| Cylindrotheca closterium (Ehrenberg) Lewin & Reimann | + | + | |||||
| Fragilaria sp. | + | + | |||||
| Grammatophora sp. | + | ||||||
| Melosira nummuloides C.A. Agardh | + | ||||||
| Melosira sp. | + | ||||||
| Navicula crucicula (Wm. Smith) Donkin | + | + | |||||
| Navicula directa (W. Smith) Ralfs in Pritchard | + | + | + | + | + | + | |
| Navicula subinflata Grunow | + | ||||||
| Navicula transitans var. derasa f. delicatula Heimdal | + | + | + | + | + | + | + |
| Navicula vanhoffennii Gran | + | + | |||||
| Navicula sp. | + | + | |||||
| Nitzschia panduriformis Gregory | + | ||||||
| Nitzschia seriata Cleve | + | + | + | + | + | + | |
| Nitzschia sp. | + | ||||||
| Pleurosigma directum Grunow in Cleve & Grunow | + | ||||||
| Thalassionema bacillare (Heiden in Heiden & Kolbe) Kolbe | + | ||||||
| Diatoms | Day 0 | Days 5–15 | |||||
|---|---|---|---|---|---|---|---|
| ASW | ASW + S | ASW + C | f/2(ASW) | f/2(ASW) + S | f/2(ASW) + C | ||
| Achnanthes subsessilis Kutzing | + | + | + | + | |||
| Amphora coffeaeformis (Agardh) Kutzing | + | + | + | + | + | + | |
| Amphora rostrata (Wm. Smith) | + | ||||||
| Chaetoceros curvisetus Ehrenberg | + | ||||||
| Cocconeis scutellum Ehrenberg | + | ||||||
| Coscinodiscus sp. | + | ||||||
| Cymbella sp. | + | + | |||||
| Grammatophora marina Kutzing | + | ||||||
| Leptocylindrus danicus Cleve | + | ||||||
| Melosira nummuloides C.A. Agardh | + | + | + | ||||
| Navicula crucicula (Wm. Smith) Donkin | + | + | + | ||||
| Navicula granii (Jorgensen) Gran | + | ||||||
| Navicula subinflata Grunow | + | + | |||||
| Navicula transitans var. derasa (Grunow, in Cleve and Grunow) Cleve | + | + | + | ||||
| Navicula transitans var. derasa f. delicatula Heimdal | + | + | + | + | + | + | + |
| Navicula sp. | + | + | + | + | |||
| Nitzschia longissima (Brebisson, in Kutzing) Ralfs in Prichard | + | ||||||
| Nitzschia seriata Cleve | + | ||||||
| Nitzschia sigma (Kutzing) Wm. Smith | + | ||||||
| Odontella aurita (Lyngbye) C.A. Agardh | + | ||||||
| Pleurosigma directum Grunow in Cleve & Grunow | + | ||||||
| Skeletonema costatum (Greville) Cleve | + | ||||||
| Diatoms | Day 0 | Days 5–15 | |||||
|---|---|---|---|---|---|---|---|
| ASW | ASW + S | ASW + C | f/2(ASW) | f/2(ASW) + S | f/2(ASW) + C | ||
| Achnanthes longipes (Agardh) | + | + | + | ||||
| Achnanthes subsessilis Kutzing | + | + | + | + | |||
| Amphora coffeaeformis (Agardh) Kutzing | + | + | + | + | + | ||
| Cocconeis scutellum Ehrenberg | + | + | + | + | |||
| Cylindrotheca closterium (Ehrenberg) Lewin & Reimann | + | ||||||
| Cymbella sp. | + | + | + | + | + | + | |
| Grammatophora marina Kutzing | + | ||||||
| Melosira nummuloides C.A. Agardh | + | + | + | ||||
| Navicula crucicula (Wm. Smith) Donkin | + | ||||||
| Navicula subinflata Grunow | + | + | + | + | |||
| Navicula transitans var. derasa f. delicatula Heimdal | + | + | + | + | + | + | + |
| Navicula vanhoffennii Gran | + | ||||||
| Navicula sp. | + | ||||||
| Nitzschia longissima (Brebisson, in Kutzing) Ralfs in Prichard | + | ||||||
| Nitzschia panduriformis Gregory | + | ||||||
| Nitzschia seriata Cleve | + | + | + | + | |||
| Nitzschia sigma (Kutzing) Wm. Smith | + | + | |||||
| Nitzschia sp. | + | ||||||
| Pleurosigma directum Grunow in Cleve & Grunow | + | ||||||
| Thalassionema bacillare (Heiden in Heiden & Kolbe) Kolbe | + | ||||||
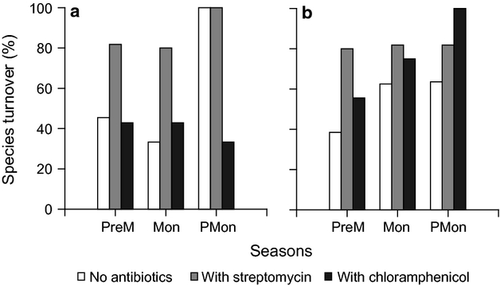
Effect of streptomycin and chloramphenicol on fouling diatom communities
Pre-monsoon
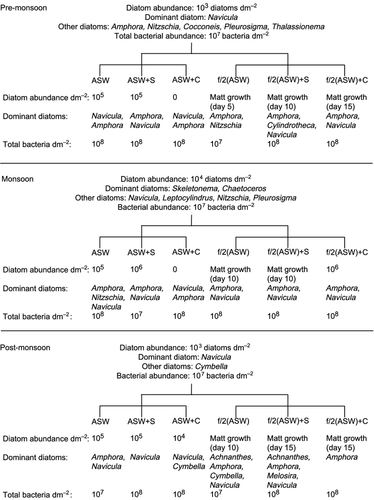
In the 2-day-old biofilm, 2.4 × 103 diatoms dm−2 were observed (Fig. 1a,b). Navicula was the dominant diatom (Fig. 2). A total of 19 species belonging to 11 genera were recorded (Table 1). Under unenriched conditions, diatom abundance increased over the incubation period in control (ASW without antibiotics) (Fig. 1a). A similar but higher increase was observed in the unenriched streptomycin treatment (Fig. 1a). A switch in dominance patterns was observed between Navicula and Amphora in ASW and between Navicula (day 5), Nitzschia (day 10) and Amphora (day 15) in ASW + S (Fig. 2). Chloramphenicol suppressed diatoms; a maximum value of 103 diatoms dm−2 was observed on day 5, followed by no growth on days 10 and 15 (Fig. 1a). Navicula and Amphora were the only two genera recorded (Fig. 2). The variation in diatom abundance in control and antibiotic treatments was statistically significant (one-way ANOVA, P = 0.0151).
Incubation under nutrient-enriched conditions led to matt diatom growth not only in control and streptomycin treatments but also in chloramphenicol (Fig. 1b). However, the extent of growth differed. Matt growth commenced earlier in control (day 5), followed by streptomycin (day 10) and chloramphenicol (day 15) (Fig. 1b). Characteristic variations in the dominant diatoms (Amphora and Navicula) were observed in streptomycin and chloramphenicol treatments (Fig. 2).
Monsoon
An average of 2.1 × 104 diatoms dm−2 were recorded in the 2-day-old biofilm (Fig. 1c,d). Skeletonema spp. and Chaetoceros spp. dominated the community (Fig. 2). In all, 22 species belonging to 14 genera were observed (Table 2). In unenriched control and streptomycin treatments, an increase in the diatom abundance (up to 105 and 106 diatoms dm−2, respectively) was observed (Fig. 1c). Chloramphenicol inhibited diatoms, with no growth on day 15 (Fig. 1c). These variations in abundance were statistically significant (one-way ANOVA, P = 0.0042) and, overall, were similar to those observed in the unenriched treatments in pre-monsoon. During monsoon, Navicula dominated up to day 10 in most unenriched treatments (Fig. 2). On day 15, Nitzschia and Amphora were dominant in control, whereas Amphora was dominant in the streptomycin treatment (Fig. 2).
In enriched control and streptomycin treatments, matt diatom growth was noticed from day 10 onwards (Fig. 1d). Unlike the unenriched chloramphenicol treatment, the enriched chloramphenicol treatment exhibited increased diatom abundance (Fig. 1d). However, these variations were not statistically significant (Kruskal–Wallis test, ns). Navicula was dominant on day 5 in most of the enriched treatments (Fig. 2). Amphora was the dominant form in the chloramphenicol treatment on days 10 and 15 (Fig. 2).
Post-monsoon
In the 2-day-old biofilm, 1.75 × 103 diatoms dm−2 were noted (Fig. 1e,f). Navicula was the dominant diatom (Fig. 2). A total of 20 species belonging to 11 genera were observed (Table 3). Unenriched control and streptomycin treatments exhibited increased diatom abundance, whereas chloramphenicol inhibited diatoms (Fig. 1e). These trends were similar to the pre-monsoon and monsoon observations and were statistically significant (one-way ANOVA, P = 0.0001). Navicula dominated the assemblages of all the unenriched treatments throughout the incubation period, with one exception (control day 15 community dominated by Amphora) (Fig. 2).
In enriched conditions, diatom abundance in the control increased progressively over the incubation period, with matt growth on day 10 (Fig. 1f). In the streptomycin and chloramphenicol treatments, matt growth was recorded on day 15 (Fig. 1f). As in unenriched conditions, these variations were statistically significant (one-way ANOVA, P = 0.0105). Navicula dominated the assemblages in all enriched treatments throughout the incubation period (Fig. 2).
Overall, species richness, evenness and diversity of the biofilm diatom communities did not show any particular trend in the streptomycin and chloramphenicol treatments (Fig. S1). Significant variation across treatments (day 5 values) was observed only for species richness in unenriched conditions in pre-monsoon (one-way ANOVA, P = 0.0367) and enriched conditions in monsoon (one-way ANOVA, P = 0.0152). Cluster analysis of the fouling diatom communities revealed two groups at 50% Bray–Curtis similarity level (Fig. S4). The groups were separated based on differences in abundance. The treatments supporting matt growth clustered together, particularly during pre-monsoon. The unenriched chloramphenicol treatments grouped together on most occasions (Fig. S4).
Of the treatments, streptomycin had the highest species turnover on most occasions (Fig. 5). The most striking period was post-monsoon, which had the highest species turnover for control and streptomycin compared with the other sampling periods; the opposite trend was observed for chloramphenicol (33%) under unenriched conditions (Fig. 5a).
Effect of streptomycin and chloramphenicol on bacterial communities and Vibrio dynamics
Pre-monsoon
Bacterial abundance in unenriched treatments showed an opposite trend when compared with diatoms (Fig. 3a). Chloramphenicol had the highest bacterial abundance, followed by control and streptomycin (Fig. 3a). Under enriched conditions, maximum bacterial abundance was noted on day 15 (Fig. 3b). Vibrio alginolyticus and V. parahaemolyticus were sensitive to chloramphenicol and streptomycin, respectively, but only in unenriched conditions (Fig. 4).
Monsoon
Bacterial abundance increased over the incubation period in all treatments except streptomycin (Fig. 3c,d). Chloramphenicol had consistently high bacterial abundance (Fig. 3c,d). Vibrio cholerae peaked on day 5 in all treatments (Fig. 4). In enriched conditions, V. alginolyticus was sensitive to chloramphenicol and was noticed only up to day 5 in control and streptomycin (Fig. 4).
Post-monsoon
Bacterial abundance in streptomycin and chloramphenicol treatments was higher than in control, on most occasions, irrespective of the nutrient enrichment levels (Fig. 3f). V. cholerae and V. parahaemolyticus were not recorded on day 0, but were noticed during the subsequent observations (Fig. 4). Vibrio alginolyticus peaked on day 5 in all unenriched treatments (Fig. 4).
Significant variation in bacterial abundance across treatments was observed during pre-monsoon (unenriched conditions) (one-way ANOVA, P = 0.0010) and in the monsoon periods (enriched conditions) (one-way ANOVA, P = 0.0082). Among the Vibrio species, only V. alginolyticus varied significantly across treatments (in pre-monsoon, unenriched conditions) (one-way ANOVA, P = 0.0280).
Correlation analyses
Pearson's correlation indicated no significant correlation between fouling diatom abundance, species richness, evenness and diversity (y-axis) and total bacteria (TBC), V. alginolyticus, V. cholera and V. parahaemolyticus (x-axis) (Table 4). Due to this, correlation analyses between specific diatom genera and bacterial groups were not performed.
| Parameters | Pearson r coefficient (P-value) | |||
|---|---|---|---|---|
| Total bacterial count (TBC) | V. alginolyticus | V. cholerae | V. parahaemolyticus | |
| Diatom abundance |
–0.4570 (0.2163) |
–0.0776 (0.8687) |
0.2977 (0.5168) |
0.4877 (0.3264) |
| Species richness |
–0.4135 (0.2686) |
0.2582 (0.5762) |
0.2955 (0.5200) |
0.6539 (0.1589) |
| Species evenness |
0.1685 (0.6647) |
0.2173 (0.6398) |
–0.4475 (0.3141) |
–0.1016 (0.8481) |
| Species diversity |
–0.1744 (0.6537) |
0.1375 (0.7688) |
–0.3302 (0.4695) |
0.4361 (0.3873) |
Effect of streptomycin and chloramphenicol on chlorophyll a and nutrient concentrations
Enriched treatments had higher initial concentrations of nitrate and phosphate compared with unenriched treatments (Fig. S3).
Pre-monsoon
An erratic trend in chlorophyll concentration was observed (Fig. S2). Matt diatom growth in enriched treatments was associated with a sharp decrease in silicate and nitrate concentration from day 10 onwards in control and streptomycin, and on day 15 in chloramphenicol (Fig. S3). In some cases, nitrate concentration peaked on day 15 (after the decrease). Matt diatom growth in the enriched chloramphenicol treatment was associated with a sharp increase in ammonia (Fig. S3).
Monsoon
A contrasting trend in chlorophyll a concentrations was observed for streptomycin and chloramphenicol treatments in unenriched and enriched conditions (Fig. S2). Under unenriched conditions, both these treatments had lower chlorophyll a concentrations. The reverse was true under enriched conditions (Fig. S2). Nutrient profiles were similar to the pre-monsoon scenario except that no spike in ammonia was observed in the enriched chloramphenicol treatment (Fig. S3), possibly due to absence of matt diatom growth.
Post-monsoon
An erratic trend in chlorophyll a concentration was observed in all unenriched treatments (Fig. S2). In the enriched treatments, chlorophyll a concentrations of the biofilms were highest on day 10, but only in control and streptomycin treatments (Fig. S2), probably reflecting matt diatom growth. Nutrient concentration profiles were similar to those observed in monsoon (Fig. S3).
Discussion
A consistent feature across all the sampling periods was that chloramphenicol inhibited diatom communities, whereas streptomycin did not (Fig. 6). These contrasting trends for both antibiotics can be understood considering their mechanisms of action. As stated earlier in the introduction, streptomycin does not seem to affect diatoms directly. This is in accordance with our results showing comparable/increased diatom abundance in streptomycin when compared with control. Additionally, streptomycin promoted growth of the non-dominant diatoms, notably Nitzschia (in unenriched treatment; pre-monsoon) and Cocconeis (in enriched treatment; monsoon). This resulted in higher species turnover in streptomycin treatments (compared with control; both unenriched and enriched) (Fig. 5). The contrasting observations with chloramphenicol (consistent inhibition of diatoms) is consistent with its inhibitory mechanism of action. Variation in species turnover was also observed in chloramphenicol treatments (Fig. 5), indicating the rate at which changes occur in fouling diatom communities. Amphora and Navicula, which dominate fouling and benthic communities in the adjoining intertidal sandflat (Mitbavkar & Anil 2006, 2007) were the only diatoms that survived after 15 days’ incubation.
It is apparent from the present study that antibiotics influence fouling diatom communities, indicating that bacteria (antibiotic producers) play an important role in modulating fouling diatom communities. The differential effect of streptomycin (non-inhibition/promotion) and chloramphenicol (inhibition) on fouling diatom communities indicates that the interactions between bacteria and fouling diatom communities are specific, depending on the type of antibacterial/antibiotic crosstalk involved and the constituent diatoms and bacteria.
Seasonal variations in the effects of streptomycin and chloramphenicol on fouling diatom communities
The trends observed for streptomycin and chloramphenicol were consistent across the sampling periods irrespective of the differences in the ‘initial’ species composition of the fouling diatom community. The initial diatom community in monsoon was dominated by centric diatoms (Skeletonema and Chaetoceros) as opposed to pennate dominance (mainly Navicula) in the pre- and post-monsoon periods. The monsoon period was also characterized by the highest diatom and bacterial abundance among the seasons. This period had the highest concentrations of nutrients (except nitrite) and low salinity. At the onset of monsoon, pennate diatoms were overtaken by opportunists such as Skeletonema and Chaetoceros, which probably bloomed in the water column and were trapped in the biofilm. Skeletonema blooms have been reported in the study area and surrounding regions during monsoon (Patil & Anil 2011). The resulting high abundance of this species was responsible for highest diatom abundance in monsoon. Chaetoceros, also dominating in monsoon, contributes to blooms during this period (Patil & Anil 2011). However, on incubation under controlled conditions, Skeletonema and Chaetoceros were overtaken by Amphora, Navicula and Nitzschia, probably due to superior competitive abilities of the latter species.
Although the effects of streptomycin and chloramphenicol on the fouling diatom communities were consistent temporally, variation in species turnover was observed. The highest species turnover was mostly observed during post-monsoon. This could be attributed to the presence of the smallest number of species in the ‘initial’ fouling diatom community (only three species – Cymbella sp., Navicula subinflata and Navicula transitans var. derasa f. delicatula) in the post-monsoon period. Among these, only Cymbella sp. and N. subinflata persisted in the antibiotic treatments. The additional species that emerged on incubation in control and antibiotic treatments (Table 3) were probably present in the initial natural biofilm, but at undetectable levels. Therefore, although not evident in the day 0 microscopic counts, the high species turnover in post-monsoon indicated the relevance of the ‘initial’ composition of the fouling diatom community in influencing the rate at which antibiotic-mediated changes occur.
Effects of streptomycin and chloramphenicol on total bacteria and Vibrio spp.
No clear trend in the effect of streptomycin on bacteria was observed. Maximum bacterial abundance was observed in the chloramphenicol treatments, but only under unenriched conditions. This was contrary to the trend observed for diatoms. In enriched conditions, bacterial abundance in most treatments was highest at the end of incubation. Correlation between diatom and bacterial parameters was not significant (Table 4). The reverse was observed in studies carried out elsewhere (details in Dobretsov & Thomason 2011). The positive/negative correlations reported in these studies can be understood in terms of the wide range of interactions between diatoms and bacteria, ranging from inhibitory to stimulatory effects (Cole 1982). However, not much is known about the community-level interactions which were the focus of this study. Additionally, as this study focuses on diatom communities, future experiments must address bacterial communities using high-resolution molecular approaches. Overall, in the present work, streptomycin and chloramphenicol did not inhibit bacteria. These observations differ from those in similar experiments by D'Costa & Anil (2011) on benthic diatom communities. In the latter study, streptomycin and chloramphenicol inhibited bacteria to <10 and 77% of control values, respectively. These differences must be related to the ‘biofilm’ mode of life conferring resistance to antibiotics, as has been reported earlier for bacteria (Costerton et al. 1999). Hall-Stoodley et al. (2004) attributed the resistance of bacteria in biofilms to three factors. First, the biofilm slime matrix has barrier properties; it helps to localize antibiotic-degrading enzymatic activities. Second, there are zones of dormant but viable cells in the biofilm which, by virtue of being metabolically inactive, are not affected by antibiotics. Third, sub-populations of resistant bacteria may exist embedded in biofilms. In fact, biofilm formation by bacteria is a means of responding to antibiotic exposure and is perhaps an evolutionary adaptation of bacteria to defend against various antibiotics of microbial origin (O'Toole & Stewart 2005). The non-inhibition of bacteria by streptomycin and chloramphenicol can also be attributed to the proliferation of the surviving bacterial fraction, in response to antibiotic degradation towards the end of the 15-day incubation period. In a separate study (D'Costa & Anil 2012), stability of streptomycin and chloramphenicol in f/2(ASW) ranged from 77 to 90% compared with day 0 values. However, the period over which antibiotic stability was assessed was limited to 7 days. Based on this, the possibility of a marginal decrease in antibiotic potency over the 15-day incubation period in this study cannot be ruled out.
In the case of Vibrio species, a clear trend was noticed for Vibrio cholerae in the ‘initial’ 2-day-old biofilm community. V. cholerae was the only Vibrio species that showed a similar trend as diatoms, i.e. maximum abundance in monsoon. Vibrio cholerae abundance in the water column in the study area is the highest in monsoon (personal observations), indicating that the bacterial community in biofilms closely reflects conditions in the planktonic phase, as has already been discussed for diatoms (Skeletonema). The high V. cholerae abundance in monsoon could be attributed to its preference for low salinity (personal observations). Its high abundance in monsoon coincided with dominance of the diatoms Chaetoceros and Skeletonema. This is contrary to Rehnstam-Holm et al. (2010) who reported a negative correlation between Chaetoceros, Skeletonema and vibrios along the southwest coast of India. However, their study was carried out during the post-monsoon season. Hence salinity is one of the ecological factors that play an important role in Vibrio spp. dynamics. Several other factors also influence Vibrio spp. dynamics, one of them possibly being bacterial antagonism, already reported for V. cholerae (Long et al. 2005), similar to the conditions expected in the antibiotic treatments. This is supported by our observations during monsoon. Vibrio cholerae was observed only up to day 5 in both unenriched and enriched control conditions but were noted subsequently in the antibiotic treatments during the same period.
Overall, notable shifts in Vibrio spp. dynamics in the antibiotic treatments were evident in pre-monsoon and monsoon (Fig. 4). However, Vibrio species did not show significant correlation with diatom parameters (Table 4). However, given the close associations between diatoms and Vibrio, these antibiotic-mediated changes in Vibrio dynamics may be reflected in the associated fouling diatom communities. Temporal variations must also be considered. Vibrio cholerae is less susceptible to antagonism at higher temperatures (Long et al. 2005). This is consistent with our observations in the warm pre-monsoon. Vibrio cholerae were observed up to day 10 in control conditions, as opposed to day 5 during monsoon, suggesting the role of temperature as a regulatory factor. Alternatively, the V. cholerae strains during pre-monsoon and monsoon could differ. Maximum Vibrio enhancement was observed in the enriched conditions in pre-monsoon and unenriched conditions in monsoon (with naturally elevated background nutrient concentrations), highlighting the relevance of nutrient concentrations for Vibrio spp., already suggested by Rehnstam-Holm et al. (2010) and references therein.
Influence of nutrient levels on the effects of streptomycin and chloramphenicol on fouling diatom communities
In enriched conditions, the higher initial nitrate and phosphate concentrations favoured diatoms with high growth rates (Amphora, Navicula), whereas co-existing species were suppressed. This led to a rapid build-up of biomass (matt diatom growth). This is reflected in (i) elevated species turnover and biofilm chlorophyll values (Fig. S2, Fig. 5), although cyanobacterial contribution to the chlorophyll a pool cannot be ruled out; (ii) depletion in nitrate and silicate concentrations (Fig. S3); and (iii) clustering of enriched control and antibiotic treatments that exhibited matt diatom growth, especially in pre-monsoon (Fig. S4). However, the time at which matt growth commenced depended on the type of antibiotic.
Interestingly, nutrient enrichment had an over-riding effect on the inhibitory action of chloramphenicol. This is clear in the cluster analysis, where unenriched and enriched chloramphenicol treatments form distinct groups, exhibiting a low degree of similarity (Fig. S4). The matt diatom growth in the enriched chloramphenicol treatment was associated with a spike in ammonia concentration in pre-monsoon, probably due to decomposition of the overgrown matt diatom growth. Visual observations indicated a white, loosely adhering film on the polystyrene slides, corroborating decomposition of the diatom film. Therefore, nutrient concentrations modulate the response of biofilm diatom communities to antibiotics.
Conclusions
Overall, the results of this study highlight the distinct differences in the effects of streptomycin and chloramphenicol on biofilm diatom communities and point to the wide range of antibiotic-mediated interactions between fouling diatom and bacterial communities. The rate at which these antibiotic-mediated effects occur depends on the ‘initial’ species composition of the biofilm diatom community. The simultaneous changes in Vibrio dynamics, especially Vibrio cholerae, may be reflected in the fouling diatom communities. Additionally, nutrient concentrations are an important driving force in determining the response of fouling diatom communities to antibiotics. However, it must be remembered that antibiotics are but a single class among a wide range of microbial products involved in mediating interactions of bacteria with other organisms; these include bacteriocins, acyl-homoserine lactones and peptidoglycan (Shank & Kolter 2009). Therefore, the range of cross-talk possible is even wider and needs to be the focus of future studies.
As the approach used in this study is novel, future studies must consider carefully the differences between in situ and experimental conditions in the laboratory. The experimental conditions consisted of low levels of grazing, static incubation conditions and high nutrient enrichment levels compared with in situ conditions. Therefore, it is likely that the species that emerged in these experiments could be different from those in natural conditions. These factors need to be taken into account when extrapolating these results to natural communities. From the anti-fouling perspective, the influence of substrate on antibiotic-mediated responses needs to be analysed. In the present study, hydrophobic polystyrene slides were used. Replication of these experiments on different substrata (including fouling-release coatings) would fill this gap. Interpretation of these interactions at the ‘microfouling’ level will form a basis for future anti-fouling technologies.
Acknowledgements
The authors are grateful to the Director, National Institute of Oceanography (NIO) for his encouragement and support. We appreciate the comments of the anonymous reviewers. We thank Ms Anisia Pereira, Ms Presila Dias, Ms Sneha Prabhudessai and Ms Sneha Naik for their help. This is a NIO Contribution No. 5374.




