Thioploca spp. sheaths as niches for bacterial and protistan assemblages
Abstract
Sulfide oxidizing bacterial mats are common in regions of the continental shelves characterized by high primary production and the resultant oxygen minimum zone. These mats are made up of several species of Beggiatoa and/or Thioploca, which oxidize sulfide that is generated in the sediment. Thioploca spp. inhabit a large polysaccharide sheath that encompasses bundles of 1–20 filaments (trichomes), each ranging from 3 to 60 μm in diameter. This sheath has been shown to be a critical component of the autecology of Thioploca. Analysis of Thioploca from cold seeps in Monterey Bay using light and transmission electron microscopy identified new and diverse microbial assemblages associated with interstitial spaces between trichomes, inside the sheath. Small diameter, non-vacuolate, filamentous prokaryotes were numerous. Amoebae, euglenozoa, ciliates and other protists of unknown affiliation were observed in sheaths. Most of the protists possessed food vacuoles and some protists showed ultrastructural evidence of endosymbionts. These observations suggest that Thioploca sheaths may serve as oases on the sea floor, providing nutritional and detoxification services to previously unrecognized microbial partners.
Introduction
Thioploca spp. are large, filamentous, vacuolate bacteria that form visible white mats overlying sulfide-rich aquatic sediments. Thioploca have the capacity to oxidize hydrogen sulfide (H2S) using nitrate (NO3−) as an electron acceptor. However, these compounds are spatially separated. Thioploca is unique in dealing with these opposing gradients of NO3− and H2S by inhabiting and traveling through the sediment within a large (mm in diameter and cm long/deep) polysaccharide sheath. Thioploca spp. have been observed in a number of coastal sea-floor environments associated with marine oxygen minimum zones (OMZs) such as the continental shelves off Chile (Schulz et al. 1996; Jørgensen & Gallardo 1999), Pakistan (Schmaljohann et al. 2001) and Monterey Bay (Ahmad et al. 1999). Partly as a function of the widespread and increasing occurrence of hypoxic environments that foster Thioploca (Teske 2010), they have been implicated as an important actor in globally significant biogeochemical cycling and remineralization that occurs at sediment water interfaces in these areas (Otte et al. 1999).
Sulfide is necessary to maintain Thioploca. Although the current conventional wisdom (Mentel & Martin 2008) holds that high sulfide concentration is not necessarily toxic to eukaryotic metabolism, it does impose restrictions on which electron acceptors are available and therefore the efficiency with which energy can be produced. Some eukaryotic organisms in or near high concentrations of sulfide deal with the sulfide through diverse symbioses. These symbiotic relationships between prokaryotes and eukaryotes have been posited to aid the eukaryotes either by providing nutritional sustenance (Goffredi et al. 2004) or by detoxifying the environment through the oxidation of the hydrogen sulfide (Buck et al. 2000).
Thioploca is a huge bacterium with an even larger physical presence in the form of the sheath and trichomes. The environment within the sheath is characterized by no or very low concentrations of O2, and low but measurable concentrations of H2S (Huettel et al. 1996). We know that another filamentous bacterium (Desulfonema sp.) can use the sheath as a physical niche and places itself proximal to the Thioploca for metabolic purposes (Fukui et al. 1999; Teske et al. 2009). Since protists have been shown to inhabit the sediment at these mats (Buck & Barry 1998; Buck et al. 2000), we hypothesized that we would also find protists within the sheath of Thioploca. We document a whole new suite of organisms found inside Thioploca spp. sheaths and discuss the possible adaptations and benefits to these organisms.
Material and Methods
Thioploca-containing sediments were collected using 6.9-cm diameter, 25-cm-long acrylic core tubes from a cold seep at 906 m in Monterey Canyon (Ahmad et al. 1999) by the remotely operated vehicle (ROV) Ventana. Individual Thioploca sheaths to be used for electron and light microscopy were separated from the sediment by gentle sieving through a 63-μm mesh. Sheaths were stained with Rose Bengal to enhance visualization of microbial assemblages associated with sheaths. Application of this stain leaves the polysaccharide sheath transparent while coloring its contents. Micrographs of Rose Bengal-stained sheaths were taken with an Olympus dissecting microscope. Individual sheaths used for transmission electron microscopy (TEM) were separated from the sediment by removing most of the sediment through a 63-μm sieve and transferred to cryovials with forceps and preserved with 0.1 m cacodylate buffered 2% glutaraldehyde in filtered seawater and embedded into thin resin sheets using standard protocols (Buck et al. 2000). Individual sheaths were excised from the resin, mounted on blocks, sectioned and stained.
Results
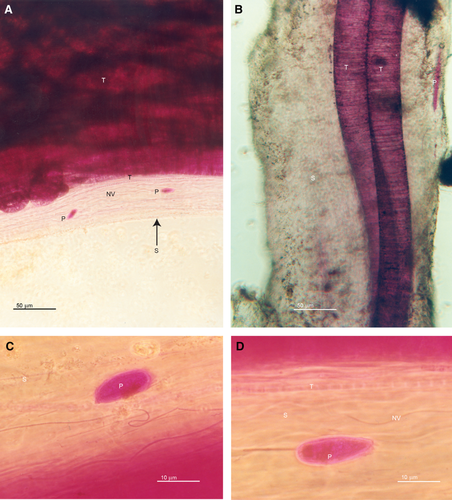
The sheaths collected in this study were white in color, 110–285 μm in diameter and up to several centimeters in length. Rose Bengal-stained sheaths were examined using light microscopy. We observed multiple diameters (3–60 μm) of Thioploca spp., small diameter (~1 μm), non-vacuolate filamentous prokaryotes, and 12 unique protists (Fig. 1A–D). The robust uptake of the Rose Bengal stain by the trichomes meant that we could only view other constituents of the sheath with light microscopy by examining the edge of the sheath away from trichomes (Fig. 1A–D). It is likely that most of the non-Thioploca constituents of the sheath are obscured. It was also quite evident by the focal depth that these non-Thioploca constituents were inside the sheath and not on the surface of the sheath.
Transmission electron microscopy of individual sheaths revealed a diversity of organisms inside the sheath. The inside of the sheath was established by locating the polysaccharide wall, which appears distinct due to refractive, non-sheath material adhering to the outside of the sheath (Fig. 2A), and exploring the side of the sheath that encompassed the trichomes. The trichomes are characterized by a large, conspicuous central vacuole (Fig. 2A). The peripheral space around the vacuole is occupied by protoplasm and by small, relatively uniform empty spaces which represent the elemental sulfur that has dissolved during the dehydration process associated with the embedding (Fig. 2A). Non-vacuolate filamentous prokaryotes, typically small in diameter (on the order of 1 μm) were abundant and scattered between the trichomes of Thioploca (Figs 1A–D and 2A–G). It is impossible to identify these small non-vacuolate prokaryotes based solely upon ultrastructure. However, we could see at least three separate morphologies/metabolisms in the TEMs. Sulfur vesicles indicate sulfide oxidizers (Fig. 2B and C). The oblique longitudinal sections of the filaments in Fig. 2C show a crenulated cell wall which is also recognizable in a cross-section (Fig. 2C). There are spiral morphologies (Fig. 2C and G) and filaments without sulfur vesicles. It is impossible to state whether the preponderance of non-filamentous prokaryotes in the sheaths imaged in the TEMs are cross-sections of filaments, or rods or cocci (Fig. 2A and C–E).

Protists were observed randomly scattered throughout the interior of the sheath. These protists (n = 12), on the order of 10s of microns in size (Figs. 2D–H) were assignable to several groups including amoebae (Fig. 2C), euglenoids (Fig. 2F), ciliates (Fig. 2H) and unidentified groups (Fig. 2E and G). The most conspicuous organelles of the protists were food vacuoles. The food vacuole is characterized by an encompassing membrane around refractive, unidentified and disorganized material (Figs 2E–H). A total of 10 food vacuoles can readily be seen in four protists (Fig. 2E–H). In some cases, protists also possessed intact bacteria reminiscent of endosymbionts (e.g. intact membranes surrounding organized prokaryotic protoplasm; Fig. 2D and F). Although large conspicuous classical mitochondria were observed in some protists (not shown), most did not possess these organelles. Protists from anoxic environments frequently lack classical mitochondria, and instead harbor alternative structures such as hydrogenosomes (van der Giezen et al. 2005; Edgecomb et al. 2010; Müller et al. 2012). We found one unidentified protist with hydrogenosome-like organelles in close association with endosymbiotic bacteria (not shown) (Gijzen et al. 1991). Not all the sheaths that we examined were found to contain protists.
Discussion
The discovery of Desulfonema sp., a relatively large diameter, non-vacuolate sulfate-reducing bacterium, residing inside the sheaths of Thioploca spp. (Fukui et al. 1999; Teske et al. 2009) as well as a number of other bacteria with diverse metabolisms (Kojima et al. 2006) demonstrates that the habitable space inside sheaths is more dynamic and complex than previously thought. We extend these observations to include diverse protistan endobionts and other associated prokaryotes.
Sheaths are critical to maintaining Thioploca assemblages, providing a means for individual Thioploca trichomes alternatively to travel into the water column to obtain nitrate and then to go into the sediment to oxidize sulfide. For the protists living in the sheath among the Thioploca and non-vacuolate filaments, the sheath potentially represents (i) a physical niche, (ii) biological protection from high concentrations of hydrogen sulfide, (iii) protection from grazing by meiofauna, and (iv) access to otherwise unexploited food resources, i.e. heterotrophic prokaryotes consuming concentrated dissolved organic carbon. We do not know which of these characteristics of Thioploca are most important to the protists. The diffusion barrier represented by the sheath (Schulz & Jørgensen 2001) coupled with the rapid oxidation of sulfide by Thioploca (Otte et al. 1999) within the sheath potentially produces a fairly benign environment for eukaryotes with respect to sulfide. Larger sheaths may be abandoned by trichomes of Thioploca because of the absence of sulfide caused by the higher diffusional barrier (Schulz & Jørgensen 2001). Larger sheaths would have lower concentrations of sulfide compared with smaller sheaths, hence they would potentially be more habitable and would also have more interstitial space. This may explain why we did not see protists in all sheaths.
The presence of multiple taxa of protists with numerous food vacuoles is suggestive of a food chain operating within Thioploca sheaths. Prokaryotes use elevated dissolved organic carbon secreted by the large Thioploca and kept at high concentration by the diffusion boundary of the polysaccharide sheath. Protists would ingest prokaryotes.
The extent to which sheath microbiota represent endemic forms or opportunistic members of the seep sediment infauna remains to be determined. Other protists in the same groups as those observed inside the sheaths have been observed in high abundance in the same sediment from which the Thioploca sheaths were harvested (Buck & Barry 1998). However, one notable difference between the seep protistan infauna and the protistan endobionts of Thioploca sheaths was the lack of epibacterial symbionts on the sheath protists (Buck et al. 2000).
Conclusions
Sheathed assemblages such as those obtained from Monterey Bay may represent a newly discovered niche for protists. The oxidation of sulfide by the large Thioploca to the benefit of smaller protists is analogous to the proposed mechanism in play with small bacterial epibionts on protists in seep sediments (Buck et al. 2000) and in other anoxic/hypoxic niches (Bernhard et al. 2000). Future studies will require more in-depth comparative analysis with expanded sample inventories that include microbial communities within the surrounding seep environment in order to determine the extent to which observed sheath assemblages define specific or opportunistic interactions. Additional explorations of the microenvironment within individual sheath structures using microelectrodes combined with phylogenetic staining or quantitative PCR methods targeting specific taxonomic or functional gene anchors could help refine hypotheses related to nutritional versus protective services and provide direct visual evidence linking specific taxonomic groups with the sheath environment.
Acknowledgements
We thank the crew of the ROV Ventana and R/V Point Lobos for assistance with sample collection and J. Yang and P. J. Whaling for excellent technical assistance. J. Krupp assisted in the EM aspects of the study. D. Patterson, D. Lynn, C. Preston and A. Rogerson participated in discussions of the identities of the protists and prokaryotes. We thank two anonymous reviewers for their detailed and helpful suggestions. This study was funded by a grant to J. P. Barry from the David and Lucille Packard Foundation to MBARI. The authors have no conflict of interests to declare.




