Methods to quantify components of the excavating sponge Cliona orientalis Thiele, 1900
Abstract
This study applied the loss after combustion (LAC) method and the acid decalcification (ADC) method to quantify different components of an excavating sponge. Samples of dried coral skeleton of Favia sp. invaded by the Indo-Pacific excavating sponge Cliona orientalis Thiele, 1900 were used. The sponge tissue penetrated the 12-mm-thick samples to approximately 10 mm. The average proportional weight of organic matter, siliceous spicules, calcareous substrate and salts in the entire samples was found to be respectively 2.5%, 4.4%, 90.5% and 2.5% of dry weight applying the LAC method, and 2.9%, 5.9%, 89.0% and 2.3% of dry weight applying the ADC method. Respective volumetric proportions of the organic matter, spicules, substrate and salts were then calculated to be 6.4%, 5.5%, 85.2% and 3.0% of volume with the LAC method, and 7.4%, 7.2%, 82.7% and 2.7% of volume with the ADC method. The LAC method showed low variability of data and is simple and fast and therefore is recommended. The ADC method generated very similar results to the LAC method. However, due to the handling involved in the ADC method, more than half of the spicules may be lost and the method is therefore not recommended unless careful data corrections are considered. In addition, the buoyant weight method was used to quantify actual substrate weight in the fresh sponge-substrate samples. This method was found to be at least 97% effective, revealing that buoyant weights can potentially be used to quantify bioerosion rates of excavating sponges. To our knowledge, this is the first study to systemically quantify organic and inorganic components of an excavating sponge and its calcareous substrate, providing improved standard methods for future studies.
Introduction
Quantification of various components of marine sponges is challenging because sponges are made up of composite materials including organic (i.e. tissue and spongin) and inorganic matter (i.e. spicules and salts). This situation is further complicated in the case of excavating sponges due to their cryptic and endolithic lifestyle, and samples always contain a significant proportion of calcareous host substrate and in some cases also microbial symbionts. The technical difficulties associated with separating these components limit research on excavating sponges when a reference unit such as biomass is needed. Currently, the extent of excavating sponges is commonly estimated using the surface area of exposed sponge tissue (e.g. Rosell et al. 1999; Schönberg 2001, 2003; Cebrian 2010; Daume et al. 2010) or the cross-sectional tissue area of substrate slabs (e.g. Bak 1976; Nava & Carballo 2008; Calcinai et al. 2011); however, both methods are inadequate for determining the three-dimensional structure or an accurate estimate of biomass for excavating sponges (see review in Schönberg 2001). A number of studies identified a relationship between the sponge structures visible on the surface and volume of internal tissue (Rosell 1994; Schönberg 2001; Daume et al. 2010; Calcinai et al. 2011) but none of these studies provided estimates of substrate-free endolithic biomass or separated different materials in excavating sponges.
One potential method to quantify various components of excavating sponges is the loss after combustion (LAC) method, which is simple and cost-effective and has been widely used to determine organic and carbonate contents of sediments, based on the assumptions that organic matter is removed at 550 °C and that carbonate evolves into carbon dioxide and oxide at 950 °C (Heiri et al. 2001). The results obtained from the LAC method usually show a low variability of data and are reliable because the involved handling and the associated error are minimal. Likewise, high temperature has been adopted to remove organic matter in free-living sponges (e.g. 500 °C in Barthel 1995 and 550 °C in Schönberg & Barthel 1997) and in the excavating sponge of Cliona celata Grant, 1826 (450 °C in Bell et al. 2002). However, in the case of excavating sponges, spicules still remain with the calcareous substrate after combustion. In this study, an attempt was made to quantify spicules and other components in an excavating sponge using the LAC method.
In addition to the LAC method, the acid decalcification (ADC) method was tested. Since the 1900s, the ADC method has been used to separate coral tissue from its skeleton (Duerden 1902, cited in Brock & Brock 1977). This method was also employed in bioerosion studies to isolate endoliths from the carbonate substrates (see review in Schönberg 2001), among others for clionaid sponges, in order to estimate their biomass, freeing the sponge tissue by dissolving calcareous substrates using hydrochloric acid or nitric acid (e.g. Rützler 1975; Rosell 1993, 1994). During other studies, the sponge tissue itself was digested in hydrogen peroxide or sodium hypochlorite and the sponge biomass was calculated from the sample weight loss after digestion (see summary in Table 1). To date, the proportions of substrate versus the sum of sponge materials have been determined but the different components of the sponges, inhabiting the substrate, have been relatively unexplored. Moreover, the accuracy of the quantitative methods has never been assessed, and there is the added complication that during substrate decalcification, parts of organic material such as sponge tissue may also be digested. Notably, the spicules adhering to this digested organic portion are likely to be lost during decalcification, possibly leading to an underestimation of the mass of spicules. In addition, formaldehyde and ethanol, which are commonly used in the fixation of clionaid sponges (Table 1), can also considerably reduce biomass and may cause a significant bias in the small weights obtained after drying (Wetzel et al. 2005). Different concentrations of reagents, durations and operating temperatures used in the fixation, decalcification or organic removal process may also have contributed to the discrepancy in results among earlier publications such as Bell et al. (2002) and Rose & Risk (1985), in which the organic to inorganic ratios were determined to be 50% in C. celata and 99% in Cliona delitrix Pang, 1973, respectively. This difference appears too big to reflect true conditions, even if the spicular content may be different among the two species.
| Clionaid sponge | Fixation | Decalcification | Removal of organic matter | Reference |
|---|---|---|---|---|
| Cliona aprica Pang, 1973 | Nil | Hydrochloric acid (NRa) | Nil | Rützler (1975) |
| Cliona caribbaea Carter, 1882 | Nil | Nil | Hydrogen peroxide (3 or 4 days) | Acker & Risk (1985) |
| Nil | Nil | Sodium hypochlorite (NR) | Rützler (2002) | |
| Cliona celata Grant, 1826 | Nil | Nitric acid (7%) | Heated nitric acid (7%) | Wesche et al. (1997) |
| Nil | Nil | 450 °C (6 hours) or nitric acid (10%) | Bell et al. (2002) | |
| Cliona delitrix Pang, 1974 | Formaldehyde (4%) | Heated hydrochloric acid (NR) | Hydrogen peroxide (50%, 2 weeks) | Rose & Risk (1985) |
| Nil | Hydrochloric acid (5%, a few days) | Nil | Ward-Paige et al. (2005) | |
| Cliona lobata Hancock, 1849 | Formaldehyde (10%) | Nitric acid (6%) | Nil | Rosell (1994) |
| Cliona orientalis Thiele, 1900 | Formaldehyde (4%, 48 hours) | Hydrochloric acid (1%, 2 hours; then 5%, 22 hours) | 550 °C (1 hour) | The present study |
| Cliona cf. orientalis Thiele, 1900b | Ethanol (95%) | Nil | Hydrogen peroxide (35%, 4 days) | Bergman (1983) |
| Cliona viridis (Schmidt, 1862)c | Formaldehyde (10%) | Nitric acid (6%) | Nil | Rosell (1993) |
| Nil | Nil | Hydrogen peroxide (NR) | Cerrano et al. (2007) | |
| Pione lampa (de Laubenfels, 1950)d | Nil | Hydrochloric acid (NR) | Nil | Rützler (1975) |
| Nil | Nil | Sodium hypochlorite (NR) | Rützler (2002) | |
| Nil | Hydrochloric acid (5%, a few days) | Nil | Ward-Paige et al. (2005) | |
| Pione vastifica (Hancock, 1849)d | Formaldehyde (10%) | Nitric acid (6%) | Nil | Rosell (1994) |
| Nil | Nitric acid (7%) | Heated nitric acid (7%) | Wesche et al. (1997) | |
| Formaldehyde (4%, 24 hours) | Sodium citrate and formic acid (NR) | Nil | Steindler et al. (2001) |
- a NR, Concentration or duration not reported.
- b Described as C. viridis in Bergman (1983).
- c Described as Cliona nigricans Schmidt, 1862 in Cerrano et al. (2007) but re-classified as C. viridis in Rosell & Uriz (1991) and Barucca et al. (2007).
- d Described as Cliona sp. in the cited literature but re-classified as Pione sp. in Rosell & Uriz (1997).
An inability to accurately quantify biomass, or other components of excavating sponges, leads to difficulties when estimating bioerosion. Moreover, most methods used to quantify bioerosion by excavating sponges are destructive (see review in Schönberg 2001). For example, bioerosion rates of Cliona spp. are commonly assessed by attaching a sponge graft to a calcareous block and determining how far the sponge penetrates the block by measuring the change in block dry weight over time (e.g. Schönberg 2002; Holmes et al. 2009). However, for the block to be dried and reweighed, the sponge graft has to be removed, allowing the data to be obtained at only one point in time. A more advanced method to study sponge erosion to date is micro-computed tomography, which allows visualization of the sponge-invaded substrates in three dimensions on live material (e.g. Schönberg & Shields 2008). However, this technology requires small samples, incurs high costs and provides limited access. In an ideal situation, the method to quantify bioerosion would be comparatively simple and cheap and would allow repeated measurements on the live animal. Since the 1960s, scientists have been measuring buoyant weight (BW) of corals, which involves underwater weighing of live corals, resulting in an estimate of the weight of coral skeleton with an accuracy of ±1% (Bak 1973, 1976; Jokiel et al. 1978; Davies 1989). Despite the simplicity and accuracy of the BW method and its potential use for determining the mass of calcareous substrate invaded by an excavating sponge, to date it has not been employed in this context. However, we regard the BW method as useful for repeated measurements on live organisms and possibly more accurate in comparison with the traditional and usually destructive methods to study bioerosion, allowing a higher resolution. We therefore wanted to test the effectiveness of BW to estimate the mass of the carbonate substrate invaded by an excavating sponge. Due to its isosmotic nature and high water content (Barnes 1980), sponge tissue is assumed to have the same density as the surrounding seawater and thus not to contribute to BW. However, because of the high density of siliceous sponge spicules, which is similar to opal (Sandford 2003), the actual weight of the calcareous substrate may be overestimated in the BW method and a correction factor may be required, which we will provide from our results.
Overall, the present study aimed to modify and adapt the LAC and ADC methods in order to calculate weight and volumetric proportions of organic and inorganic contents of the Indo-Pacific excavating sponge Cliona orientalis Thiele, 1900, a sponge representing all the methodological difficulties described above. We also assessed the methods for their advantages and disadvantages, notably for bias that can be introduced by handling. In addition, the effectiveness of using BW to estimate mass of the calcareous substrate invaded by C. orientalis was evaluated by comparing the BW results with results on the actual weight of the substrate calculated through the LAC and ADC methods. To our knowledge, this is the first study to systemically quantify the organic and inorganic contents of an excavating sponge and the calcareous substrate it inhabits.
Material and Methods
Sample collection
Cliona orientalis was collected from invaded skeletons of Favia sp. at 1.5 m depth off Peel Island, Moreton Bay, Australia during low tide using a hammer and chisel. Samples were immediately transported to the laboratory at the University of Queensland, frozen at −20 °C and later carefully cut to small cylinders with a height of 12 mm and a diameter of approximately 10 mm. Only a thin layer (<1 mm) of continuous tissue of C. orientalis was situated on the surface, while most of the sponge tissue penetrated the substrate to approximately 10 mm. Other endolithic organisms were not visible. The taxonomic identity of C. orientalis was confirmed by spicule morphology following standard methods (Schönberg 2000). In this study, all dried sponge-substrate samples (i.e. the substrate of Favia coral skeletons with its upper part invaded by C. orientalis) were assumed to comprise four components: the organic content (i.e. sponge tissue plus its dinoflagellate symbiont Symbiodinium sp.), siliceous spicules, calcareous substrate and salts. All samples, after each process of drying or combustion at 100, 550 or 950 °C, were cooled in a desiccator before being weighed at room temperature at 22 °C. All weights, including buoyant weights, were measured using the same electronic balance to 0.1 mg accuracy (A200S, Sartorius, Göttingen, Germany). Three preliminary studies were carried out to optimize the processes of drying, desalination and combustion, and to determine the effects of temperature, formaldehyde and hydrochloric acid on the weights of organic matter, spicules or substrate in the sponge-substrate samples. Afterwards, sponge components were quantified in the main studies.
Preliminary studies
Optimization for drying, desalination and combustion processes
Ten defrosted sponge-substrate samples were reconditioned in artificial seawater with a salinity of 34‰ for 3 h, drip-dried on tissue paper, weighed (wet weight; WW), and then dried at 100 °C. All samples were weighed again at the 0.5th, 1st, 2nd, 3rd, 6th, 12th, 18th and 24th h during the drying period in order to determine the optimal incubation time to obtain a constant dry weight including salts (DSW). The completely dried samples were individually rinsed in 200 ml of distilled water for 5 min to remove the salts, drip-dried, dried at 100 °C and then reweighed, obtaining the desalinated dry weight (DW). To ensure recapture of all particles during the rinsing process, the water was filtered through a GF/F glass microfiber filter paper (pore size: 0.7 μm; Whatman, Kent, UK), which was pre-dried at 100 °C, pre-weighed and weighed again after the filtration and drying. The obtained weights were compared with the blank filter weights to determine the weight of any loose materials. The procedures were repeated six times to determine the optimal rinsing frequency to achieve a constant DW.
After that, the desalinated samples were transferred into pre-weighed crucibles, keeping a sample to crucible ratio of approximately 10% by weight. Combustion proceeded in a muffle furnace (Eurotherm 91e, Lenton, Market Harborough, UK) at 550 °C for 120 min and then at 950 °C for further 120 min. All samples were reweighed at the 8th, 15th, 30th, 60th and 120th min of each combustion period in order to determine the optimal combustion time to obtain a constant ash weight at 550 °C and at 950 °C (AW550, AW950). Blank crucibles were also combusted at 550 and 950 °C but only weighed before and at the 60th and 120th min during each combustion period to ascertain constant crucible weight.
Effects of temperature on the weights of spicules and substrate
Ten defrosted sponge-substrate samples were decalcified in 5% hydrochloric acid for 24 h and rinsed in distilled water. Then organic matter in the decalcified sponge samples was digested in 12.5% sodium hypochlorite for 3 days. The supernatant was carefully decanted and the remaining solution with the layer of spicules was washed out with 100 ml of distilled water and filtered through a GF/F filter, which was pre-combusted at 550 °C and pre-weighed. The filter, which was coated with the spicules after filtration, was dried at 100 °C overnight, weighed, combusted at 550 °C for 1 h to test the temperature effect on clean spicules, and then reweighed. Weight changes in the spicules were compared by subtracting the pre-combusted weight of the filter from the corresponding weights of the dried and 550 °C combusted filters with spicules. Similarly, 10 pieces of blank GF/F filters were subjected to the same process as a control. Further combustion at 950 °C was not attempted during this process as it exceeds the temperature tolerance of the GF/F filter. To determine the weight change in spicules from 550 °C to 950 °C, 10 extra samples of spicules were extracted from Cliona orientalis in pre-weighed crucibles at 550 °C following the ADC method described below in more detail. The crucibles containing spicules were then weighed before and after further combustion at 950 °C for 1 h.
In addition, fragments of skeleton of Favia sp. without sponge tissue were used to study the effects of temperature on the substrate material. Ten skeleton cylinders (12 mm in height; 10 mm in diameter) were desalinated, dried, weighed and then cleaned in 12.5% sodium hypochlorite for 48 h. All samples were rinsed, dried and reweighed, and the resulting weight reduction indicated the weight of associated organic material, including microendoliths if present. The skeleton cylinders were then transferred in pre-weighed crucibles and weighed before and after 1 h combustion at 550 °C, and then after 1 h combustion at 950 °C to determine the temperature effect on the substrate material.
Effects of formaldehyde, hydrochloric acid, and handling on the weights of organic matter, spicules and substrate
During the ADC method, formaldehyde was used to minimize the digestive effect of the subsequently used hydrochloric acid on Cliona orientalis during decalcification. A respective preliminary study was divided into two parts. Part A aimed to determine the shortest possible fixation time in formaldehyde which provided the greatest resistance against the subsequent digestive effect of hydrochloric acid on organic matter and spicules of the sponge during decalcification. The subsequently observed loss of spicules determined in Part A is here interpreted as caused by both chemical effect and handling error. Hence, Part B aimed to assess the real effects of formaldehyde and hydrochloric acid on spicules using a free-living clionaid sponge that did not require fixation and decalcification for the extraction of spicules. The difference in the loss of spicules obtained from Part A and Part B was attributed to the handling effect with the ADC method.
In Part A, desalinated and pre-weighed sponge-substrate samples containing C. orientalis were divided into 10 groups (n = 3), which were separately fixed in 40 ml of 4% formaldehyde for 0, 3, 6, 12, 24, 48, 72, 120, 168 and 336 h, respectively. All fixed samples were rinsed in distilled water and decalcified in 1% hydrochloric acid for 2 h and then in 5% of the acid for further 22 h. All decalcified samples were rinsed, dried, weighed (DWDecal) and then combusted at 550 °C for 1 h to remove organic matter and leave behind the spicules. Samples were weighed again (AWDecal550). The values of AWDecal550 were corrected according to our earlier results on the reduced weight of spicules due to combustion at 550 °C. The difference between DWDecal and the corrected AWDecal550 yielded ash-free dry weight, i.e. weight of organic matter after the fixation-decalcification treatment. Moreover, our results from the LAC approach were used to calculate the initial weights of spicules and organic matter in the desalinated sponge-substrate samples used in Part A. Net weight changes in the sponge spicules and organic matter after the whole fixation-decalcification treatment were determined by comparing the corrected AWDecal550 to the calculated initial weight of spicules, and comparing the ash-free dry weight to the calculated initial weight of organic matter, respectively. Similarly, 10 pieces of pre-cleaned Favia skeleton cylinders without sponge tissue were subjected to the same fixation-decalcification treatment in order to determine the effects of formaldehyde and hydrochloric acid on weight of the host substrate.
In Part B, the free-living Spheciospongia papillosa Ridley & Dendy, 1886 was sampled from Ningaloo Reef, Western Australia. Spheciospongia papillosa is a very close and putatively congeneric relative of C. orientalis and has the same types of spicules (Y. Ise, pers. comm.). Ten samples of S. papillosa were combusted at 550 °C to remove organic matter. The extracted spicules were then re-suspended in distilled water and filtered through GF/F filters, which were pre-combusted at 550 °C and pre-weighed. Each filter coated with the spicules was dried, weighed and then placed in 4% formaldehyde for 48 h. Each filter was collected and the formaldehyde solution was then filtered through the same respective filter for each sample, spicule-coated side up. The filtrate and subsequently 100 ml of distilled water were filtered through the same filter again to recover spicules. The filters coated with spicules were dried and reweighed. Each filter was then placed in 1% hydrochloric acid for 2 h. Additional acid was added to increase the concentration to 5% and the exposure continued for further 22 h. The solution of hydrochloric acid, the filtrate and subsequently 100 ml of distilled water were filtered again through the same filter, which was then dried and reweighed. The putative effects of formaldehyde and hydrochloric acid on the spicules were determined by the weight changes in the filters with spicules before and after the corresponding exposures. Similarly, 10 pieces of blank GF/F filters were subject to the same process as a control. Moreover, 10 extra samples of S. papillosa spicules were exposed to distilled water instead of the diluted formaldehyde or hydrochloric acid following the same procedures. The percentage loss of spicules after the distilled water treatment indicated the impact due to distilled water or handling error during the processes in Part B.
Main studies
Buoyant weight method and pretreatment
 (1)
(1) (2)
(2)Results from the preliminary studies were used to employ optimal treatment times and durations in the following. After the BW measurements, the sponge-substrate samples were drip-dried prior to WW measurements. All samples were then dried at 100 °C for 6 h and then weighed again (DSW). Each dried sample was rinsed in 200 ml of distilled water for 5 min and then drip-dried. The process was repeated four times to remove salts. The desalinated samples were dried again (DW) and the difference between DSW and DW yielded the weight of salts (SW). Ten replicates of the desalinated samples were then subjected to the LAC method, and 10 other samples were subjected to the ADC method (Fig. 1).
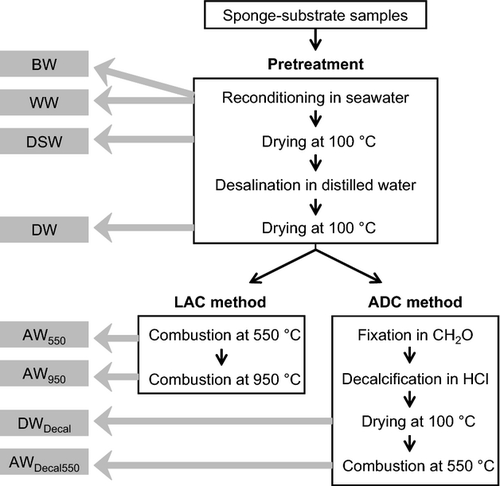
Loss after combustion method
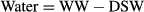 (3)
(3)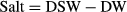 (4)
(4)
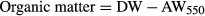 (5)
(5)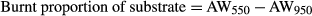 (6)
(6)Acid decalcification method
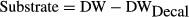 (7)
(7)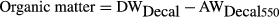 (8)
(8)Data analysis
In the present study, Cliona orientalis penetrated down to approximately 10 mm in the 12-mm-thick coral samples, i.e. 83% of each sample was sponge-invaded. Actual weights of organic matter, spicules, coral substrate and salts in each sample were expressed in percentages relative to DSW of the entire sample calculated through the LAC or ADC method. In addition, to compare our results with those using 100% sponge-invaded substrates in the previous studies, the present results of the weights of substrate and salts were corrected to 83% in order to eliminate the effects of sponge-free substrate and its associated salts prior to the re-calculation of weight proportions of the four components.
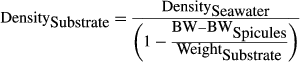 (9)
(9)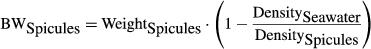 (10)
(10)The effectiveness of the BW method was evaluated by correlating both the uncorrected BW and corrected BW (i.e. BW−BWSpicules) to the weight of substrate obtained by the LAC or ADC method using linear regression. Statistical procedures were carried out using the statistical software package SPSS 16.0 (SPSS, Chicago, IL, USA).
Results
Optimization for drying, desalination and combustion processes
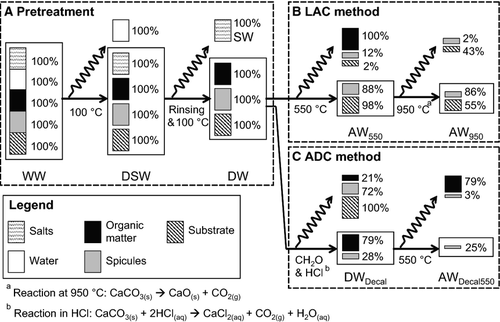
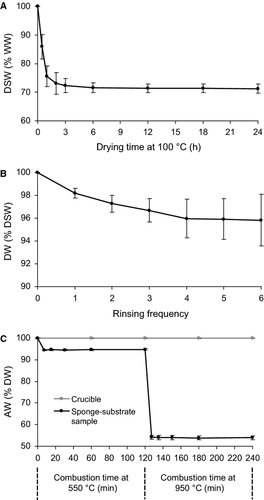
When drying the sponge-substrate samples at 100 °C, DSW stabilized after 6 h at 71.5% WW ± 1.9% (mean ± SD) (Fig. 3a), which was used as the optimal drying time in this study. During the desalination process, a constant DW, i.e. 96.0% DSW ± 1.7%, was observed in the samples after four cycles of rinsing in distilled water and drying (Fig. 3b). The associated standard deviation gradually increased with the rinsing frequency due to introduced handling error. Nevertheless, material loss into the rinsing water was less than 1% DW after four cycles of rinsing and hence negligible. For combustion, constant AWs of 94.6% DW ± 0.3% and 54.0% DW ± 1.0% were obtained after 15 min at 550 °C and 950 °C, respectively (Fig. 3c). The weight of crucibles remained unchanged, i.e. 100% of DW (SD < 0.01%), after a 4-h combustion. For the main studies, a 1-h combustion time was used.
Effects of temperature on the weights of spicules and substrate
The effect of combustion at 550 °C on the GF/F filters was less than a 1% loss of the original weight and therefore negligible. By contrast, the weight of the spicules isolated from Cliona orientalis and collected on GF/F filters was reduced to 88.3% of the initial spicule weight ±1.9% at 550 °C. Another 10 samples of spicules were extracted from C. orientalis in pre-weighed crucibles at 550 °C following the ADC method and further combusted at 950 °C. The weight of spicules after combustion at 950 °C was found to be 97.0% of that at 550 °C ± 1.6%. Hence, by combining the two results, the mean weight of spicules of C. orientalis was reduced to 88% of the initial spicule weight when combusted at 550 °C and further to 86% (i.e. 88% × 97%) when combusted at 950 °C. On the other hand, the weight of organics enclosed in the coral skeleton, possibly including microendoliths, contributed less than 1% of the weight of the substrate and was thus negligible. At 550 °C, the substrate material decreased to 97.9% of the initial substrate weight ± 0.2%, and then to 55.1 ± 0.3% at 950 °C. The obtained results were used to develop the correction factors used in Equations 5, 6 and 8 (Fig. 2b and 2c).
Effects of formaldehyde, hydrochloric acid, and handling on the weights of organic matter, spicules or substrate
In Part A, a longer fixation time in formaldehyde tended to preserve a larger amount of biomass of Cliona orientalis against the subsequent digestive effect of hydrochloric acid. Formaldehyde fixations lasting longer than 48 h led to the stabilization of the mean weights of the organic matter at 78.5% of the initial tissue weight ± 1.3% (Fig. 4a) and of the spicules at 28.0% of the initial spicule weight ± 0.7% (Fig. 4b) following decalcification. Hence, to preserve the largest amount of biomass using the shortest possible fixation time, 48-h fixation in formaldehyde was used for the main studies. On the other hand, whereas 100% of the substrate material was dissolved in hydrochloric acid, the effect of formaldehyde on the substrate was less than a 1% loss of the initial substrate weight and thus negligible. The obtained results were used to develop the correction factors used in Equation 7 (Fig. 2c).
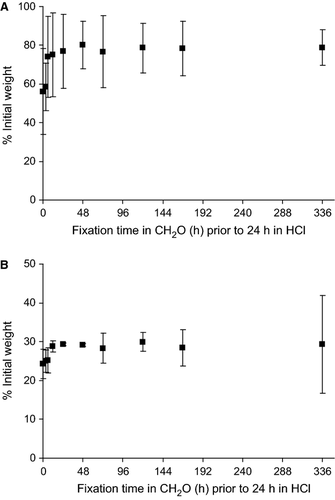
The large reduction in spicule weight (72%) found in Part A was due to both chemical effect and the handling error associated with the ADC method. Hence, Part B was conducted to differentiate the effects of formaldehyde, hydrochloric acid and handling on the mass of spicules. In Part B, spicules from Spheciospongia papillosa were isolated and collected on GF/F filters. The perceived effect of formaldehyde and hydrochloric acid on the blank filters was less than 1% reduction of the initial filter weight and thus negligible. The weight of the S. papillosa spicules was reduced to 94.3% of the initial spicule weight ± 6.0% after the 48-h fixation in formaldehyde and further to 85.2 ± 7.3% after the subsequent decalcification in hydrochloric acid. When the spicules were exposed to distilled water instead of formaldehyde or hydrochloric acid following the same procedures, the weight of the spicules decreased to 95.8% of the initial spicule weight ± 4.6%, indicating approximately 4% loss of spicules associated with the effects of distilled water and handling when transferring the spicules between solutions and filters. Hence, formaldehyde has a minimal effect on spicules, as the loss of spicules due to formaldehyde was similar to that due to distilled water.
Approximately 9% (i.e. 94% minus 85%) loss of spicules was associated with the exposure to hydrochloric acid. However, up to 4% of spicule loss was earlier interpreted as due to distilled water or handling error when transferring the samples, and thus the digestive effect of hydrochloric acid on the mass of spicules may actually range from 5% to 9%. Hence, the loss of spicules caused by handling with the ADC method was calculated to range between 63% and 67% by subtracting the amount of spicules remaining in Part A and the chemical reduction of spicule weight estimated in Part B (i.e. 100% initial spicule weight – 28% spicule weight remaining after processing – 0% spicule loss from formaldehyde – 5–9% spicule loss from hydrochloric acid).
Proportions of organic matter, spicules, substrate and salts
From the LAC method, we calculated proportional weight of organic matter, spicules, coral substrate and salts to be 2.5 ± 0.3%, 4.4 ± 0.9%, 90.5 ± 1.3% and 2.5 ± 0.4% of DSW, respectively, in the 83% sponge-invaded substrates (Fig. 5a). The proportion of organic matter in relation to total biomass (i.e. organic matter plus spicules) in Cliona orientalis was determined to be 36.7 ± 3.7%. Using the ADC method, the proportional weight of organic matter, spicules, substrate and salts were calculated to be 2.9 ± 1.4%, 5.9 ± 2.3%, 89.0 ± 2.2% and 2.3 ± 0.4% of DSW, respectively, including the corrections as described above (Fig. 5b), and the respective organic matter to biomass ratio was 33.6 ± 15.5%. The water content in the entire cores of the fresh sponge-substrate samples was found to be 25.0% WW ± 2.6% and 24.2% WW ± 3.3% using the LAC method and ADC method, respectively.
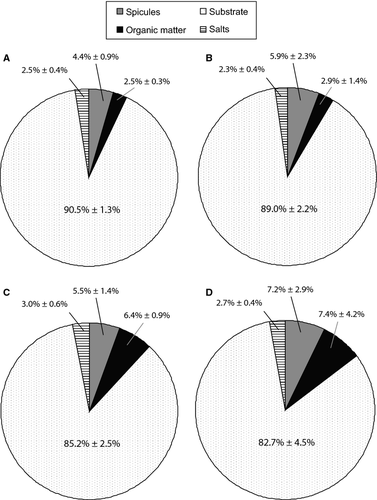
To compare the results to previous studies that used 100% sponge-invaded material, our data were corrected. The proportional weight of organic matter and spicules, and the 83%-corrected values for coral substrate and salts, were recalculated to be 3.0 ± 0.4%, 5.2 ± 1.1%, 89.3 ± 1.5% and 2.5 ± 0.4% of DSW, respectively, with the LAC method, and 3.4 ± 1.6%, 6.9 ± 2.7%, 87.5 ± 2.5% and 2.2 ± 0.4% of DSW, respectively, with the ADC method.
Actual weights of organic matter, spicules, substrate and salts in the 83% sponge-invaded substrates were converted into volumes using corresponding densities. Respective densities of the substrate were calculated from the LAC and ADC methods to be 2.74 ± 0.24 g·cm−3 and 2.80 ± 0.38 g·cm−3 respectively. Volumetric proportions of organic matter, spicules, substrate and salts were calculated to be 6.4 ± 0.9%, 5.5 ± 1.4%, 85.2 ± 2.5% and 3.0 ± 0.6% of the volume of the dried sponge-substrate samples, respectively, using the LAC method (Fig. 5c), whereas those for the ADC method were calculated to be 7.4 ± 4.2%, 7.2 ± 2.9%, 82.7 ± 4.5% and 2.7 ± 0.4%, respectively, in the 83% sponge-invaded substrates (Fig. 5d).
Evaluation of the buoyant weight method
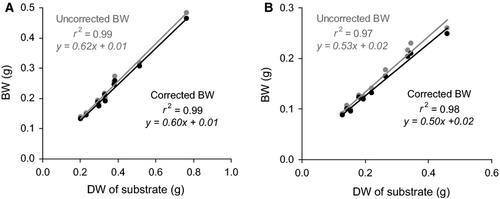
The BW method proved to be effective in the quantification of the mass of coral substrate invaded by Cliona orientalis. All regressions between BWs and the substrate weights obtained from the LAC and ADC methods were significant (P < 0.001). It appeared to be unnecessary to correct the BW values for the spicular content. Correlating BW to coral substrate weight, r2 for the LAC method was 0.99 regardless of whether the uncorrected or corrected BW was used (Fig. 6a). In the ADC method, r2 was 0.97 using the uncorrected BW and could only be improved by 1% when the corrected BW was used (i.e. r2 = 0.98, Fig. 6b).
Discussion
The present study provides improved standard methods to quantify different components of excavating sponges. Overall, the LAC method and ADC method generated very similar results. The vast majority of the mass in the Favia samples containing Cliona orientalis is calcareous substrate (approximately 90%), while sponge organic matter, siliceous spicules and salts together contribute to approximately 10% of total mass of the samples regardless of whether the 83% or 100% sponge-invaded sample was used in the calculation. In addition, BW was demonstrated effectively to estimate actual substrate weight in the fresh sponge-substrate samples, indicating that BW can potentially be used to quantify bioerosion rates of excavating sponges. However, each method has its own advantages and disadvantages, and thus the application of each method depends on the aim and design of the experiment.
Loss after combustion method
In the LAC method, the weight of the spicules in Cliona orientalis was reduced to 88% at 550 °C and further to 86% at 950 °C. The results were similar to the weight losses in siliceous spicules from the demosponges Suberites domuncula and Pseudospongosorites suberitoides when being heated to 550 and 950 °C (Sandford 2003). We interpret the observed weight loss as a reduction of chemically bound water and the combustion of the organic thread in the centre of each spicule. In comparison, a slightly reduced weight (by 2%) of the Favia skeleton was observed at 550 °C, possibly indicating the loss of the skeleton's organic matrix usually embedded in corals (Allemand et al. 2011). The mean weight loss in the coral skeleton from 550 to 950 °C, i.e. 44%, revealed the proportion of carbon dioxide evolving from calcium carbonate, matching the molecular mass ratio of CO2 (44 g·mol−1) to CaCO3 (100 g·mol−1) (Heiri et al. 2001).
The results obtained from the LAC method generally showed low variability of means. Indeed, the LAC method is simple and fast, allowing quantification of endolithic biomass, and is thus recommended as a routine method to quantify different components of excavating sponges. Repeating the combustion process resulted in highly accurate data (Fig. 3c), therefore most of the variation in the LAC method appeared to have been generated during the pretreatment process and may be due to the inherent biological variation among the sponge-substrate samples. A possible drawback of the LAC method is the difficulty of removing other endolithic organisms prior to quantification. It potentially leads to a slight overestimation of biomass and slightly higher variability of data if the amount of endolithic organisms inhabiting the substrate samples is not determined. However, substrate occupied by C. orientalis is not usually inhabited by other macroendoliths, and the live cover by the sponge prevents new colonization with microendoliths (C.H.L. Schönberg, pers. obs). Moreover, the weight of the non-sponge organics found in the coral skeleton was determined to be minimal, and we therefore assume that the results from this study are reliable. Other disadvantages of the LAC method include the removal of organic matter prior to quantification and the difficulty in separating spicules from the final ash containing calcium oxide, limiting further analysis of the samples.
Acid decalcification method
In comparison with the LAC method, the higher variability of means obtained with the ADC method was probably due to greater room for error introduced by handling and more calculation assumptions. Nevertheless, after taking all this into account, the ADC method generated very similar results compared with the LAC method and is recommended only if careful data correction is considered. However, the ADC method is superior to the LAC method in that it allows a quantitative separation of all excavating sponge materials from calcareous substrate, which was not previously demonstrated. Another advantage of the ADC method is the possible isolation of non-sponge organics that may include macroendoliths or microendoliths during the decalcification process. Now that all above-listed materials can be separated and quantified, a logical consequence would be to also estimate the amount of symbiotic dinoflagellates, which were at present not differentiated from the organic matter of C. orientalis, in order to address questions associated with the relative role of symbionts in excavating sponges.
During the ADC method, only 28% of the sponge spicules remained behind after the fixation-decalcification treatment. Whereas up to 9% of spicular matter may have been digested by hydrochloric acid, approximately 63–67% of the spicules were lost due to handling. This significant loss of spicules was likely associated with the instability of the digested portion of organic matter in the fixation-decalcification treatment. Spicules still adhering to this disintegrating organic portion were at high risk to detach and may have been washed away during the transfers of samples between solutions. The mass ratio of organic matter to spicules is approximately 1:2 in C. orientalis and thus two units of spicules may be lost with every unit of digested tissue. Moreover, the risk of losing spicules was increased because spicules in clionaid sponges occur in a far denser arrangement at the surface than in the body of the sponges, where they are held together by only a minimum of tissue material (see Fig. 6E, 6F, 8B and 8D in Schönberg 2000). The surface is the first to disintegrate in any maceration process, and this is likely to incur the largest bias. In addition, spicules are easily blown off and lost, once isolated and dry, and handling at this stage poses a significant risk. Rose & Risk (1985) isolated spicules from Cliona delitrix by dissolving the whole sponge-substrate samples in heated hydrochloric acid and found that spicules only contributed about 1% of the sponge biomass (versus ≥63% in C. orientalis in this study), possibly revealing a major loss of spicules due to handling.
Buoyant weight method
Using BW, Jokiel et al. (1978) and Davies (1989) found predicted coral skeletal weights to be accurate to within 1% in the corals Acropora humilis, Fungia scutaria, Montipora verrucosa, Pocillopora damicornis and Pocillopora verrucosa. Likewise, the BW method presently proved to be effective in indicating the actual weight of coral substrate invaded by Cliona orientalis (r2 ≥ 0.97). Improvement of data was negligible when correcting for the weight of spicules, suggesting that the correction was unnecessary for C. orientalis and probably for other excavating sponges with similar spicule to tissue ratios. In the present study, only dead sponge samples were used, but the BW method can be adopted for use on live excavating sponges, serving as a relatively simple and cheap method to quantify sponge erosion in comparison with the traditional and usually destructive methods described in the Introduction. Time-series data on bioerosion can be obtained by repeatedly measuring BWs of the same substrate sample invaded by an excavating sponge if care is taken during handling, avoiding loss of fragments. Because sponge tissue literally carries no weight underwater, a decreasing BW of a sample indicates the bioerosion rate caused by the sponge, providing information for long-term monitoring programs on coral reef health and carbonate cycling.
Comparison with previous studies
Available literature on proportions of organic and inorganic contents in Cliona spp. is limited. Only Bergman (1983), Acker & Risk (1985) and Rose & Risk (1985) have provided data on biomass in 100% sponge-invaded substrate samples, whereas to date no information on volumetric proportions is available. In Bergman (1983), Acker & Risk (1985) and Rose & Risk (1985), hydrogen peroxide was used to digest organic matter, and the sponge mass was calculated from the weight loss after the digestion and rinsing. However, spicules were likely washed away during the process before the samples being weighed (as discussed above) and thus the results reported in these studies are presently interpreted as total biomass, i.e. organic matter plus spicules. In the present study, when 100% sponge-invaded substrate was used, the sum of organic matter and spicules in C. orientalis was 8% with the LAC method and 10% with the ADC method. The results are comparable to the values of total biomass reported for the Caribbean Cliona caribbaea Carter, 1882 (6%; Acker & Risk 1985), a very close relative of C. orientalis, and Cliona delitrix (5%; Rose & Risk 1985). Indeed, our results on C. orientalis are very similar to that on Cliona cf. orientalis collected on the Australian Great Barrier Reef [10%; Bergman 1983; as Cliona viridis (Schmidt, 1862)]. This suggests that the present calculations and correction factors, in particular those for the spicules, are suitable for quantitative separation of the sponge components. In Bergman (1983), Acker & Risk (1985) and Rose & Risk (1985), partial weights of organic matter and spicules were not differentiated and thus further comparison is not appropriate.
Any discrepancy in results among studies on material compositions in bioeroding sponges may be due to the use of different sponge species and host substrates, i.e. Favia sp. in the present study versus Montastrea spp. in Acker & Risk (1985) and Rose & Risk (1985) (species of the coral substrate not reported in Bergman 1983). Growth or excavating capability, and hence putatively biomass, of C. orientalis was demonstrated to increase in denser calcareous substrates with lower pore volumes (Schönberg 2002, 2003). In addition, a further discrepancy may occur if salt content is not investigated in the sponge samples. Marine sponges are assumed to be isosmotic to surrounding seawater, and the amount of salts in the sponges is thus proportional to ambient salinity (Barnes 1980). In the present study, salinity was around 34‰ at the sampling site, and in consequence the salt weight in C. orientalis could be as high as the organic weight or half of the weight of spicules. If salts are not differentiated from the sponge components, they can erroneously contribute to biomass and introduce bias (Schönberg & Barthel 1997). Methods used in Bergman (1983), Acker & Risk (1985) and Rose & Risk (1985) not only removed organic matter and spicules, but also salts. As a consequence, their calculated values for sponge biomass included the mass of salts and may be overestimated.
Conclusions
A risk of losing materials by handling exists in component analyses of excavating sponges. This risk can be minimized but not always be avoided, and correction factors have to be determined for these lost materials. Great care must be taken as all the calculations and corrections will increasingly add variation to the results. Overall, where it is sufficient, we recommend the more reliable LAC method for quantification of different components in clionaid sponges and suggest the use of BW to estimate bioerosion rates of excavating sponges. The ADC method is not recommended, unless careful data correction is considered, and the mass of sponge tissue or spicules has to be distinguished from that of the substrate.
Acknowledgements
We thank E. Lewis, N. Van Dyck and K. Townsend at the Moreton Bay Research Station, and W. Loh and N. Kongjandtre at The University of Queensland (UQ) for field assistance. This study was funded by Australian Research Council (ARC) Linkage Grant LP0775303 to S. Dove, ARC Centre of Excellence Grant CE0561435 to O. Hoegh-Guldberg and S. Dove, and The UQ Research Scholarship and The International Society for Reef Studies Graduate Fellowship to J. K. H. Fang. A permit from the Department of Environment and Resource Management (QS2010/MAN79) was provided to conduct this study.




