Development of Liver-Heart Score for Early Detection of Myocardial Contractile Dysfunction in Cirrhosis by Strain Imaging
Funding: Dr. Marcel Razpotnik received a promotional award for young investigators from the Austrian Society of Ultrasonography for the cirrhotic cardiomyopathy project and a research award from the Medical-Scientific Society for Carinthia and East Tyrol.
ABSTRACT
Aim
Cirrhotic cardiomyopathy is characterised by myocardial dysfunction in patients with cirrhosis in the absence of other cardiac conditions. We aimed to develop and validate a scoring system to identify patients at high risk for reduced global longitudinal strain, a newly proposed marker of myocardial dysfunction in the updated diagnostic criteria for cirrhotic cardiomyopathy.
Methods
Prospectively recruited patients with cirrhosis in the training and validation groups underwent identical hepatological and cardiological evaluations, including strain echocardiography. Risk factors for myocardial dysfunction were identified using logistic regression.
Results
In a cohort of 452 consecutive patients, 278 were excluded due to non-cirrhotic cardiomyopathy or conditions potentially affecting strain measurements. The prevalence of reduced global longitudinal strain was 9.8% (13/133) in the training group and 19.5% (8/41) in the validation group. Multivariate logistic regression revealed BMI ≥ 28 kg/m2 (OR 7.02), CAP > 260 dB/m (OR 8.53), and age > 57 years (OR 4.68) as independent predictors of reduced myocardial contractility. These variables were combined and weighted based on their beta coefficients to develop the Liver-heart score (CAP > 260 dB/m [2 pts], BMI ≥ 28 kg/m2 [2 pts], age > 57 years [1 pt]). The AUC-ROC was 0.84 in the training and 0.83 in the validation cohort. A Liver-heart score of 5 points was associated with increased mortality, observed at 2 years (44.4% vs. 17.3%) and the end of the follow-up period (66.7% vs. 37.7%, HR 1.3, p < 0.01).
Conclusion
The Liver-heart score can accurately rule out reduced myocardial contractility and may be useful for risk stratification in cirrhotic patients.
Abbreviations
-
- ALD
-
- Alcoholic Liver Disease
-
- ALT
-
- Alanine Aminotransferase
-
- AST
-
- Aspartate Aminotransferase
-
- AUC
-
- Area Under the Curve
-
- BMI
-
- Body Mass Index
-
- CACS
-
- Coronary Artery Calcium Score
-
- CAD
-
- Coronary Artery Disease
-
- CAP
-
- Controlled Attenuation Parameter
-
- CCM
-
- Cirrhotic Cardiomyopathy
-
- CCTA
-
- Coronary Computed Tomography Angiography
-
- CI
-
- Cardiac Index
-
- CP
-
- Child-Pugh Class
-
- DBP
-
- Diastolic Blood Pressure
-
- DD
-
- Diastolic Dysfunction
-
- ECG
-
- Electrocardiogram
-
- EF
-
- Ejection Fraction
-
- GLS
-
- Global Longitudinal Strain
-
- HBV
-
- Hepatitis B Virus
-
- HCC
-
- Hepatocellular Carcinoma
-
- HCV
-
- Hepatitis C Virus
-
- HT
-
- Hypertension
-
- INR
-
- International Normalised Ratio
-
- IVSEDd
-
- Interventricular Septum End-Diastolic Diameter
-
- LAVI
-
- Left Atrial Volume Index
-
- LDL
-
- Low-Density Lipoprotein
-
- LSM
-
- Liver-Stiffness Measurement
-
- LV
-
- Left Ventricular
-
- LVEDd
-
- Left Ventricular End-Diastolic Diameter
-
- MASLD
-
- Metabolic Dysfunction-Associated Steatotic Liver Disease
-
- NPV
-
- Negative Predictive Value
-
- NT-pro BNP
-
- N-terminal pro–B-type natriuretic peptide
-
- PPV
-
- Positive Predictive Value
-
- pQTc
-
- Prolongation of Corrected QT Interval
-
- PTCA
-
- Percutaneous Transluminal Coronary Angioplasty
-
- PVT
-
- Portal Vein Thrombosis
-
- SBP
-
- Systolic Blood Pressure
-
- SV
-
- Stroke Volume
-
- SVRI
-
- Systemic Vascular Resistance Index
-
- TE
-
- Transient Elastography
-
- TIPS
-
- Transjugular Intrahepatic Portosystemic Shunt
Summary
- Elevated BMI, increased CAP, and advanced age are independent predictors of subclinical myocardial dysfunction in cirrhosis.
- The Liver–heart score can accurately rule out reduced myocardial contractility and may be useful for risk stratification of cirrhotic patients.
- CCM, reduced GLS, and a Liver-heart score of 5 points are associated with a significantly higher risk of death.
1 Introduction
Chronic liver disease may induce subclinical myocardial dysfunction in the absence of other cardiac conditions, potentially influencing patient prognosis and outcomes following interventions [1].
Recent evidence, as reported by Liu et al., suggests that the underlying pathogenic and pathophysiological mechanisms are rooted in two distinct pathways: (1) factors related to portal hypertension, hyperdynamic circulation, gut bacterial/endotoxin translocation, and the resulting inflammatory response; (2) liver insufficiency with alterations in the synthesis or metabolism of proteins, lipids, carbohydrates, bile acids, and hormones [2, 3]. As its own entity, cirrhotic cardiomyopathy (CCM) can be diagnosed only when other primary or secondary cardiomyopathies have been excluded.
Research indicates that approximately 50% of individuals with cirrhosis exhibit signs of cardiac dysfunction, the clinical significance of which remains uncertain [4]; furthermore, cardiovascular complications post-liver transplantation are fatal in 7%–21% of cases [5].
Autopsy studies have consistently shown that cardiac histopathological findings in cirrhosis are not contingent upon the aetiology of the underlying liver condition [6, 7].
While previous studies have demonstrated a correlation between hepatic steatosis and increased cardiovascular mortality in patients with metabolic dysfunction-associated steatotic liver disease (MASLD), comprehensive data on cirrhotic cohorts remain limited. Notably, fatty liver alterations have been associated with cardiovascular mortality in up to 16% of cases, exhibiting a dose-dependent relationship [8]. The most recent multidisciplinary European MASLD guideline recommends a risk-adapted, stepwise cardiovascular workup in individuals with MASLD considered for liver transplantation, due to the significantly increased pre-, peri-, and post-operative cardiovascular morbidity and mortality in this population [9]. It is important to note that patients with alcohol-associated liver cirrhosis also have a high burden of concomitant cardiometabolic risk factors [10], rendering them at risk for cardiovascular morbidity and mortality.
The revisited diagnostic criteria for CCM now recommend the routine assessment of global longitudinal strain (GLS) alongside left ventricular (LV) ejection fraction (EF) to evaluate systolic function in patients with cirrhosis [11]. Analysis of speckle patterns offers high sensitivity to early myocardial alterations that precede overt heart failure symptoms and enhances the diagnostic capability for CCM.
However, the mechanisms contributing to GLS impairment in the cirrhotic heart have yet to be fully elucidated. It is crucial to recognise that GLS is not a specific marker for CCM; instead, it reflects the influence of prevalent cardiovascular risk factors or coexisting cardiac conditions. Furthermore, the availability of strain echocardiography is not universal in routine clinical practice, and the routine interdisciplinary collaboration between cardiologists and hepatologists is not always established.
A simple clinical tool would be advantageous for identifying cirrhotic patients at an elevated risk for myocardial dysfunction, particularly those who would benefit most from further cardiological evaluation.
Our research aimed to develop and validate a predictive model based on a scoring system, utilising routinely obtained patient characteristics, laboratory, and clinical variables, including liver stiffness measurements (LSM) and controlled attenuation parameter (CAP) by vibration-controlled transient elastography. The model is designed to reliably differentiate patients with liver cirrhosis at risk for reduced global longitudinal strain from those with a low probability of myocardial dysfunction.
2 Methods
2.1 Study Design and Participants
The study cohort comprised consecutive patients with liver cirrhosis, irrespective of aetiology, prospectively assessed at a university-affiliated tertiary care hospital in Austria. Recruitment occurred during inpatient and outpatient visits between January 2019 and July 2022 as part of a collaborative effort between the Departments of Gastroenterology, Hepatology, and Cardiology. All participants underwent initial screening, which included a comprehensive medical history and physical examination, followed by a thorough evaluation of hepatic and cardiac function (Figure 1).
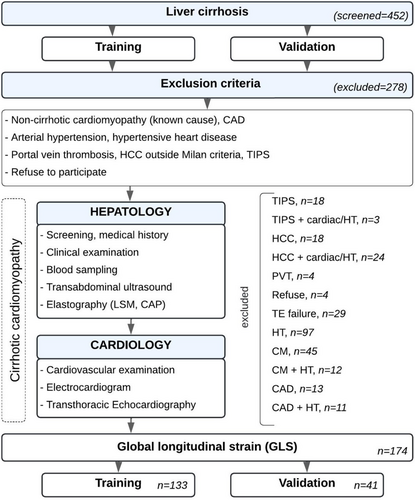
In addition, we included an independent validation cohort of prospectively examined consecutive patients, recruited after completing the training group, who underwent the same diagnostic procedures.
Consistent with the definition of cirrhotic cardiomyopathy, which is diagnosed by ruling out other potential etiologies [12] and to ensure a more accurate evaluation of the liver-heart axis, individuals with pre-existing non-cirrhotic cardiomyopathy, such as ischemic, valvular or hypertensive heart disease-conditions that could impair strain measurements-were excluded from the final analysis.
Patients were also excluded if they had a history of portal vein thrombosis, transjugular intrahepatic portosystemic shunt (TIPS) implantation, hepatocellular carcinoma outside Milan criteria, failed transient elastography measurements, or refused to participate in the study (Figure 1).
Enrolled participants underwent longitudinal follow-up with regular clinical evaluations and liver function tests tailored to individual patient conditions. Mortality rates were systematically recorded throughout this period.
Written consent was obtained from all participating subjects. This study adhered to the principles of the 2013 Declaration of Helsinki and was approved by the local ethics committee (Ethikkommission Kärnten No. A21/19).
2.2 Clinical and Laboratory Parameters
Data on personal medical history, alcohol consumption, smoking habits, and medication usage were systematically collected. Hypertension was defined based on persistently high resting blood pressure (> 140/90 mmHg) or ongoing antihypertensive treatment. Individuals were classified as obese when their body mass index (BMI) exceeded 30 kg/m2.
The laboratory evaluation encompassed a complete blood count, electrolytes, serum creatinine, glomerular filtration rate (GFR), prothrombin time (PT), international normalised ratio (INR), albumin, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), N-terminal pro–B-type natriuretic peptide (NT-proBNP).
2.3 Assessment of Cardiac Function
A 12-lead electrocardiogram (ECG) was recorded for all participants before echocardiographic evaluation. The QT interval was adjusted for heart rate using Fridericia's formula to calculate the corrected QT interval (QTc). QTc durations > 450 ms in males and > 470 ms in females were classified as abnormal [13].
The conventional echocardiographic examinations adhered to the latest recommendations of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) [14], and were conducted by EACVI-certified cardiologists using a Vivid E95 (General Electronic, Boston, USA) system with a 2.5 MHz probe.
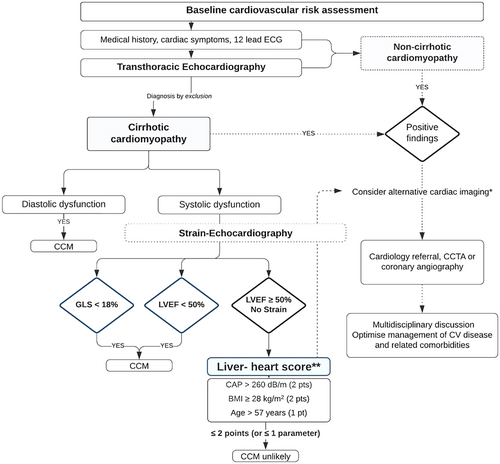
The systolic dysfunction of the left ventricle was defined according to the 2019 CCM Consortium recommendations as LV ejection fraction (EF) ≤ 50% or reduced absolute value of global longitudinal strain that is more negative than −18% [11]. To avoid confusion in the present study, changes in GLS are described using absolute values. For instance, a GLS < 18 refers to strain values that are more positive (e.g., a GLS of −16%) than −18%.
EF was calculated based on the volumetric difference between the end-diastolic and end-systolic phases by tracing the myocardium and ventricle cavity interface in apical four- and two-chamber views using the biplane Simpson's method.
The Cardiac Index (CI) was computed by multiplying the stroke volume by the heart rate to obtain Cardiac Output, which was then divided by the body surface area. Hyperdynamic circulation (HC) was defined as CI > 4.0 L/min/m2 or Systemic Vascular Resistance Index (SVRI) < 1970 dynes-sec/cm–5/m2, which was derived from echocardiographic findings.
Individuals with reduced myocardial contractility were further assessed either by a coronary calcium scan calculating the Agatston score or invasively by coronary angiography in case coronary artery disease was clinically suspected.
For the assessment of the diastolic function, mitral inflow velocities during early (E) and late (A) diastole were obtained by pulsed Doppler from the apical four-chamber view by placing the sample volume (2–3 mm) at the tip of the mitral leaflets. The myocardial motion pattern (early diastolic mitral annular velocity, e′) was assessed using septal tissue Doppler in the same apical four-chamber view.
Left atrial volume was measured using the biplane area-length method and was indexed for body surface area (LAVI). Tricuspid regurgitation (TR) velocity was obtained using continuous-wave Doppler from an apical four-chamber view.
Hepatologist-centered recommendations for diastolic dysfunction in cirrhotic patients have been adopted from current ASE/EACVI guidelines [15], incorporating four key parameters: septal e' velocity < 7 cm/s, E/e′ ≥ 15, LAVI > 34 mL/m2, and TR velocity > 2.8 m/s. The presence of more than two criteria indicates elevated LV filling pressure and is considered diagnostic of CCM [11]. Cases meeting only two of the four diagnostic criteria were classified as having ‘indeterminate’ diastolic function. These cases were further evaluated using additional parameters, including the E/A ratio, deceleration time, and isovolumetric relaxation time, within the clinical context of patient factors such as age, volume overload, and NT-proBNP values.
2.4 Strain Echocardiography
A detailed quantitative study of myocardial function was performed offline using speckle-tracking echocardiography (STE) by an EACVI-certified investigator with Echo Pac computer software Version v203 (General Electric, Boston, USA).
Global longitudinal strain (GLS) measurements were made from the three standard apical views and averaged over three consecutive cardiac cycles. After optimising image quality, the frame with a clearly defined endocardium was selected.
The region of interest was automatically drawn and manually adjusted to the wall thickness, excluding the papillary muscles. The results were presented as Bull's-ye plots (Figure S1).
2.5 Assessment of Liver Function
Abdominal ultrasound was performed on all patients using a 3.5-MHz transducer (Hitachi Arietta V70) by an experienced hepatologist dedicated to liver sonography and blinded to the echocardiographic data at the time of examination.
As previously reported, fatty liver was diagnosed sonographically based on a combination of parenchymal brightness, liver-to-kidney contrast difference, deep echo attenuation due to fat, and blurring of vessel walls [16].
Cirrhosis was diagnosed in patients with impaired liver function based on the following criteria: (1) Liver stiffness > 15 kPa by transient elastography (TE), confirmed by a second measurement, or (2) Liver stiffness 10–15 kPa by TE, accompanied by clinical or imaging evidence of portal hypertension [17].
Portal hypertension was diagnosed non-invasively based on the presence of characteristic clinical and imaging signs: gastro-oesophageal varices, ascites, portosystemic collateral vessels, or splenomegaly with concurrent thrombocytopenia.
2.6 Transient Elastography
Liver stiffness and controlled attenuation parameter (CAP) were measured using the FibroScan 502 Touch device (EchoSens, Paris, France) by a specially trained operator (with over 1000 examinations) in the supine position after an overnight fast. Based on the patient's BMI, the appropriate probe size (M or XL) was chosen and positioned between intercostal spaces over the right hepatic lobe. The median value of 10 valid acquisitions at the same spot was recorded to represent liver stiffness in kilopascals (kPa).
The variability of liver stiffness measurements (LSM) was described by the interquartile range (IQR)-to-median ratio with values ≤ 0.3 considered reliable. Using the same signal and parenchymal volume, ultrasound attenuation was calculated and expressed in decibels per meter (dB/m).
2.7 Statistics
The Shapiro–Wilk test was used to assess the normal distribution of the data. Continuous parameters with a normal distribution were described by mean values and standard deviations, non-parametric by median values and range intervals, and categorical parameters by frequencies (percentages). Depending on the data distribution, parametric (Student's t-test) or non-parametric (Kruskal-Wallis) tests were used to determine the significant differences between these variables. Univariate logistic regression was used to identify liver measurements and patient characteristics associated with reduced global longitudinal strain. Variables with significant associations on univariate analysis were selected for multivariate logistic regression. The combination of variables yielding the highest area under the receiver-operating characteristic curves (AUROC) were selected for the final model. These variables were then weighted based on their respective beta coefficients to develop a simple scoring system to predict reduced myocardial contractility—the Liver-heart score.
The Liver-heart score was validated using an independent, prospectively obtained dataset of consecutive cirrhotic patients who underwent the same diagnostic procedures as those in the training group. Additionally, a sensitivity analysis was conducted using 5-fold cross-validation on the combined training and validation cohorts to assess the consistency of the model's predictive performance.
To evaluate the impact of parameters on mortality, we utilised a Cox regression model and reported hazard ratios (HRs) adjusted for revascularisation performed during the follow-up period. A p-value < 0.05 was considered to indicate statistical significance in all tests. Statistical analyses were performed using IBM SPSS software (Version 29.0.0.0).
3 Results
3.1 Study Population Characteristics
Of the 452 patients initially screened, 174 patients were included in the final analysis (Figure 1). Table 1 summarises the clinical, laboratory, and echocardiographic characteristics of the study population.
| Group | Training | Validation | p |
|---|---|---|---|
| n = 133 | n = 41 | ||
| Age (years) | 57.1 ± 10.3 | 58.5 ± 8.2 | 0.41 |
| Male gender | 80 (60.2%) | 23 (56.1%) | 0.77 |
| BMI (kg/m2) | 25.6 ± 4.1 | 26.8 ± 6.3 | 0.17 |
| Aetiology | |||
| ALD | 95 (71.4%) | 23 (56.1%) | 0.10 |
| MASLD | 8 (6%) | 5 (12.2%) | 0.33 |
| Other | 30 (22.6%) | 13 (31.7%) | 0.33 |
| Child-pugh class | |||
| A | 64 (48.1%) | 15 (36.6%) | 0.27 |
| B | 37 (27.8%) | 20 (48.8%) | 0.02 |
| C | 32 (24.1%) | 6 (14.6%) | 0.28 |
| Portal hypertension | 119 (89.5%) | 30 (73.2%) | 0.01 |
| Varices | 79 (59.4%) | 23 (56.1%) | 0.85 |
| MELD score | 10 (6–31) | 14 (6–27) | 0.08 |
| LSM (kPa) | 33.4 (9.9–75) | 37.7 (11.2–75) | 0.54 |
| CAP (dB/m) | 263.1 ± 62.7 | 256.7 ± 60.2 | 0.64 |
| Bilirubin (mg/dL) | 1.1 (0.2–20.2) | 1.8 (0.2–13.4) | 0.34 |
| ALT (U/L) | 31 (7–352) | 28 (12–83) | 0.13 |
| AST (U/L) | 53 (15–459) | 51 (24–117) | 0.53 |
| Platelets (109/L) | 100 (20–380) | 99 (33–595) | 0.44 |
| INR | 1.2 (1.0–2.4) | 1.3 (1.0–2.4) | 0.98 |
| Albumin (g/dL) | 3.6 ± 0.7 | 3.5 ± 0.6 | 0.26 |
| NT-pro BNP (pg/mL) | 118 (17.4–7788) | 83 (21–682) | 0.53 |
| Creatinine (mg/dL) | 0.8 (0.5–2.8) | 0.8 (0.5–2.1) | 0.59 |
| Cholesterol (mg/dL) | 142 (37–620) | 133 (56–279) | 0.58 |
| Triglycerides (mg/dL) | 90 (31–1114) | 101 (52–233) | 0.23 |
| LDL (mg/dL) | 76 (15–262) | 68 (15–230) | 0.82 |
| Diabetes | 20 (15%) | 6 (14.6%) | 0.85 |
| Hyperlipidemia | 14 (10.5%) | 4 (9.8%) | 0.87 |
| Smoking | 70 (52.6%) | 19 (46.3%) | 0.59 |
| Beta-Blocker | 66 (49.6%) | 20 (48.8%) | 0.93 |
| Statins | 9 (6.8%) | 2 (4.9%) | 0.94 |
| SBP (mmHg) | 126 ± 17 | 124 ± 17 | 0.54 |
| DBP (mmHg) | 73 ± 10 | 71 ± 9.8 | 0.28 |
| LVEDd (mm) | 46.7 ± 5.2 | 47.9 ± 5.5 | 0.22 |
| IVSEDd (mm) | 11 ± 2.1 | 10.5 ± 1.8 | 0.17 |
| LVEF (%) | 60.9 ± 6.3 | 61.5 ± 7.4 | 0.61 |
| LV-GLS (%) | −21.6 ± 2.9 | −20.2 ± 3.1 | 0.01 |
| CI (L/min/m2) | 2.9 (1.6–7.2) | 3.3 (2.4–6.2) | 0.03 |
| SV (ml) | 76.9 ± 20.5 | 82.6 ± 12 | 0.25 |
| SVRI (dynes-sec/cm–5/m2) | 2433 ± 787 | 1826 ± 426 | 0.02 |
| Hypercirculation | 35 (26.3%) | 11 (26.8%) | 0.89 |
| E/e′ Ratio | 9.2 (5.2–24) | 8.9 (5.7–18.2) | 0.86 |
| LAVI (ml/m2) | 26.4 (11.6–69.6) | 29.8 (12.1–64.1) | 0.05 |
| e′ velocity (cm/s) | 8.3 ± 2.2 | 7.6 ± 1.7 | 0.07 |
| TR velocity (m/s) | 2.1 ± 0.6 | 2.3 ± 0.5 | 0.27 |
| pQTc | 42 (31.6%) | 13 (31.7%) | 0.86 |
- Abbreviations: ALD, Alcoholic Liver Disease; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BMI, Body Mass Index; CAP, Controlled Attenuation Parameter; CI, Cardiac Index; DBP, Diastolic Blood Pressure; e′, early septal diastolic mitral annular velocity; EF, Ejection Fraction; GLS, Global Longitudinal Strain; INR, International Normalised Ratio; IVSEDd, Interventricular Septum End-Diastolic Diameter; LAVI, Left Atrial Volume Index; LDL, Low-Density Lipoprotein; LSM, Liver-Stiffness Measurement; LV, Left Ventricular; LVEDd, Left Ventricular End-Diastolic Diameter; MASLD, Metabolic Dysfunction-Associated Steatotic Liver Disease; NT-pro BNP, N-terminal pro–B-type natriuretic peptide; pQTc, Prolongation of Corrected QT Interval; SBP, Systolic Blood Pressure; SV, Stroke Volume; SVRI, Systemic Vascular Resistance Index; TR, tricuspid valve regurgitation.
In the training group, significantly more patients were classified as Child-Pugh B and exhibited clinically significant portal hypertension compared to the validation group, with nearly half showing at least one feature of liver decompensation. Ascites was present in 45 cases (33.8%), including large, therapy-refractory accumulations in 14 patients (10.5%).
3.2 Prevalence of Cirrhotic Cardiomyopathy and Cardiological Assessment
The prevalence of cirrhotic cardiomyopathy among the participants in the training group was 18.8%, as determined using the 2019 CCC diagnostic criteria. Reduced global longitudinal strain, assessed by speckle-tracking echocardiography, was identified in 13 (9.8%) of the cirrhotic patients in the training group and 8 (19.5%) in the validation group (p = 0.16). Only two presented with reduced LVEF ≤ 50%, indicating that most patients with end-stage liver disease have a subclinical contractile dysfunction.
The mean absolute value of GLS in the validation group was lower than in the training group (20.2% ± 3.1% vs. 21.6% ± 2.9%, p = 0.01), reflecting the non-balanced distribution of covariates, such as aetiology and haemodynamic-related factors between the groups.
In the training group, 10 patients (7.5%) met more than two diagnostic criteria for diastolic dysfunction. An additional 18 patients (13.5%) presented with exactly two criteria and were classified as having “indeterminate” diastolic function. Following comprehensive echocardiographic assessment using additional parameters, 7 patients from the indeterminate group were reclassified as having diastolic dysfunction, resulting in an overall prevalence of 12.8%. Five patients with reduced GLS were also diagnosed with diastolic dysfunction.
Patients presenting reduced GLS underwent further evaluation for the presence of coronary artery disease (CAD) (Figure 2). Among the cirrhotic patients in the training group, a confirmed or suspected CAD, indicated by elevated Agatston scores, was identified in four individuals. In three cases, CAD was definitively excluded. Additionally, three patients died before a cardiological assessment could be conducted within the study period, two declined the examinations, and one developed atrial fibrillation during follow-up (Table 2).
| Patient | Diagnosis | BMI | CAP | LSM | CP | DD | CAD work-up/outcome |
|---|---|---|---|---|---|---|---|
| 61 M | HBV | 27,8 | 266 | 27 | A | 1 | CACS 414, PTCA of RCA |
| 58 M | ALD | 29,7 | 271 | 21,5 | C | 0 | CACS 98, PTC: CAD excluded/TIPS in follow-up |
| 58 W | MASLD | 36,1 | 271 | 72 | A | 0 | CACS 501; no PTCA/TIPS in follow-up |
| 77 W | ALD | 28,6 | 281 | 26 | A | 1 | ICMP; PTC (no stent) |
| 71 W | MASLD | 31,9 | 288 | 13 | A | 0 | CACS 0 |
| 58 M | ALD | 28,7 | 291 | 12,5 | C | 1 | CACS 1400/died; obduction (right heart and acute liver failure) |
| 72 M | ALD | 24,7 | 302 | 19,2 | B | 0 | No CAD work-up/atrial fibrillation in follow-up |
| 63 M | ALD, HCC | 33,1 | 314 | 9,7 | A | 0 | Died before CAD work-up |
| 63 M | ALD | 35,6 | 330 | 75 | B | 0 | Died before CAD work-up/Covid-19 |
| 61 M | HCV | 31,1 | 375 | 27 | A | 0 | CACS 425, PTCA of LAD and LCX |
| 46 M | HCV | 31,2 | 385 | 70,6 | B | 0 | PTC: CAD excluded |
- Abbreviations: ALD, Alcoholic Liver Disease; BMI, Body Mass Index; CACS, Coronary Artery Calcium Score; CAD, Coronary Artery Disease; CAP, Controlled Attenuation Parameter in dB/m; CP, Child-Pugh Class; DD, Diastolic Dysfunction; HBV, Hepatitis B Virus; HCC, Hepatocellular Carcinoma; HCV, Hepatitis C Virus; LSM, Liver Stiffness Measurement in kPa; MASLD, Metabolic Dysfunction-Associated Steatotic Liver Disease; PTCA, Percutaneous Transluminal Coronary Angioplasty.
3.3 Association of Hepatic Steatosis, Fibrosis, and Clinical Characteristics With GLS
The global longitudinal strain demonstrated a positive correlation with the controlled attenuation parameter in cirrhotic patients (r = 0.268, p < 0.01). In contrast, liver stiffness measurements did not reveal significant differences between patients with and without reduced myocardial contractility.
| Analysis | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | ß | SE | OR (95% CI) | p | ß | SE | OR (95% CI) | p |
| Male gender | 0.87 | 0.68 | 2.38 (0.62–9.10) | 0.18 | ||||
| Age > 57 years | 1.77 | 0.79 | 5.9 (1.25–27.66) | 0.01 | 1.54 | 0.89 | 4.68 (0.82–26.75) | 0.08 |
| BMI ≥ 28 kg/m2 | 2.54 | 0.70 | 12.67 (3.24–49.52) | < 0.01 | 1.95 | 0.78 | 7.02 (1.51–32.56) | 0.01 |
| Alcoholic aetiology | −0.99 | 0.60 | 0.37 (0.12–1.20) | 0.10 | ||||
| Diabetes | 0.03 | 0.81 | 1.03 (0.21–5.04) | 0.97 | ||||
| Hyperlipidemia | 0.49 | 0.83 | 1.64 (0.32–8.27) | 0.57 | ||||
| Smoking | −1.36 | 0.69 | 0.26 (0.07–1.00) | 0.03 | ||||
| Beta-blockers | −0.88 | 0.62 | 0.41 (0.12–1.42) | 0.15 | ||||
| Statins | 0.93 | 0.85 | 2.55 (0.48–13.50) | 0.31 | ||||
| LSM by TE > 15 kPa | 0.16 | 1.10 | 1.18 (0.14–10.21) | 0.88 | ||||
| LSM by TE > 25 kPa | 1.02 | 0.82 | 2.79 (0.56–13.83) | 0.17 | ||||
| LSM by TE > 40 kPa | −0.09 | 0.68 | 0.92 (0.24–3.46) | 0.90 | ||||
| CAP > 260 dB/m | 2.43 | 1.07 | 11.36 (1.38–93.24) | < 0.01 | 2.14 | 1.12 | 8.53 (0.96–76.21) | 0.05 |
| Child-Pugh C | −0.06 | 0.69 | 0.9 (0.24–3.65) | 0.93 | ||||
| MELD ≥ 10 | −0.11 | 0.59 | 0.89 (0.28–2.81) | 0.85 | ||||
| Portal hypertension | −1.09 | 0.73 | 0.33 (0.08–1.41) | 0.16 | ||||
| Ascites | −1.12 | 0.79 | 0.33 (0.07–1.54) | 0.11 | ||||
| Varices | −0.25 | 0.59 | 0.78 (0.25–2.46) | 0.67 | ||||
| AST/ALT ≤ 2 | 0.97 | 0.79 | 2.65 (0.56–12.53) | 0.18 | ||||
| INR > 1.5 | −0.93 | 1.07 | 0.39 (0.04–3.19) | 0.33 | ||||
| Platelets < 50 109/L | −0.46 | 1.08 | 0.63 (0.08–5.23) | 0.65 | ||||
| Albumin < 3.5 g/dL | 0.05 | 0.59 | 1.06 (0.34–3.33) | 0.93 | ||||
| Albumin < 3.0 g/dL | 0.47 | 0.79 | 1.60 (0.33–7.65) | 0.54 | ||||
| Cholesterol < 200 mg/dL | −0.09 | 1.09 | 0.92 (0.11–7.80) | 0.94 | ||||
| LDL < 100 mg/dL | 0.33 | 0.64 | 1.39 (0.40–4.87) | 0.60 | ||||
| Triglycerides < 150 mg/dL | 0.10 | 0.81 | 1.10 (0.22–5.41) | 0.91 | ||||
| NT-pro BNP ≥ 125 pg/mL | 0.54 | 0.62 | 1.72 (0.51–5.85) | 0.38 | ||||
| QTc prolongation | 0.17 | 0.85 | 1.18 (0.22–6.32) | 0.84 | ||||
- Abbreviations: ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BMI, Body-Mass Index; CAP, Controlled Attenuation Parameter; INR, International Normalised Ratio; LDL, Low-Density Lipoprotein; LSM, Liver-Stiffness Measurement; NT-pro BNP, N-terminal pro–B-type natriuretic peptide; TE, Transient Elastography.
Table 3 summarises the clinical parameters identified as potential risk factors for reduced GLS in cirrhotic patients. Patients aged > 57 years showed a 5.9-fold increased risk of reduced myocardial contractility (p=0.01). A BMI ≥ 28 kg/m2 was associated with an OR of 12.7, and a CAP > 260 dB/m yielded an OR of 11.4, with both parameters remaining significant in multiple regression analysis.
Liver stiffness measurements > 25 kPa, determined using transient elastography, were associated with a 2.8-fold increase in the risk of reduced GLS; however, this association did not achieve statistical significance in regression analysis (β = 1.02, p = 0.17).
3.4 Prediction of Reduced GLS and Model Building
Independent variables significantly correlated with the reduced GLS were entered into the multiple logistic regression and weighted based on their respective beta coefficients (Table 3). Subsequently, these weighted variables were combined into a composite scoring algorithm, termed the Liver-heart score, designed to differentiate between cirrhotic patients with and without reduced global longitudinal strain.
Various combinations of CAP > 260 dB/m, BMI ≥ 28 kg/m2, age > 57 years, and smoking were selected for multiple logistic regression and assessed using AUC. The optimal model included CAP > 260 dB/m, BMI ≥ 28 kg/m2 and age > 57 years, yielding an AUC of 0.84. On multivariable analysis, smoking was not independently associated with myocardial dysfunction (Beta-Coefficient: −0.47, 95% CI: 0.87–0.59, p = 0.59). Including smoking in the model reduced its predictive performance (AUC of 0.83) and was therefore not included in the final model.
Based on the beta coefficients for the final model, CAP > 260 dB/m and BMI ≥ 28 kg/m2 were each assigned 2 points, and age > 57 years 1 point, to derive the Liver-heart score, which can reach a maximum value of 5 points.
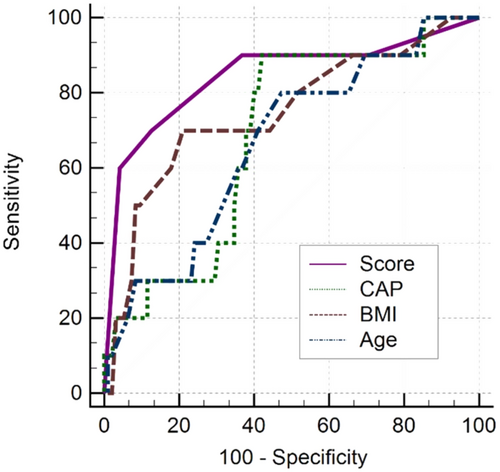
By applying a cut-off value of > 2 points, we observed a sensitivity of 92.3% (95% CI: 64%–99.8%) and a specificity of 66.7% (95% CI: 57.5%–75%). Among the 81 patients scoring ≤ 2 points, only one (1.2%) was incorrectly classified, suggesting a high negative predictive value (98.8%, 95% CI: 93.3%–100%) for ruling out myocardial dysfunction in cirrhotic patients using the proposed scoring system (Table 4, Figure 3).
| Variable | Cutoff | Group | AUC (95% CI) | Se (%) | Spe (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| CAP | > 262 | Training | 0.68 (0.58–0.77) | 90.0 | 57.9 | 18.4 | 98.2 |
| Validation | 0.67 (0.46–0.84) | 80.0 | 50.0 | 28.6 | 90.9 | ||
| BMI | > 27.6 | Training | 0.78 (0.70–0.85) | 76.9 | 79.2 | 28.6 | 96.9 |
| Validation | 0.68 (0.51–0.82) | 50.0 | 78.6 | 40.0 | 84.6 | ||
| Age | > 57 | Training | 0.65 (0.56–0.73) | 84.6 | 51.7 | 15.9 | 96.9 |
| Validation | 0.64 (0.47–0.79) | 62.5 | 58.1 | 27.8 | 85.7 | ||
| Scorea | > 2 | Training | 0.84 (0.77–0.90) | 92.3 | 66.7 | 23.1 | 98.8 |
| Validation | 0.83 (0.65–0.94) | 80.0 | 75.0 | 57.1 | 90.0 |
- Abbreviations: AUC, Area Under the Curve; BMI, Body Mass Index; CAP, Controlled Attenuation Parameter; NPV, Negative Predictive Value; PPV, Positive Predictive Value; Se, Sensitivity; Spe, Specificity.
- a Liver-heart score: CAP > 260 dB/m (2 pts) + BMI ≥ 28 kg/m2 (2 pts) + Age > 57 years (1 pt).
For a high cut-off of > 3 points, a sensitivity of 53.9% (95% CI: 25.1%–80.8%), a specificity of 90% (95% CI: 83.2%–94.7%), and a negative predictive value of 94.7% (95% CI: 88.9%–98%) were observed.
3.5 Validation of Results
The prediction score was validated using an independent group of 41 prospectively recruited cirrhotic patients, adhering to the same inclusion criteria and diagnostic algorithms as the training group. The AUC remained high in this cohort at 0.83 (95% CI: 0.65–0.94, p < 0.001). For a cut-off of > 2 points, the analysis revealed a sensitivity of 80% (95% CI: 44.4%–97.5%), a specificity of 75% (95% CI: 53.3%–90.2%), and a negative predictive value of 90.0% (95% CI: 67.5%–98.9%). Two out of 27 patients with a score ≤ 2 points were incorrectly classified. Using a cut-off of > 3 points, the results showed a sensitivity of 50% (95% CI: 18.7%–81.3%), a specificity of 91.7% (95% CI: 73%–99%), and a negative predictive value of 81.5% (95% CI: 61.5%–93.9%) (Table 4).
3.6 Sensitivity Analysis
As a sensitivity analysis, the multiple logistic regression model's performance with the selected variables was further assessed using 5-fold cross validation on the combined training and validation cohorts, with an average AUC of 0.86 (95% CI: 0.80–0.93), demonstrating the consistency of the model's performance across various folds.
3.7 Survival Analysis
During a median follow-up of 40 months (range: 0.5–65), all-cause mortality was 34.5% (46/133). Four patients who developed malignancy (two hepatocellular carcinomas, one lung cancer, and one colon cancer) and subsequently died from tumour progression were excluded from the survival analysis. Patients diagnosed with CCM were associated with higher mortality (55% vs. 28.4%) compared to those with cirrhosis but negative echocardiographic findings (HR 2.5, 95% CI: 1.2–4.9, p = 0.01). Another strong predictor of higher mortality in the Cox regression analysis was the Child-Pugh classification (HR: 2.8, 95% CI: 1.5–5.3, p < 0.01). In the multivariable model, both variables remained significant, suggesting an independent association of CCM with mortality.
The mortality rate was also higher among patients with cirrhosis and reduced absolute GLS values (53.8% vs. 30.2%, HR: 2.2, 95% CI: 1–4.7, p = 0.05), although only seven events were reported in this subgroup (Figure 4).

Additionally, a Liver-heart score of 5 points was associated with increased mortality, observed at both 2-year follow-up (44.4% vs. 17.3%) and the end of the follow-up period (66.7% vs. 37.7%, HR: 1.3, 95% CI: 1.1–1.5, p < 0.01).
4 Discussion
Delayed recognition of myocardial dysfunction in cirrhotic patients carries unfavourable prognostic implications; therefore, improving diagnostic approaches to identify those at risk for cardiovascular (CV) events early in the disease course is desirable and should be encouraged [18].
Cirrhotic cardiomyopathy presents with a diverse range of structural and functional cardiac abnormalities, including electrophysiological changes in asymptomatic individuals, variations in cardiac output, and clinical manifest heart failure. The diagnostic workup of this condition requires a methodical exclusion of other prevalent cardiac pathologies, employing criteria similar to those applied in our study. CCM has previously been associated with hepatorenal syndrome, major CV events, and increased mortality, particularly during the post-liver transplant period [19]. A recent study utilising revised consensus criteria for CCM reported a hazard ratio of 2.6 (95% CI: 1.2 to 5.5) for CV events following liver transplantation [20].
The reported prevalence of CCM according to these revisited criteria is estimated at approximately 20%, and may increase up to 47% when metabolic factors, fatty liver disease, and alcohol abuse coexist [20]. However, the existing data have been inconsistent across studies, partly due to a critical error in the initial publication of the updated diagnostic criteria. The original publication suggested both an absolute GLS < 18% and > 22% as markers of systolic dysfunction, with the upper threshold later removed in an erratum [21]. This error contributed to several studies reporting an inaccurately high prevalence of CCM [4 22]. For instance, Cesari et al. reported an overall CCM prevalence of 29%, with systolic dysfunction defined by either reduced or elevated GLS-accounting for 25%, regardless of cirrhosis aetiology or the presence of ascites. Consistent with our findings, advanced diastolic dysfunction was identified in 10% of cases, although no information was provided on “indeterminate” diastolic function [22]. In contrast, Izzy and colleagues, applying the same criteria, identified reduced GLS in only two patients (1.7%) but reported a much higher prevalence of diastolic dysfunction (33.3%) compared to other authors, suggesting significant methodological differences between these studies [20]. In another study, only 4.5% of patients met more than two diagnostic criteria for diastolic dysfunction, while 11.1% were classified as having indeterminate diastolic function. After further evaluation, 6 of the 10 patients in this indeterminate group were reclassified as having diastolic dysfunction, resulting in a prevalence of 11.1%, closely reflecting our results [23]. The lack of clear recommendations for managing these cases, which often rely on less reliable additional parameters, represents a limitation in the accurate diagnosis of CCM.
GLS has been proposed as the main parameter for routine clinical assessment of systolic function in cirrhotic patients and should be utilised whenever possible. However, the additional strain measurements can be time-consuming and the required software is not universally available, particularly in resource-limited settings. Furthermore, collaboration between haepatology and cardiology departments is not always well-established.
Values below 20.5% were linked to a six-fold increase in the incidence of heart failure following liver transplantation [20]. In another cohort study, GLS at rest was identified as an independent predictor of mortality among liver transplant candidates over a three-year follow-up period [24].
This study demonstrates a significant correlation between hepatic steatosis and subclinical myocardial dysfunction in patients with liver cirrhosis, irrespective of the disease's aetiology. For the first time, we identified a linear relationship between the degree of hepatic steatosis, quantified by the controlled attenuation parameter, and myocardial contractility as measured by global longitudinal strain. Our analysis further confirms that this correlation persists as statistically significant even after adjustment for shared cardiovascular risk factors, such as elevated BMI. These findings underscore the profound and independent impact of hepatic steatosis on myocardial contractility in cirrhotic patients. Targeting coexisting hepatic steatosis in liver cirrhosis with the objective of enhancing myocardial contractility represents a potentially paradigm-shifting strategy in the clinical management of these patients. Such a strategy would integrate therapeutic interventions that address the multifaceted nature of cirrhosis, potentially redefining treatment protocols and improving patient outcomes.
Our findings are supported by previous studies, which have demonstrated that the degree of hepatic steatosis in patients with both alcoholic and non-alcoholic fatty liver disease is independently associated with impaired cardiac function, even after adjusting for confounding factors such as type 2 diabetes, age, gender, and BMI [25]. Although we found no correlation between liver stiffness values and either reduced GLS or overall mortality in our cirrhotic cohort, a prospective study on critically ill patients demonstrated that liver stiffness exceeding 18 kPa at ICU admission was associated with increased ICU and long-term mortality, even in non-cirrhotic patients [26].
Obesity has been identified as a significant risk factor for reduced GLS in cirrhotic patients, corroborating previous findings in liver transplant candidates [24] and supported by population-based studies [27]. Our analysis revealed that patients with a BMI of 28 kg/m2 or higher exhibited significantly lower absolute GLS values compared to those with a BMI below this cutoff (−20.4% vs. −21.9%, p < 0.01).
Utilising the positive correlations observed among parameters routinely assessed during hepatological examinations, a novel scoring system, termed the Liver-heart score, was developed to differentiate cirrhotic patients at risk for reduced global longitudinal strain from those with a low probability of myocardial dysfunction. This predictive model demonstrated excellent diagnostic accuracy, with a sensitivity of 92.3% and a negative predictive value of 98.5%. Cirrhotic patients with a score of 2 or less seem to be at low risk for myocardial dysfunction, with only 1.2% of such cases misclassified. Those exhibiting a CAP below 260 dB/m are categorised as low risk for cardiac dysfunction, regardless of BMI or age.
Furthermore, cirrhotic patients with a Liver-heart score > 3 points were found to be nine times more likely to exhibit reduced GLS compared to those with lower scores. 60% of patients meeting all three criteria demonstrated reduced GLS, indicating that this subgroup might particularly benefit from comprehensive cardiological assessment. However, practitioners must keep in mind that the Liver-heart score cannot replace a conventional echocardiographic examination, as it is still essential to evaluate diastolic function, particularly in cirrhotic patients with pre-existing cardiac conditions or hypertensive disease.
The score is intended to complement existing diagnostic tools in clinical practice. It can be easily calculated using readily available parameters during routine assessments of cirrhotic patients, and is useful particularly in those with no prior cardiac history and negative findings on conventional echocardiography. Furthermore, it could serve as a decision support tool to optimise the utilisation of often limited resources.
Patients exhibiting reduced GLS underwent further evaluation for coronary artery disease using either a coronary calcium scan to calculate the Agatston score or coronary angiography, based on their clinical presentation. CAD was confirmed or suspected in four individuals, while three were definitively cleared of the condition. Additionally, three patients died before a cardiological assessment could be conducted within the study period, two declined the examinations, and one developed atrial fibrillation during follow-up. Overall, a clinically significant outcome was observed in 72.7% of evaluated cirrhotic patients with reduced GLS, underscoring the critical importance of early detection of impaired GLS and highlighting the potential impact of the score.
A prospective study by Wang et al. assessed long-term clinical outcomes in patients screened for fatty liver disease who subsequently underwent coronary angiography. They reported that patients with MASLD exhibited a significantly higher risk of developing coronary stenosis (84.6% vs. 64.1%; p < 0.001) and an increased need for percutaneous coronary intervention. In this cohort, older age and diabetes emerged as independent predictors of cardiovascular events [28]. Additionally, a recent animal study highlighted that advanced liver fibrosis in mice induces oxidative stress, vascular inflammation, and dysfunction, potentially contributing to the development of coronary artery disease [29].
In addition to pro-inflammatory and pro-coagulant mediators, metabolic factors critically influence the pathogenetic mechanism underlying the interaction between liver and heart function. A comprehensive study demonstrated that the degree of hepatic steatosis, along with impaired fatty acid and glucose metabolism and insulin resistance in the myocardium, was associated with cardiac dysfunction [30]. Moreover, impairment of GLS in cirrhotic patients could also be mediated by factors such as epicardial adipose tissue, which has been previously linked to liver fibrosis and steatosis, cardiac dysfunction, and the development of CAD [31].
Strain imaging has been shown to identify LV dysfunction earlier than conventional echocardiography, thereby offering a new perspective on the diagnostics of CCM and the potential for early therapeutic interventions before irreversible myocardial dysfunction occurs [32]. A peak GLS in the range of −20% is considered normal in a healthy person, with lower absolute values indicating a higher probability of myocardial dysfunction. Generally, a lower limit of normal in the range of −18% is accepted and has recently been adopted in the hepatological criteria [11 14]. However, the application of these standard values to cirrhotic patients is limited by systemic vasodilation and decreased afterload, leading to a hyperdynamic circulation that complicates the interpretation of GLS values. Consequently, higher absolute cutoff values have been considered when assessing LV contractility in cirrhotic patients compared to the general population [20]. Our results show significantly lower absolute GLS values in the validation group compared to the training group (20.2% vs. 21.6%, p = 0.01), reflecting the unbalanced distribution of covariates such as aetiology, Child-Pugh classification, and haemodynamic factors including portal hypertension, cardiac index, and systemic vascular resistance index between the groups. The fact that our model performed well on the validation cohort, despite these differences, underscores its robustness and its ability to reflect real-world clinical scenarios with heterogeneous patient characteristics. Future studies should address the impact of hypercirculatory changes on GLS values and the prevalence of CCM, particularly in the context of covariates such as age, BMI and CAP.
Our well-characterised prospective study has some limitations. The strict exclusion criteria are consistent with the current definition of CCM, which is diagnosed by ruling out other possible etiologies [12]. However, this approach may introduce selection bias. Hypertension-mediated cardiac dysfunction, a common cause of impaired GLS in the general population, was excluded along with hypertension-related heart disease, which is prevalent in unselected cohorts. This was intended to facilitate a more accurate examination of the liver-heart axis. The meta-analysis conducted by Tadic et al. demonstrated that GLS is more sensitive than conventional ejection fraction measurements in detecting hypertension-induced cardiac damage [33].
A prospective validation cohort better reflects real-world scenarios by accounting for possible temporal changes in patient populations, diagnostic practices, and referral pathways. The aim of our study was to develop a predictive model that can be prospectively applied in clinical practice, making the use of a prospective validation cohort both more generalisable and clinically relevant compared to randomised validation. Furthermore, retrospective 5-fold cross-validation of the regression model with the selected variables in the combined cohort demonstrated consistently high predictive performance.
Nevertheless, external validation in more heterogeneous populations, including diverse ethnicities and utilising various healthcare systems and software versions, is essential to ensure the score's applicability and reliability.
In summary, we identified simple-to-measure clinical predictors for cirrhotic cardiomyopathy as determined by reduced GLS and developed a predictive model termed the Liver-heart score. This model was subsequently validated in an independent cohort, demonstrating excellent performance in differentiating patients with reduced GLS from those with a low probability of myocardial dysfunction. The relevance of the incorporated parameters for cardiac dysfunction is supported by previous well-conducted studies. The Liver-heart score may enhance the utility of conventional echocardiography by enabling more precise cardiovascular risk stratification in cirrhotic patients. It may also facilitate timely referral for advanced diagnostics or intervention in contexts where specialised testing is inaccessible.
Author Contributions
Conceptualization: M.R., S.B., M.P-R.; Methodology: M.R., S.B., M.P-R., H.A., F.T., C.E.; Investigation: M.R., S.B., P.W., P.H., M.H., M.F.; Data Curation: M.R., S.B., P.W.; Formal Analysis: S.B., M.R., N.W.; Writing original draft: M.R., S.B., M.P.-R.; Critical revision of the manuscript: F.T., M.D., C.E., A.W., R.M., N.W.; Project administration: M.R.; Supervision: M.P-R., F.T.
Acknowledgements
Assistance with the study: none. Presentation: the preliminary data of the manuscript were presented during the United European Gastroenterology Week 2022 in Vienna.
Ethics Statement
This study adhered to the principles of the 2013 Declaration of Helsinki and was approved by the local ethics committee (Ethikkommission Kärnten No. A21/19).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




