Germination of phytoplankton resting cells from surface sediments in two areas of the Southern Chinese coastal waters
Abstract
To understand the role of phytoplankton resting cells in the outbreak of algal blooms, particularly harmful algal blooms, surface sediments were collected monthly from April 2007 to March 2008 from two bays near the international ports in the Southern Chinese coastal waters. Sieved sediments were incubated for 20 and 40 days, and germinated vegetative cells were observed. Altogether, 97 taxa were recorded, of which 50 were diatoms and 35 dinoflagellates. Vegetative cells of cyanobacteria, chlorophytes, dictyophytes, euglenophytes, haptophytes, and raphidophytes were also observed. Centric diatoms such as Chaetoceros, Melosira, Skeletonema, and Thalassiosira dominated. Scrippsiella, Gymnodinium, and Alexandrium were common dinoflagellate taxa. Diatom spores germinated in samples from all seasons but were abundant in the autumn and winter samples. Low numbers of dinoflagellate cells germinated in the winter samples. The nanophytoplankton taxa, Gymnodinium corii and Chrysochromulina sp., which have not been recorded in the previous phytoplankton surveys, were abundant, suggesting either their new appearance in the water column or perhaps that they were overlooked in routine phytoplankton monitoring due to their small sizes. Vegetative cells of harmful or potentially harmful taxa were germinated, and some of them such as Amphidinium, Gambierdiscus, Ostreopsis, and Coolia have not previously been reported in the study area. Based on the results of the incubation of sediments from the two bays near the international ports, it is suggested that international shipping increases the risk of the introduction of new phytoplankton species and thus promotes the incidence of harmful algal blooms.
Introduction
A wide variety of phytoplankton species, including diatoms, dinoflagellates, cyanobacteria, chlorophytes, euglenophytes, haptophytes, and raphidophytes, are known to form spores, cysts or other types of resting stages (hereafter referred to as resting cells) as a part of their life history (Head 1996; McQuoid & Hobson 1996). Resting cells are dormant stages produced in response to certain physical-chemical conditions (McQuoid & Hobson 1996). Once resting cells are formed, they sink to the sea floor and become a part of the benthos. Resting cells have thick walls made of refractory organic matter or silicic matter and thus preservation is generally high in sedimentary marine environments (Zonneveld et al. 2008). Sometimes, temporary resting stages may also be produced by vegetative cells when environmental conditions are unfavorable but revert to the motile stages when conditions again become favorable (Figueroa & Bravo 2005). The temporary resting stages are considered to be non-zygotic, and are not resistant to long-time storage (Bravo et al. 2010). Therefore, resting cells are generally regarded as dormant resting stages with a mandatory dormancy period (Anderson & Wall 1978). Resting cells repopulate waters if resuspended and exposed to suitable light, temperature, and nutrients after a period of a forced dormant stage (McQuiod 2002). They serve as a survival strategy to overcome unfavorable conditions (Dale 1983; Patil & Anil 2008). Such seeding from resting cells may affect the phytoplankton community structure, play an important role in species dispersal (Anderson et al. 2003), and initiate blooms of phytoplankton that include harmful species (e.g. Kremp 2001; Smayda 2002; Genovesi et al. 2009).
Harmful algal blooms (HABs) have occurred frequently in marine environments. The global increases in the frequency, duration and geographic scope of HABs have been attributed to anthropogenic nutrient enrichment of coastal waters (Anderson et al. 2002), the introduction of non-indigenous harmful algal species via ships' ballast water (Hallegraeff & Gollasch 2006; Smayda 2007), and changing climate patterns (Hallegraeff 2010; Kosten et al. 2012). The Southern China coast has been one of the fastest developing regions in the world over the past two decades. During this time the region has suffered frequent HABs (Qi et al. 2004; Wang et al. 2009). The high nutrient levels and subtropical climate in this area provide favorable conditions for the growth of phytoplankton (Wang et al. 2009). Also, several large international ports are located in this area, including three of the 10 largest in the world: Port Hong Kong, Port Guangzhou and Port Shenzhen. Intensive shipping increases the risk of introduction of exotic species. Actually, many HABs were first reported in this area and then expanded to other coastal regions (Qi et al. 2004). There are many studies of the distribution of phytoplankton resting cells in Southern Chinese coastal waters especially of dinocysts (Wang et al. 2004, 2007, 2010; Fu et al. 2011). Within the rich spore/cyst assemblages in the surface sediments, HAB species have been found to be abundant (Wang et al. 2004). However, there is limited information concerning the germination of resting cells (Kang et al. 2009).
We hypothesized that intensive international shipping in the Southern Chinese coastal waters may help spread the HAB species and thus increase the occurrence of HABs. In this study, surface sediment samples were collected monthly from April 2007 to March 2008 from Dapeng Bay and Daya Bay, where Port Shenzhen and Port Huizhou, respectively, are located. Sediments were incubated at the nutrient levels, salinity, temperature and light intensity of the local seawater, and germinated phytoplankton, which refers to both germinated phytoplankton cells and their offspring after incubation, was observed. Meanwhile, the phytoplankton community structure in surface water, resting cells in sediments and water parameters were analysed as well. The objective of this study was to examine the germination of phytoplankton resting cells and subsequent growth of vegetative cells, and to obtain a better understanding of their potential for promoting HABs.
Study Areas
The study areas, Dapeng Bay and Daya Bay, are located at the northeast part of the South China Sea in Guangdong Province. The Pearl River is to the west of the two bays. The climate is mild, wet and subtropical with an annual mean air temperature of 22 °C. The coldest months are January and February, with a monthly mean air temperature of about 15 °C, and the hottest months are July and August, with a monthly mean air temperature around 29 °C. The annual salinity usually varies between 22 and 34, and does not fluctuate significantly. The flood season is between May and October, and the dry season from November to the following April.
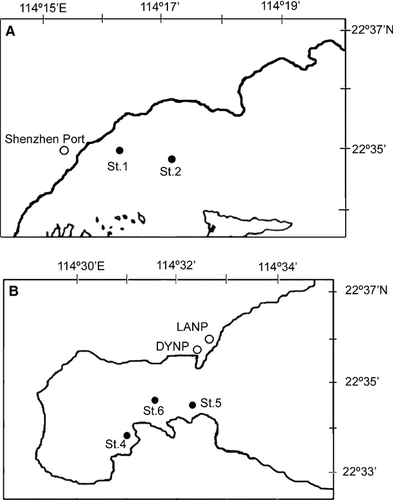
Dapeng Bay is located close to two big cities: Hong Kong and Shenzhen. It is also the location of the Shenzhen Port, which is the fourth largest container port in the world. The port began operation in 1995 and has undergone rapid development over the last decade. This bay has been highly enriched and HABs have occurred frequently (Qi & Huang 1997). However, limited information about phytoplankton has been available during the past decade, especially following the operation of the Shenzhen Port.
Daya Bay is located between Shenzhen city and Huizhou city. It is one of the largest gulfs and important aquacultural areas along the Southern Chinese coast. Port Huizhou, the largest petroleum and natural gas storage and transportation port in Southern China, is in the northwest part of the bay. It is also the site of two nuclear power plants: the Daya Bay Nuclear Power Plant and the LingAo Nuclear Power Plant. This bay is strongly enriched due to an increase in nutrient loading since the 1990s. Inorganic nitrogen levels have increased four- to five-fold during the past decade, resulting in a 40-fold increase in N:P (Wang et al. 2009). The phytoplankton biomass has increased dramatically also, and harmful species have become more prevalent (Qi et al. 2004). For example, this is a site of recurring episodes of paralytic shellfish poisoning (PSP; Anderson et al. 1996). Local shellfish were reported to contain the highest PSP toxin levels along the Chinese coast (Lin et al. 1999).
Methods
Water sampling and analysis
Monthly sampling of water and sediment samples was carried out from April 2007 to March 2008 at two stations (St. 1 and St. 2) in Dapeng Bay and three stations (St. 3, St. 4, and St. 5) in Daya Bay (Fig. 1). The location and sediment characteristics of the five stations are listed in Table 1.
| Station | Location | Longitude | Latitude | Water depth (m) | Sediment character (face) |
|---|---|---|---|---|---|
| St. 1 | Inshore area near to resident town of Dapeng Bay | 114°16′18″ E | 22°35′01″ N | 3.2 | Dark gray sandy clay |
| St. 2 | Inshore area of Dapeng Bay | 114°17′03″ E | 22°34′40″ N | 5.3 | Brown mud-sand |
| St. 3 | Fish cage area of Daya Bay | 114°31′00″ E | 22°33′48″ N | 4.6 | Dark gray sandy clay |
| St. 4 | Shell fishery area of Daya Bay | 114°32′20″ E | 22°34′30″ N | 5.0 | Brown mud-sand |
| St. 5 | Control area of Daya Bay | 114°31′28″ E | 22°34′38″ N | 9.7 | Brown sand-mud |
Water samples were collected from sub-surface (0.5 m below the sea surface) and supra-bottom (0.5 m above the sea bed) with a Niskin sampler. Water temperature, salinity, dissolved oxygen (DO), and pH value were recorded with an YSI meter (YSI-561; YSI Incorporated, Ohio, USA) at the sampling site. The Secchi disc depth was also measured during sampling. The subsamples for chlorophyll a (Chl-a) and dissolved nutrients were filtered in the field through pre-combusted Whatman GF/F glass-fiber filters (0.7-μm pore size). The filters were used for the Chl-a analysis and the filtrates for the dissolved-nutrient analysis. Nutrients including DIN (the sum of NO3-N, NO2-N, and NH4-N), DIP (PO4-P) and DSi (SiO3-Si), and Chl-a were analysed following standard procedures (SOC 1991). For phytoplankton enumeration, a known volume (1 ml) of water sample was fixed with Lugol's iodine for microscopic analysis. The phytoplankton species were enumerated in triplicate and were identified on the basis of the identification keys (Taylor 1987; Fukuyo et al. 1990; Tomas 1997; Hallegreaff et al. 2003).
Sediment sampling
Sediment samples were collected with a light gravity corer designed by the Tokyo University Fisheries Oceanography Laboratory (TFO corer). The top sediments (0–2 cm) from three cores collected during each sampling were put together in a sealed tight bag. These samples were stored in the dark at 4 °C for over 2 months until further treatment, to allow most of the dinocysts to go through the requisite dormant resting stage, and to ensure that most of the viable cells in these sediments were resting cells and not vegetative cells or temporary rest stages.
Sediment treatment and incubation
As the focus of this study was on harmful dinocysts and their potential HABs, sieved sediments were used for germination. Samples were processed by sonication and sieving. The 0–2-cm sediment samples of triplicate cores (about 3.14 cm3 for each core) were gently sonicated (50 Hz) for approximately 30 s in a beaker with 50 ml filtered seawater in a water bath sonicate. As most dinocysts are between 20 and 125 μm, the aliquot was sieved and washed with filtered seawater through a 125-μm mesh and onto a 20-μm mesh. The slurry remaining on the 20-μm mesh was washed in a beaker and collected in a plastic tube, with a final sample volume of 15 ml in filtered seawater. These treated samples were used for germination and analysis of resting cells.
A known volume (2 ml) of treated sediment samples in triplicate was suspended in 30 ml of modified f/2 culture media (Guillard 1973) prepared in autoclaved, filtered, aged local seawater. The nitrogen (N), phosphorus (P) and silica (Si) concentrations in the media were modified to 35.7 μm N, 2.38 μm P and 35.7 μm Si, which correspond to the nutrient concentrations in the local seawater (Wang et al. 2009). Other elements were at the same levels as in the f/2 culture media. As the water temperatures in local seawater were between 20 °C and 30 °C, with an annual mean of 25.4 °C (Wang et al. 2009), incubation was carried out at 25 ± 1 °C. Irradiance was 100 μmol photons·m−2 per s, produced by fluorescent tubes with a 12:12 L/D photoperiod.
A pre-experiment was done to determine the suitable incubation duration. Ten samples from April and May 2007 were incubated for about 2 months and observed every 2 days until there was a precipitous decrease of abundance and diversity of phytoplankton in cultures. The results showed that both diversity and abundance of germinated phytoplankton were significantly lower at the beginning of 20 days (P < 0.05, by Duncan's multiple range test). The abundance was constant (P > 0.05) but species diversities decreased significantly after 40 days of incubation (P < 0.05). Thus, two groups of three replicate flasks for each sample were incubated for 20 and 40 days, respectively, according to the pre-experiment results. After incubation, 20 ml of culture was pipetted from the flasks without stirring up the sediment. Because of the huge workload for phytoplankton analysis, the triplicate cultures were combined and preserved with 1% Lugol's iodine. The fixed samples were concentrated to 5 ml by sedimentation.
Observation of germinated phytoplankton
Phytoplankton species were identified and counted from aliquots of 0.5–1.0-ml sub-samples by Leica DMIRB microscopy (Leica Microsystems Ltd., Wetzlar, Germany). Observation usually was conducted at 200× magnification, or at larger magnifications of 400× or 600× when the exterior or inner constructions of algal cells were unclear. At least three observations were conducted for each sample, and generally over 1000 cells and at least a minimum of 200 cells were observed in each sample with confidence limits of 6% and ±14%, respectively (Anderson & Throndsen 2003). The abundance of germinated phytoplankton was presented as cells·cm−3 of sediment. Some species were identified to the genus level, others to species. One of the dominant species was identified as Gymnodinium corii Schiller by sequences of the internal transcribed spacers of ribosomal DNA (ITS rRNA gene) and divergent domains 1 and 2 (D1–D2) of the large subunit ribosomal DNA (LSU rRNA gene).
Resting cells in sediments
Resting cells from the sieved subsamples (0.5–1 ml) were analysed and counted in a 1-ml counting chamber using a Leica DMIRB inverted microscope at 400× magnification.
Some data concerning resting cells, water parameters and phytoplankton have been published elsewhere (Wang et al. 2010; Fu et al. 2011) and are presented here to establish their relationships with germinated phytoplankton.
Data analysis
Similarity between sampling stations and between periods was analysed. The fourth root-transformed abundance data of germinated phytoplankton, phytoplankton in surface water, resting cells in surface sediments, and raw data of water environmental parameters were used to construct a lower triangular dissimilarity matrix using Euclidean distance. This matrix was then subjected to clustering by hierarchical cluster analysis with IBM SPSS 19.0. A Pearson correlation test was performed to evaluate the relationships of germinated phytoplankton communities with resting cells in sediments, phytoplankton in surface water and the environmental factors.
Results
Hydrographic conditions
The means and ranges of environmental parameters in these two bays during the survey are listed in Table 2. Surface water temperature ranged from a maximum of 30.7 °C in Dapeng Bay and 29.1 °C in Daya Bay (July 2007) to a minimum of 14.6 °C and 13.1 °C (February 2008), respectively. Thermal stratification, as judged by the difference in surface and bottom temperatures, was well developed in all stations in June with the temperature differences of 3.2–6.5 °C, but existed only in St. 5 between May and September. Water was vertically mixed during the rest of the months. Salinity in surface water ranged between a high of 33.8 (Dapeng Bay) and 34.5 (Daya Bay) in May 2007 and a low of 25.8 in July 2007 in Dapeng Bay and 28.4 in June 2007 in Daya Bay. The low salinity in the surface layer recorded in June–August was due to freshwater influx during the flood season.
| St. 1 Annual mean (range) | St. 2 Annual mean (range) | St. 3 Annual mean (range) | St. 4 Annual mean (range) | St. 5 Annual mean (range) | |
|---|---|---|---|---|---|
| Water temperature (°C) | |||||
| Surface | 23.6 (14.7–30.6) | 23.5 (14.6–30.7) | 23.0 (13.5–28.2) | 23.4 (13.9–29.1) | 23.3 (13.1–28.9) |
| Bottom | 22.8 (14.1–29.5) | 22.1 (14.3–30.2) | 23.0 (11.6–27.4) | 22.8 (11.7–27.9) | 22.8 (12.1–27.5) |
| Salinity | |||||
| Surface | 31.4 (28.6–33.5) | 31.1 (25.8–33.8) | 32.4 (28.4–34.4) | 32.6 (29.3–34.5) | 32.6 (29.6–34.4) |
| Bottom | 31.6 (29.1–33.7) | 31.3 (26.0–33.9) | 32.3 (28.4–34.4) | 32.4 (28.5–34.5) | 32.8 (28.6–34.6) |
| Dissolved oxygen (mg·l−1) | |||||
| Surface | 7.1 (3.9–10.7) | 7.2 (4.1–10.4) | 5.5 (4.0–6.7) | 7.6 (5.8–11.2) | 8.5 (5.2–12.7) |
| Bottom | 5.8 (3.2–8.3) | 6.0 (3.1–7.9) | 4.7 (3.0–7.3) | 6.1 (4.4–8.7) | 5.9 (3.4–9.8) |
| pH value | |||||
| Surface | 8.2 (7.8–8.6) | 8.2 (7.9–8.7) | 8.3 (8.0–9.3) | 8.5 (8.1–8.8) | 8.4 (8.0–8.8) |
| Bottom | 8.2 (7.7–8.6) | 8.2 (7.8–8.8) | 8.3 (8.0–9.3) | 8.4 (8.1–8.7) | 8.4 (7.9–8.8) |
| Secchi disc depth (m) | 1.3 (0.9–1.6) | 1.7 (1.3–2.8) | 2.4 (1.5–3.8) | 2.1 (1.4–3.4) | 1.9 (0.7–2.6) |
| DIN (μm) | |||||
| Surface | 27.1 (1.8–59.5) | 15.5 (1.5–32.4) | 12.2 (1.7–20.2) | 6.8 (3.2–13.3) | 6.8 (1.0–14.5) |
| DIP (μm) | |||||
| Surface | 1.01 (0.05–2.64) | 0.50 (0.03–1.15) | 0.40 (0.03–1.23) | 0.17 (0.04–0.39) | 0.18 (0.04–0.51) |
| DSi (μm) | |||||
| Surface | 9.8 (5.2–14.3) | 11.0 (5.8–17.1) | 10.4 (5.6–15.4) | 6.7 (4.2–12.4) | 6.2 (4.0–9.3) |
The DO concentrations in surface water were 3.9 mg·L−1 (June 2007)–10.7 mg·L−1 (May 2007) in Dapeng Bay and 4.0 mg·L−1 (September 2007)–12.7 mg·L−1 (June 2007) in Daya Bay. DO concentrations in bottom waters were lower than those in surface waters. The maximum difference was observed during the flood season. Water transparency measured by Secchi disc ranged between 1.3 and 2.4 m, far less than the water depth (Table 1), which indicated the presence of aphotic environments in the bottom water and sea bed.
Nutrient concentrations (DIN, DIP, and DSi) were higher in Dapeng Bay than in Daya Bay. Maximum nutrient concentrations were found during the flood season (June–September), and low concentrations were recorded in dry winter season.
Phytoplankton from the water column
Altogether 172 phytoplankton species (115 diatoms, 39 dinoflagellates, and 18 others) were recorded in Dapeng Bay, and 175 (114 diatoms, 36 dinoflagellates, and 25 others) in Daya Bay. Average cell numbers of phytoplankton were comparable among stations, and varied from 117 to 175 cells·ml−1 (Table 3). Diatoms dominated the phytoplankton community in both bays, accounting for an average of >85% of total cell numbers. Dinoflagellates were the second most abundant group, contributing an average of 3.5–11.8% of the total phytoplankton. Among them, Chaetoceros spp., Skeletonema costaum, Thalassiosira spp., Pseudo-nitzschia spp., Leptocylindrus danicus and Thalassionema nithschioides were predominate diatoms, and dinoflagellate species Ceratium spp., Prorocentrum spp., Scrippsiella trochoidea, and Alexandrium tamarense occurred commonly. Concentration of Chl-a varied from a low of 0.7 μg·L−1 to a high of 12.6 μg·L−1.
| St. 1 Annual mean (range) | St. 2 Annual mean (range) | St. 3 Annual mean (range) | St. 4 Annual mean (range) | St. 5 Annual mean (range) | |
|---|---|---|---|---|---|
| Diatom cell number | 143 (2–1041) | 157 (4–1315) | 113 (44–2723) | 144 (35–3921) | 148 (24–3640) |
| Dinoflagellate cell number | 3 (0.1–116) | 5 (1–147) | 1 (2–40) | 4 (4–95) | 7 (5–466) |
| Total cell number | 153 (9–1193) | 175 (11–1435) | 117 (47–2740) | 149 (40–4018) | 157 (37–3684) |
| Diatom proportion | 95.5 (78.3–100) | 92.9 (74.9–96.2) | 89.1 (58.8–99.4) | 90.5 (58.3–99.1) | 86.2 (48.9–99.8) |
| Dinoflagellate proportion | 3.5 (0–15.2) | 6.1 (1.5–20.8) | 8.6 (0.5–40.6) | 8.4 (0.5–41.7) | 11.8 (0.2–45.5) |
| Chl-a (μg·l−1) | 3.6 (1.3–12.6) | 4.9 (0.9–11.4) | 2.7 (1.0–5.9) | 3.7 (0.7–9.1) | 3.4 (1.2–8.3) |
Resting cells from the surface sediments
Altogether 83 species (51-dinocysts, 29-diatoms/diatom spores and three other groups) were recorded from the surface sediments of the five stations. Diatom spores of Chaetoceros spp., Skeletonema costatum, Thalassiosira spp., and benthic species Navicula and Pleurosigma occurred abundantly in sediments. Cyst of Scrippsiella trochoidea was the most abundant dinocyst, and cysts of the HAB species such as Alexandrium spp., Cochlodinium sp., and Lingulodinium polyedrum occurred commonly as well. Some dinocysts, such as cysts of Pyrodinium bahamense, Ensiculifera spp., and Fragilidium spp., whose vegetative cells had not been recorded previously in studying area, were observed during the survey.
Annual means and ranges of cell number and proportion of resting stages in surface sediments are listed in Table 4. The annual average number of total resting cells ranged from 672 cells·cm−3 (St. 5) to 1711 cells·cm−3 (St. 3). Diatoms were abundant in St. 3 and dinocysts in St. 5. Diatom spores contributed the majority of the resting stage assemblages with average proportions of 56.1–80.7%, and dinocysts contributed an average of 15.6–43.9%.
| St. 1 Annual mean (range) | St. 2 Annual mean (range) | St. 3 Annual mean (range) | St. 4 Annual mean (range) | St. 5 Annual mean (range) | |
|---|---|---|---|---|---|
| Diatom spore number | 1013 (99–2369) | 475 (59–1026) | 1290 (257–6317) | 581 (21–1125) | 411 (132–1362) |
| Dinocyst number | 102 (6–211) | 173 (33–421) | 214 (95–421) | 163 (7–365) | 260 (79–513) |
| Total resting cell | 1203 (118–2566) | 881 (197–3237) | 1711 (411–6475) | 750 (103–1323) | 672 (263–1461) |
| Diatom spore proportion | 80.7 (47.3–100) | 63.4 (4.9–90.7) | 68.7 (26.0–97.6) | 76.2 (59.8–89.3) | 56.1 (23.5–93.2) |
| Dinocyst proportion | 15.6 (0–37.5) | 29.3 (9.3–70.0) | 21.8 (2.4–58.1) | 23.2 (10.7–38.1) | 43.9 (5.4–76.5) |
Germinated phytoplankton
All sediment samples from the two bays contained phytoplankton resting cells, which germinated into vegetative cells during the incubation. Altogether 97 taxa were recorded, but the number of species was likely to be higher because not all taxa were identified to species level. Seventy-three taxa were recorded in cultures of samples from two stations of Dapeng Bay, and 81 taxa in three stations of Daya Bay. Diatoms and dinoflagellates were the most diversified groups, with 50 and 35 taxa observed, respectively. Vegetative cells in other groups such as cyanobacteria, chlorophytes, dictyophytes, euglenophytes, haptophytes, and raphidophytes occurred as well.
Centric diatoms such as Chaetoceros, Melosira, Skeletonema, and Thalassiosira, and benthic diatoms such as Navicula dominated. Scrippsiella trochoidea was the most abundant bloom dinoflagellate during incubation. Alexandrium tamarense, Gymnodinium corii, Cochlodinium sp., and Heterocapsa spp. also occurred commonly. Other HAB species such as Amphidinium, Gambierdiscus toxicus, Ostreopsis sp., Coolia sp., Chattonella marina, and Heterosigma akasiwo were observed occasionally. A haptophyte species, Chrysochromulina sp., was abundant in some cultures.
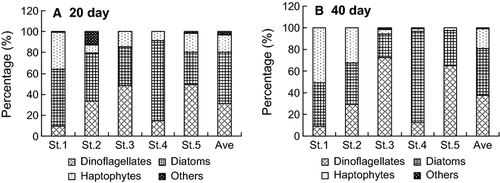
The species compositions of germinated phytoplankton were comparable after 20 and 40 days of incubation (Fig. 2). Diatoms were the largest group quantitatively, with average proportions of 49.3% and 43.2% after 20 and 40 days of incubation, respectively. Dinoflagellates were the second largest group, contributing 31.1% and 37.7%, respectively. Haptophytes contributed significantly, with average proportions of 16.5% and 18.5% after 20 and 40 days of incubation, respectively. Species compositions varied among stations; high proportions of haptophytes were recorded at the two stations in Dapeng Bay, especially after 40-day incubation. Dinoflagellates dominated in St. 3 and St. 5, whereas diatoms predominated in St. 4.
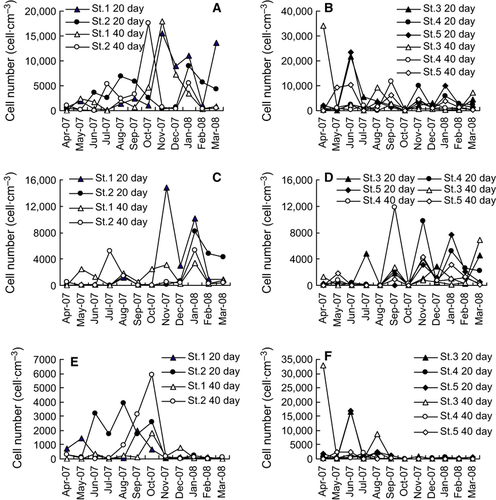
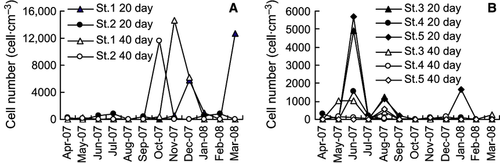
The germinated phytoplankton cell numbers were comparable in the two areas, the average cell numbers being 4193 and 3428 cells·cm−3 (20 days) and 3185 and 3401 cells·cm−3 (40 days) in Dapeng Bay and Daya Bay, respectively. However, seasonal changes in the number of germinated phytoplankton cells were different between the two sites (Fig. 3A and B). Peak abundances occurred in autumn and winter samples from Dapeng Bay, but in spring and summer samples from Daya Bay. Cell densities were low in St. 4 (Table 5). The average cell numbers were 4856, 3530, 3704, 2465, and 4116 cells·cm−3 in the five stations for the 20-day incubation, and 3468, 3102, 5041, 1980, and 3182 cells·cm−3 for 40-day incubation, respectively (Table 5).
| 20-day incubation | 40-day incubation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| St. 1 | St. 2 | St. 3 | St. 4 | St. 5 | St. 1 | St. 2 | St. 3 | St. 4 | St. 5 | |
| Diatoms | 2642 | 1629 | 1379 | 1897 | 1298 | 1386 | 1182 | 1073 | 1663 | 1036 |
| Dinoflagellates | 471 | 1181 | 1798 | 368 | 2010 | 304 | 908 | 3655 | 255 | 2077 |
| Haptophytes | 1696 | 283 | 518 | 195 | 733 | 1776 | 1003 | 228 | 47 | 59 |
| Total phytoplankton | 4856 | 3530 | 3704 | 2465 | 4116 | 3468 | 3102 | 5041 | 1980 | 3182 |
Diatom spores germinated in all seasons, and were abundant in autumn and winter samples (Fig. 3C and D). Cell numbers of diatoms were higher in 20-day incubation than in 40-day incubation, with the averages of 1769 and 1268 cells·cm−3, respectively. Diatoms were abundant in St. 1 and St. 4, where dinoflagellate cell numbers were lower. The diatom Chaetoceros was the most abundant, but Skeletonema and Thalassiosira were also common.
Peak abundances of germinated dinoflagellates occurred in samples between April and October (Fig. 3E and F). Few cells germinated in the late autumn and winter samples. The germination of dinoflagellates mostly occurred in the summer–autumn samples in Dapeng Bay, but in the spring–summer samples in Daya Bay. The maximum abundance in Daya Bay, recorded as 32,821 cells·cm−3 in the sample from St. 3 in April 2008, was significantly higher than that in Dapeng Bay, recorded as 5888 cells·cm−3 in samples from St. 2 in October 2007. Cell numbers of germinated dinoflagellates were lowest in samples collected from St. 4 (average 311 cells·cm−3) and next from St. 1 (average 387 cells·cm−3). Dinoflagellate cell numbers were comparable in 20- and 40-day incubations with averages of 1165 and 1440 cells·cm−3, respectively.
Scrippsiella trochoidea was the dominant dinoflagellate species, and showed the same pattern of cell number variations as total dinoflagellates (Fig. 4A and B). This species contributed more than 50% of total dinoflagellates in most cultures, with an average of 56%, and its average contribution to total germinated phytoplankton was as high as 19%.
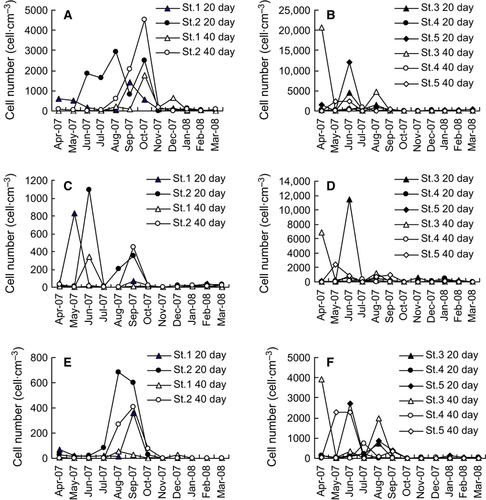
Gymnodinium corii was a common dominant species, particularly in the spring and summer samples (Fig. 4C and D). Gymnodinium corii was more abundant in samples from Daya Bay, with maximum cell numbers of 1089 and 11,442 cells·cm−3 in Dapeng Bay and Daya Bay, respectively.
Alexandrium spp., the main species producing PSP toxins, occurred in high numbers. Alexandrium tamarense was the most common species. Cell numbers were higher in Daya Bay (Fig. 4E and F), with a maximum number of 3923 cells·cm−3 from St. 3 in April 2007 in the 40-day incubation.
The haptophyte species Chrysochromulina occurred abundantly in some cultures; however, peak abundances did not occur synchronously for the 20- and 40-day incubations (Fig. 5). Higher numbers of Chrysochromulina were recorded in cultures of samples from Dapeng Bay.
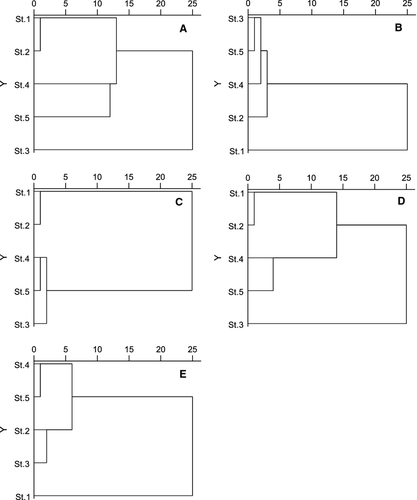
Cluster analysis
Similarity analysis between sampling stations is illustrated in Fig. 6. Cluster analysis based on the abundance of germinated phytoplankton in the 20-day incubation divided the five stations into one group (St. 1, St. 2, and St. 4) and two ungrouped stations (Fig. 6A). For 40-day incubation, four stations were clustered together in one large group, and St. 1 was ungrouped (Fig. 6B). Cluster analysis based on phytoplankton in surface water divided the five stations into two groups (Fig. 6C), one group including two stations in Dapeng Bay (St. 1 and St. 2) and the other group the three stations in Daya Bay (Sts 3–5). The cluster results based on resting stages in surface sediments were similar to those of germinated phytoplankton in the 20-day incubation, in which St. 1, St. 2, and St. 4 grouped together, and St. 5 and St. 3 were ungrouped (Fig. 6D). In a dendrogram based on water parameters, St. 4, St. 5, and St. 2 grouped together, and St. 3 and St. 1 were ungrouped.
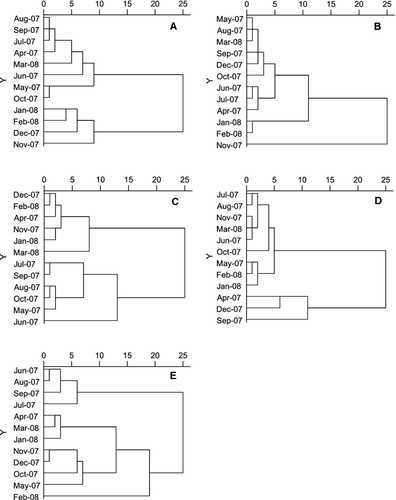
Cluster dendrograms of the sampling months are illustrated in Fig. 7. Twelve months were divided into two groups based on the abundance of germinated phytoplankton in the 20-day incubation (Fig. 7A). The first group included all months from spring to autumn (April–October 2007, and March 2008). The second group included months from November 2007 to the following February, which represented the dry cold season. A cluster dendrogram based on 40-day incubated germinated phytoplankton was similar to that based on 20-day incubation, which included three groups (Fig. 7B). One large group included all months of the first group in figure 7A with the addition of December 2007, a second group was represented by the coldest months (January and February, 2008), and November 2007 was ungrouped alone. The phytoplankton community in surface water divided the 12 months into two groups (Fig. 7C), one including all months in the dry season (November to the following April), and the other the flood season months from May to October. A cluster dendrogram based on resting cells included two groups (Fig. 7D), one large group including 9 months, and the other group consisting of April, December, and September. Concerning environmental parameters (Fig. 7E), the four rainiest months (July–September) were grouped together, and all months in the dry season (November to the following April) and the two adjacent months (May and October) were grouped together.
Correlations of germinated phytoplankton with environmental parameters, vegetative phytoplankton and resting cells
The results of Pearson correlations are presented in Table 6. The abundance of germinated diatoms and dinoflagellates after 20 days of incubation showed a significant positive correlation with the corresponding resting cells. However, no significant relationships were observed between germinated phytoplankton after 40 days of incubation and resting cells. Furthermore, the germinated phytoplankton showed significant positive relationships not only with the corresponding planktonic cells but also with the total phytoplankton in the water column.
| Germinated phytoplankton | Resting cells in surface sediments | Phytoplankton in surface water | ST | SS | DIN | DIP | DSi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diatoms | Dinos | Others | Total | Diatoms | Dinos | Others | Total | ||||||
| 20-day incubation | |||||||||||||
| Diatoms | 0.430 ** | −0.191 | 0.047 | −0.253 | 0.533 ** | −0.095 | −0.494** | 0.520 ** | −0.504** | 0.032 | −0.050 | −0.090 | −0.135 |
| Dinos | 0.145 | 0.400 * | −0.206 | 0.193 | 0.155 | 0.421 * | 0.467 ** | 0.421 * | 0.284 * | −0.357** | 0.029 | 0.034 | 0.052 |
| Others | −0.018 | 0.054 | −0.179 | −0.011 | 0.153 | 0.340 * | 0.393 * | 0.504 ** | 0.229 | −0.381** | 0.194 | −0.025 | 0.086 |
| Total | 0.103 | 0.191 | 0.101 | 0.141 | 0.584 ** | −0.134 | 0.031 | 0.408 * | −0.197 | −0.313* | 0.045 | 0.007 | −0.050 |
| 40-day incubation | |||||||||||||
| Diatom | 0.227 | −0.036 | 0.184 | −0.053 | 0.560** | −0.244 | −0.369* | 0.557 ** | −0.398* | 0.057 | 0.053 | −0.130 | −0.083 |
| Dinos | −0.012 | 0.252 | 0.032 | 0.079 | 0.096 | 0.501 ** | 0.445 ** | 0.504 ** | 0.374 * | −0.185 | 0.126 | −0.125 | 0.080 |
| Others | 0.043 | 0.026 | 0.197 | 0.069 | 0.067 | −0.082 | 0.413 * | 0.080 | 0.337 * | −0.058 | 0.281 | 0.060 | 0.031 |
| Total | 0.028 | 0.280 | 0.284 | 0.287 | −0.452* | 0.089 | 0.270 | 0.544* | 0.104 | −0.151 | 0.237 | −0.162 | 0.034 |
- **P < 0.01, *P < 0.05. Significance level >95% is indicated in boldface.
- Dinos, dinoflagellates; others, other phytoplankton groups except for diatoms and dinoflagellates; total, overall phytoplankton; ST, surface temperature; SS, surface salinity.
The abundance of germinated diatoms declined with increasing water temperature, whereas dinoflagellates and the other phytoplankton groups increased with water temperature and decreased with salinity. However, there were no significant correlations between the germinated phytoplankton and nutrients.
Discussion
Characteristics of the germinated phytoplankton community
Phytoplankton studies showed that phytoplankton communities in the two sea areas were dominated by diatoms, which contributed over 85% of the total cell numbers (Table 3). However, in germinated phytoplankton, the diatoms contributed <50%. As the purpose of this study was to understand the potential of germination and growth of harmful flagellate species, samples were screened through a 20-μm sieve. Some small diatom spores might pass through the sieve, resulting in relatively low numbers of diatom spores in sediments (Table 4) and germinated vegetative cells in cultures. However, the species richness of germinated diatoms was higher than that of the observed resting spores in sediments (Wang et al. 2010; Fu et al. 2011). Since taxonomic studies of diatom spores are limited, many spores might have been overlooked during the observations, even though those missing spores germinated during the incubation.
The species richness of germinated dinoflagellates was lower than that of dinocysts. The germination of dinocysts is controlled not only by environmental conditions (Keafer et al. 1992), but also by an endogenous biological rhythm (Anderson & Keafer 1987; Matrai et al. 2005). For some dinocysts, a required maturation period may also restrict germination to a particular season (Anderson & Keafer 1987; Kremp & Anderson 2000; Matrai et al. 2005). In our study, low numbers of dinoflagellate cells germinated in the winter samples. Furthermore, most dinocysts require a 2-week to 6-month dormant resting period before the onset of germination (Ishikawa & Taniguchi 1994; Kremp & Anderson 2000; Tobin & Horner 2010). Although our samples had been stored under cold, dark conditions for over 2 months to ensure that dormancy conditions were met, some cysts still might not have matured. Meanwhile, some dinocysts might have germinated but their vegetative cells were outcompeted by other species because of their low growth rates. This explains why cysts of heterotrophic dinoflagellates such as Protoperidinium spp. were abundant in sediment samples (Wang et al. 2010; Fu et al. 2011) even though their vegetative cells were seldom observed in germinated phytoplankton.
The germination of resting cells provides the initial cells in the upper water column. However, only when the vegetative cells multiply successfully to a certain population size does this species have the opportunity to survive and become established in natural seawater. Therefore, our results represent the vegetative cells which successfully survived and subsequently multiplied after germination. Those which had germinated but could not survive through incubation, were not included in our germinated phytoplankton community. Due to the different conditions in the laboratory and natural seawater, the germinated phytoplankton community structure might differ from that in natural communities.
Potential effects of experimental design on germinated phytoplankton community
Our sediments were stored in tightly closed bags for over 2 months before incubation to ensure most of the resting stages passed through the compulsory dormant stage, and to restrain the viability of vegetative diatoms and temporary resting stages. As not all resting stages require light for germination, oxygenic storage conditions will allow some cysts to germinate, such as those of dinoflagellates (Anderson et al. 1987) and haptophytes (Imai & Yamaguchi 2012). Therefore, anoxic conditions are generally used for storage of resting stage samples because anoxia prevents germination (Anderson et al. 1987). However, as some resting cells have a lower germination potential after exposure to anoxia and high sulfide concentrations (Kremp & Anderson 2000), storage in anoxic conditions may also affect germination (Pertola et al. 2006). Meanwhile, the bottom water and benthic base were not anoxic in the study areas (Table 2), and the storage conditions in this study were comparable to those of natural benthic conditions (dark, with low oxygenic condition).
Although the sediment samples were sieved through a 20-μm mesh in this study, nano species (<20 μm) dominated in germinated phytoplankton. To prevent the disruption of resting cells in sediment, low frequency water bath sonication (50 Hz) was used to separate resting cells from sediment particles. Quite a number of cells <20 μm might not have been separated from larger sediment particles, and might have been retained in the samples. Some clogging of the 20 μm mesh might have occurred incidentally trapping smaller cells. Phytoplankton cell counts in this study included not only vegetative cells that geminated from resting cells but also those that multiplied during the 20- and 40-day incubation periods. Nano species multiplied quickly after germination, and became dominant in cultures.
The duration of the incubation may affect the germinated phytoplankton structure. It was reported that the maximum density of germinated dinoflagellates occurred between 3 and 5 days of incubation, and dinoflagellates almost disappeared and were replaced by diatoms after 2 weeks of incubation (Persson 2002). The low growth rate of dinoflagellates caused the shift of the phytoplankton community from dinoflagellates to diatoms (Persson 2002). However, the phytoplankton composition showed no significant changes between the 20- and 40-day incubation periods in our study, and the contributions of different groups of phytoplankton were comparable (Fig. 2, Table 5). This is partly because the predominant dinoflagellate species in this study have their own competitive advantages. For example, Scrippsiella trochoidea, the most common and abundant dinoflagellate species, won the phytoplankton competition by its high adaptability, coupled with a quick germination response and a short dormancy period (Wang et al. 2007). Another dominant dinoflagellate, Gymnodinium corii, is competitive with diatoms, possibly due to the small size and high growth rate.
Factors influencing the germinated phytoplankton
Results showed that germinated phytoplankton abundance was positively correlated with phytoplankton in the water column (Table 6), revealing benthic–pelagic coupling between the germination of resting stages and vegetative cells in water column. The germination of resting stages and their resultant vegetative cells have been reported to play an important role as seed populations and the bloom occurrence in dinoflagellate Scrippsiella hagaii (Kremp 2001), raphidophyte Chattonella (Imai & Yamaguchi 2012), and diatoms Chaetoceros and Skeletonema (Patil & Anil 2008). As the cultured phytoplankton not only included those germinated but also reflected the subsequent growth of germinated species, resting cells in sediments showed a positive correlation with germinated phytoplankton only in the 20-day incubation, but no significant relationship with those in the 40-day incubation (Table 6).
The production of resting stages is affected by a variety of environmental factors including nutrients, light, temperature, salinity, and also internal regulation (Kremp et al. 2009; Figueroa et al. 2011). Many authors have reported nitrogen depletion to be the most important environmental variable causing dormancy in marine diatoms and dinoflagellates (McQuoid & Hobson 1996; Patil & Anil 2008; Kremp et al. 2009). However, in this study, nutrients showed no significant correlations with germinated phytoplankton. As the areas studied were located in an inshore enriched embayment where nutrient concentrations were high and the growth of phytoplankton rarely limited (Wang et al. 2009), nutrients would not induce dormancy.
As the germination was undertaken in laboratory conditions at a certain temperature, light intensity, and nutrient concentrations, the germination of phytoplankton is largely determined by the abundance and composition of resting cells in the sediments. Resting cells are mostly produced to overcome unfavorable environmental conditions, such as nutrient limitation, low water temperature, and decreased light intensity (Dale 1983; McQuoid & Hobson 1996; Figueroa et al. 2011). The numbers of germinated diatoms decreased with warming water temperature; however, germinated dinoflagellates increased (Table 6), which suggests that low temperatures accelerated the formation of diatom spores, and high temperatures induced encystment. The area studied is located in Southern China, which experiences typical subtropical weather. Water temperatures are above 25 °C for most of the year, and usually above 30 °C in summer, with the coldest water temperature about 13 °C (Table 2). Since dinoflagellates do not grow well at high temperatures (Wang et al. 2009; Roder et al. 2012), cyst-producing dinoflagellates choose encystment to survive through the hot summer. As the high temperatures occur synchronously with low salinity in flood season, the levels of germinated dinoflagellates declined with an increase in salinity as well (Table 6). Figueroa et al. (2011) also observed that the sexuality in the population of Alexandrium minutum increased significantly as salinity decreased and temperature increased. On the other hand, diatoms adapt to growth at high temperatures, and the peak abundance of diatoms occurred successively during the summer–autumn seasons in the study areas (Wang et al. 2009). The low temperature, decreasing light intensity, and low nutrient levels in winter accelerate the formation of diatom spores (McQuoid & Hobson 1996).
The results of cluster analysis of sampling stations showed quite a good correspondence of germinated phytoplankton in the 20-day incubation and the resting cells in surface sediments (Fig. 6), which was also shown in the correlation analysis (Table 6). Meanwhile, two stations (St. 1 and St. 2) in Dapeng Bay were generally grouped together or were ungrouped (Fig. 6). The results indicated that phytoplankton community structure including vegetative and benthic communities differed between sea areas. From the cluster analyses of the sampling months, the 12 months were generally divided into two groups – the flood season group (May–October) and the dry season group (November to the following April), which suggests that rainfall and subsequently low salinity and high nutrients might play important roles in the seasonal succession of phytoplankton.
The germination of resting cells and HABs
Scrippsiella trochoidea was the predominant species in germinated dinoflagellates in this study. It is also the dominant dinoflagellate and cyst type in Chinese coastal waters, especially in Southern China (Wang et al. 2007). It has bloomed almost every year in our study areas since its first bloom in 1998 (SOA 1998–2009). Its blooms have occurred worldwide in coastal zones as well (Ishikawa & Taniguchi 1994). This species has a great facility for cyst formation and germination (Wang et al. 2007). High abundances of S. trochoidea occurred in the spring–autumn samples in this study, in agreement with its reported bloom seasons (Wang et al. 2007). The results suggest that a rich cyst bed and high germination efficiency contribute to the initial seeding of blooms of S. trochoidea and result in the recurrent blooms in this area.
Gymnodinium corii, a nano species about 10 μm in diameter, was abundant in this study. It was observed in the germinated phytoplankton in cultures of trap sediments from Daya Bay in our previous study, and was identified by scanning electric microscopy and analysis of rRNA gene sequences (Kang et al. 2009). This species has not been reported in previous phytoplankton surveys in Chinese coastal waters. Since the small-sized dinoflagellates are difficult to identify and are not usually determined to the species level in routine monitoring, it is hard to know whether the species is a newcomer or not. A Gymnodinium sp. of similar size and shape as G. corii was abundant in water samples from Daya Bay in May 1998, but it was not identified to species level (Wang et al. 2001). There is limited literature on G. corii and, up to now, no resting cyst has been reported for the species. However, because of its mass appearance in germinated phytoplankton, it would seem that G. corii is cyst-forming. The life history, cyst formation, and cyst shape of G. corii require further study.
Chrysochromulina, which has not been recorded in previous phytoplankton surveys, was abundant in the germinated phytoplankton in this study. We assume therefore that this species exists in water column of the study areas. However, due to its small size it is inconspicuous and easily missed in routine phytoplankton monitoring (<5 μm). Moestrup & Thomsen (2003) have claimed that some species of Chrysochromulina are potentially toxic. The high occurrence of this species in germinated phytoplankton in both bays in this study suggests it has potential as a bloom-former.
The eruption of blooms of rare and novel harmful species and blooms of previously undescribed species are common features of the global increase in HABs (Smayda 2007). Three mechanisms potentially underlie the HAB phenomenon: (i) increasing nutrient enrichment of global coastal waters, (ii) global warming and associated climatic perturbations, and (iii) global dispersal and redistribution of bloom species in ballast water (Smayda 2007). The coasts of Southern China have been experiencing these global trends, with high nutrient level, warming climate, and intensive international shipping. Therefore the risks for HABs have increased. The results of this study showed that vegetative cells of numerous harmful taxa such as Alexandrium tamarense, Gymnodinium, Cochlodinium, Chattonella, and Hetereosigma germinated. Furthermore, toxic taxa such as Amphidinium, Gambierdiscus, Ostreopsis, and Coolia, which have not previously been reported in the study areas, were present. Although it is hard to say whether these taxa are newcomers, it is a fact that new harmful species and new HABs have increased sharply in Chinese coastal waters since the year 2000. The coast of Southern China is the prime region for the first appearance of most of the undescribed species and new blooms in China (Qi et al. 2004). Based on the results of the incubation of sediments from the two bays near the international ports, it is suggested that international shipping increases the risk of the introduction of new phytoplankton species and thus promotes the incidence of HABs.
Acknowledgements
The authors gratefully acknowledge Dr. Larry B. Liddle of Long Island University, USA, for reviewing the manuscript. This work was supported by National Key Technology R & D Program of China (2012BAC07B05), and the National Natural Foundation of China (No. 41076093).




