Microvesicle Tissue Factor Procoagulant Activity Is Elevated and Correlated With Disease Severity in Patients With Cirrhosis
Handling Editor: Dr. Alejandro Forner
Funding: The authors received no specific funding for this work.
ABSTRACT
Background and Aims
Tissue factor-expressing microvesicles (MV-TF) have been found to correlate with thrombotic complications in various diseases. Simultaneously, there is expanding research regarding the effect of the coagulation cascade on liver fibrosis progression. The aim of our manuscript was to evaluate MV-TF activity in patients with cirrhosis and its correlation with disease severity.
Methods
We prospectively enrolled 82 patients [11 with cirrhosis and hepatocellular cancer (Group 1), 50 with cirrhosis (Group 2) and 21 controls (Group 3)]. Extensive workup for disease staging and exclusion criteria was undertaken. Exclusion criteria included thrombophilia, history of thrombosis, recent hospitalisation, ongoing infection, alcohol dependence, cancer, haematological diseases and use of anticoagulant, antiplatelet or contraceptive drugs. Plasma tissue factor antigen concentration and MV-TF activity were assessed.
Results
MV-TF showed median values of 4.03 [1.57], 3.17 [1.59] and 2.26 [1.23] pg/mL in Groups 1, 2 and 3, respectively. There was a statistically significant difference between Groups 1 and 3 (p < 0.001) and Groups 2 and 3 (p = 0.003), while Group 1 had higher values than Group 2 without statistical significance (p = 0.088). In Group 2, the patients' Child-Pugh (CP) stage was A in 56%, B in 26% and C in 18% of cases. MV-TF activity significantly correlated with decompensated cirrhosis (p = 0.005) and higher CP stage (p = 0.011). Finally, MV-TF activity significantly correlated with 12-month mortality (p = 0.021).
Conclusions
MV-TF activity is elevated in patients with cirrhosis, showing a significant correlation with disease severity. MV-TF may play a role in the procoagulant imbalance of liver cirrhosis and their contribution in disease progression should be studied further.
Abbreviations
-
- CP
-
- Child-Pugh
-
- DeCi
-
- decompensated cirrhosis
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- HCC
-
- hepatocellular cancer
-
- MVs
-
- microvesicles
-
- MV-TF
-
- tissue factor-expressing microvesicles
-
- PARs
-
- protease-activated receptors
-
- PVT
-
- portal vein thrombosis
-
- TF
-
- tissue factor
-
- TFag
-
- tissue factor antigen
Summary
- This study shows that the procoagulant activity of tissue factor-expressing microvesicles, implicated in the pathophysiology of thrombosis in multiple diseases, is elevated and correlated with disease severity in stable patients with cirrhosis.
1 Introduction
For many years, liver cirrhosis was thought to be a disease purely connected with bleeding complications, with patients with cirrhosis being characterised as ‘auto-anticoagulated’ due to perceived alterations of the coagulation cascade that favour bleeding [1]. However, over the last years, there has been a drastic shift towards a ‘rebalanced’ hemostatic profile, where coagulant and anticoagulant factors are both severely depleted, and therefore, this sensitive balance can be easily unsettled towards both thrombosis and bleeding [2, 3]. Moreover, there is emerging data that anticoagulation may even confer a survival benefit in patients with cirrhosis [4, 5].
Tissue factor (TF) is one of the most significant mediators of the coagulation cascade. Factor VII binds to TF and signals the start of the coagulation pathway by activating factors IX and X and eventual clot formation [6]. Initially, there was the conception that TF cannot be present in the blood without inducing the coagulation cascade due to its high procoagulant potential and is only present in the subendothelium, shifting to the intravascular space in the event of vessel wall disruption. However, there have been many studies in a variety of diseases that have established that TF is expressed by a variety of circulating cells and, even more importantly, by microvesicles (MVs) [7, 8], small fragments of the membrane of various cell types which maintain a significant role in cell signalling and thrombus formation [9-12].
Microvesicle TF (MV-TF) procoagulant activity has been studied in multiple diseases, especially in patients with cancer. In both cancer patients and patients with systematic or infectious diseases, numerous studies have shown a significant correlation between high MV-TF levels and the subsequent occurrence of thrombotic complications and mortality [13, 14]. There have also been studies in patients with cirrhosis showcasing increased MV-TF levels, albeit in a variety of clinical states (infection, acute kidney injury, hepatocellular cancer) [15, 16].
Due to the fact that high MV-TF levels are seen in a variety of disorders, it has been challenging to identify MV-TF activity that is directly related to the pathophysiology of cirrhosis and not to comorbidities or acute complications. Such activity may reflect the activation of the coagulation cascade in liver cirrhosis and its potential role in the progression of liver disease [17-19], as highlighted by growing evidence of the use of anticoagulation as potential therapeutic tools [20-23].
The aim of our study is to determine MV-TF activity levels in patients with stable cirrhosis and contemplate their relevance in terms of disease severity, disease progression, thrombotic complications and mortality.
2 Methods
2.1 Study Design
This was a comprehensive two-part study designed to evaluate plasma TF antigen (TFag) and MV-TF activity in patients with cirrhosis. There was an initial case–control part where patients with cirrhosis +/− hepatocellular cancer (HCC) were compared to controls with regards to TFag and MV-TF activity concentrations, with the additional investigation of whether TFag and MV-TF concentrations were correlated with the severity of cirrhosis in patients without HCC. Finally, there was a prospective cohort part, where TFag and MV-TF concentrations were investigated as potential predictors of 1-year survival in patients with cirrhosis and without HCC. Regarding the study endpoints, the primary endpoint was defined as the comparison of MV-TF activities of patients with cirrhosis and healthy controls. In contrast, secondary endpoints included comparisons of TFag concentrations between patients with cirrhosis and healthy controls, MV-TF and TFag concentrations in relation to severity of cirrhosis and correlation of MV-TF activity with survival, portal vein thrombosis and decompensation at 6 and 12 months.
2.2 Study Sample Calculation
For the calculation of the study sample, we relied on a recent study which provides data on the differences between patients with cirrhosis and healthy individuals in terms of the plasma activity of tissue factor-expressing microvesicles [15]. The MV-TF values of cirrhotic and healthy patients were used, with the desired statistical difference (effect size) calculated as 0.87. The power of the study was set at 90%, and the significance level was set at 5%. Regarding group allocation, due to further analysis of the patients with cirrhosis, it was considered desirable to have a 3/1 (cirrhosis/healthy) group allocation. Based on the above and the use of the G*Power software (Copyright: Universität Düsseldorf) [24], the desired sample size was calculated to be 82 subjects (61 with cirrhosis, 21 healthy).
2.3 Study Population
Consecutive patients with cirrhosis and controls that fulfilled the inclusion and exclusion criteria were enrolled. The inclusion criterion for patients with cirrhosis was the histologic or radiologic diagnosis of cirrhosis, aided by laboratory parameters. Patients with decompensated cirrhosis were defined by the history of an event of decompensation, specifically variceal bleeding, ascites or hepatic encephalopathy. Patients with HCC and cirrhosis were also included in an effort to determine whether increased MV-TF activity is mainly attributed to the presence of cancer or severe liver disease. Controls were either hepatitis B patients under treatment or healthy individuals, both with laboratory and radiologic evidence of absence of advanced fibrosis (shear-wave elastography with liver stiffness measurement < 9 kPa [25]). Extended exclusion criteria were used in order to exclude other diseases that were likely to influence TF concentrations and ensure that there were no acute complications of cirrhosis. Exclusion criteria included (i) Active cancer or history of cancer (other than HCC for patients with cirrhosis), (ii) diagnosis of haematological disease, (iii) diagnosis of thrombophilia disorder, (iv) history of portal vein thrombosis (PVT) or other episode of thrombosis, (v) hospitalisation within the previous month, (vi) use of anticoagulant or antiplatelet drugs, (vii) use of contraceptives, (viii) active bacterial infection, (ix) alcohol abuse in the last 3 months and (x) chronic kidney disease (defined as creatinine > 1.5 mg/dL). All subjects evaluated underwent baseline extensive laboratory and radiological examinations and medical history interviews to ensure suitability. Laboratory examinations included prothrombotic mutations (factor V Leiden, G20210A), lupus anticoagulant, anticardiolipin and β2glycoprotein-1 antibodies. Subjects who tested positive for either of these parameters were excluded. Additionally, patients diagnosed with PVT at baseline ultrasound were excluded.
2.4 Study Parameters and Follow-Up
All patients with cirrhosis underwent an abdominal ultrasound with screening for PVT and HCC at baseline, along with laboratory workup for disease staging according to Child-Pugh (CP) and MELD scores and coagulation parameters. Patients were followed up every 3 months for at least 1 year. New diagnoses of HCC, PVT occurrence, decompensating events, hospitalisations and deaths were recorded.
2.5 Blood Collection and Sample Preparation
The process of sample preparation was done according to international guidelines [26, 27]. Venous blood was drawn with a 21G needle from overnight fasting patients and was transferred to a sodium citrate tube, with the first sample used for routine coagulation measurements and four tubes collected from each patient for platelet-free plasma preparation. The tubes were kept in a vertical position during transport and were processed within 30 min after venipuncture. To acquire platelet-free plasma, whole blood sodium citrate tubes were centrifuged (2500 g/15 min), and the supernatants were transferred to empty tubes and centrifuged (2500 g/15 min), accordingly. The resulting supernatants were collected (without collecting plasma closer than 10 mm from the pellet) and stored at −80°C for further analysis.
2.6 Tissue Factor Antigen Assay
For the quantitative determination of TF in platelet-free plasma samples collected from the patient and control groups in our study, we used a solid phase-sandwich enzyme immunoassay technique (Human Coagulation Factor III/Tissue Factor Quantikine ELISA, R&D Systems/Bio-Techne). Platelet-free plasma samples of 61 patients and 19 control subjects were analysed. The resulting calibration curve (4-parameter logistic curve fit) allowed us to deduce the TF concentration in plasma samples (pg/mL).
2.7 Microvesicle Tissue Factor Activity Assay
For the determination of the procoagulant activity of MV-TF in plasma samples, we used an enzyme-linked immunosorbent assay (ELISA) method (ZYMUPHEN MP-TF/Ref 521196, Hyphen BioMed/CoaChrom Diagnostica GmbH). Platelet-free plasma samples from 61 patients and 21 control subjects were analysed. In the wells of a microplate, a coated monoclonal antibody, specific for an epitope of the extracellular region of TF, captured the MV-TFs present in the samples, calibrators and controls.
Subsequently, after overnight incubation and wash steps of the plate, adding factors VIIa and X into the reaction mixture resulted in the formation of the TF-VIIa complex and the activation of factor X (FXa). A chromogenic FXa substrate was added, and colour developed proportionately to the amount of MV-TF captured in the initial step. The colour development ended with the addition of a stop solution reagent, and the colour intensity was photometrically measured at a wavelength of 405 nm using a Thermo Scientific Multiskan FC Microplate Photometer (Thermo Scientific Multiskan FC Microplate Photometer, Thermo Fisher Scientific), (SkanIt Software 6.0.2 Research Edition, Thermo Fisher Scientific). From the resulting calibration curve, the MV-TF concentration in plasma samples (pg/mL) was deduced.
2.8 Ethics
The study was conducted in compliance with the Declaration of Helsinki and all individuals provided written informed consent before enrolment. The study was approved by the Ethics Committee of the School of Medicine of the Aristotle University of Thessaloniki (Protocol number: 1.502/19.10.2021).
2.9 Statistical Analysis
Statistical analysis was performed with the SPSS 28 software package (IBM Corp.). The Shapiro–Wilk test was used to test the normality of the investigated variables. Variables with normal distribution were expressed as mean ± standard deviation and variables without normal distribution as median [IQR]. Chi-square tests were performed to compare frequencies between different groups. Student's t-tests and ANOVA were performed for comparisons between normally distributed variables, while Mann–Whitney and Kruskal–Wallis tests were performed for comparisons between not normally distributed variables.
3 Results
A total of 97 patients with cirrhosis were evaluated for study inclusion, with 36 patients being excluded. Eighty-two subjects were enrolled in the study, 11 with cirrhosis and HCC (Group 1), 50 with cirrhosis without HCC at baseline (Group 2) and 21 controls (Group 3). Demographic data are reported in Table 1. Most subjects were male in all groups, with the mean age of each group being 62.8 ± 10.6, 59.3 ± 10.3 and 59.1 ± 14.2 years, respectively. There was no statistical difference between groups in terms of baseline demographics and liver disease severity (between Groups 1 and 2). The chief aetiology of cirrhosis among both groups A and B was alcohol-related cirrhosis, with 22% of subjects in Group 1 and 36.4% in Group 2 having two or more etiological factors. With regard to Group 2, half of the patients had decompensated cirrhosis (DeCi), with the majority of patients being categorised as CP A (56%). Decompensation was due to alcohol-related cirrhosis in the majority of patients (56%). The primary type of decompensating event was ascites (64%), followed by variceal bleeding (28%) and hepatic encephalopathy (8%).
| Group | Cirrhosis-HCC (n = 11) | Cirrhosis (n = 50) | Control (n = 21) | p |
|---|---|---|---|---|
| Gender (male/female) | 9/1 | 29/21 | 12/9 | 0.108 |
| Age (years) | 62.8 ± 10.6 | 59.3 ± 10.3 | 59.1 ± 14.2 | 0.626 |
| BMI (kg/m2) | 27.2 ± 4.2 | 28 ± 5.2 | 24.4 ± 2.7 | 0.075 |
| Aetiology (n, %) |
6 ALC (54.5%) 5 HBV (45.5%) 2 MASH (18.2%) |
28 ALC (56%) 11 HBV (22%) 10 HCV (20%) 8 MASH (16%) |
0.865 | |
| Decompensated cirrhosis (n, %) | 8/11 (72.7%) | 25/50 (50%) | 0.171 | |
| Child-Pugh stage (n, %) |
6 C-P A (54.5%) 4 C-P B (36.4%) 1 C-P C (9.1%) |
28 C-P A (56%) 13 C-P B (26%) 9 C-P C (18%) |
0.675 | |
| MELD | 10.6 ± 2.4 | 11.5 ± 4.5 | 0.38 | |
| BCLC stage (n, %) |
5 BCLC A (45.5%) 4 BCLC B (36.4%) 2 BCLC C (18.2%) |
- Abbreviations: ALC, alcoholic cirrhosis; BCLC, Barcelona liver clinic cancer; BMI, body mass index; C-P, Child-Pugh; HBV, hepatitis B virus; HCC, hepatocellular cancer; HCV, hepatitis C virus; MASH, metabolic associated steatohepatitis; SD, standard deviation.
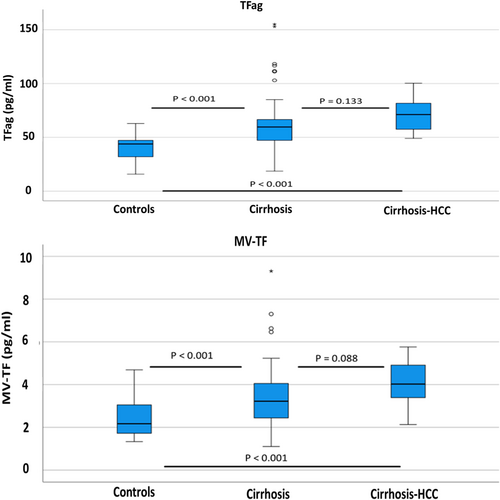
There was a significant difference between groups regarding TFag and MV-TF activity values. TFa median values were 71.2 [25.1] pg/mL, 59.7 [20.5] pg/mL and 44.7 [13.5] pg/mL in Groups 1, 2 and 3, respectively. There was a statistically significant difference between Groups 1 and 3 (p < 0.001) and Groups 2 and 3 (p < 0.001), while Group 1 had higher values than Group 2 without statistical significance (p = 0.133). MV-TF activity showed median values of 4.03 [1.57] pg/mL, 3.17 [1.59] pg/mL and 2.26 [1.23] pg/mL in Groups 1, 2 and 3, respectively. There was a statistically significant difference between Groups 1 and 3 (p < 0.001) and Groups 2 and 3 (p = 0.003), while Group 1 had higher values than Group 2 without statistical significance (p = 0.088). TFag and MV-TF activity values for each group are presented in Figure 1.
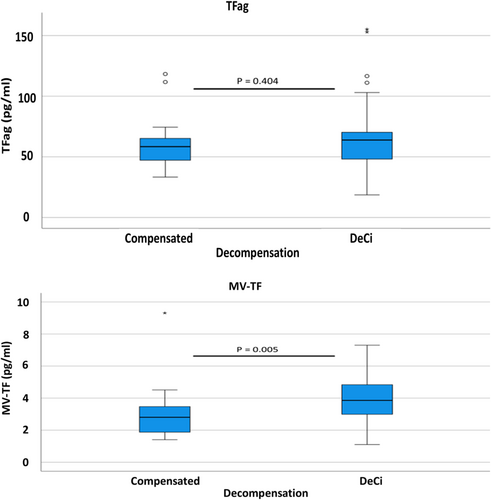
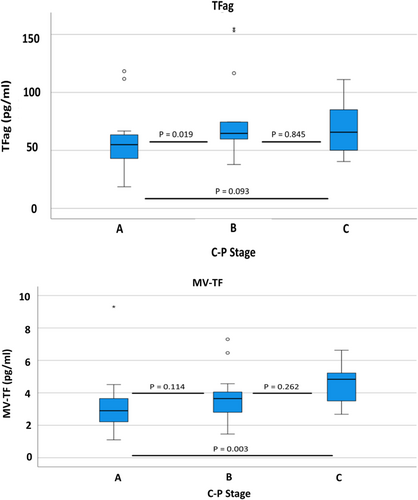
With regard to the severity of liver cirrhosis in Group 2, there was a significant correlation of decompensation with MV-TF activity (p = 0.005) but not TFag (p = 0.404). Patients with DeCi had significantly higher median MV-TF activity values [3.75 (1.76) pg/mL] than compensated patients [2.81 (1.59) pg/mL]. MV-TF activity and TFag values according to decompensation status are depicted in Figure 2. Additionally, there was a significant correlation between the CP stage and both MV-TF activity (p = 0.011) and TFag (p = 0.037). Patients with Child Pugh A, B and C cirrhosis had MV-TF activity values of 2.9 [1.46], 3.65 [1.54] and 4.84 [1.92] pg/mL, respectively. MV-TF activity and TFag values according to the CP stage are depicted in Figure 3.
Furthermore, with respect to patients' mortality in Group 2, there was no statistically significant correlation of either TFag (0.102) or MV-TF activity (0.621) with death at 6 months. However, in the Group 2 patients with surveillance data available at 12 months [n = 46, mortality: 7/46 (15.2%)], higher MV-TF activity significantly correlated with death at 12 months (p = 0.021), whereas TFag was not (p = 0.079). There was no significant difference in terms of PVT (n = 2) and decompensation (n = 3) incidence at 12 months, according to TFag and MV-TF levels. Finally, there was a significant positive correlation of TFag and MV-TF activity values (r = 0.29, p = 0.009).
4 Discussion
Our study has shown that there are significantly elevated levels of both TFag and MV-TF activity in patients with cirrhosis, with or without HCC. Moreover, due to meticulous exclusion criteria, these elevations could be attributed to the pathophysiology of the underlying disease without being influenced by comorbidities or acute complications of cirrhosis. Furthermore, MV-TF levels are significantly correlated with the severity of cirrhosis and mortality of these patients.
There has been significant interest in the components of the coagulation cascade in patients with cirrhosis, with many studies focusing on the procoagulant potential of microparticles. Campello et al. reported that only patients with cirrhosis and concomitant HCC had higher MV-TF levels compared to healthy controls [15]. Conversely, Rautou et al. showed that patients with decompensated cirrhosis (Child-Pugh B-C) have significantly higher MV-TF levels than healthy controls, while compensated patients (Child-Pugh A) did not [16]. In that study, both acutely hospitalised patients and patients with stable disease were included. Other studies have assessed plasma MV-TF concentration with flow cytometry in patients with acute complications of cirrhosis, such as infections and acute kidney injury [28, 29]. These studies have shown an acute elevation of MV-TF concentration during acute kidney injury and infections but failed to show a correlation between these elevations and thrombotic complications.
Limitations of our study include the small sample size, especially in terms of patients with HCC. Patients with HCC were not independently included in sample calculation, which resulted in our study having insufficient power to detect differences between HCC and non-HCC patients. Furthermore, a larger sample size would enable us to better detect differences between different groups and according to cirrhosis severity, as well as provide a higher number (only three patients developed PVT in 1 year of follow-up) of thrombotic complications to test their relationship with high TFag and MV-TF activity levels. However, the sample size is partly a result of the strict exclusion criteria that we placed. These criteria were necessary to avoid random elevations of TFag and MV-TF activity due to other diseases or acute complications of cirrhosis. Another limitation is the fact that there are multiple assays (in-house or commercial) for the measurement of MV-TF activity, with differences in terms of sample preparation, sensitivity, specificity and calculated MV-TF values [30-33]. This makes it difficult to compare results among studies using different methods. However, a comparative study by the International Society on Thrombosis and Hemostasis has shown that functional assays have higher sensitivity and specificity than flow cytometry studies for MV-TF detection [34]. The primary strength of our study is its design, which excluded a significant number of patients with diseases, medical histories, treatments and recent illnesses that had the potential to influence TFag and MV-TF values. Therefore, we can safely claim that TFag and MV-TF levels in our study reflect almost solely the procoagulant dynamics of stable liver cirrhosis.
As expected, since high MV-TF activity has been mainly associated with various neoplastic conditions [35-37], patients with HCC had higher MV-TF activity, though not statistically significant, due to their small sample. Additionally, our study shows that higher MV-TF activity levels are present in DeCi without HCC and contribute to the hypercoagulability of the disease. This elevation may also explain the significantly higher incidence of thrombotic complications (apart from PVT) in CP B or C patients compared to CP A patients [38, 39]. In terms of explaining the origin of TF-expressing microvesicles in patients with cirrhosis, many studies have indicated that the most probable effector is systematic inflammation and endotoxemia that induce TF expression, mainly from monocytes [40-42].
However, the most crucial question that arises from our study is whether the elevated MV-TF activity observed in patients with cirrhosis contributes to the progression of liver fibrosis and portal hypertension. There have been two main mechanisms that connect increased expression of TF to hepatic fibrogenesis. The parenchymal extinction theory maintains that enhanced TF activity promotes thrombin generation and clot formation, leading to micro-infarcts that impede blood flow and induce hepatic congestion, ischemia and hepatocyte loss [19, 43, 44]. On the other hand, thrombin interacts with protease-activated receptors (PARs), leading to increased hepatic stellate cell activation [17, 19, 45]. Both of these mechanisms are tied to TF's enhanced activation of the coagulation cascade and subsequent enhanced thrombin generation in patients with cirrhosis [46-50]. Our study contributes to the mounting evidence that the coagulation cascade plays a significant role in the evolution of hepatic fibrosis and, therefore, that anticoagulation may have a therapeutic role in patients with cirrhosis.
There are many unanswered questions with regard to the impact of MV-TF activity in the progression of cirrhosis and portal hypertension. Future studies should include a larger sample to better evaluate the incidence of thrombotic complications and mortality. Future directions may also include blood sampling from the portal or hepatic veins to more accurately identify the effects of TF in the liver microenvironment or evaluate MV-TF activity before and post transjugular intrahepatic portosystemic shunt insertion due to its effect on portal hypertension and endotoxemia, which may influence MV-TF activity levels [51].
To conclude, our study has shown that MV-TF activity and TFag levels are significantly elevated in patients with cirrhosis and correlated with disease severity. More extensive studies should be conducted to further evaluate its relationship with thrombotic complications and disease progression. Enhanced MV-TF activity is another piece of the puzzle of the correlation of procoagulant mechanisms with liver fibrosis, further promoting the concept of therapeutic coagulation in patients with cirrhosis.
Author Contributions
Adonis A. Protopapas: conceptualisation, methodology, formal analysis, investigation, writing – original draft, writing – review and editing, Visualisation. Anna Takardaki: methodology, formal analysis, validation, investigation, writing – review and editing, visualisation. Nefeli Protopapa: methodology, investigation, resources, writing – review and editing. Ioanna Papagiouvanni: methodology, validation, resources, writing – review and editing. Andreas N. Protopapas: conceptualisation, methodology, validation, resources, writing – review and editing, supervision. Lemonia Skoura: conceptualisation, validation, writing – review and editing, supervision. Christos Savopoulos: conceptualisation, methodology, validation, writing – review and editing, supervision. Ioannis Goulis: conceptualisation, methodology, validation, writing – review and editing, supervision, project administration. All authors approved the final version of the article, including the authorship list.
Acknowledgements
The authors have nothing to report.
Ethics Statement
The study was conducted in compliance with the Declaration of Helsinki and all individuals provided written informed consent before enrollment. The study was approved by the Ethics Committee of the School of Medicine of the Aristotle University of Thessaloniki (Protocol number: 1.502/19.10.2021).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author.




