Embryonic development and intracapsular feeding in Hexaplex trunculus (Gastropoda: Muricidae)
Abstract
Spawning, phases of embryonic development, intracapsular feeding mechanism and development mode of banded murex Hexaplex trunculus (Linnaeus, 1758) were examined using specimens from the Aegean Sea. In addition, the numbers and characteristics of non-viable nurse eggs during different phases were examined in relation to the development phases of viable embryos. Females spawned between 59 and 162 egg capsules containing 306.76 ± 50.74 eggs. Trochophore larvae first appeared on the 15th day after spawning. Nurse egg consumption began on the 17th day after spawning when the embryos reached the early veliger stage. In the beginning, veligers consumed the nurse eggs by mechanically disintegrating them with velar cilia movement. From the 18th day after spawning, embryos began to consume whole nurse eggs, although mechanical disintegration continued until hatching. Viable embryos consumed the most developed nurse eggs first. The average number of nurse eggs consumed per embryo was 24.67 by the end of the intracapsular period. The average number of hatchlings was 11.95 ± 3.81 per capsule with 1321.48 ± 133.1 μm shell length. According to our observations H. trunculus shows dispersal polymorphism, with most of the hatchlings completing metamorphosis after a short planktonic non-feeding period (up to 2 days), while others metamorphose prior to hatching. Planktonic hatchlings had both foot and well developed four-lobed velum and minimum 1 3/4 whorls. Both hatchling types could be seen in the capsule mass from the same female.
Introduction
In lecithotrophic caenogastropods the most common extra-embryonic energy sources are nurse eggs or nurse embryos (Thorson 1950; Collin & Spangler 2012), which exist together with the viable embryos in special capsules (Hyman 1967; Poulin et al. 2001). Nurse eggs or nurse embryos are consumed by the viable embryos (oophagy or adelphophagy) (Gallardo 1976; Pechenik 1979; Chaparro & Paschke 1990; Elgar & Crespi 1992; Kamel et al. 2010) until metamorphosis is complete, or nearly complete, in the capsule (Bouchet 1989; Krug 2007). The amount consumed influences hatching size (Spight 1976; Gallardo 1979; Rivest 1983; see Collin 2003) as well as the developmental mode (Hadfield et al. 1972; Krug 2009). As a result, relatively small or large lecithotrophic individuals hatch from the capsule as swimming larvae (Johannesson 1988; Bouchet 1989) that have greater dispersal potential (Collin 2012) or as crawling juveniles (D'Asaro 1970, 1986; Meirelles & Mattews-Cascon 2005; Averbuj & Penchaszadeh 2009) that can better avoid the risks of planktonic life (Krug 1998, 2001).
Eventually, the intracapsular processes determine post-larval characteristics (Rivest 1983; Krug 2001, 2009; Collin et al. 2007; Brante et al. 2009). However, information about intracapsular processes, especially intracapsular feeding mechanisms and nurse egg-viable embryo characteristics is very limited (Radwin & D'Attilio 1976; Rivest 1983; Collin & Spangler 2012). Here we investigated larval development of Hexaplex trunculus (Gastropoda: Muricidae). The main objectives of this study were (i) to provide detailed information about the intracapsular structures that change in number and characteristics through interaction during the intracapsular period, (ii) to examine the feeding behaviors of the embryos through the consumption of nurse eggs, and (iii) to monitor the larval developmental stages of H. trunculus. In addition, we aimed to present additional data to earlier studies (Bandel 1975; Vasconcelos et al. 2004; Lahbib et al. 2009) on the spawning process and dispersal strategies of this species.
Hexaplex trunculus was selected as the ideal species for investigation of intracapsular development. H. trunculus is an ecologically important species as muricid gastropods are important predators in marine benthic communities (Menge 1974), and are successful invaders throughout the worlds seas (see Morton 2008); H. trunculus is one of the most common members of the Mediterranean muricids (Morton et al. 2007), is an opportunistic generalist predator (Peharda & Morton 2006) and is a heavy consumer of several bivalve species, especially Mytilus galloprovincialis and Ostrea edulis (Sawyer et al. 2009). Furthermore the commercial harvest of H. trunculus has increased significantly in recent years, especially in Mediterranean countries (Benović 1997; Peharda & Morton 2006; Vasconcelos et al. 2008).
Material and methods
Adult H. trunculus individuals were collected in early January from the Urla seashore, Izmir (38°22′ N, 26°47′ E) and taken to the laboratories located at Urla, Izmir. Similar-sized specimens were placed in 500-l tanks which had running seawater. Individuals were fed with Mytilus galloprovincialis during an adaptation period; the specimens that fed were assumed to have adapted to laboratory conditions and were included in the experiments. The selected specimens were transferred into two indoor 500-l tanks with running seawater, aeration and natural photoperiod in February. Individuals were fed ad libitum with live M. galloprovincialis throughout the experiments. Some individuals were transferred into aquaria filled with running seawater and aeration for photographing, video recordings and observation. All the tanks and aquaria had net-covered tops to avoid possible escapes, and all transfers were made by hand to limit stress. Fecal material and other debris was removed daily. Due to the constant flow of sea water, water temperature in holding tanks was similar to natural conditions. During April and May holding rank water temperature was measured at 18.2 ± 1.9 °C and 22.4 ± 2.6 °C. Salinity ranged from 35.5 to 36.5 during the duration of the experiment.
From the first day of spawning until hatching eight to ten egg capsules were randomly removed each day from capsule masses that had spawned simultaneously in each holding tank. Collected egg capsules were immediately transported to the laboratory for examination. Egg capsules were measured with an ocular micrometer under a stereomicroscope using D'Asaro's (1986) method: the maximum distance between apex and basal membranes was taken as length and the widest distance between the lateral walls as width.
Intracapsular inclusions were classified and analyzed separately: ‘Egg’ (e) refers to anything before cleavage, including both fertilized and unfertilized eggs; the stages between Egg and the end of blastomere development (blastomeres forming) were classified in a single category the ‘s1 phase’ (s1); inclusions which had completed blastomere development were called ‘s2 phase’ (s2); inclusions that degenerated or dissolved at any stage of intracapsular development were labeled ‘degenerated structures’ (d); inclusions that continued to develop after the blastula stage and until the end of the larval period were called ‘viable embryos’ (ve). Nurse eggs referred to all intracapsular inclusions (e, s1, s2, and d structures) except viable embryos (ve). Abnormally developed embryos were not independently classified; but were included in the calculations as normal embryos because of their consumption of nurse eggs.
Intracapsular structures were counted and classified under an Olympus BX51 (Olympus Corporation, Tokyo, Japan) microscope with an Olympus DP25 digital camera and measured with tpsdig2 and Olympus CELLSENS computer software.
In eggs and s1–s2 phase inclusions, the distance between the edges was taken as diameter or length (L) and width (W). The length (L) and width (W), respectively, were taken as the distances between the lateral edge points of the cephalic region in trochophore and early veliger stages, and as the distance between the apex and the end point of siphonal canal and the maximum width of the shell in veliger and pediveliger. Daily growth increments were calculated by measuring the shell or the cephalic region in length or in width according to the following equation: G = (L1 – L0) (t1 – t0)−1, where L0 and L1 are shell (or cephalic region) length (or width) at times t0 and t1, respectively. The number of nurse eggs consumed per embryo was calculated by dividing the mean number of e + s1 + s2 + d per capsule (before the begining of nurse egg consumption) by the mean number of viable embryos per capsule. After the first day of hatching, only the embryos or inclusions in the capsules were measured and only the embryos or inclusions from closed capsules were counted and added to calculations. For each capsule, the first day of hatching was taken as hatching time.
Results
Spawning
Spawning started on 2 May at 19 °C water temperature. A total of 39 females with shell lengths between 57 and 67.3 mm (62.65 ± 2.58) spawned. Females deposited their capsules on the walls of the tanks or on the other capsule masses. At the end of the process, capsule masses created sponge-like masses. Some capsules (<15) that did not attach to any surface were found on the floors of holding tanks after spawning was completed.
Females spawned up to 162 capsules in one session, the average being 108.24 ± 26.62 per female (minimum 59, maximum 162). While most females spawned all their capsules in one session, a few females spawned a second time depositing no more than 40 egg capsules. The spawning process lasted 16–48 h (30.18 ± 12.56 h). Average spawning frequency was 3.58 capsules per hour.
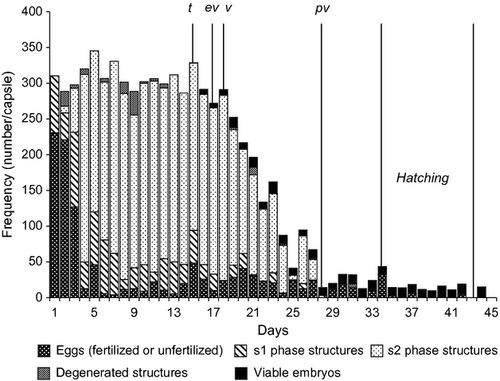
Egg capsules were flat, convex on one side, and trapezoid to rectangular in shape (Fig. 1a–c). The colors changed from cream white-yellow to yellow-dark yellow and light purple during development. Average capsule length was 5.32 ± 0.5 mm (min. 4.1, max. 6.5) and average capsule width was 4.68 ± 0.47 mm (min. 3.5 mm, max. 5.8 mm). In most of the specimens, length was greater than width (L/W = 1.14 ± 0.07) and were not independent of each other (y = 0.75x + 0.66; R2 = 0.66, P < 0.01).

Intracapsular inclusions and development
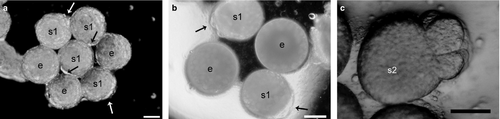
Fertilized eggs started to reach s1 phase on the first day post-spawning and reached s2 phase on the third after spawning. Most of the eggs did not develop past s2 phase and a small number of the eggs did not reach s1 phase. Hence, eggs, s1 and s2 phase inclusions, degenerated inclusions and viable embryos could exist together within the same capsule simultaneously (Fig. 1b). The total number (until starting of nurse egg consumption) of all intracapsular inclusions (e, s1, s2, d and ve) per capsule was 306.76 ± 50.74 (min. 220, max. 444). The total number of intracapsular inclusions per capsule and the capsule sizes were significantly correlated (Pearson correlation = 0.418, P < 0.001).
Eggs were almost round and could be seen with the naked eye from the first day of spawning (Fig. 2a and b); the average diameter was 235.7 ± 14 μm (min. 192 μm, max. 256 μm; n = 1730). Egg diameter did not correlate with capsule size (Pearson correlation = −0.0787, P = 0.673). Egg diameter did not change during the intracapsular period except for degeneration or development (d or s1) (ANOVA; df = 3; f = 0.297; P = 0.827). The average length during s1 phase (Fig. 2a and b) was 262.7 ± 17.6 μm (min. 208 μm, max. 304 μm; n = 1300 from 95 capsules) and the average width was 211.5 ± 16.5 μm (min. 176 μm, max. 256 μm). The average length and width of s2 phase inclusions (Fig. 2c) were 285.45 ± 19.63 μm (min. 240, max. 320 μm) and 199.03 ± 17.19 μm (min. 160, max. 240).
Between the 6th and 14th days of spawning (from the last observation of blastomere development to the first appearance of the viable embryos) the numbers of respective intracapsular inclusions did not change (Kruskal–Wallis Test, P > 0.1). In this stable period, average number of eggs were 14.76 ± 20.06, s1 phase = 40.55 ± 36.3, s2 phase = 245.16 ± 59.12 and d = 7.78 ± 19.13 per capsule (Fig. 3). With the appearance of the viable embryos, the numbers of other intracapsular inclusions (e, s1, s2 and d) began to decrease as a result of nurse egg consumption, and except in a few cases all of them vanished before hatching. The first intracapsular inclusion to vanish was s2 (27th day post-spawning), on the 28th day s1, on the 31st day d, and on the 42nd day eggs completely vanished (Fig. 3).
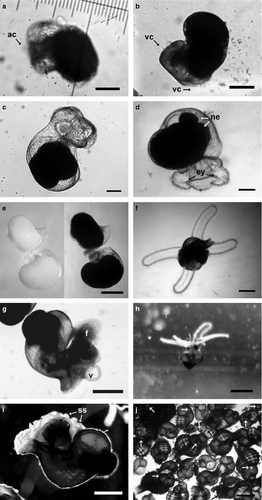
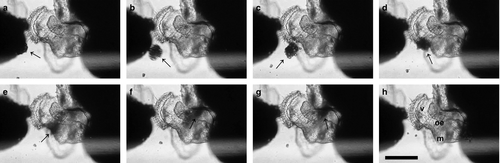
Viable embryos were detected first on the 15th day post-spawning as trochophore larvae and differed from other inclusions by their cilia induced rotational movement (Fig. 4a). Viable embryos did not feed on nurse eggs or any other inclusion at the trochophore stage. At the 17th day post-spawning, embryos had reached the early veliger stage and nurse egg consumption began. A bi-lobed short velum was the first deterministic structure of the veliger phase, and was identified by the formation and movement of the velar cilia (Fig. 4b and c). During the development of early veliger, veliger and pediveliger stages, embryos continued to consume nurse eggs until hatching. In the beginning, veligers consumed nurse eggs in pieces that were mechanically produced by the beating of velar cilia. Beginning on the 18th day post-spawning, embryos started to swallow nurse eggs whole (Fig. 4d) although mechanical disintegration of nurse eggs (Fig. 5) continued until hatching, especially for egg clusters (see Supporting Information). The average number of nurse eggs consumed per embryo was 24.67 during the intracapsular period. Cannibalism was not observed among healthy embryos while they remained in the capsule. However, embryos with broken shells (dead or alive) were always consumed by healthy embryos (see Supporting Information).
The average number of viable embryos was 0.17 ± 0.41 (min. 0, max. 1) on the 15th day of post-spawning (Fig. 3), with length of 226 μm and width of 192 μm (Fig. 6). From the 19th day post-spawning to hatching the average number of viable embryos per capsule was 11.95 ± 3.81 (min. 5, max. 23). The number of viable embryos per capsule did not change from the 19th day post-spawning to hatching during the intracapsular period (Kruskal–Wallis test: χ2 = 23.167; df = 20; P = 0.281). Eventually, between five and 23 individuals (11.95 ± 3.81, n = 198) hatched per capsule; the embryo/nurse eggs ratio (number of ve/number of e + s1 + s2 + d) was 0.041. The number of embryos per capsule among different egg masses was found to be significantly constant at α = 0.05 (Kruskal–Wallis test).
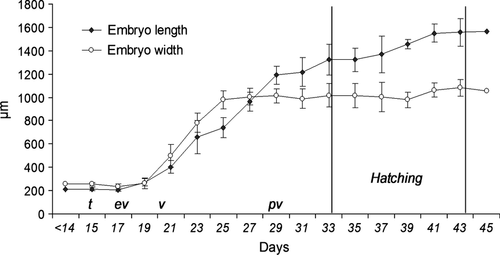
The average shell (protoconch) increments were found to be 46.78 ± 8.56 μm·day−1 in length and 38.20 ± 8.02 μm day−1 in width between the 15th and the 43th days of spawning. The embryo reached the veliger phase on the 20th day post-spawning (Fig. 4d) and started to resemble the adults after the 22nd day (Fig. 4e). On the 24th day post-spawning, embryos reached protoconch 11/4 and the eyes were observed for the first time. Embryos reached the pediveliger phase at the 28th day of post-spawning with minimum 11/2 protoconch whorl, and with shell pigmentation starting. In this phase, a well developed four-lobed velum (Fig. 4f) and the foot existed together (Fig. 4g). While elongation of the velum continued until hatching, the foot began to be in use effectively before hatching.
Hatching times were highly variable (between 34 and 43 days). Some individuals escaped from the capsule, while others remained in it. For each capsule, the first day of hatching was taken as hatching time because the escape holes were open and the individuals were able to leave the capsules.
Hatched individuals measured an average of 1321.48 ± 133.1 μm shell length and 1013.34 ± 99.23 μm shell width, and maximum and minimum sizes were 1040–1520 and 800–1200 μm in length and width, respectively (Fig. 6). Although most of the individuals hatched as swimming planktonic veligers, some hatched as crawling juveniles and both types could be found in the egg mass laid by a single female. Planktonic hatchlings presented a minimum 13/4 whorl, with both a foot and a well developed four-lobed velum. A small number of crawling juveniles were able to swim via a still existing velum, but most had no velum (Fig. 4g and h). Swimming veligers were never observed to feed on plankton. Velum disappearance and permanent settling started 2 days after hatching. Shell sculpturing was observed at that time (Fig. 4i). Immediately after settling, the first drilling behavior was observed (see Supporting Information) and following this an intense cannibalism occurred settled juveniles (Fig. 4j) due to the lack of food in the test environment.
Discussion
Prior to spawning, feeding rates of all individuals declined, especially between March and May. A decline in feeding activity during the breeding season has been reported for some gastropod species including Littorina littorea (Williams, 1970), Nucella lapillus (Feare, 1970) and Buccinum undatum (Martel et al., 1986; Himmelman & Hamel, 1993; Brokordt, 2003). The average number of eggs (nurse + embryonic) per capsule was quite different from the findings of Vasconcelos et al. (2004) and Lahbib et al. (2009) (Table 1). Positive correlations between female sizes and eggs per capsule have been reported before in H. trunculus (Lahbib et al., 2009) and in many other species, e.g. Crepipatella dilatata (Gallardo, 1976; Chaparro et al., 1999) and Conus pennaceus (Perron & Corpuz, 1982). However, female size was not varied enough to explain such differences among the three studies. The differences in eggs per capsule and also egg capsules per female (Table 1) may be associated with the different conditions of the females, differences of climatic parameters in sample sites that could induce different metabolic responses (Cumplido et al. 2011) or, briefly, heterochronic plasticity as suggested before to be a cause of intraspecific variations of larval development in some invertebrates (Levin et al. 1991; Russo & Patti 2005). However, we cannot provide a clear explanation without comparing a good deal of additional data such as environmental fluctuations in laboratory-sampling sites, which can change the maternal effects (Crean & Marshall 2009; Krug 2009).
| present study | Vasconcelos et al. (2004, 2008) | Lahbib et al. (2009) | Lahbib et al. (2009)a | |
|---|---|---|---|---|
| female size (mm) | 62.65 ± 2.58 | 64.70 ± 7.31 | 58.3 ± 8.4 | 52.5 ± 5 |
| capsules per female | 108.24 ± 26.62 | 118 ± 89 | 158.80 ± 48.30 | 142.60 ± 69.59 |
| size of capsule (mm) | ||||
| length | 5.32 ± 0.5 | 5.5 ± 0.5 | 4.8 | 4.9 |
| width | 4.68 ± 0.46 | 4.7 ± 0.4 | 4.4 | 4.7 |
| eggs per capsule | 306.76 ± 50.74 | 723 ± 66 | 376.5 ± 36.7 | 236.5 ± 8.87 |
| egg diameter (μm) | 235.7 ± 14 | 240 ± 8 | – | – |
| embryos per capsule | 11.95 ± 3.81 | – | 17b | 11.5b |
| time to hatching (days) | 34–43 | 30–32 | 52 | 56 |
| hatchling length | 1321.48 ± 133.1 | 1640 ± 220 | – | – |
- a Specimens from different site.
- b Modified from the original paper.
The number of eggs (nurse + embryonic) per capsule increased with capsule size but egg size was not correlated with capsule size. These results are compatible with many studies of caenogastropods including muricidae, e.g. Harding et al. (2007) in Rapana venosa. Even though the water temperature, salinity and pH measurements in the laboratory were similar to field measurements (Urla seashore), spawning duration or frequency can differ in the field depending on external and social factors, such as greater communal spawning events. But the number of eggs (nurse and embryonic) per capsule and embryos per capsule were similar to the samples collected in the field (M. Güler, unpublished data).
Generally, intracapsular inclusions are classified as embryos, nurse eggs or nurse embryos (Thorson 1950; Gallardo 1976, 1979; Perron 1981; Spight 1981; Deslous-Paoli & Héral 1986; Chaparro et al. 1999; Collin 2003; Oyarzun et al. 2011), nutritive or non-nutritive fluid (Bayne 1968; Clark & Jensen 1981; Penchaszadeh & Miloslavich 2001) and nutritive smears (Krug 2009). To monitor the dynamic and versatile structure of intracapsular inclusions of H. trunculus it was necessary to further classify the nurse eggs as well: eggs, s1 phase (which resulted from eggs that arrested their development in the blastula) and s2 phase (which resulted from eggs that arrested their development after the completion of the blastula), although all of these inclusions were consumed as an extra-embryonic food source by viable embryos.
The viable embryos began ingesting nurse eggs when they reached early veliger (17 days after spawning). In muricidae, nurse egg consumption has been reported to begin at the veliger stage in Nucella emarginata (LeBoeuf, 1971), at the early trochophor estage in N. crassilabrurn (Gallardo, 1979). In other families, nurse egg consumption has been reported to begin at the trochophore stage in Chorus giganteus (Gallardo & Cancino, 2009), at the early veliger in Trophon geversianus (Cumplido et al., 2011), and also at the trochophore stage in Searlesia dira (Rivest, 1983) and the veliger stage in Buccinanops cochlidium (Averbuj & Penchaszadeh, 2009). Several types of ingestion of nurse eggs have been suggested, such as degradation by rotation of the nurse eggs, mechanical destruction of the eggs and ingestion of particles, and the ingestion of the entire nurse egg (Fioroni 1967). According to our observations, intracapsular ingestion of H. trunculus can be described as (i) mechanical disintegration of the eggs via the ciliary movement of the velum and the ingestion of the particles, (ii) ingestion of already disintegrated detached (disintegrated by other embryos or degenerated structures) particles or (iii) ingestion of entire nurse egg (see Supporting Information). Detached pieces were brought into the mouth by the flow created from by velar movement. Rivest (1981) and Hadfield & Iaea (1989) interpreted the existence of modifications in ciliation as an adaptation for feeding on nurse eggs; according to Moran (1997) this is a specialization for feeding on intracapsular nutrition.
Although the viable embryos consumed all of the structures in the capsules, the fastest and the most intense consumption rates were clearly recorded for s2. Despite existing in the highest number by far, s2 was the first intracapsular inclusion to disappear (Fig. 3). Given this situation, it can be suggested that s2 phase inclusions are the original nurse eggs and viable embryos prefer to consume them first. We also observed this preference behavior. In this case there must be a preference mechanism. It is hard to say that embryos act like a ‘search-and-destroy predator’ and we did not find any proof of that, but it is possible to suggest a couple of theories concerning this situation. First, s2 is an attractive stage to eat. Rivest (1983) suggested that the micromeres of the nurse eggs of Searlesia dira (Buccinidae) have an aid function in the feeding process, providing a ‘handle’ for early embryos. If this is true in the case of H. trunculus, embryos select their prey after direct encounters with the nurse eggs and continue to move if they first encounter nurse eggs with an unfavorable structure (adapted from a feeding selection theory in sea stars, Sommer et al. 1999); only after s2 disappear in the nearby area will the embryos proceed to eat other eggs. Gibson (1997) documented in Boccardia proboscidea (Annelida: Spionidae) that nurse eggs and both planktotrophic and adelphophagic larvae can exist in the same capsule. However, Oyarzun et al. (2011) did not observe that larvae selected nurse eggs or siblings within capsules, but ate whatever was closest to their mouths.
Secondly, a kind of passive selection could have occurred. Dupouy (1964) observed in Theodoxus fluviatilis (Neritidae) some agglutinated nurse egg masses in the capsule. We detected similar circumstances in H. trunculus. According to our observations, intracapsular structures of H. trunculus have the tendency to form clusters in time, except s2 (see Supporting Information). It is possible that embryos eat intracapsular inclusions randomly and consumption of the clustered inclusions is probably more difficult than single inclusions, thus clustered inclusions (e, s1, d) disappear later in the capsule. The second theory seems more logical but clustered inclusions do not form at the beginning of the nurse egg consumption process. The first is valid only if s2 is assumed to have preferable qualities for consumption. In any case, advanced phase nurse eggs (s2) were eliminated first.
We did not observe cannibalism among normal healthy embryos, and the number of viable embryos per capsule did not change during the intracapsular period; however, embryos with broken shells (veliger to pediveliger) were always consumed by healthy embryos. Embryos could not attack or drill healthy siblings during the intracapsular period. Drilling activity did not start until a few days after hatching (see Supporting Information). Thus, embryos can consume large and entire inclusions but embryonic shells appear to provide protection from other embryos during the intracapsular period. Most of the shell damages and breakages observed in the trials probably resulted from our handling activities and it is unlikely that this situation will occur in the field. However, such unwanted damage paved the way for getting some additional information about the feeding abilities of embryos and functions of the protoconch.
In lecithotrophic caenogastropods the most common extra-embryonic energy sources are nurse eggs (in oophagy) that fail to develop or nurse embryos (in adelphophagy) that are consumed by the other embryos before development is complete (see Collin & Spangler 2012). All of the consumed inclusions that were observed in this study showed an arrested development before the beginning of the intracapsular feeding process. Therefore intracapsular development of H. trunculus meets the definition of oophagy rather than adelphophagy, even though the structures called s1 and s2 in this study developed to different stages of blastomere.
Generally, oophagic or adelphophagic development induces highly variation in hatchling sizes (Collin 2003) and such variation could be considered to be an adaptation for larval/post larval survival (Rivest 1983; Krug 2009). Variations in the hatching size were documented in many species that provide extra-embryonic food during embryonic development as well as in some members of the family muricidae (Spight 1976; Gallardo 1979; Rivest 1983; Hoagland 1986; Chaparro et al. 1999; Moran & Emlet 2001; Lloyd & Gosselin 2007). In the present study the sizes of H. trunculus hatchlings ranged from 1040 to 1520 μm. It is probable that the variation in hatching size resulted from variation in the nurse egg/embryo ratios (Rivest 1983; Averbuj & Penchaszadeh 2009) or from different numbers of embryos per capsule (Spight 1976; Averbuj & Penchaszadeh 2009). Chaparro et al. (1999) suggested in Crepidula dilatata that hatching size is a function of the number of nurse eggs consumed by each embryo, which in turn is governed by female sizes. But in this present study, female size did not vary very much. Some embryos remained in egg capsules without feeding even though they could escape; Chaparro & Paschke (1990) observed similar behavior in C. dilatata; which may be another cause of the differences in the hatching size.
Vasconcelos et al. (2004, 2008) defined H. trunculus as a direct-developer and as a species that has highly restricted mobility. In our study, some recently hatched individuals were observed to fit to this definition after hatching as crawling juveniles. However, H. trunculus showed dispersal polymorphism in which most of the hatchlings herein observed completed metamorphosis after a short planktonic non-feeding period (up to 2 days) while others metamorphosed prior to hatching. It is likely that the different hatching types in H. trunculus are a result of variations in the hatching time or the hatching size (Chester 1996) and crawling juveniles are the later form of the pediveliger larvae. This kind of polymorphism may be an adaptive tactic for post-hatching survival but is not a case of poecilogony because females do not produce two different types of viable embryos (Bouchet 1989; Krug 2009).
Radwin & D'Attilio (1976) asked whether encapsulated eggs that arrested development were genetically predetermined to abort or is their abortion accidental and random. Our conclusion is that the aborted development of nurse eggs does not occur randomly. We suggest that if the abortion occurred randomly there would probably have been greater variation in the number of viable embryos from different capsules or capsules from different females. In the present study, the number of embryos per capsule was not significantly different among capsules from the same female and among capsules from different females.




