Effectiveness and Safety of Glecaprevir/Pibrentasvir in Italian Children and Adolescents With Chronic Hepatitis C: A Real-Word, Multicenter Study
Funding: The authors received no specific funding for this work.
Handling Editor: Alessio Aghemo
ABSTRACT
Background & Aims
Glecaprevir/Pibrentasvir (GLE/PIB) has been approved by the European Medicine Agency (EMA) and by the US Food and Drug Administration (US-FDA) for the treatment of children and adolescents from 3 years of age with chronic hepatitis C virus (CHC) infection. The aim of this study was to confirm the real-world effectiveness and safety of GLE/PIB in children and adolescents (3 to < 18 years old) with CHC.
Methods
This prospective, multicentre study involved 11 Italian centres. Children and adolescents (from 3 to < 18 years of age) received a weight-based dose (up to 300/120 mg) of GLE/PIB once daily for 8 weeks. The effectiveness endpoint was sustained virological response 12 weeks after the end of treatment (SVR12). Safety was assessed by adverse events (AE) and clinical/laboratory data.
Results
Sixty-one patients (median age 12 years, interquartile range 5) were enrolled and treated between June 2020 and October 2023. Genotype distribution was as follows: 24/61 genotype 1 (39.4%), 13/61 genotype 2 (21.3%), 18/61 genotype 3 (29.5%) and 6/61 genotype 4 (9.8%). Sixty (98.4%) patients completed treatment and follow-up. SVR12 was obtained by 60/61 patients (98.4%). One patient died because of an oncological illness while on treatment. AE occurred in 13.1% of the patients, were mild and no patients prematurely stopped treatment.
Conclusions
This study confirmed the real-life effectiveness and safety of the 8-week therapy with GLE/PIB for treatment of CHC in children and adolescents.
Summary
- Glecaprevir/Pibrentasvir is a direct-acting antiviral drugs combination with pangenotypic activity.
- It was approved by the European Medicines Agency and US Food and Drug Administration for adolescents aged 12–17 years with chronic hepatitis C virus infection in 2019 and for children aged 3–11 years in 2021.
- The high efficacy and the optimal safety profiles of glecaprevir/pibrentasvir in children and adolescents have been demonstrated by the Phase 2/3 DORA study.
- The present multicenter prospective collaborative study confirmed for the first time the efficacy and safety of the 8-week therapy with GLE/PIB for treatment of chronic hepatitis C in children and adolescents in real life.
Abbreviations
-
- AE
-
- adverse events
-
- ALT
-
- alanine transaminase
-
- AST
-
- aspartate transaminase
-
- CHC
-
- chronic hepatitis C virus
-
- DAA
-
- direct-acting antiviral
-
- EMA
-
- European Medicine Agency
-
- ESPGHAN
-
- European Society for Paediatric Gastroenterology, Hepatology and Nutrition
-
- GLE/PIB
-
- glecaprevir/pibrentasvir
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis c virus
-
- IQR
-
- interquartile range
-
- LSM
-
- liver stiffness measurement
-
- RBV
-
- ribavirin
-
- SVR12
-
- sustained virological response 12 weeks after the end of treatment
-
- US-FDA
-
- US Food and Drug Administration
-
- WHO
-
- World Health Organization
1 Introduction
Hepatitis C virus (HCV) infection is highly prevalent with 58 million people worldwide chronically infected, of which 3.2 million are children and adolescents [1]. Vertical transmission is the main route of HCV acquisition in children worldwide. While 25%–40% of vertically infected children may clear HCV infection spontaneously in the first 4 years of life, 60%–75% of them will develop chronic infection [2-5]. Chronic HCV infection has usually a mild-symptomatic course in childhood; however, the progression of disease and its related complications (such as fibrosis, cirrhosis and hepatocellular carcinoma [HCC]) have been reported [6-11]. Chronic HCV infection impacts the quality of life of children and adolescents [12-14] and its cure has been associated with a rapid improvement in both clinical and psychosocial health features [15-17].
The recently updated World Health Organization (WHO) and European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines recommend that all children and adolescents with HCV infection who are older than 3 years of age should undergo treatment with direct-acting antiviral (DAA) regimens [18-21]. Glecaprevir/Pibrentasvir (GLE/PIB) was the first pangenotypic anti-HCV regimen approved by the European Medicines Agency (EMA) and US Food and Drug Administration (US-FDA) for adolescents in 2019 and for children between 3 and 12 years old in 2021 [22].
The clinical efficacy and safety of GLE/PIB for the treatment of HCV were evaluated in 47 adolescents and 80 children aged 3–17 enrolled in the Phase 2/3 DORA study parts 1 and 2, respectively [23, 24].
The aim of the present prospective multicenter study was to describe the real-world effectiveness and safety of GLE/PIB treatment in a cohort of Italian children and adolescents with chronic HCV infection.
2 Materials And Methods
This ongoing multicenter, real-world, observational study was designed and conducted in 11 Italian paediatric hepatology and infectious diseases referral centers.
Patients aged 3–17 years, who had chronic HCV infection and who were treated with GLE/PIB were prospectively enrolled between June 2020 and October 2023. Study eligibility represented real-world circumstances, including patients without cirrhosis and with compensated cirrhosis, and those who were treatment-naïve or who had been previously treated with pegylated interferon (PEG-IFN)-based regimen with ribavirin (RBV). Exclusion criteria included age of the patients ≥ 18 years and unavailable informed consent.
Fibrosis or cirrhosis were evaluated based on clinical signs and symptoms, and/or the mean liver stiffness measurement (LSM) detected by transient elastography (Fibroscan). Fibrosis was staged as follows: no or mild fibrosis (F0-F1; LSM < 7.2 kPa), moderate fibrosis (F2; LSM 7.2–9.7 kPa), severe fibrosis (F3; LSM 9.8–14.5 kPa) and cirrhosis (F4; LSM > 14.5 kPa) [25].
For the purpose of this study, adolescents were defined as those aged ≥ 12 years and children as those between 3 and 11 years of age.
Adolescents received the fixed-dose combination of daily GLE/PIB (300/120 mg), children received a paediatric formulation of GLE/PIB at a weight-based daily dose [150/60 mg for weight ≥ 12 kg and < 20 kg; 200/80 mg for weight ≥ 20 kg and < 30 kg; 250/100 mg for weight ≥ 30 kg and < 45 kg] for 8 or 12 weeks depending on the presence of cirrhosis and previous treatment history. The paediatric formulation was made of granules to be administered by mixing with a small amount (1–2 teaspoons) of soft food such as Greek yogurt, peanut butter, jam or hazelnut spread. The pharmaceutical company had no role in this study.
All participants underwent physical examination and laboratory testing including blood count cells, transaminases levels (alanine transaminase [ALT]; aspartate transaminase [AST]), kidney function test (creatinine; azotemia), and HCV-RNA quantitation at baseline, Week 2, 4 and at the end-of-treatment. Sustained virologic response was defined as undetectable HCV-RNA at Week 12 after the end of treatment (SVR12). Biochemical tests were determined using commercially available kits. HCV-RNA was measured using a real-time reverse transcription-polymerase chain reaction assay, with a lower limit equal to 12 IU/mL. The quantitative analysis of RNA was performed using an Amplicor HCV Monitor (Roche Diagnostic Systems, Branchburg, NJ), as previously reported [26].
The effectiveness endpoint was the percentage of patients attaining SVR12. Safety and tolerability of GLE/PIB therapy were evaluated by monitoring adverse events (AE), laboratory values, physical examination findings and vital signs.
2.1 Statistical Analysis
Data were managed and analysed using SPSS (V.25TH) software. Continuous variables were expressed as median and interquartile range (IQR). Categorical or non-normally distributed variables were expressed as percentages and frequency. Difference in medians was calculated using the Wilcoxon Signed Rank Test.
2.2 Ethics
The study was conducted in accordance with the Good Clinical Practise Guidelines and the ethical principles of the Declaration of Helsinki. The study protocol was approved by the local Ethics Committee or institutional review board for each trial centre. Informed consent form was obtained for each patient by parents or legal guardians.
3 Results
3.1 Baseline Evaluation of Study Cohort
During the study period, 61 patients were evaluated and enrolled (43 > 11 years of age; 15 > 5 years; 3 3–5 years). Median age at enrollment was 12 years (IQR 5). Thirty-seven were male (60.7%). Genotype distribution was: 24 children infected with genotype 1 (39.4%), 13 with genotype 2 (21.3%), 18 with genotype 3 (29.5%) and 6 with genotype 4 (9.8%). For all patients (61/61) the route of transmission was vertical. No patient was co-infected with human immunodeficiency virus or hepatitis B virus. Sixty patients (98.4%) had no or mild fibrosis (F0-F1) and one 3-year-old patient with HCV genotype 1 infection was diagnosed with fibrosis F3 (LSM 12 kPa) [25]. Fifty-nine patients were treatment-naïve and 2 genotype 3-infected patients (aged 6 and 17 years) were previously treated with PEG-IFN plus RBV.
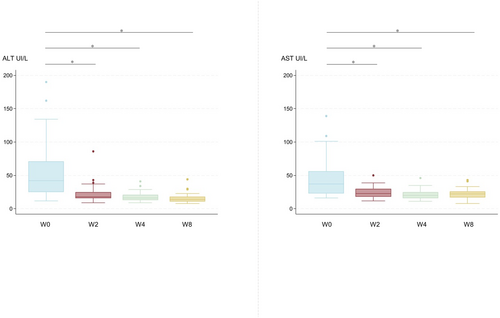
Median serum ALT and AST levels at baseline were 41 UI/L (IQR 43.25) and 37 UI/L (IQR 31) respectively.
Demographic and laboratory characteristics of patients at baseline are shown in Table 1.
| n (%) or median (IQR) | |
|---|---|
| Age, years | 12 (5) |
| Gender | |
| Male | 37 (60.7%) |
| Female | 24 (39.3%) |
| HCV genotype | |
| 1 | 24 (39.4%) |
| 2 | 13 (21.3%) |
| 3 | 18 (29.5%) |
| 4 | 6 (9.8%) |
| ALT (UI/L) | 41 UI/L (IQR 43.25) |
| AST (UIL) | 37 UI/L (IQR 31) |
| Fibrosis score | |
| No or mild fibrosis (F0-F1) | 61 (98.4%) |
| Moderate fibrosis | 0 |
| Severe fibrosis | 1 (1.6%) |
| Cirrhosis | 0 |
| HCV-treatment story | |
| Naïve | 59 (96.7%) |
| PEG-INF + RBV | 2 (3.3%) |
| HBV/HIV co-infection | 0 |
- Abbreviations: ALT = alanine transaminase, AST = aspartate transaminase, HCV = hepatitis C virus, IQR = interquartile range, PEG-INF = pegylated-interferon, RBV = ribavirin.
3.2 Effectiveness
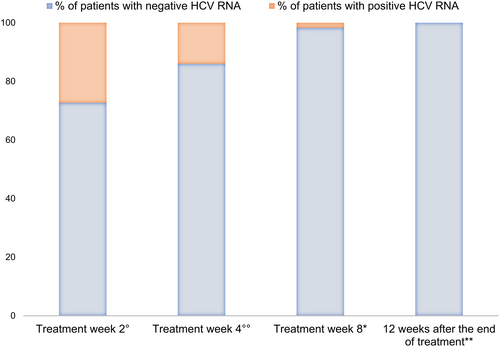
All treatment-naïve patients (59/61) received GLE/PIB therapy for 8 weeks. For the two treatment-experienced patients infected with genotype 3, GLE/PIB was scheduled for 8 weeks rather than 16 weeks as for clinician decision. Treatment was started at a median age of 7.5 years (IQR 3) for children and 13 years (IQR 3) for adolescents. All the patients have completed the therapy. The HCV-RNA was negative in 32/44 tested patients at Week 2 and in 43/50 tested patients at Week 4; 60/61 have achieved SVR12. One patient, affected by metastatic nasopharyngeal carcinoma, died 1 week after completing the 8-weeks-course of therapy due to disease progression.
Figure 1 summarise virological responses at the different treatment weeks. ALT and AST levels decreased during treatment (p < 0.0001; Figure 2).
3.3 Safety and Tolerability
All children and adolescents were able to take GLE/PIB granules (18/61; 29.5%) or tablets (43/61; 70.5%). No children refused to swallow the granule formulation, including in the younger cohort. AE occurred in 13.1% of patients (8/61): headache (4/8; 50%), weakness (3/8; 37.5%), abdominal pain (3/8; 37.5%), diarrhoea (1/8; 12.5%), nausea (2/8; 25%) and/or vomit (2/8; 25%). No AE were serious or required treatment withdrawal (Table 2).
| n (%) | |
|---|---|
| Adverse event | 8 (13.1) |
| Headache | 4 (50) |
| Weakness | 3 (37.5) |
| Abdominal pain | 3 (37.5) |
| Diarrhoea | 1 (12.5) |
| Nausea | 2 (25) |
| Vomit | 2 (25) |
| ALT/AST elevation | 0 (0) |
| Serious adverse event | |
| Deaths* | 1 (1.6) |
| Withdrawal of therapy | 0 |
- Abbreviations: ALT = alanine transaminase, AST = aspartate transaminase.
- *Death due to metastatic nasopharyngeal carcinoma progression, 1 week after completing the 8-weeks-course of therapy.
4 Discussion
The present study shows the high effectiveness and safety of GLE/PIB for treatment of children and adolescents with chronic HCV infection in real life.
Despite the indolent or mildly symptomatic course of the infection, individuals, both adults and children, with early-acquired HCV exhibit a significantly diminished health status compared to the uninfected population [27]. Data indicate that chronic HCV infection in children has a detrimental impact on the quality of life and cognitive function. The infection not only increases caregiver stress and strains the family system but is also associated with a poorer health status. Furthermore, it may lead to impaired psychosocial and cognitive functioning [12-14].
Three studies have delved into the impact of DAA treatment on the quality of life of children and adolescents, revealing that treatment is linked to an improvement in health-related quality of life scores after achieving SVR12 [15-17]. These findings underscore that the influence of chronic HCV infection on quality of life should be considered a compelling indication for initiating treatment in children, alongside the traditional goals such as clearing HCV viremia and preventing potential progression of liver disease.
During the IFN era, treating children as young as 3 years old was a subject of intense debate, and guidelines from WHO, ESPGHAN, and other major international societies uniformly recommended delaying treatment in younger children until DAAs were approved and available [21]. The recently updated WHO and ESPGHAN guidelines now advocate for the use of pangenotypic regimens as the first choice for the treatment in all children and adolescents [18-21, 28, 29]. Given the recommendation for universal treatment of HCV-infected children and adolescents, it becomes imperative to assess the real-world effectiveness and safety of DAA regimens, particularly in low- and middle-income countries where the burden of infection is highest [30].
GLE/PIB is a pangenotypic combination regimen for treatment of chronic HCV infection, currently approved by FDA and EMA for children and adolescents aged 3–17 years. Parts 1 and 2 of the DORA study parts 1 and 2 demonstrated the high efficacy and safety of this regimen in 47 adolescents (median age 14 years, range 12–17) and 80 children (n = 80; median age 7 years, range 3–11). The SVR12 was 100% in adolescents, 96% in children aged 3–5 years, 100% in children aged 6–8 years and 93% in patients aged 9–11 years. None of the two parts of the study reported AE led to drug discontinuation or serious [23, 24]. Only one real-world study from Japan reported the efficacy and safety of GLE/IB in 22 treatment-naïve and 3 treatment-experienced patients with a mean age of 13 years who received the treatment for 8 (24/25) or 12 weeks (1/25 GT 2b, PEG-INF plus RBV treatment-experienced). The SVR12 was 96%. The most common AE were observed in 24% of patients and included pruritus, rash, abdominal pain, nausea, herpes zoster infection, transient elevation of serum creatinine, and transient elevation of serum indirect bilirubin. Serious AE or AE leading to drug discontinuation were not observed [31].
The present study is the first real-world demonstrating the efficacy and safety of the GLE/PIB combination for 8 weeks of therapy in Italian children and adolescents chronically infected with HCV.
The SVR was 98.4% with only one child not achieving SVR12. No virological relapse was observed. These results suggest that therapy with GLE/PIB for 8 weeks in children and adolescents with HCV infection is effective and safe, independently of the treatment-status history and the fibrosis status of the patient. SVR12 was obtained also in the two treatment-experienced patients infected with genotype 3, who received a shortened 8 weeks treatment course of GLE/PIB. Shortened treatment has been demonstrated to effective for children treated with sofosbuvir/ledipasvir although the findings of the present study are insufficient to suggest 8 weeks of treatment with GLE/PIB for treatment-experienced and cirrhotic patients [32].
The paediatric formulation of GLE/PIB had the advantage of being palatable, guaranteeing a high compliance even in the youngest patients. Compliance with treatment and the ability to take the oral medication has been highlighted as crucial before starting treatment in the younger age cohort of children between 3 and 5 years [20]. The lowest efficacy rates reported in paediatric studies on the use of DAA for children was for children younger than 5 years and was secondary to non-adherence and early treatment discontinuation [33]. Difficulty in taking the oral medication by young children was a major limitation to the treatment efficacy and the ability of children to swallow the medications is a requirement for initiating therapy.
The most common AE recorded by the patients' parents included mild or moderate headache, weakness, abdominal pain, diarrhoea, nausea and/or vomiting. These symptoms were observed in ≥ 10% of patients and resolved in a few days after starting therapy. No patients presented serious adverse effects or interrupted GLE/PIB treatment.
In conclusion, the present study confirmed that GLE/PIB administered for 8 weeks in children and adolescents with HCV infection aged 3–17 years is safe, highly effective, and well-tolerated. The use of this combination can be implemented in routine clinical practise.
Author Contributions
Mariangela Stinco and Chiara Rubino contributed to investigation, methodology, data curation, writing-original draft and writing-review and editing; Giuseppe Indolfi, Sandra Trapani and Elisa Bartolini contributed to supervision and writing-review and editing. All authors contributed to methodology and data curation, followed the patients, collected the data and contributed to the final manuscript. All authors had final responsibility for the decision to submit for publication.
Acknowledgements
The authors have nothing to report. Open access publishing facilitated by Universita degli Studi di Firenze, as part of the Wiley - CRUI-CARE agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




